Modulation of the Dipole Potential of Model Lipid Membranes with Phytochemicals: Molecular Mechanisms, Structure–Activity Relationships, and Implications in Reconstituted Ion Channels
Abstract
:1. Introduction
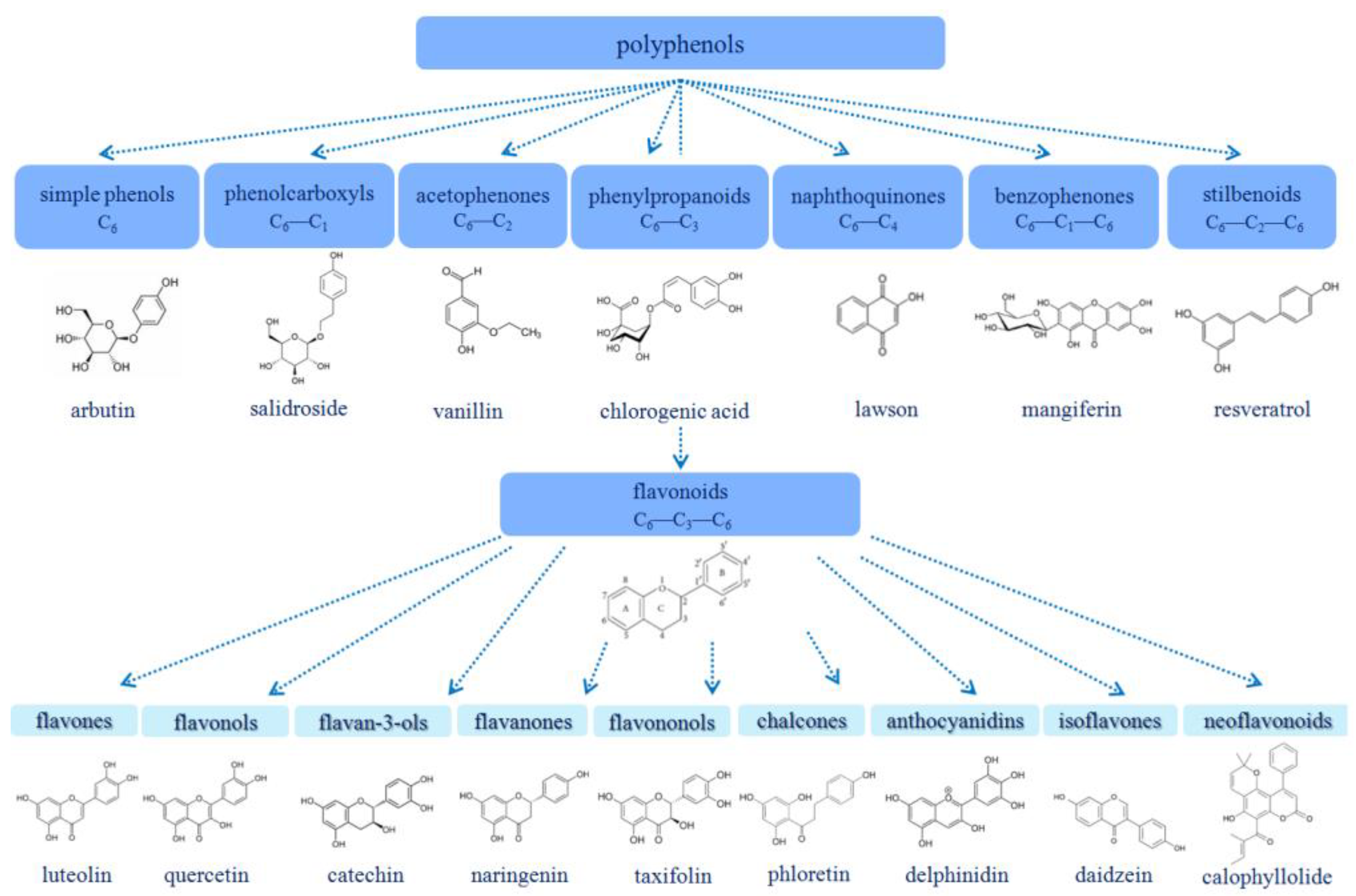
1.1. Polyphenols
1.2. Alkaloids
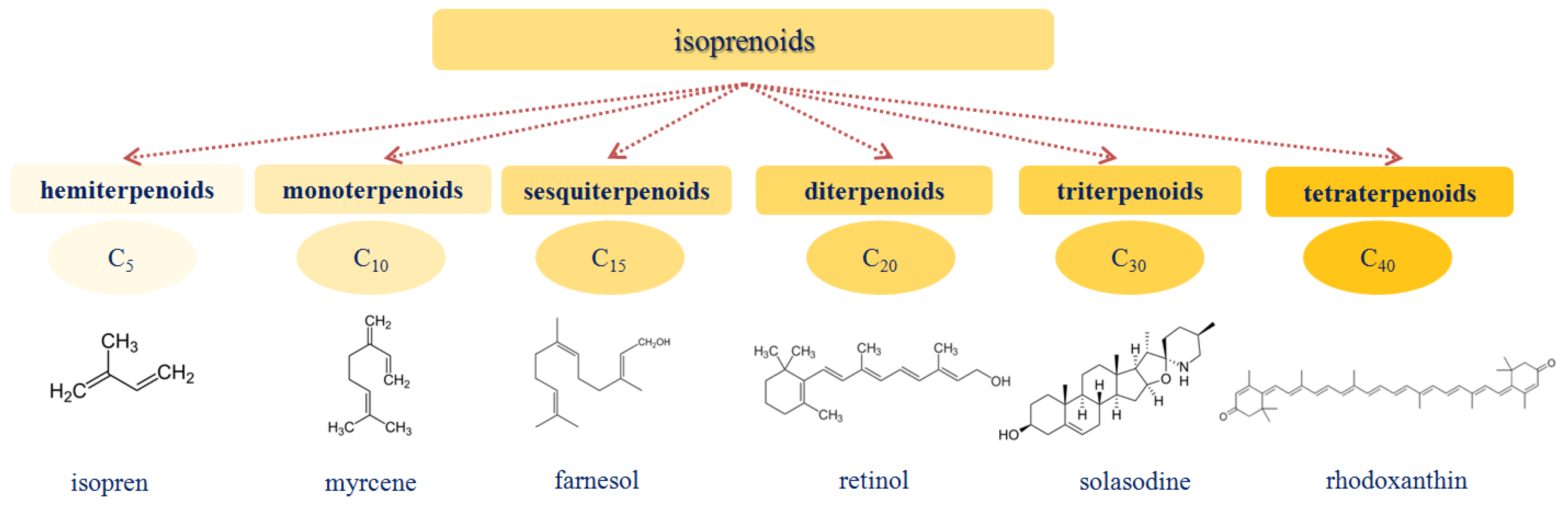
1.3. Isoprenoids
2. Phytochemicals Alter the Electrical Properties of the Model Lipid Membranes
- (1)
- The ability of chalcones to reduce the boundary/dipole potential increases in the following order: 4′-hydroxychalcone ≈ isoliquiritigenin (about −40 mV) ≤ cardamonin ≈ licochalcone A (about −60 ÷ −70 mV) < butein −120 mV). Despite the lower lipophilicity of butein among the other tested chalcones, its great efficiency might be explained by its higher dipole moment, which is probably related to the electron density shift in the A and B rings produced by the four hydroxyl groups.
- (2)
- The chalcone butein and the dihydrochalcone phloretin are almost equally effective (the Δφb(max) values coincide within the estimation error). This might indicate that the presence/absence of a double bond in the propane fragment linking the phenolic rings in the molecule of butein/phloretin, which significantly affects the mobility of the rings relative to each other, is not of key importance (Figure 5a).
- (3)
- The chalcone isoliquiritigenin and the flavanone liquiritigenin are almost equally effective (Δφb(max) values coincide within the measurement error), indicating that cyclization (the formation of a heterocycle) does not practically affect the ability of the compounds to modify the potential jump at the membrane–aqueous solution interface (Figure 5a).
