Mathematical Modeling of the Influence of the Karman Vortex Street on Mass Transfer in Electromembrane Systems
Abstract
1. Introduction
2. Materials and Methods
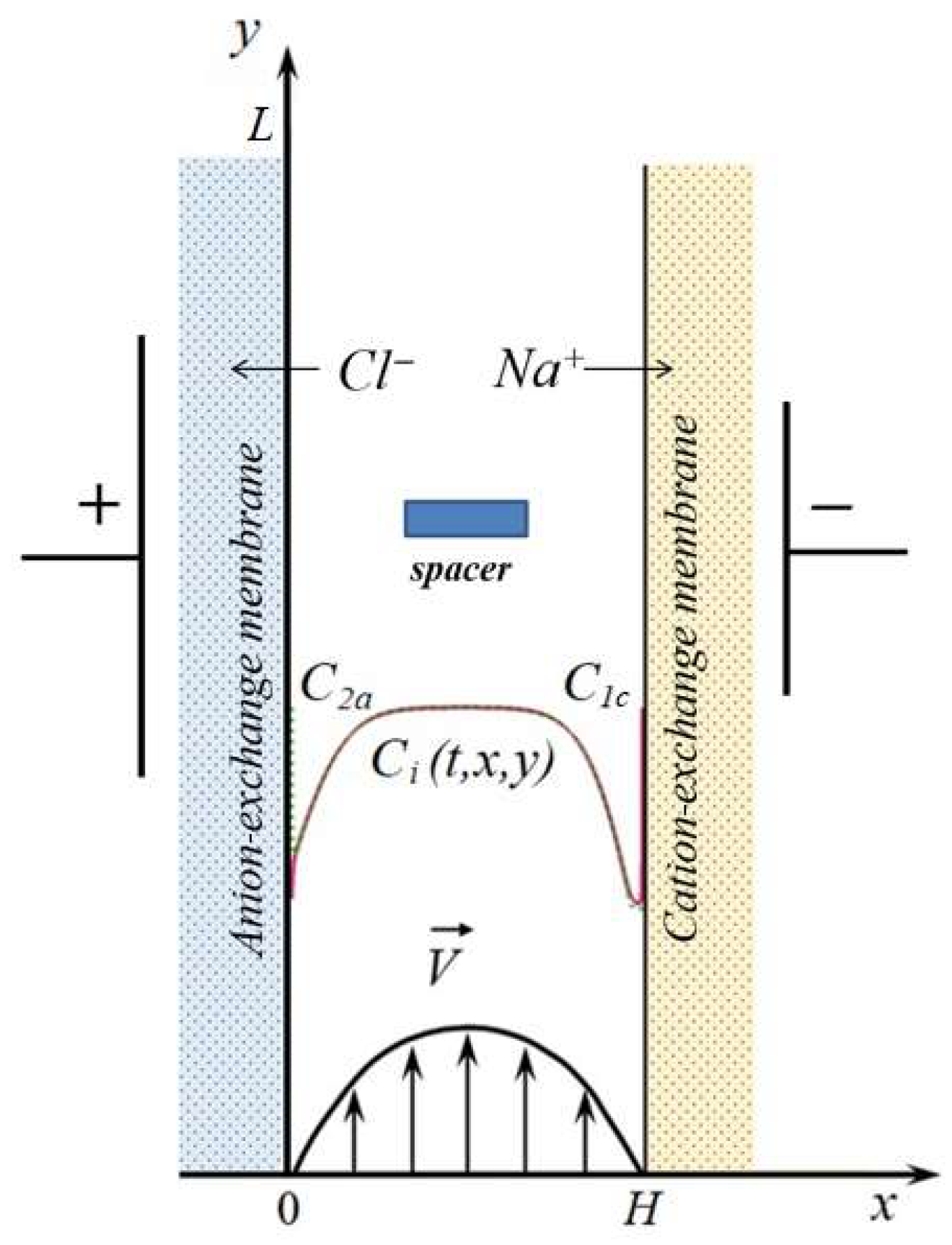
2.1. Mathematical Model
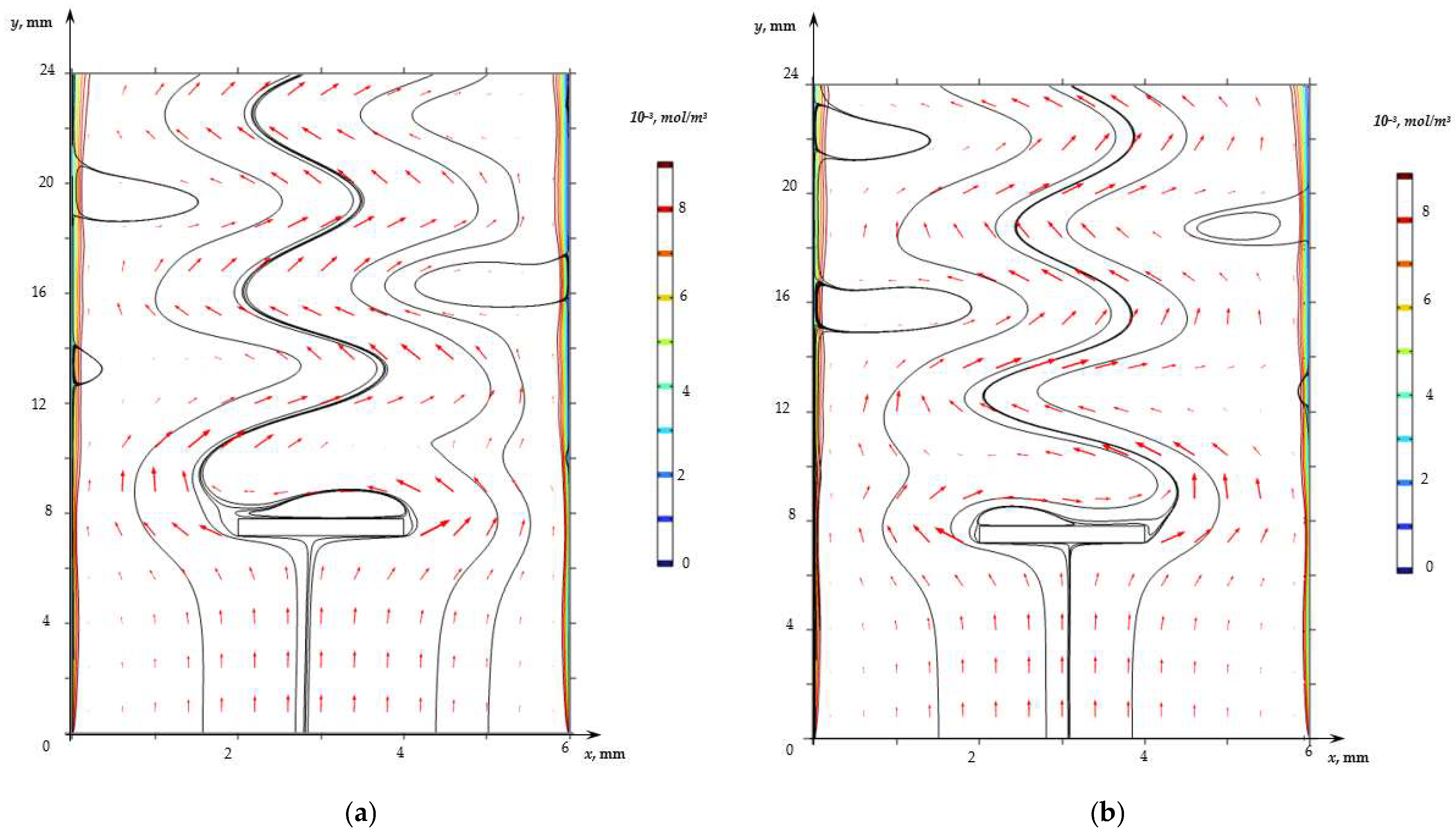
- (a)
- At the electrolyte solution/anion-exchange membrane interface ():
- (b)
- At the electrolyte solution/cation-exchange membrane interface ():
- (c)
- At the channel inlet ():
- (d)
- At the channel outlet ():
- (e)
- At the boundaries of the spacer, the no-slip condition is applied, and the equality to zero of the ion fluxes normal to the boundaries and the components of the electric field strength. Thus, a non-conductive spacer is considered.
- (f)
- The initial conditions are taken as consistent with the boundary conditions.
