Electrophoretic Deposition and Characterization of Thin-Film Membranes Li7La3Zr2O12
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of the Electrolyte
2.2. Preparation and Characterization of LLZ Suspensions for EPD
2.3. Electrophoretic Deposition and Characterization of LLZ Films
2.4. Characterization of Electrochemical Properties of Annealed LLZ Coatings—Impedance Spectroscopy
3. Results and Discussions
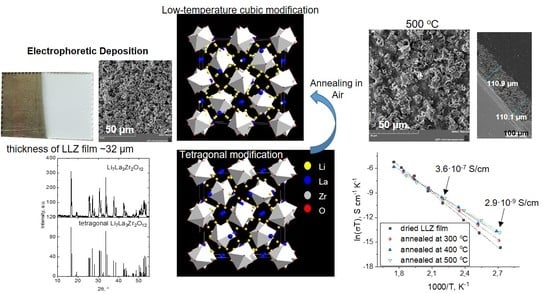
3.1. Characterization of the Initial Powder of Electrolyte and Suspensions Based on It
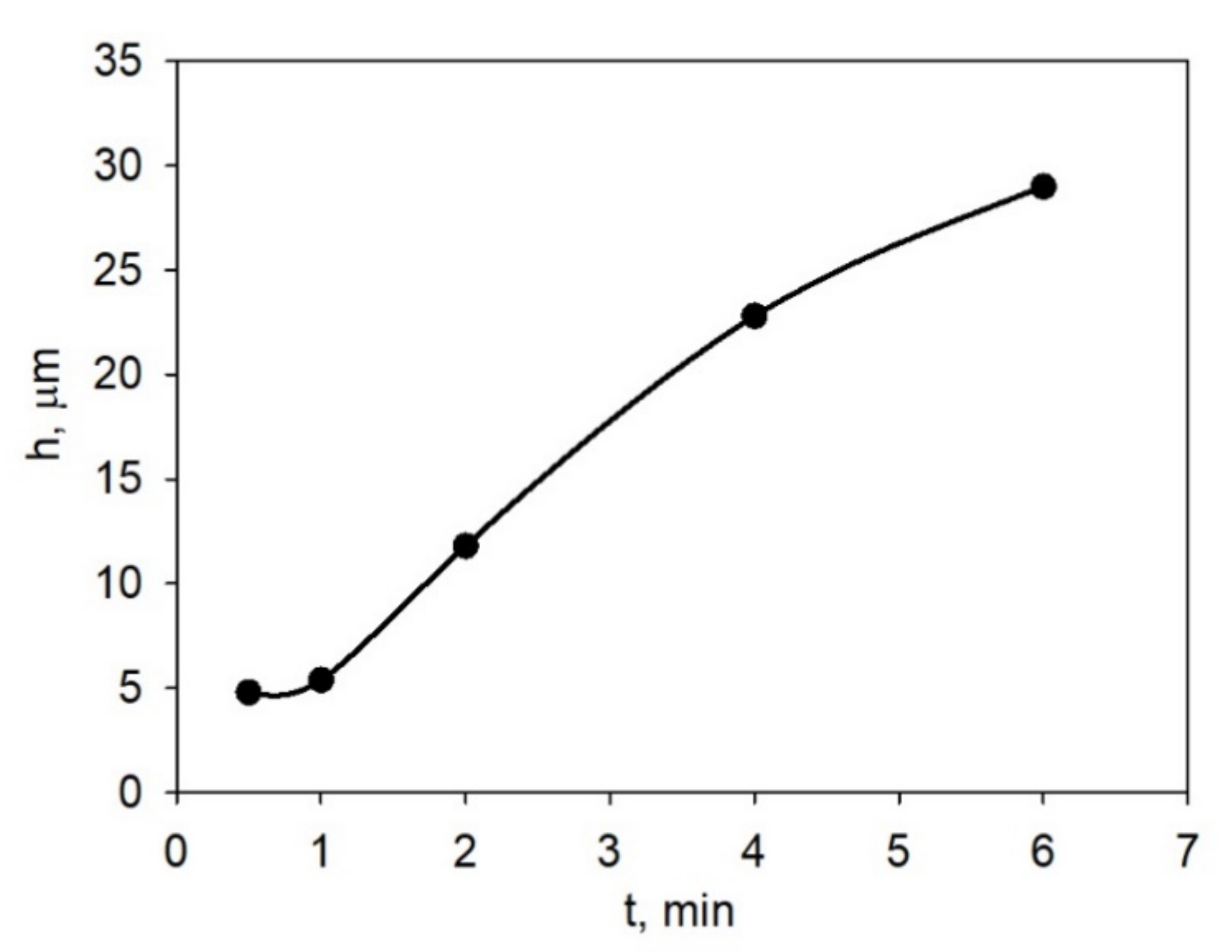
3.2. Electrophoretic Deposition from LLZ Suspension on Ti Substrate
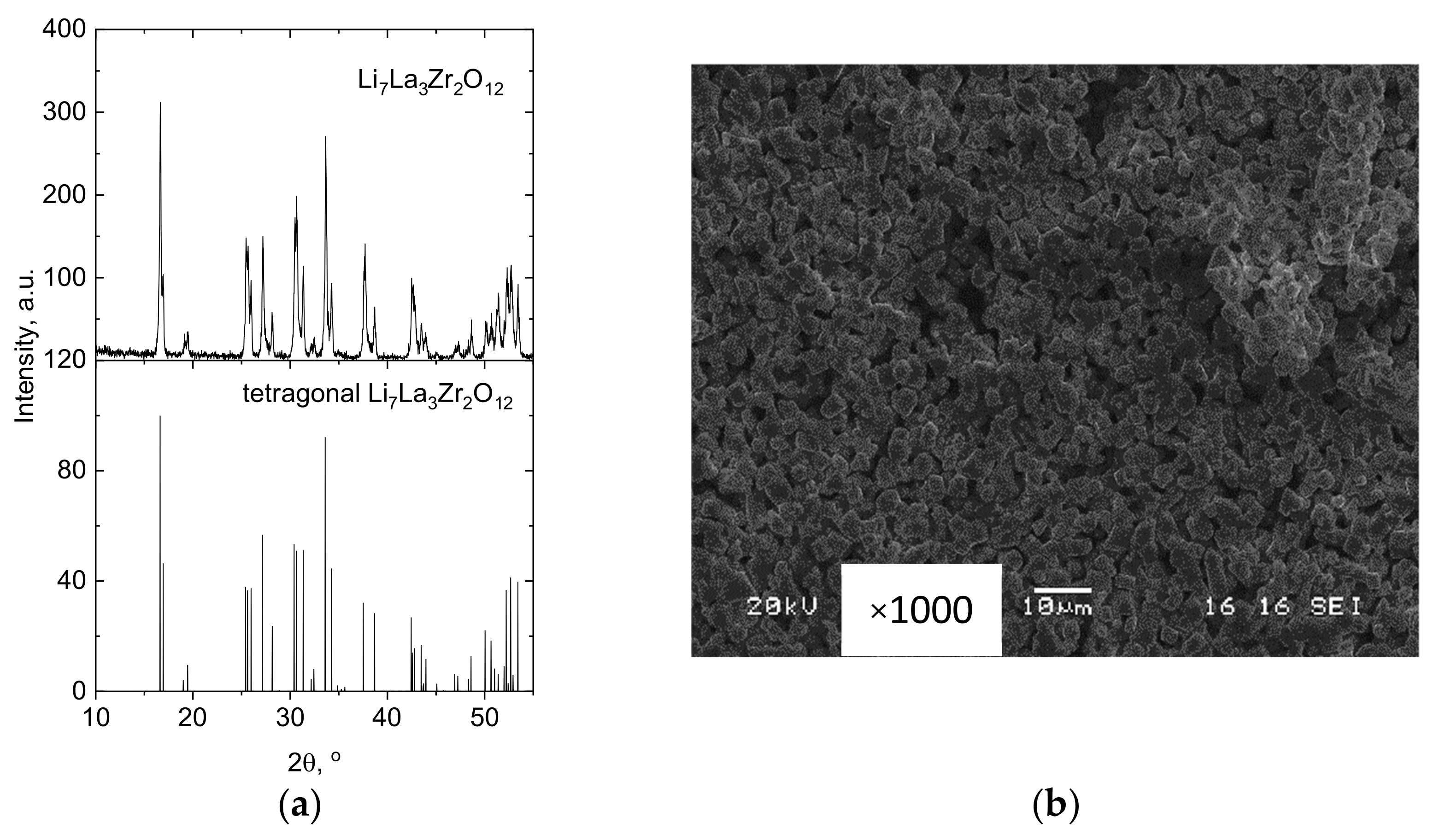
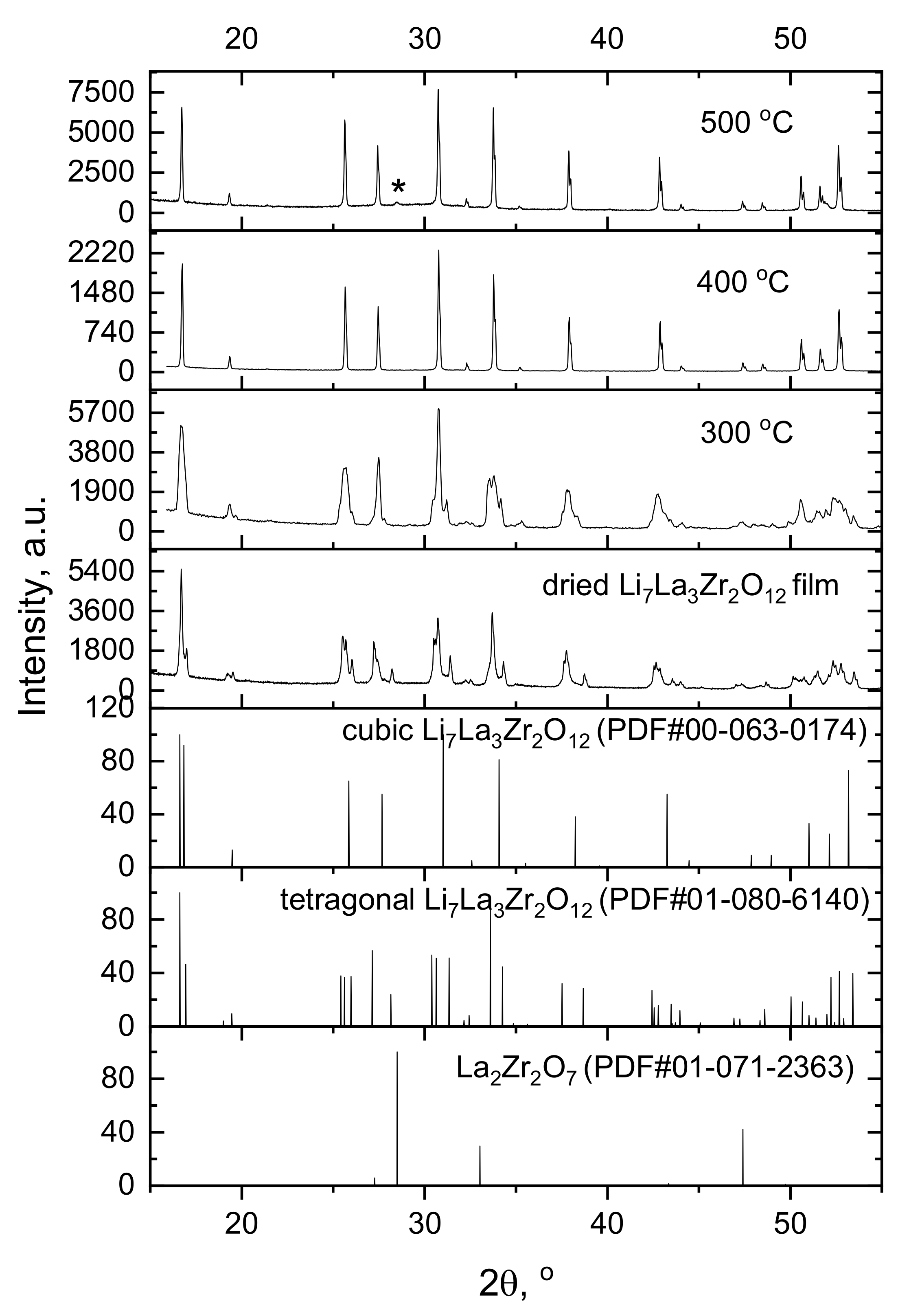
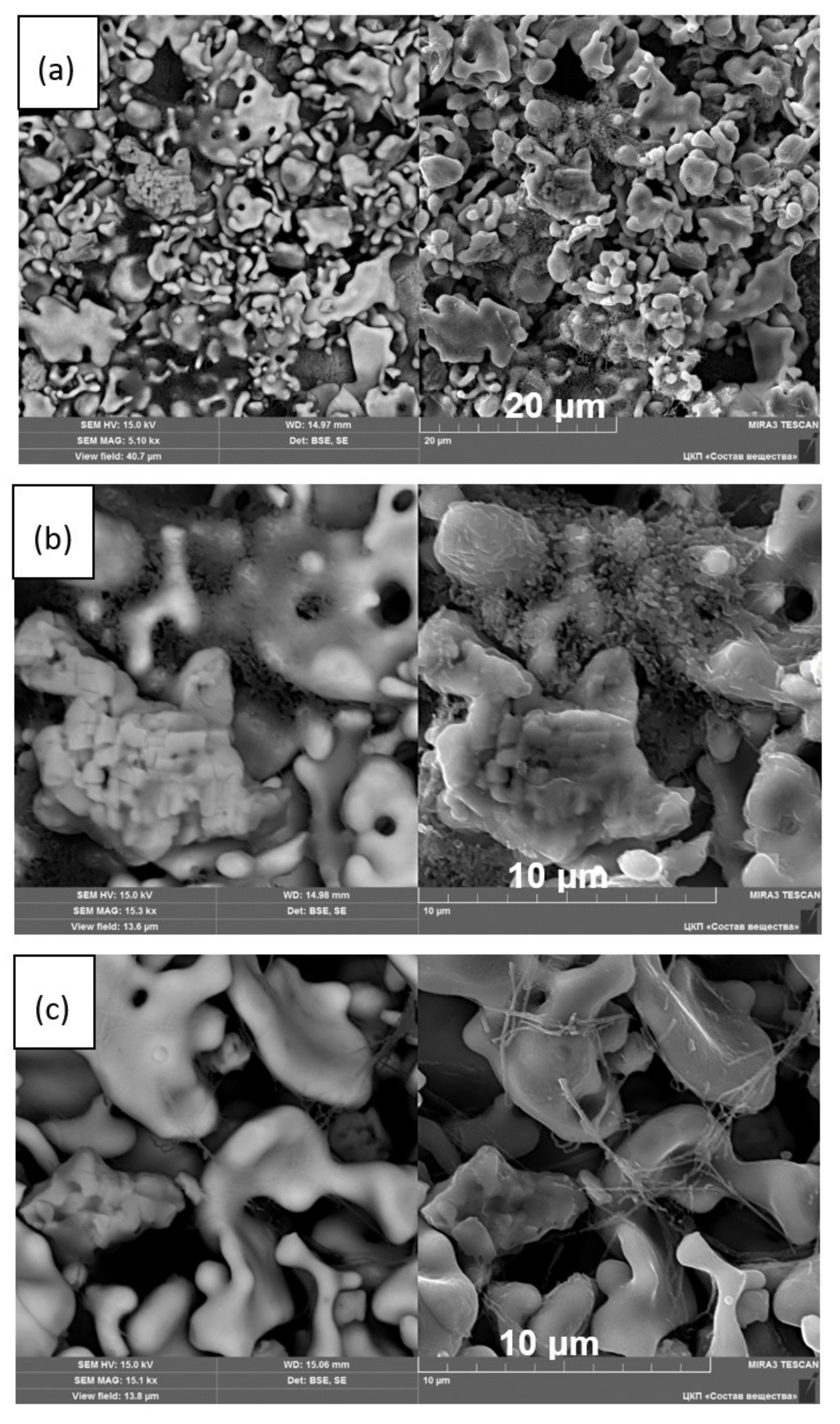
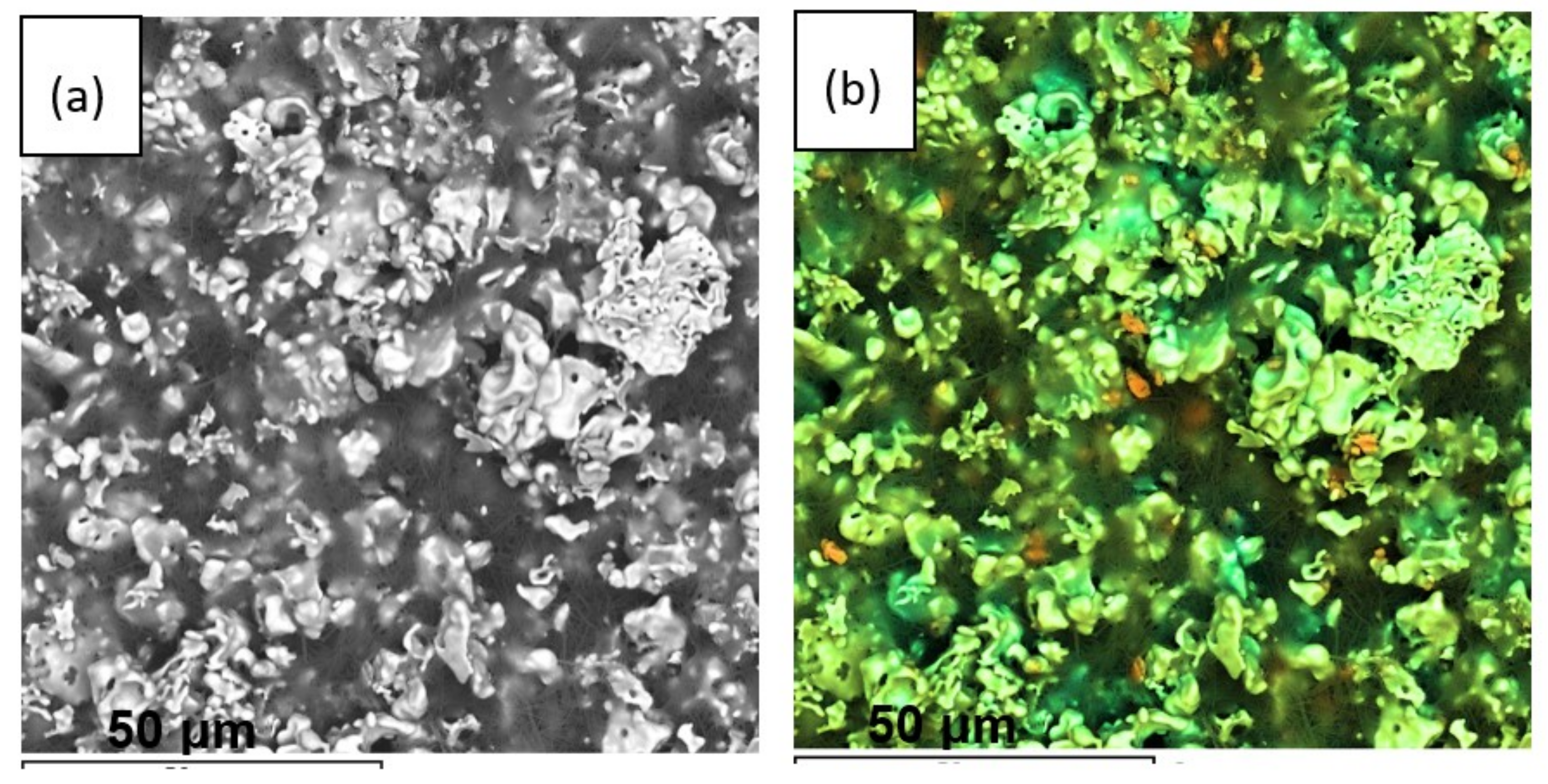
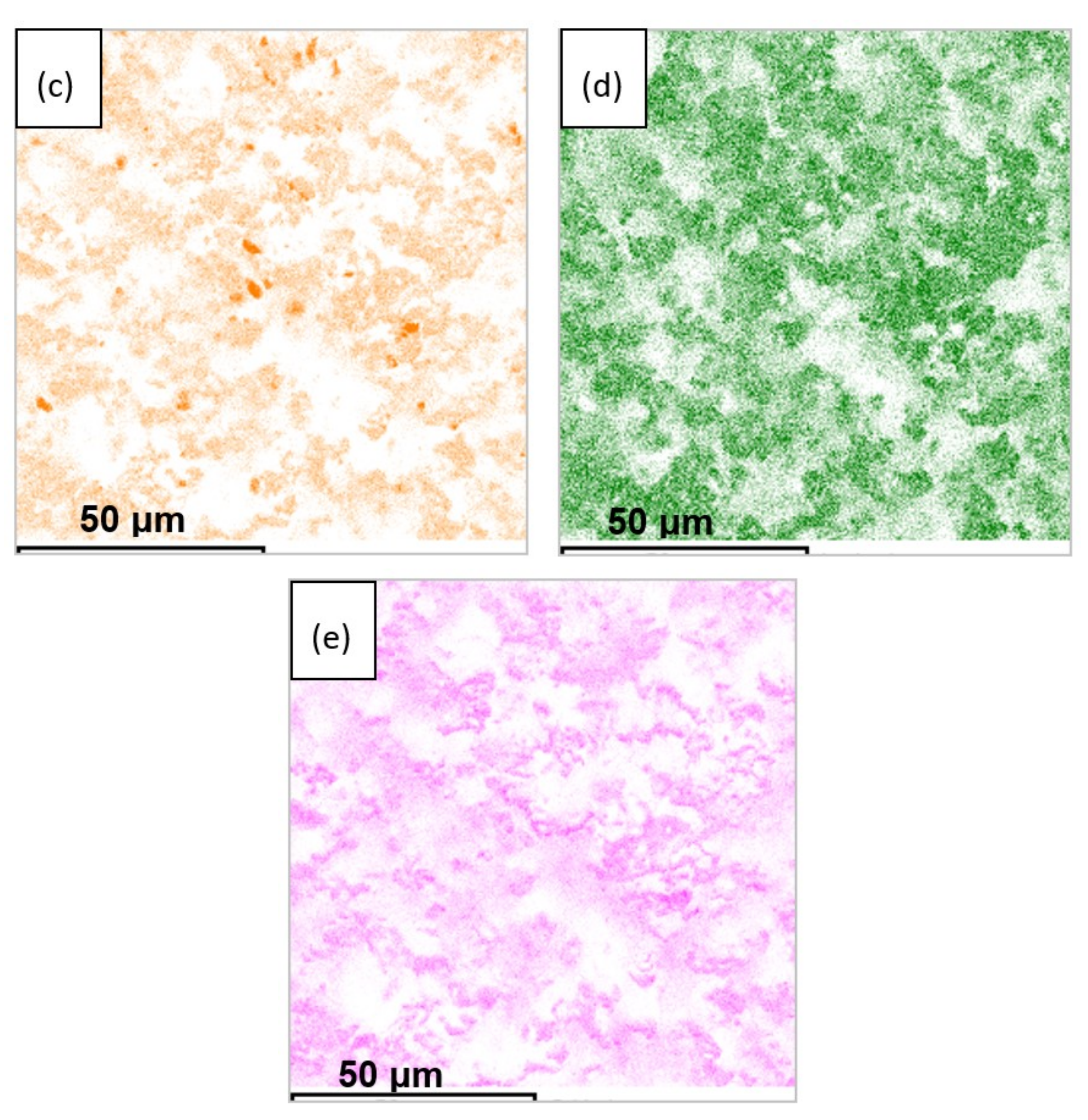
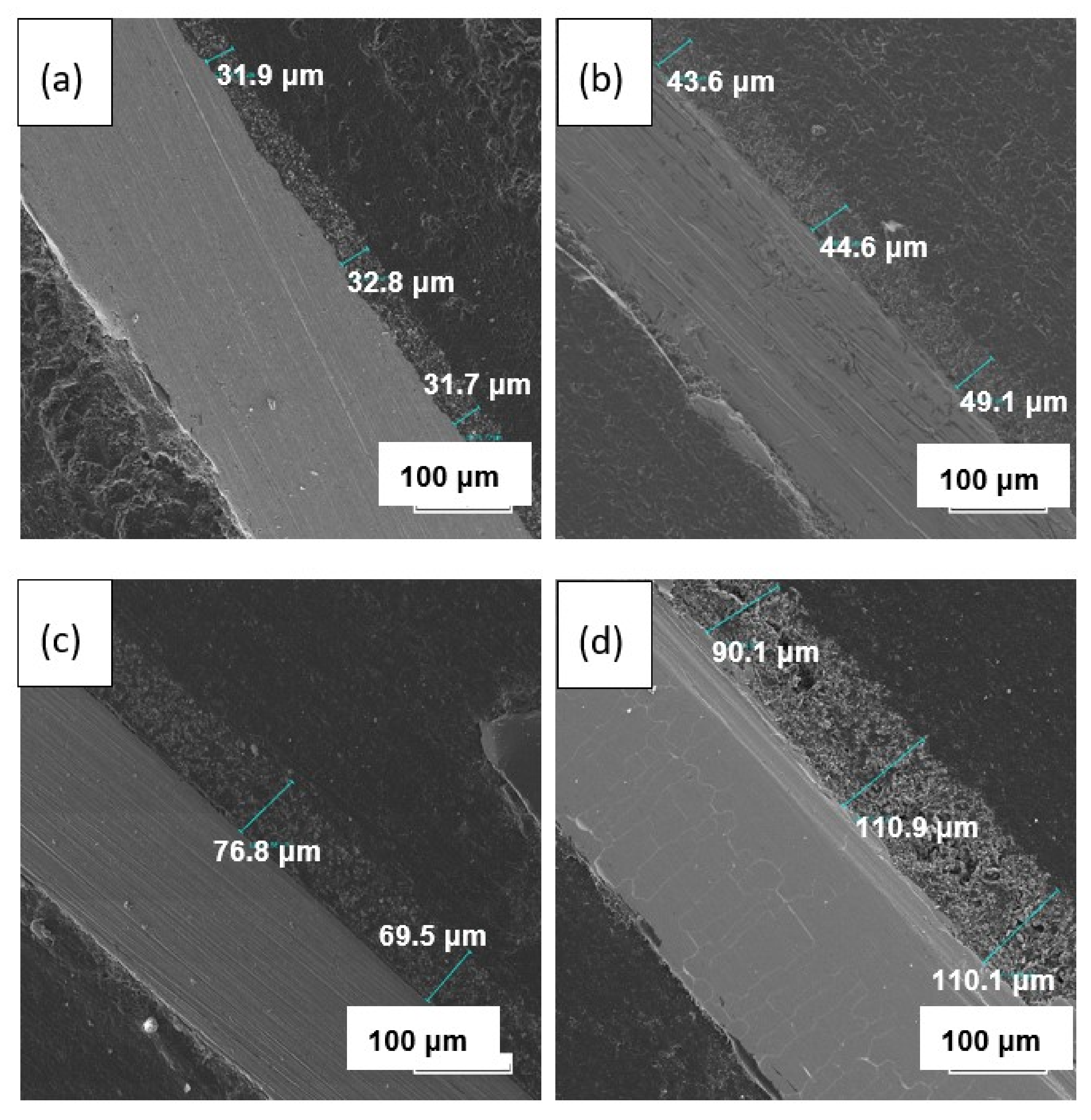
3.3. XRD and Microstructural Studies of LLZ Coatings
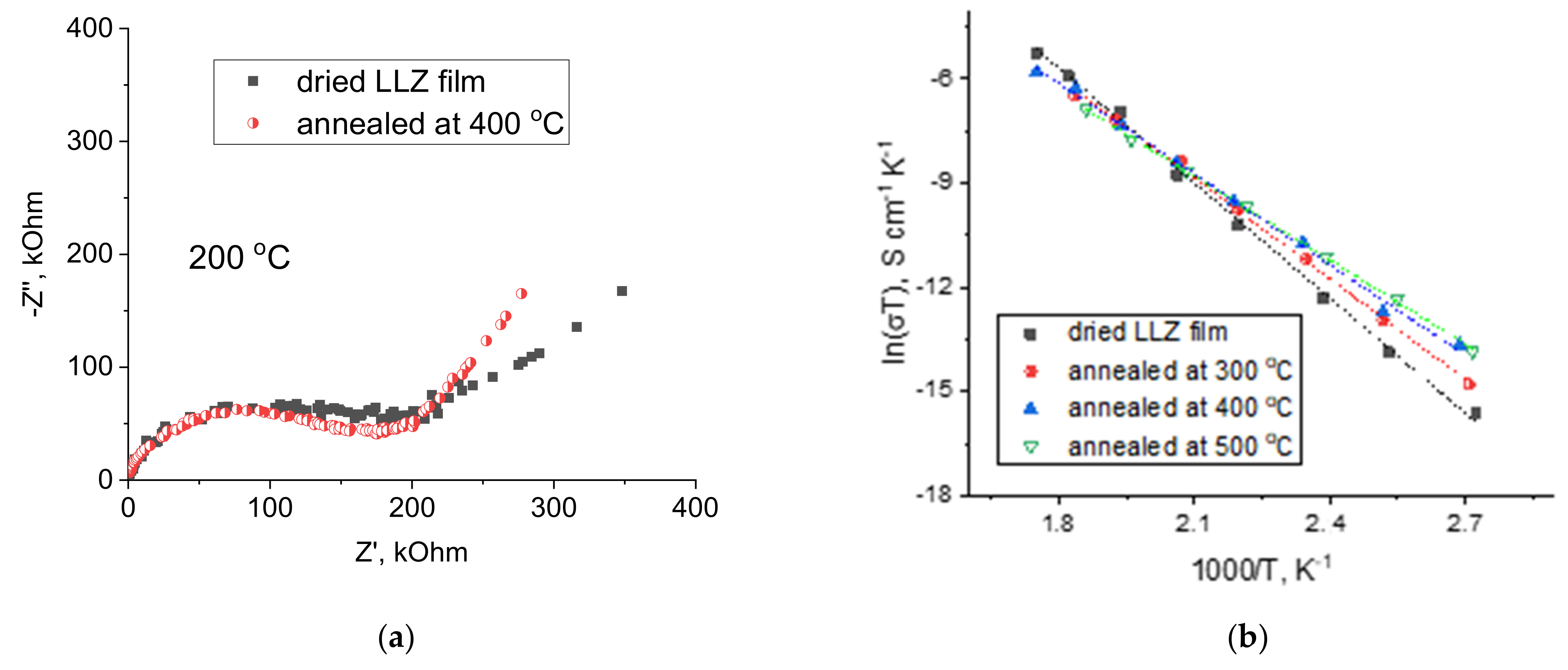
3.4. Lithium-Ion Conductivity Studies of LLZ Coatings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kong, L.; Wang, L.; Zhu, J.; Bian, J.; Xia, W.; Zhao, R.; Lin, H.; Zhao, Y. Configuring solid-state batteries to power electric vehicles: A deliberation on technology, chemistry and energy. Chem. Commun. 2021, 57, 12587–12594. [Google Scholar] [CrossRef] [PubMed]