- (4)
- The exclusion of the carbonyl group from the structure (catechin compared to taxifolin, Δφb(max) does not exceed 6 mV) does not affect the compound’s dipole-modifying effect (Figure 5a).
- (5)
- The inclusion of an additional OH group in the molecules of flavanones (naringenin compared to liquiritigenin, Δφb(max) ≈ −70 mV)), flavonols (myricetin compared to quercetin, Δφb(max) ≈ −100 mV)), and stilbenoids (piceatannol compared to resveratrol, Δφb(max) ≈ −10 mV) does not alter the dipole-modifying properties of the compounds (Figure 5b). This is not true in the case of chalcones/dihydrochalcones (phloretin (about −150 mV) compared to isoliquiritigenin (about −40 mV)), or isoflavones (genistein (about −70 mV) compared to daidzein (about −20 mV)) (Figure 5c).
- (6)
- The methylation of the hydroxyl group in the B-ring of biochanin A compared to genistein leads to a significant potentiation of the dipole-modifying ability of isoflavones (Figure 5c).
- (7)
- The reduction of the double bond in the heterocycle eliminates the dipole-modifying ability of the compound (taxifolin (about 0 mV) compared to quercetin (about −100 mV)) (Figure 5d). This effect can be explained by the difference in the dipole moments of the structurally related flavononols and flavonols.
- (8)
- The replacement of the oxidized propane chain connecting the two aromatic rings in the chalcone butein with the diene chain in the stilbenoid piceatannol eliminates the dipole-modifying properties (Figure 5d).