2.2. Numerical Implementation
2.3. Parameters Used in Computations
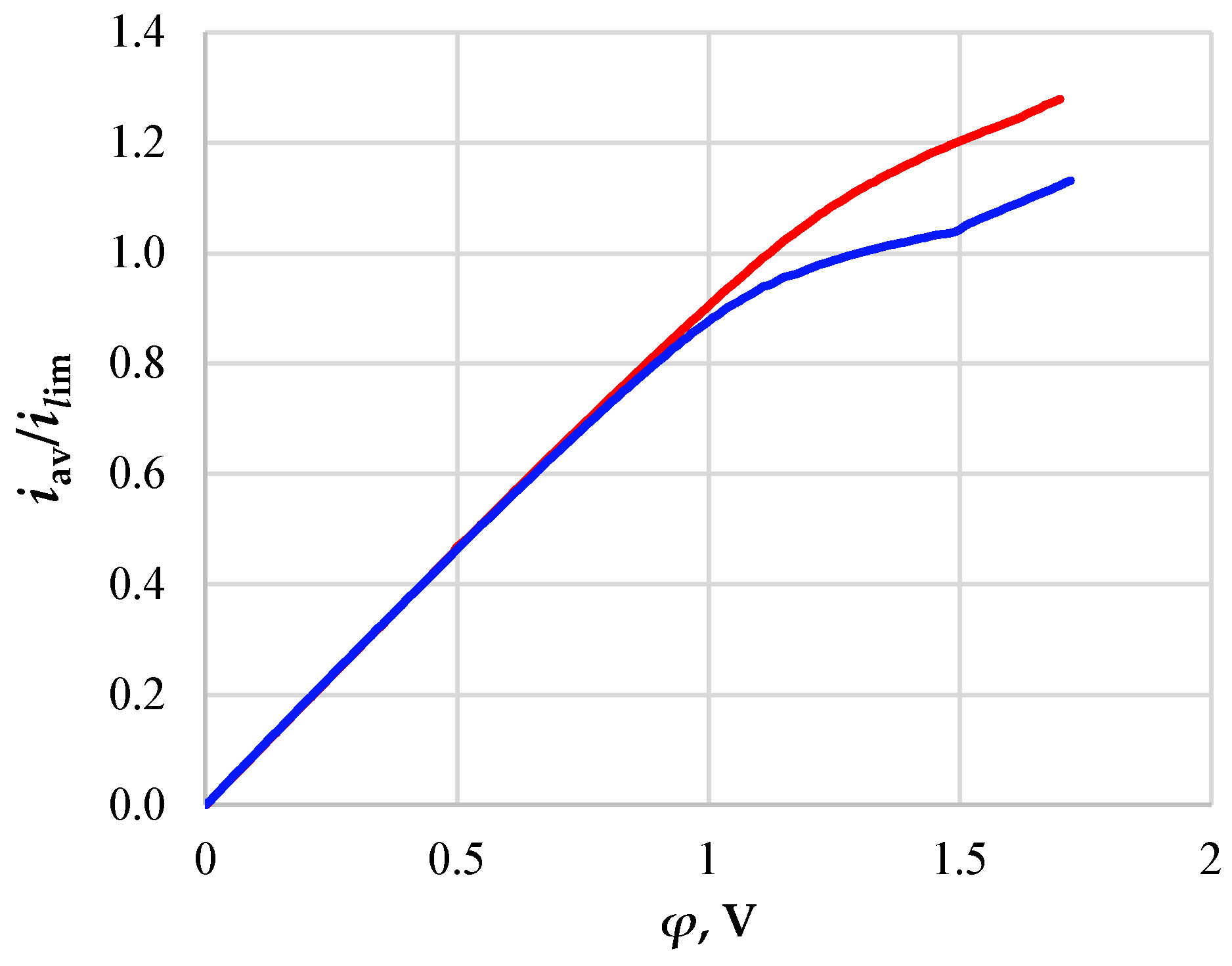
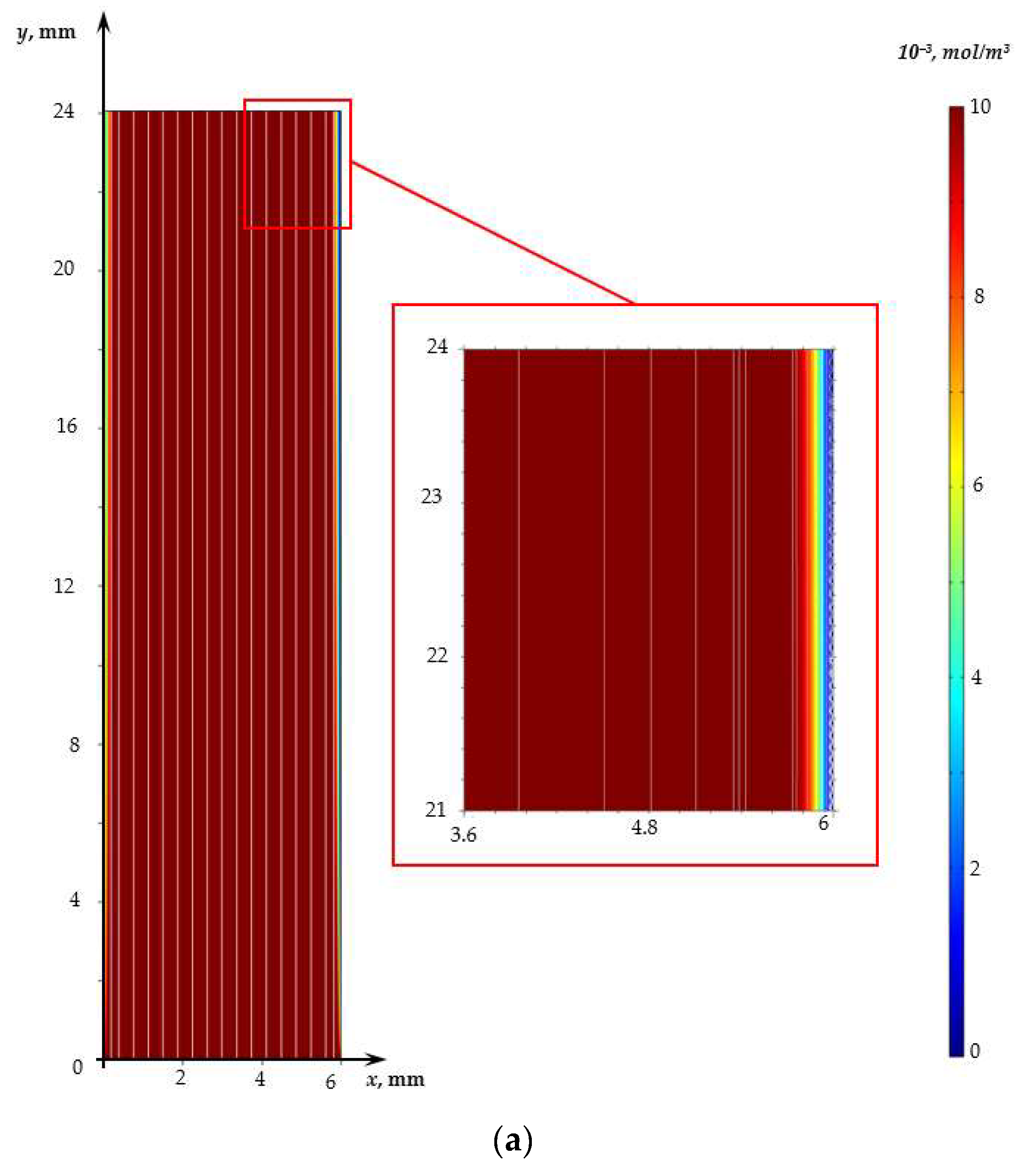
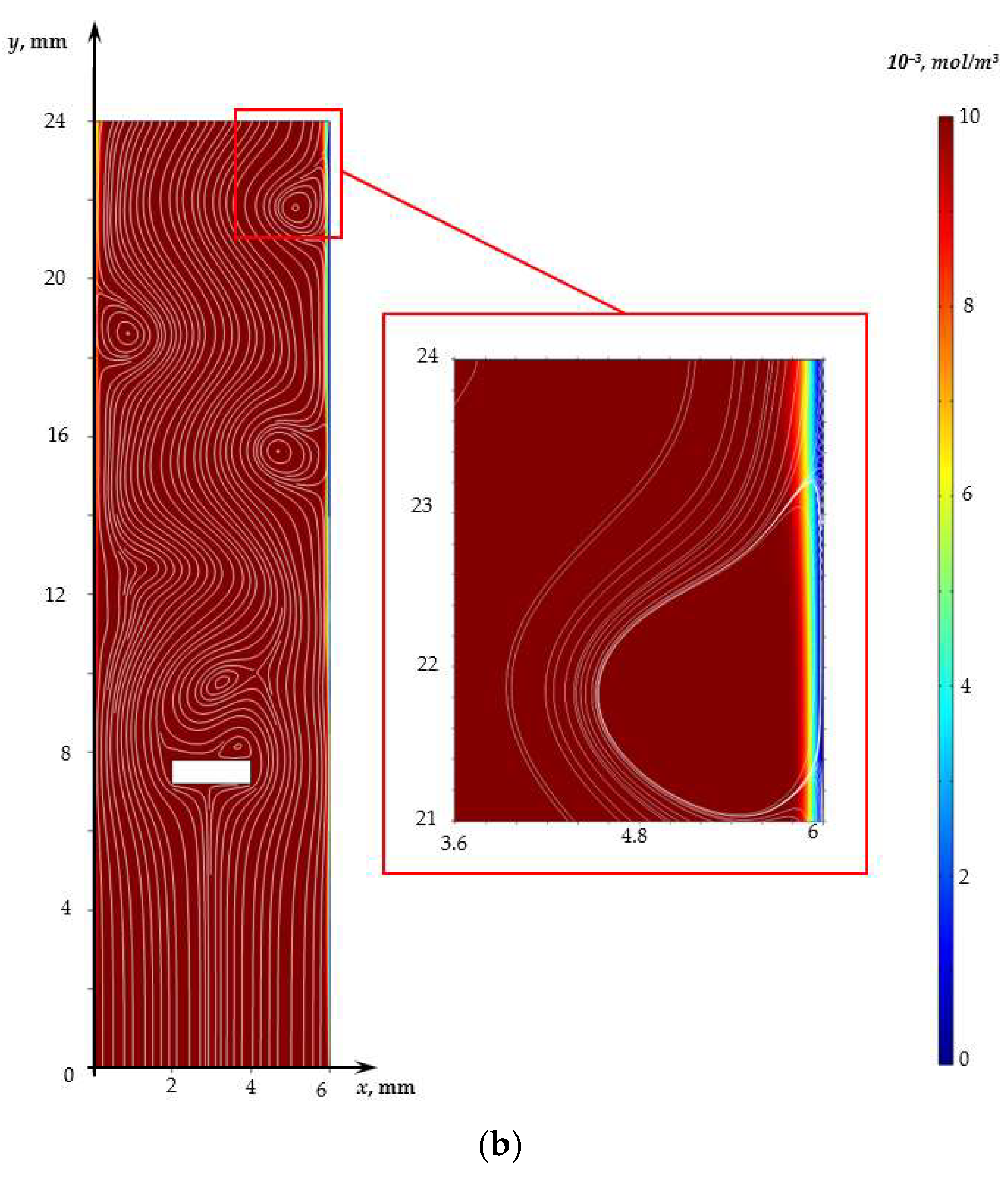
3. Results and Discussion
- -
- The initial section of linear growth (at the potential jump up to 1 V), which is characterized by a rather high concentration of ions in the region near the membrane;
- -
- The area of the “plateau” of the limiting current (between approximately 1 V and 1.5 V), which describes the saturation of the current corresponding to the almost complete depletion of ions at the membrane surface;
- -
- The section of the overlimiting current due to the development of electroconvection (with the potential jump greater than 1.5 V).
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoekstra, A.Y.; Wiedmann, T.O. Humanity’s unsustainable environmental footprint. Science 2014, 344, 1114–1117. [Google Scholar] [CrossRef]
- Petersen, L.; Heynen, M.; Pellicciotti, F. Freshwater Resources: Past, Present, Future. Int. Encycl. Geogr. 2016, 3, 1–12. [Google Scholar]
- Dinar, A.; Tieu, A.; Huynh, H. Water scarcity impacts on global food production. Glob. Food Secur. 2019, 23, 212–226. [Google Scholar] [CrossRef]
- Mekonnen, M.; Hoekstra, A.Y. Four billion people facing severe water scarcity. Sci. Adv. 2016, 2, 1500323. [Google Scholar] [CrossRef]
- Gude, V.G. Desalination and water reuse to address global water scarcity. Rev. Environ. Sci. Bio/Technol. 2017, 16, 591–609. [Google Scholar] [CrossRef]
- Djehdian, L.A.; Chini, C.M.; Marston, L.; Konar, M.; Stillwell, A.S. Exposure of urban food-energy-water (FEW) systems to water scarcity. Sustain. Cities Soc. 2019, 50, 101621. [Google Scholar] [CrossRef]
- Jones, E.; Qadir, M.; van Vliet, M.T.; Smakhtin, V.; Kang, S.M. The state of desalination and brine production: A global outlook. Sci. Total Environ. 2019, 657, 1343–1356. [Google Scholar] [CrossRef]
- Ali, A.; Tufa, R.A.; Macedonio, F.; Curcio, E.; Drioli, E. Membrane technology in renewable-energy-driven desalination. Renew. Sustain. Energy Rev. 2018, 81, 1–21. [Google Scholar] [CrossRef]
- Tzanakakis, V.A.; Paranychianakis, N.V.; Angelakis, A.N. Water Supply and Water Scarcity. Water 2020, 12, 2347. [Google Scholar] [CrossRef]
- Doornbusch, G.; van der Wal, M.; Tedesco, M.; Post, J.; Nijmeijer, K.; Borneman, Z. Multistage electrodialysis for desalination of natural seawater. Desalination 2021, 505, 114973. [Google Scholar] [CrossRef]