- Bates, A.M.; Preger, Y.; Torres-Castro, L.; Harrison, K.L.; Harris, S.J.; Hewson, J. Are solid-state batteries safer than lithium-ion batteries? Joule 2022, 6, 742–755. [Google Scholar] [CrossRef]
- Wu, J.; Yuan, L.; Zhang, W.; Li, Z.; Xie, X.; Huang, Y. Reducing the thickness of solid-state electrolyte membranes for high-energy lithium batteries. Energy Environ. Sci. 2021, 14, 12–36. [Google Scholar] [CrossRef]
- Balaish, M.; Gonzalez-Rosillo, J.C.; Kim, K.J.; Zhu, Y.; Hood, Z.D.; Rupp, J.L.M. Processing thin but robust electrolytes for solid-state batteries. Nat. Energy 2021, 6, 227–239. [Google Scholar] [CrossRef]
- Garbayo, I.; Struzik, M.; Bowman, W.J.; Pfenninger, R.; Stilp, E.; Rupp, J.L.M. Glass-Type Polyamorphism in Li-Garnet Thin Film Solid State Battery Conductors. Adv. Energy Mater. 2018, 8, 1702265. [Google Scholar] [CrossRef]
- Campanella, D.; Belanger, D.; Paolella, A. Beyond garnets, phosphates and phosphosulfides solid electrolytes: New ceramic perspectives for all solid lithium metal batteries. J. Power Source 2021, 482, 228949. [Google Scholar] [CrossRef]
- Zheng, F.; Kotobuki, M.; Song, S.; Lai, M.O.; Lu, L. Review on solid electrolytes for all-solid-state lithium-ion batteries. J. Power Source 2018, 389, 198–213. [Google Scholar] [CrossRef]
- Yaroslavtsev, A.B. Solid electrolytes: Main prospects of research and development. Rus. Chem. Rev. 2016, 85, 1255–1276. [Google Scholar] [CrossRef]