- (9)
- All glycosides are less effective at modulating the boundary potential than the related aglycones (phlorizin (about −90 mV) vs. phloretin (about −150 mV); rutin (about −40 mV) vs. quercetin (about −100 mV); and genistin (about −10 mV) vs. genistein (about −70 mV)) (Figure 5e).
- (1)
- The xanthine derivatives caffeine, pentoxifylline, 3,9-dimethylxanthine, and 7-(β-hydroxyethyl)theophylline do not affect the potential jump at the membrane–aqueous solution interface. The ability of the other tested xanthines to reduce the boundary/dipole potential increases in the following series: 1,7-dimethylxanthine ≈ 3-isobutyl-1-methylxanthine (about −20 mV) ≤ theophylline (about −40 mV). It can be assumed that the orientation of the dipole moment of xanthines relative to the normal membrane surface, which strongly depends on the type and localization of the hydrophobic substituents, is of decisive importance (Figure 6a).
- (2)
- The pronounced ability of the benzylamines capsaicin and dihydrocapsaicin to influence the membrane boundary/dipole potential (about −120 mV) can be associated with their high lipophilicity and polarity. Moreover, the saturation of the side chain (dihydrocapsaicin compared to capsaicin) is irrelevant for the dipole-modifying properties of benzylamines (Figure 6b).
- (3)
- The derivatives of β-phenylethylamine, synephrine and hordenine are almost equally effective (Δφb(max) values coincide within the measurement error), indicating that the presence of an additional OH group in the side chain (synephrine compared to hordenine) does not affect the compounds’ dipole-modifying effect (−30 ÷ −40 mV) (Figure 6b).
- (4)
- One can also note a significant decrease in the boundary/dipole potential in the presence of quinine, piperine, melatonin, colchicine, and conessine. The absence of information on several structurally similar compounds does not allow one to draw any strictly defined conclusions, and only some trends can be noted. The significant dipole-modifying activity of the effects of quinine and melatonin (about −30 mV) might be related to their structurally close quinoline and indole fragments. The ability of piperine to reduce the boundary/dipole potential (about −50 mV) might be associated with its piperidine fragment and is unlikely to be related to the piperonyl moiety, which is also present in the structure of inactive berberine. The attachment of dimethylamine to the A ring of the steroid core in the molecule of conessine instead of the hydroxyl group in the molecules of solasodine and solanidine might be responsible for the slight dipole-modifying effect of the first molecule (about −20 mV).
- (1)
- Contrary to polyphenols, all glycosylated analogs (saponins: digitonin, tribulosin, dioscin, and escin) are more effective in modulating the membrane boundary potential (Δφb(max) = −20 ÷ −50 mV)) than the corresponding aglycones (sapogenins: diosgenin, uvaol, lupeol, betulin, solasodine, and solanidine) (Δφb(max) does not exceed −6 mV) (Figure 7).
- (2)
- Dipole-modifying effects do not depend on the structure of the sapogenin (steroid or triterpenoid). Steroids (diosgenin, solasodine, and solanidine) and triterpenoids (uvaol, lupeol, and betulin) are all ineffective (Figure 7).
3. The Role of Phytochemicals in the Formation and Functioning of the Ion Channels Formed by Anti-Microbial Agents
- (1)
- As expected, a reduction in the membrane dipole potential causes a decrease in the conductance of anionic channels and an increase in the conductance of cationic pores. The flavonoids phloretin and genistein, the alkaloids capsaicin and dihydrocapsaicin, and the steroid saponin tribulosin, which drastically reduce the membrane dipole potential, (Table 1) lead to an increase in the amplitude of the GrA channels, but the observed changes are small due to the significant (about 80%) shielding of the dipole potential in the aqueous pore of the GrA channel [125,144,145]. The other tested phytochemicals that are not characterized by significant dipole-modifying effects (Table 1), such as the alkaloids pentoxifylline, piperine, and synephrine and the triterpenoid sapogenin lupeol, do not practically change the conductance of the GrA channels (Table 2).The opposite effects are observed in the cases of the SrE and AmB channels (Table 2). The dipole-potential-diminishing polyphenols phloretin, myricetin, butein, and naringenin (Table 1) cause a significant reduction in SrE pore conductance (Table 2). The changes are not expressed due to about a 90% shielding of the membrane dipole potential in the SrE pore [145]. The high shielding of the dipole potential in the SrE pore practically eliminates the effect of the phytochemicals that reduce the dipole potential by less than 50 mV, such as 4′-hydroxychalcone, cardamonin, liquiritigenin, licochalcone A, resveratrol, pentoxifylline, piperine, and synephrine (Table 1 and Table 2). The schematic representation of the mechanism of action of phloretin in the conductance of the single GrA and SrE channels is presented in Figure 9a,b.The double-length AmB channels are more sensitive to the changes in the bilayer dipole potential probably due to lower shielding (about 60%) in the aqueous pore: phloretin and quercetin, which decrease the dipole potential by more than 100 mV, lead to a 2–3-fold decrease in AmB conductance (Table 1 and Table 2).