- Golubenko, D.V.; Yaroslavtsev, A.B. Effect of current density, concentration of ternary electrolyte and type of cations on the monovalent ion selectivity of surface-sulfonated graft anion-exchange membranes: Modelling and experiment. J. Membr. Sci. 2021, 635, 119466. [Google Scholar] [CrossRef]
- Kim, B.; Kwak, R.; Kwon, H.J.; Pham, V.S.; Kim, M.; Al-Anzi, B.; Lim, G.; Han, J. Purification of High Salinity Brine by Multi-Stage Ion Concentration Polarization Desalination. Sci. Rep. 2016, 6, 31850. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, S.; Kwak, R. Controlling ion transport with pattern structures on ion exchange membranes in electrodialysis. Desalination 2021, 499, 114801. [Google Scholar] [CrossRef]
- Kovalenko, A.V.; Evdochenko, E.; Stockmeier, F.; Köller, N.; Uzdenova, A.; Urtenov, M.A.K. Influence of spacers on mass transport in electromembrane desalination systems. J. Phys. Conf. Ser. 2021, 2131, 022011. [Google Scholar] [CrossRef]
- Rubinstein, I. Electroconvection at an electrically inhomogeneous permselective interface. Phys. Fluids A 1991, 3, 2301. [Google Scholar] [CrossRef]
- Rubinstein, I.; Zaltzman, B. Electro-osmotically induced convection at a permselective membrane. Phys. Rev. E 2000, 62, 2238–2251. [Google Scholar] [CrossRef] [PubMed]
- Zaltzman, B.; Rubinstein, I. Electro-osmotic slip and electroconvective instability. J. Fluid Mech. 2007, 579, 173–226. [Google Scholar] [CrossRef]
- Kovalenko, A.; Uzdenova, A.; Urtenov, M. Theoretical Investigation of the Phenomenon of Space Charge Breakdown in Electromembrane Systems. Membranes 2022, 12, 1047. [Google Scholar] [CrossRef]
- Kovalenko, A.V.; Wessling, M.; Nikonenko, V.V.; Mareev, S.A.; Moroz, I.A.; Evdochenko, E.; Urtenov, M.K. Space-Charge breakdown phenomenon and spatio-temporal ion concentration and fluid flow patterns in over-limiting current electrodialysis. J. Membr. Sci. 2021, 636, 119583. [Google Scholar] [CrossRef]
- Uzdenova, A.; Kovalenko, A.; Urtenov, M. Theoretical Analysis of Electroconvection in the Electrodialysis Desalination Channel under the Action of Direct Current. Membranes 2022, 12, 1125. [Google Scholar] [CrossRef]