- Ramakumar, S.; Deviannapoorani, C.; Dhivya, L.; Shankar, L.S.; Murugan, R. Lithium garnets: Synthesis, structure, Li+ conductivity, Li+ dynamics and applications. Prog. Mater. Sci. 2017, 88, 325–411. [Google Scholar] [CrossRef]
- Lobe, S.; Dellen, C.; Windmüller, A.; Tsai, C.L.; Vondahlen, F.; Uhlenbruck, S.; Guillon, O. Challenges regarding thin film deposition of garnet electrolytes for all-solid-state lithium batteries with high energy density. Ionics 2018, 24, 2199–2208. [Google Scholar] [CrossRef]
- Tan, J.; Tiwari, A. Fabrication and Characterization of Li7La3Zr2O12 Thin Films for Lithium Ion Battery. ECS Solid State Lett. 2012, 1, Q57–Q60. [Google Scholar] [CrossRef]
- Lobe, S.; Dellen, C.; Finsterbusch, M.; Gehrke, H.G.; Sebold, D.; Tsai, C.-L.; Uhlenbruck, O.; Guillon, O. Radio frequency magnetron sputtering of Li7La3Zr2O12 thin films for solid-state batteries. J. Power Source 2016, 307, 684–689. [Google Scholar] [CrossRef]
- Hanft, D.; Exner, J.; Moos, R. Thick-films of garnet-type lithium ion conductor prepared by the Aerosol Deposition Method: The role of morphology and annealing treatment on the ionic conductivity. J. Power Source 2017, 361, 61–69. [Google Scholar] [CrossRef]
- Chen, R.J.; Huang, M.; Huang, W.Z.; Shen, Y.; Lin, Y.H.; Nan, C.W. Sol–gel derived Li–La–Zr–O thin films as solid electrolytes for lithium-ion batteries. J. Mater. Chem. A 2014, 2, 13277–13282. [Google Scholar] [CrossRef]