- (2)
- In contrast to the very modest changes in the conductance of the GrA and SrE channels with the decrease in the membrane dipole potential, the changes in the lifetime of the channels are more dramatic (Table 2). Phytochemicals that diminish the membrane dipole potential might induce a several-fold increase in the dwell time of the GrA channels and a more than 100-fold reduction in the lifetime of the SrE pores. The authors of the cited publications in Table 2 attributed the changes in the lifetime of the channels to the fact that the gating particles cross the region of the potential jump during the opening/closing of the channels.
- (3)
- A decrease in the membrane dipole potential causes a significant increase in the steady-state transmembrane currents induced by SrE, PmB, and NiS and a decrease in the pore-forming activity of SuF and CeC. Taking into account that the molecules of SrE, PmB, and NiS possess a positive net charge while SuF has a negative charge, the observed changes in the transmembrane current might be rationalized by the assumption that pore formation includes the immersion of the cations/anions of the channel-forming agents into the lipid bilayer. The decrease in the membrane dipole potential facilitates the incorporation of the cations of SrE, PmB, and NiS and inhibits the introduction of the SuF anions (Figure 9c). Despite the net positive charge of the CeC molecules, a decrease in their pore-forming ability with the diminishing membrane dipole potential might be explained by the embedment of the C-terminal domain of CeC into the lipid bilayer by its negative pole [116] (Figure 9d).
4. Conclusions and Outlook
- (i).
- Phytochemicals are able to change the membrane dipole potential through two different methods: an alteration in the membrane hydration (flavonols and saponins) and an incorporation of polar plant molecules into the membrane (chalcones/dihydrochalcones, piperine, and benzylamines).
- (ii).
- The most significant structural features that determine the effect of phytochemicals on the membrane dipole potential include the following:
- -
- The glycosylation of sapogenin and flavonoid molecules;
- -
- The oxidation of the hydrocarbon fragment connecting the two phenolic rings in polyphenol molecules;
- -
- The double bond in the C-ring of flavonoids;
- -
- The localization of the hydrophobic substituents in xanthine molecules.
- (iii).
- The decrease in the membrane dipole potential with a phytochemical’s addition leads to moderate changes in the conductance of single ion-selective channels and to dramatic alterations in the lifetime and number of pores formed by anti-microbial agents.
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Al Mamari, H.H. Phenolic compounds: Classification, chemistry, and updated techniques of analysis and synthesis. In Phenolic Compounds, 1st ed.; Badria, F., Ed.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Badimon, L. Effects of polyphenol intake on metabolic syndrome: Current evidences from human trials. Oxid. Med. Cell Longev. 2017, 2017, 5812401. [Google Scholar] [CrossRef] [PubMed]
- West, T.; Atzeva, M.; Holtzman, D.M. Pomegranate polyphenols and resveratrol protect the neonatal brain against hypoxic-ischemic injury. Dev. Neurosci. 2007, 29, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Kampa, M.; Nifli, A.P.; Notas, G.; Castanas, E. Polyphenols and cancer cell growth. Rev. Physiol. Biochem. Pharmacol. 2007, 159, 79–113. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, P.; Jagadeesan, R.; Sekaran, S.; Dhanasekaran, A.; Vimalraj, S. Flavonoids: Classification, function, and molecular mechanisms involved in bone remodelling. Front. Endocrinol 2021, 12, 779638. [Google Scholar] [CrossRef]
- Luiza Koop, B.; Nascimento da Silva, M.; Diniz da Silva, F.; Thayres Dos Santos Lima, K.; Santos Soares, L.; José de Andrade, C.; Ayala Valencia, G.; Rodrigues Monteiro, A. Flavonoids, anthocyanins, betalains, curcumin, and carotenoids: Sources, classification and enhanced stabilization by encapsulation and adsorption. Food Res. Int. 2022, 153, 110929. [Google Scholar] [CrossRef]