- Kovalenko, A.V.; Uzdenova, A.M.; Sukhinov, A.I.; Chubyr, N.O.; Urtenov, M.K. Simulation of galvanic dynamic mode in membrane hydrocleaning systems taking into account space charge. In Proceedings of the XV AIP International Scientific-Technical Conference “Dynamics of Technical Systems” (DTS-2019), Rostov-on-Don, Russia, 11–13 September 2019; Volume 2188. [Google Scholar]
- Pismenskiy, A.; Urtenov, M.; Kovalenko, A.; Mareev, S. Electrodialysis desalination process in conditions of mixed convection. Desalination Water Treat. 2015, 56, 3211–3213. [Google Scholar] [CrossRef]
- Mareev, S.A.; Butylskii, D.Y.; Kovalenko, A.V.; Petukhova, A.V.; Pismenskaya, N.D.; Nikonenko, V.V.; Dammak, L.; Larchet, C. Accounting for the concentration dependence of electrolyte diffusion coefficient in the Sand and the Peers equations. Electrochim. Acta 2016, 195, 85–93. [Google Scholar] [CrossRef]
- Długołȩcki, P.; Gambier, A.; Nijmeijer, K.; Wessling, M. Practical potential of reverse electrodialysis as process for sustainable energy generation. Environ. Sci. Technol. 2009, 43, 6888–6894. [Google Scholar] [CrossRef]
- Strathmann, H. Electrodialysis, a mature technology with a multitude of new applications. Desalination 2010, 264, 268–288. [Google Scholar] [CrossRef]
- Sonin, A.A.; Isaacson, M.S. Optimization of flow design in forced flow electrochemical systems, with special application to electrodialysis. Ind. Eng. Chem. Process Des. Dev. 1974, 13, 241–248. [Google Scholar] [CrossRef]
- Balster, J.; Pünt, I.; Stamatialis, D.; Wessling, M. Multi-layer spacer geometries with improved mass transport. J. Membr. Sci. 2006, 282, 351–361. [Google Scholar] [CrossRef]
- Winograd, Y.; Solan, A.; Toren, M. Mass transfer in narrow channels in the presence of turbulence promoters. Desalination 1973, 13, 171–186. [Google Scholar] [CrossRef]
- Kim, Y.; Walker, W.S.; Lawler, D.F. Electrodialysis with spacers: Effects of variation and correlation of boundary layer thickness. Desalination 2011, 274, 54–63. [Google Scholar] [CrossRef]
- La Cerva, M.L.; Liberto, M.D.; Gurreri, L.; Tamburini, A.; Cipollina, A.; Micale, G.; Ciofalo, M. Coupling CFD with a one-dimensional model to predict the performance of reverse electrodialysis stacks. J. Membr. Sci. 2017, 541, 595–610. [Google Scholar] [CrossRef]
- Burmasheva, N.V.; Prosviryakov, E.Y. Exact solution for stable convective concentration flows of a couette type. Comput. Contin. Mech. 2020, 13, 337–349. [Google Scholar] [CrossRef]
- Burmasheva, N.V.; Prosviryakov, E.Y. Exact Solution for Couette-Type Steady Convective Concentration Flows. J. Appl. Mech. Tech. Phys. 2021, 62, 155–166. [Google Scholar] [CrossRef]