- Tadanaga, K.; Egawa, H.; Hayashi, A.; Tatsumisago, M.; Mosa, J.; Aparicio, M.; Duran, A. Preparation of lithium ion conductive Al-doped Li7La3Zr2O12 thin films by a sol–gel process. J. Power Source 2015, 273, 844–847. [Google Scholar] [CrossRef]
- Kalinina, E.G.; Pikalova, E.Y. New trends in the development of electrophoretic deposition method in the solid oxide fuel cell technology: Theoretical approaches, experimental solutions and development prospects. Russ. Chem. Rev. 2019, 88, 1179–1219. [Google Scholar] [CrossRef]
- Pikalova, E.; Kalinina, E. Electrophoretic deposition in the solid oxide fuel cell technology: Fundamentals and recent advances. Renew. Sustain. Energy Rev. 2019, 116, 109440. [Google Scholar] [CrossRef]
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (EPD). Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Van der Biest, O.O.; Vandeperre, L.J. Electrophoretic deposition of materials. Annu. Rev. Mater. Sci. 1999, 29, 327–352. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Kalinina, E.G. Solid oxide fuel cells based on ceramic membranes with mixed conductivity: Improving efficiency. Russ. Chem. Rev. 2021, 90, 703–749. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Kalinina, E.G. Place of Electrophoretic Deposition Among Thin-Film Methods Adapted to the Solid Oxide Fuel Cell Technology: A Short Review. Int. J. Energy Prod. Manag. 2019, 4, 1–27. [Google Scholar] [CrossRef]