- Pecyna, P.; Wargula, J.; Murias, M.; Kucinska, M. More than resveratrol: New insights into stilbene-based compounds. Biomolecules 2020, 10, 1111. [Google Scholar] [CrossRef]
- Athar, M.; Back, J.H.; Kopelovich, L.; Bickers, D.R.; Kim, A.L. Multiple molecular targets of resveratrol: Anti-carcinogenic mechanisms. Arch. Biochem. Biophys. 2009, 486, 95–102. [Google Scholar] [CrossRef]
- Kerem, Z.; Bilkis, I.; Flaishman, M.A.; Sivan, L. Antioxidant activity and inhibition of alpha-glucosidase by trans-resveratrol, piceid, and a novel trans-stilbene from the roots of Israeli Rumex bucephalophorus L. J. Agric. Food Chem. 2006, 54, 1243–1247. [Google Scholar] [CrossRef]
- Murias, M.; Handler, N.; Erker, T.; Pleban, K.; Ecker, G.; Saiko, P.; Szekeres, T.; Jäger, W. Resveratrol analogues as selective cyclooxygenase-2 inhibitors: Synthesis and structure-activity relationship. Bioorg. Med. Chem. 2004, 12, 5571–5578. [Google Scholar] [CrossRef]
- Gehm, B.D.; McAndrews, J.M.; Chien, P.Y.; Jameson, J.L. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc. Natl. Acad. Sci. USA 1997, 94, 14138–14143. [Google Scholar] [CrossRef] [PubMed]
- Kraft, T.E.; Parisotto, D.; Schempp, C.; Efferth, T. Fighting cancer with red wine? Molecular mechanisms of resveratrol. Crit. Rev. Food Sci. Nutr. 2009, 49, 782–799. [Google Scholar] [CrossRef] [PubMed]
- Szkudelska, K.; Szkudelski, T. Resveratrol, obesity and diabetes. Eur. J. Pharmacol. 2010, 635, 1–8. [Google Scholar] [CrossRef]
- Petrovski, G.; Gurusamy, N.; Das, D.K. Resveratrol in cardiovascular health and disease. Ann. N. Y. Acad. Sci. 2011, 1215, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Bastianetto, S.; Ménard, C.; Quirion, R. Neuroprotective action of resveratrol. Biochim. Biophys. Acta. 2015, 1852, 1195–1201. [Google Scholar] [CrossRef]
- Martens, S.; Mithöfer, A. Flavones and flavone synthases. Phytochemistry 2005, 66, 2399–2407. [Google Scholar] [CrossRef]
- Boniface, P.K.; Elizabeth, F.I. Flavones as a privileged scaffold in drug discovery: Current developments. Curr. Org. Synth. 2019, 16, 968–1001. [Google Scholar] [CrossRef]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food sources, bioavailability, metabolism, and bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef]
- Jeong, S.H.; Kim, H.H.; Ha, S.E.; Park, M.Y.; Bhosale, P.B.; Abusaliya, A.; Park, K.I.; Heo, J.D.; Kim, H.W.; Kim, G.S. Flavones: Six selected flavones and their related signaling pathways that induce apoptosis in cancer. Int. J. Mol. Sci. 2022, 23, 10965. [Google Scholar] [CrossRef]
- Chagas, M.D.S.S.; Behrens, M.D.; Moragas-Tellis, C.J.; Penedo, G.X.M.; Silva, A.R.; Gonçalves-de-Albuquerque, C.F. Flavonols and flavones as potential anti-inflammatory, antioxidant, and antibacterial compounds. Oxid. Med. Cell Longev. 2022, 2022, 9966750. [Google Scholar] [CrossRef]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef] [PubMed]
- López-Lázaro, M. Distribution and biological activities of the flavonoid luteolin. Mini Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Junior, A.G.; Tolouei, S.E.L.; Dos Reis Lívero, F.A.; Gasparotto, F.; Boeing, T.; de Souza, P. Natural agents modulating ACE-2: A review of compounds with potential against SARS-CoV-2 infections. Curr. Pharm. Des. 2021, 27, 1588–1596. [Google Scholar] [CrossRef] [PubMed]
- Malikov, V.M.; Yuldashev, M.P. Phenolic compounds of plants of the Scutellaria L. genus. Distribution, structure, and properties. Chem. Nat. Comp. 2002, 38, 358–406. [Google Scholar] [CrossRef]
- Woźniak, D.; Lamer-Zarawska, E.; Matkowski, A. Antimutagenic and antiradical properties of flavones from the roots of Scutellaria baicalensis georgi. Food/Nahrung 2004, 48, 9–12. [Google Scholar] [CrossRef]