- Burmasheva, N.V.; Prosviryakov, E.Y. On Marangoni shear convective flows of inhomogeneous viscous incompressible fluids in view of the Soret effect. J. King Saud Univ. –Sci. 2020, 32, 3364–3371. [Google Scholar] [CrossRef]
- Burmasheva, N.V.; Privalova, V.V.; Prosviryakov, E.Y. Layered Marangoni convection with the Navier slip condition. Sadhana-Acad. Proc. Eng. Sci. 2021, 46, 55. [Google Scholar] [CrossRef]
- Ershkov, S.V.; Prosviryakov, E.Y.; Burmasheva, N.V.; Christianto, V. Towards understanding the algorithms for solving the Navier–Stokes equations. Fluid Dyn. Res. 2021, 53, 044501. [Google Scholar] [CrossRef]
- Aristov, S.N.; Prosviryakov, E.Y. A new class of exact solutions for three-dimensional thermal diffusion equations. Theor. Found. Chem. Eng. 2016, 50, 286–293. [Google Scholar] [CrossRef]
- Gurreri, L.; Battaglia, G.; Tamburini, A.; Micale, G.; Ciofalo, M. Multi-physical modelling of reverse electrodialysis. Desalination 2017, 423, 52–64. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, H.; Li, Z.; Wang, W. Chemistry pumps: A review of chemically powered micropumps. Lab A Chip 2016, 16, 1797–1811. [Google Scholar] [CrossRef] [PubMed]
- Tadimeti, J.G.D.; Kurian, V.; Chandra, A.; Chattopadhyay, S. Corrugated membrane surfaces for effective ion transport in electrodialysis. J. Membr. Sci. 2016, 499, 418–428. [Google Scholar] [CrossRef]
- Vasil’eva, V.; Shaposhnik, V.; Grigorchuk, O. Local mass transportduring electrodialysis with ion-exchange membranes and spacers. Russ. J. Electrochem. 2001, 37, 1164–1171. [Google Scholar] [CrossRef]
- Kim, I.H.; Chang, H.N. Experimental study of mass transportaround a turbulence promoter by the limiting current method. Int. J. Heat Mass Transf. 1983, 26, 1007–1016. [Google Scholar] [CrossRef]
- Fischl, D.; Hanson, K.; Muller, R.; Tobias, C. Mass transfer enhancement by small flow obstacles in electrochemical cells. Chem. Eng. Commun. 1985, 38, 191–207. [Google Scholar] [CrossRef]
- Długołȩcki, P.; Dabrowska, J.; Nijmeijer, K.; Wessling, M. Ion conductive spacers for increased power generation in reverse electrodialysis. J. Membr. Sci. 2010, 347, 101–107. [Google Scholar] [CrossRef]
- Balster, J.; Stamatialis, D.; Wessling, M. Membrane with integrated spacer. J. Membr. Sci. 2010, 360, 185–189. [Google Scholar] [CrossRef]