- Shri Prakash, B.; Pavitra, R.; Senthil Kumar, S.; Aruna, S.T. Electrolyte bi-layering strategy to improve the performance of an intermediate temperature solid oxide fuel cell: A review. J. Power Source 2018, 381, 136–155. [Google Scholar] [CrossRef]
- Hosomi, T.; Matsuda, M.; Miyake, M. Electrophoretic deposition for fabrication of YSZ electrolyte film on non-conducting porous NiO–YSZ composite substrate for intermediate temperature SOFC. J. Eur. Ceram. Soc. 2007, 27, 173–178. [Google Scholar] [CrossRef]
- Das, D.; Basu, R.N. Electrophoretic Deposition of Zirconia Thin Film on Nonconducting Substrate for Solid Oxide Fuel Cell Application. J. Am. Ceram. Soc. 2014, 97, 3452–3457. [Google Scholar] [CrossRef]
- Kalinina, E.; Shubin, K.; Pikalova, E. Electrophoretic Deposition and Characterization of the Doped BaCeO3 Barrier Layers on a Supporting Ce0.8Sm0.2O1.9 Solid-State Electrolyte. Membranes 2022, 12, 308. [Google Scholar] [CrossRef]
- Besra, L.; Compson, C.; Liu, M. Electrophoretic Deposition of YSZ Particles on Non-Conducting Porous NiO–YSZ Substrates for Solid Oxide Fuel Cell Applications. J. Am. Ceram. Soc. 2006, 89, 3003–3009. [Google Scholar] [CrossRef]
- Pikalova, E.; Osinkin, D.; Kalinina, E. Direct Electrophoretic Deposition and Characterization of Thin-Film Membranes Based on Doped BaCeO3 and CeO2 for Anode-Supported Solid Oxide Fuel Cells. Membranes 2022, 12, 682. [Google Scholar] [CrossRef]