- Osada, M.; Imaoka, S.; Funae, Y. Apigenin suppresses the expression of VEGF, an important factor for angiogenesis, in endothelial cells via degradation of HIF-1alpha protein. FEBS Lett. 2004, 575, 59–63. [Google Scholar] [CrossRef]
- Formica, J.V.; Regelson, W. Review of the biology of quercetin and related bioflavonoids. Food Chem. Toxicol. 1995, 33, 1061–1080. [Google Scholar] [CrossRef]
- Ren, J.; Lu, Y.; Qian, Y.; Chen, B.; Wu, T.; Ji, G. Recent progress regarding kaempferol for the treatment of various diseases. Exp. Ther Med. 2019, 18, 2759–2776. [Google Scholar] [CrossRef]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef]
- Massi, A.; Bortolini, O.; Ragno, D.; Bernardi, T.; Sacchetti, G.; Tacchini, M.; De Risi, C. Research progress in the modification of quercetin leading to anticancer agents. Molecules 2017, 22, 1270. [Google Scholar] [CrossRef]
- Park, K.S.; Chong, Y.; Kim, M.K. Myricetin: Biological activity related to human health. Appl. Biol. Chem. 2016, 59, 259–269. [Google Scholar] [CrossRef]
- Jo, S.; Kim, H.; Kim, S.; Shin, D.H.; Kim, M.S. Characteristics of flavonoids as potent MERS-CoV 3C-like protease inhibitors. Chem. Biol. Drug Des. 2019, 94, 2023–2030. [Google Scholar] [CrossRef] [PubMed]
- Mehany, T.; Khalifa, I.; Barakat, H.; Althwab, S.A.; Alharbi, Y.M.; El-Sohaimy, S. Polyphenols as promising biologically active substances for preventing SARS-CoV-2: A review with research evidence and underlying mechanisms. Food Biosci. 2021, 40, 100891. [Google Scholar] [CrossRef] [PubMed]
- Mak, J.C. Potential role of green tea catechins in various disease therapies: Progress and promise. Clin. Exp. Pharmacol. Physiol. 2012, 39, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Majo, D.D.; Giammanco, M.; Guardia, M.L.; Tripoli, E.; Giammanco, S.; Finotti, E. Flavanones in Citrus fruit: Structure–antioxidant activity relationships. Food Res. Int. 2005, 38, 1161–1166. [Google Scholar] [CrossRef]
- D’Archivio, M.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources and bioavailability. Ann. Ist. Super. Sanita 2007, 43, 348–361. [Google Scholar]
- Zhang, J. Flavonoids in grapefruit and commercial grapefruit juices: Concentration, distribution, and potential health benefits. Proc. Fla. State Hort. Soc. 2007, 120, 288–294. [Google Scholar]
- Kim, T.H.; Kim, G.D.; Ahn, H.J.; Cho, J.J.; Park, Y.S.; Park, C.S. The inhibitory effect of naringenin on atopic dermatitis induced by DNFB in NC/Nga mice. Life Sci. 2013, 93, 516–524. [Google Scholar] [CrossRef]
- Kim, Y.W.; Zhao, R.J.; Park, S.J.; Lee, J.R.; Cho, I.J.; Yang, C.H.; Kim, S.G.; Kim, S.C. Anti-inflammatory effects of liquiritigenin as a consequence of the inhibition of NF-kappaB-dependent iNOS and proinflammatory cytokines production. Br. J. Pharmacol. 2008, 154, 165–173. [Google Scholar] [CrossRef]
- Liu, R.T.; Zou, L.B.; Lü, Q.J. Liquiritigenin inhibits Abeta(25-35)-induced neurotoxicity and secretion of Abeta(1-40) in rat hippocampal neurons. Acta Pharmacol. Sin. 2009, 30, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Bengmark, S.; Mesa, M.D.; Gil, A. Plant-derived health: The effects of turmeric and curcuminoids. Nutr. Hosp. 2009, 24, 273–281. [Google Scholar] [PubMed]
- Selway, J.W.T. Antiviral activity of flavones and flavans. In Plant Flavonoids in Biology and Medicine: Biochemical, Pharmacological, and Structure-Activity Relationships; Cody, V., Middleton, E., Harborne, J.B., Eds.; Alan R. Liss, Inc.: New York, NY, USA, 1986; pp. 521–536. [Google Scholar]
- Nakayama, T.; Yamada, M.; Osawa, T.; Kawakishi, S. Suppression of active oxygen-induced cytotoxicity by flavonoids. Biochem. Pharmacol. 1993, 45, 265–267. [Google Scholar] [CrossRef]