- Kim, B.; Choi, S.; Pham, V.S.; Kwak, R.; Han, J. Energy efficiency enhancement of electromembrane desalination systems by local flow redistribution optimized for the asymmetry of cation/anion diffusivity. J. Membr. Sci. 2017, 524, 280–287. [Google Scholar] [CrossRef]
- Liang, Y.Y.; Fimbres Weihs, G.A.; Fletcher, D.F. CFD study of the effect of unsteady slip velocity waveform on shear stress in membrane systems. Chem. Eng. Sci. 2018, 192, 16–24. [Google Scholar] [CrossRef]
- Foo, K.; Liang, Y.Y.; Weihs, G.A.F. CFD study of the effect of SWM feed spacer geometry on mass transfer enhancement driven by forced transient slip velocity. J. Membr. Sci. 2019, 597, 117643. [Google Scholar] [CrossRef]
- Foo, K.; Liang, Y.Y.; Tan, C.K.; Fimbres Weihs, G.A. Coupled effects of circular and elliptical feed spacers under forced-slip on viscous dissipation and mass transfer enhancement based on CFD. J. Membr. Sci. 2021, 637, 119599. [Google Scholar] [CrossRef]
- Milton, V.D. An Album of Fluid Motion, 10th ed.; Parabolic Press: Stanford, CA, USA, 1982; p. 176. [Google Scholar]
- Urtenov, M.K.; Uzdenova, A.M.; Kovalenko, A.V.; Nikonenko, V.V.; Pismenskaya, N.D.; Vasil’eva, V.I.; Sistat, P.; Pourcelly, G. Basic mathematical model of overlimiting transfer enhanced by electroconvection in flow-through electrodial-ysis membrane cells. J. Membr. Sci. 2013, 447, 190–202. [Google Scholar] [CrossRef]
- Uzdenova, A.M.; Kovalenko, A.V.; Urtenov, M.A.K. Mathematical Models of Electroconvection in Electromembrane Systems, 1st ed.; Publishing House of KCHGU: Karachaevsk, Russia, 2011; p. 154. (In Russian) [Google Scholar]
- Kwak, R.; Pham, V.S.; Lim, K.M.; Han, J. Shear flow of an electrically charged fluid by ion concentration polarization: Scaling laws for electroconvective vortices. Phys. Rev. Lett. 2013, 110, 114501. [Google Scholar] [CrossRef]
- Nikonenko, V.V.; Mareev, S.A.; Pis’menskaya, N.D.; Uzdenova, A.M.; Kovalenko, A.V.; Urtenov, M.K.; Pourcelly, G. Effect of electroconvection and its use in intensifying the mass transfer in electrodialysis (Review). Russ. J. Electrochem. 2017, 53, 1122–1144. [Google Scholar] [CrossRef]
- Chubyr, N.O.; Kovalenko, A.V.; Urtenov, M.A.K. Two-Dimensional Mathematical Models of Binary Electrolyte Transfer in Membrane Systems (Numerical and Asymptotic Analysis), 1st ed.; Publishing House of Kuban State Technological University: Krasnodar, Russia, 2012; p. 132. (In Russian) [Google Scholar]