- Hajizadeh, A.; Shahalizade, T.; Riahifar, R.; Yaghmaee, M.S.; Raissi, B.; Gholam, S.; Aghaei, A.; Rahimisheikh, S.; Ghazvini, A.S. Electrophoretic deposition as a fabrication method for Li-ion battery electrodes and separators—A review. J. Power Source 2022, 535, 231448. [Google Scholar] [CrossRef]
- Il’ina, E.A.; Andreev, O.L.; Antonov, B.D.; Batalov, N.N. Morphology and transport properties of the solid electrolyte Li7La3Zr2O12 prepared by the solid-state and citrate-nitrate methods. J. Power Source 2012, 201, 169–173. [Google Scholar] [CrossRef]
- Kalinina, E.; Pikalova, E.; Ermakova, L.; Bogdanovich, N. Challenges of Formation of Thin-Film Solid Electrolyte Layers on Non-Conductive Substrates by Electrophoretic Deposition. Coatings 2021, 11, 805. [Google Scholar] [CrossRef]
- Pantoja-Pertegal, J.L.; Díaz-Parralejo, A.; Marcías-García, A.; Sánchez-González, J.; Cuerda-Correa, E.M. Design, preparation, and characterization of Yttria-Stabilized Zirconia (YSZ) coatings obtained by electrophoretic deposition (EPD). Ceram. Int. 2021, 47, 13312–13321. [Google Scholar] [CrossRef]
- Kalinina, E.G.; Pikalova, E.Y. Modifying Suspensions for the Electrophoretic Deposition of BaCe0.5Zr0.3Y0.1Yb0.1O3–δ Solid Electrolyte. Russ. J. Phys. Chem. A 2021, 95, 1942–1947. [Google Scholar] [CrossRef]