- Song, Z.; Shanmugam, M.K.; Yu, H.; Sethi, G. Butein and its role in chronic diseases. Adv. Exp. Med. Biol. 2016, 928, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.R.; Prasad, S.; Sung, B.; Aggarwal, B.B. The role of chalcones in suppression of NF-κB-mediated inflammation and cancer. Int. Immunopharmacol. 2011, 11, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Loa, J.; Chow, P.; Zhang, K. Studies of structure-activity relationship on plant polyphenol-induced suppression of human liver cancer cells. Cancer Chemother. Pharmacol. 2009, 63, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Kato, M.; Suzuki, M.; Asanuma, K.; Aso, Y.; Ikeda, S.; Ishigai, M. Pharmacokinetic and pharmacodynamic modeling of the effect of an sodium-glucose cotransporter inhibitor, phlorizin, on renal glucose transport in rats. Drug Metab. Dispos. 2011, 39, 1801–1807. [Google Scholar] [CrossRef]
- vom Dahl, S.; Haussinger, D. Characterization of phloretin-sensitive urea export from the perfused rat liver. Biol. Chem. Hoppe Seyler 1996, 377, 25–37. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Calhau, C.; Morais, R.M.; Pintado, M.E. Anthocyanin extraction from plant tissues: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3072–3083. [Google Scholar] [CrossRef]
- de Sousa Moraes, L.F.; Sun, X.; Peluzio, M.D.C.G.; Zhu, M.J. Anthocyanins/anthocyanidins and colorectal cancer: What is behind the scenes? Crit. Rev. Food Sci. Nutr. 2019, 59, 59–71. [Google Scholar] [CrossRef]
- Yoon, G.A.; Park, S. Antioxidant action of soy isoflavones on oxidative stress and antioxidant enzyme activities in exercised rats. Nutr. Res. Pract. 2014, 8, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E., Jr.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Liu, R.H.; Lin, S.; Zhang, P.Z.; Chen, L.Y.; Huang, H.L.; Mei, D.Y. Neoflavonoids and their pharmacological activities in Dalbergia genus. Zhongguo Zhong Yao Za Zhi 2017, 42, 4707–4715. (In Chinese) [Google Scholar] [CrossRef]
- Mukerjee, S.; Saroja, T.; Seshadri, T.R. Dalbergichromene: A new neoflavonoid from stem-bark and heartwood of Dalbergia sissoo. Tetrahedron 1971, 27, 799–803. [Google Scholar] [CrossRef]
- Aniszewski, T. Alkaloids—Secrets of Life. Alkaloid Chemistry, Biological Significance, Applications and Ecological Role; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar] [CrossRef]
- Dey, P.; Kundu, A.; Kumar, A.; Gupta, M.; Lee, B.M.; Bhakta, T.; Dash, S.; Kim, H.S. Analysis of alkaloids (indole alkaloids, isoquinoline alkaloids, tropane alkaloids). Rec. Adv. Nat. Prod. Anal. 2020, 505–567. [Google Scholar] [CrossRef]
- Sultana, S.; Asif, H.M. Review: Medicinal plants combating against hypertension: A green antihypertensive approach. Pak. J. Pharm. Sci. 2017, 30, 2311–2319. [Google Scholar] [PubMed]
- Tsuchiya, H. Anesthetic agents of plant origin: A review of phytochemicals with anesthetic activity. Molecules 2017, 22, 1369. [Google Scholar] [CrossRef]
- Qiu, S.; Sun, H.; Zhang, A.H.; Xu, H.Y.; Yan, G.L.; Han, Y.; Wang, X.J. Natural alkaloids: Basic aspects, biological roles, and future perspectives. Chin. J. Nat. Med. 2014, 12, 401–406. [Google Scholar] [CrossRef]
- Tiwari, R.; Latheef, S.K.; Ahmed, I.; Iqbal, H.M.N.; Bule, M.H.; Dhama, K.; Samad, H.A.; Karthik, K.; Alagawany, M.; El-Hack, M.E.A.; et al. Herbal immunomodulators—A remedial panacea for designing and developing effective drugs and medicines: Current scenario and future prospects. Curr. Drug. Metab. 2018, 19, 264–301. [Google Scholar] [CrossRef]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; Dewick, P.M., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2002; p. 552. [Google Scholar]
- Pelletier, S.W. (Ed.) Alkaloids: Chemical and Biological Perspectives, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 1–542. [Google Scholar]
- MacNeil, S.D.; Rotenberg, B.; Sowerby, L.; Allen, B.; Richard, L.; Shariff, S.Z. Medical use of cocaine and perioperative morbidity following sinonasal surgery-A population study. PLoS ONE 2020, 15, e0236356. [Google Scholar] [CrossRef]