- Nikonenko, V.V.; Kovalenko, A.V.; Urtenov, M.K.; Pismenskaya, N.D.; Han, J.; Sistat, P.; Pourcelly, G. Desalination at overlimiting currents: State-of-the-art and perspectives. Desalination 2014, 342, 85–106. [Google Scholar] [CrossRef]
- Urtenov, M.K.; Kovalenko, A.V.; Sukhinov, A.I.; Chubyr, N.O.; Gudza, V.A. Model and numerical experiment for calculating the theoretical current-voltage characteristic in electro-membrane systems. IOP Conf. Ser. Mater. Sci. Eng. 2019, 680, 012030. [Google Scholar] [CrossRef]
- Kovalenko, A.V.; Urtenov, M.K. Analysis of the theoretical CVC of electromembrane systems. E3S Web Conf. 2020, 224, 02010. [Google Scholar] [CrossRef]
- Gudza, I.V.; Urtenov, M.K.; Kovalenko, A.V.; Chubyr, N.O. Analysis of the theoretical current-voltage characteristic in electromembrane systems. In Proceedings of the Ion Transport in Organic and Inorganic Membranes, Sochi, Russia, 20–25 September 2021; pp. 113–114. [Google Scholar]
- Kovalenko, A.V. Mathematical Modeling of Transfer Processes in Electromembrane Systems. Ph.D. Thesis, North Caucasian Federal University, Stavropol, Russia, 24 December 2019. [Google Scholar]
- Comsol Multiphysics Reference Manual. Available online: https://doc.comsol.com/6.0/doc/com.comsol.help.comsol/COMSOL_ProgrammingReferenceManual.pdf (accessed on 1 March 2023).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uzdenova, A.; Kovalenko, A.; Prosviryakov, E.; Urtenov, M. Mathematical Modeling of the Influence of the Karman Vortex Street on Mass Transfer in Electromembrane Systems. Membranes 2023, 13, 394. https://doi.org/10.3390/membranes13040394
Uzdenova A, Kovalenko A, Prosviryakov E, Urtenov M. Mathematical Modeling of the Influence of the Karman Vortex Street on Mass Transfer in Electromembrane Systems. Membranes. 2023; 13(4):394. https://doi.org/10.3390/membranes13040394
Chicago/Turabian StyleUzdenova, Aminat, Anna Kovalenko, Evgeniy Prosviryakov, and Makhamet Urtenov. 2023. "Mathematical Modeling of the Influence of the Karman Vortex Street on Mass Transfer in Electromembrane Systems" Membranes 13, no. 4: 394. https://doi.org/10.3390/membranes13040394
APA StyleUzdenova, A., Kovalenko, A., Prosviryakov, E., & Urtenov, M. (2023). Mathematical Modeling of the Influence of the Karman Vortex Street on Mass Transfer in Electromembrane Systems. Membranes, 13(4), 394. https://doi.org/10.3390/membranes13040394