- Khanali, O.; Rajabi, M.; Baghshahi, S.; Ariaee, S. Suspension medium’s impact on the EPD of nano-YSZ on Fecralloy. Surf. Eng. 2017, 33, 310–318. [Google Scholar] [CrossRef]
- Chen, F.; Liu, M. Preparation of yttria-stabilized zirconia (YSZ) films on La0.85Sr0.15MnO3 (LSM) and LSM–YSZ substrates using an electrophoretic deposition (EPD) process. J. Eur. Ceram. Soc. 2001, 21, 127–134. [Google Scholar] [CrossRef]
- Kokal, I.; Somer, M.; Notten, P.H.L. Sol-gel synthesis and lithium ion conductivity of Li7La3Zr2O12 with garnet-related type structure. Solid State Ion. 2011, 185, 42–46. [Google Scholar] [CrossRef]
- Kuhn, A.; Narayanan, S.; Spencer, L.; Goward, G. Li self-diffusion in garnet-type Li7La3Zr2O12 as probed directly by diffusion-induced 7Li spin-lattice relaxation NMR spectroscopy. Phys. Rev. B 2011, 83, 09430201. [Google Scholar] [CrossRef]
- Toda, S.; Ishiguro, K.; Shimonishi, Y.; Hirano, A.; Takeda, Y.; Yamamoto, O.; Imanishi, N. Low temperature cubic garnet-type CO2-doped Li7La3Zr2O12. Solid State Ion. 2013, 233, 102–106. [Google Scholar] [CrossRef]
- Larraz, G.; Orera, A.; Sanjuan, M.L. Cubic phases of garnet type Li7La3Zr2O12: The role of hydration. J. Mater. Chem. A 2013, 1, 11419–11428. [Google Scholar] [CrossRef]
- Xie, H.; Li, Y.; Goodenough, J.B. Low-temperature synthesis of Li7La3Zr2O12 with cubic garnet-type structure. Mat. Res. Bull. 2012, 47, 1229–1232. [Google Scholar] [CrossRef]
- Pershina, S.V.; Il’ina, E.A.; Reznitskikh, O.G. Phase composition, density and ionic conductivity of the Li7La3Zr2O12—Based composites with LiPO3 glass addition. Inorg. Chem. 2017, 56, 9880–9891. [Google Scholar] [CrossRef]





| UST, min | Zeta Potential, mV | pH |
|---|---|---|
| 5 | +6 | 6.4 |
| 25 | −6 | 7.1 |
| 125 | −6 | 6.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyalin, E.; Il’ina, E.; Kalinina, E.; Antonov, B.; Pankratov, A.; Pereverzev, D. Electrophoretic Deposition and Characterization of Thin-Film Membranes Li7La3Zr2O12. Membranes 2023, 13, 468. https://doi.org/10.3390/membranes13050468
Lyalin E, Il’ina E, Kalinina E, Antonov B, Pankratov A, Pereverzev D. Electrophoretic Deposition and Characterization of Thin-Film Membranes Li7La3Zr2O12. Membranes. 2023; 13(5):468. https://doi.org/10.3390/membranes13050468
Chicago/Turabian StyleLyalin, Efim, Evgeniya Il’ina, Elena Kalinina, Boris Antonov, Alexander Pankratov, and Danil Pereverzev. 2023. "Electrophoretic Deposition and Characterization of Thin-Film Membranes Li7La3Zr2O12" Membranes 13, no. 5: 468. https://doi.org/10.3390/membranes13050468
APA StyleLyalin, E., Il’ina, E., Kalinina, E., Antonov, B., Pankratov, A., & Pereverzev, D. (2023). Electrophoretic Deposition and Characterization of Thin-Film Membranes Li7La3Zr2O12. Membranes, 13(5), 468. https://doi.org/10.3390/membranes13050468