- Kluska, M.; Marciniuk-Kluska, A.; Prukała, D.; Prukała, W. Analytics of quinine and its derivatives. Crit. Rev. Anal. Chem. 2016, 46, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Tawari, S.; Mundhada, D.; Nadeem, S. Protective effect of berberine, an isoquinoline alkaloid ameliorates ethanol-induced oxidative stress and memory dysfunction in rats. Pharmacol. Biochem. Behav. 2015, 136, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Hussain, G.; Rasul, A.; Anwar, H.; Aziz, N.; Razzaq, A.; Wei, W.; Ali, M.; Li, J.; Li, X. Role of plant derived alkaloids and their mechanism in neurodegenerative disorders. Int. J. Biol. Sci. 2018, 14, 341–357. [Google Scholar] [CrossRef] [PubMed]
- Pawar, K.S.; Mastud, R.N.; Pawar, S.K.; Pawar, S.S.; Bhoite, R.R.; Bhoite, R.R.; Kulkarni, M.V.; Deshpande, A.R. Oral curcumin with piperine as adjuvant therapy for the treatment of COVID-19: A randomized clinical trial. Front. Pharmacol. 2021, 12, 669362. [Google Scholar] [CrossRef] [PubMed]
- Gasparyan, A.Y.; Ayvazyan, L.; Yessirkepov, M.; Kitas, G.D. Colchicine as an anti-inflammatory and cardioprotective agent. Expert. Opin. Drug Metab. Toxicol. 2015, 11, 1781–1794. [Google Scholar] [CrossRef]
- Mikolajewska, A.; Fischer, A.L.; Piechotta, V.; Mueller, A.; Metzendorf, M.I.; Becker, M.; Dorando, E.; Pacheco, R.L.; Martimbianco, A.L.C.; Riera, R.; et al. Colchicine for the treatment of COVID-19. Cochrane Database Syst. Rev. 2021, 10, CD015045. [Google Scholar] [CrossRef]
- Clark, R.; Lee, S.H. Anticancer properties of capsaicin against human cancer. Anticancer Res. 2016, 36, 837–843. [Google Scholar]
- Isah, T. Anticancer alkaloids from trees: Development into drugs. Pharmacogn. Rev. 2016, 10, 90–99. [Google Scholar] [CrossRef]
- Hashmi, M.A.; Khan, A.; Farooq, U.; Khan, S. Alkaloids as cyclooxygenase inhibitors in anticancer drug discovery. Curr. Protein Pept. Sci. 2018, 19, 292–301. [Google Scholar] [CrossRef]
- Nikolic, N.C.; Stankovic, M.Z. Solanidine hydrolytic extraction and separation from the potato (Solanum tuberosum L.) vines by using solid-liquid-liquid systems. J. Agric. Food Chem. 2003, 51, 1845–1849. [Google Scholar] [CrossRef]
- Bushway, R.J.; Savage, S.A.; Ferguson, B.S. Inhibition of acetyl cholinesterase by solanaceous glycoalkaloids and alkaloids. Am. Potato J. 1987, 64, 409–413. [Google Scholar] [CrossRef]
- Järvinen, R. Carotenoids, retinoids, tocopherols and tocotrienols in the diet; the Finnish Mobile Clinic Health Examination Survey. Int. J. Vitam. Nutr. Res. 1995, 65, 24–30. [Google Scholar] [PubMed]
- Simon, J.E. Essential oils and culinary herbs. In Advances in New Crops; Janick, J., Simon, J.E., Eds.; Timber Press: Portland, OR, USA, 1990; pp. 472–483. [Google Scholar]
- Gao, J.M.; Wu, W.J.; Zhang, J.W.; Konishi, Y. The dihydro-beta-agarofuran sesquiterpenoids. Nat. Prod. Rep. 2007, 24, 1153–1189. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.R. Diterpenoids. Nat. Prod. Rep. 2009, 26, 1156–1171. [Google Scholar] [CrossRef] [PubMed]
- Connolly, J.D.; Hill, R.A. Triterpenoids. Nat. Prod. Rep. 2010, 27, 79–132. [Google Scholar] [CrossRef] [PubMed]
- Connolly, J.D.; Hill, R.A. Triterpenoids. Nat. Prod. Rep. 2007, 24, 465–486. [Google Scholar] [CrossRef]
- Bangham, A.D.; Horne, R.W.; Glauret, A.M.; Dingle, J.T.; Lucy, J.A. Action of saponin on biological cell membranes. Nature 1962, 196, 952–955. [Google Scholar] [CrossRef]
- Gestetner, B.; Assa, Y.; Henis, Y.; Tencer, Y.; Rotman, M.; Birk, Y.; Bondi, A. Interaction of leucerne saponins with steroids. Biochim. Biophys. Acta 1972, 270, 181–187. [Google Scholar] [CrossRef]
- Vinarova, L.; Vinarov, Z.; Atanasov, V.; Pantcheva, I.; Tcholakova, S.; Denkov, N.; Stoyanov, S. Lowering of cholesterol bioaccessibility and serum concentrations by saponins: In vitro and in vivo studies. Food Funct. 2015, 6, 501–512. [Google Scholar] [CrossRef]
- Bruneton, J. Pharmacognosie-Phytochimie, Plantes Médicinales, Lavoisier 4e éd, revue et augmentée; Tec & Dac-Editions Médicinales Internationals: Paris, France, 2009; p. 1288. [Google Scholar]
- Dinda, B.; Debnath, S.; Mohanta, B.C.; Harigaya, Y. Naturally occurring triterpenoid saponins. Chem. Biodivers. 2010, 7, 2327–2580. [Google Scholar] [CrossRef]
- Langi, P.; Kiokias, S.; Varzakas, T.; Proestos, C. Carotenoids: From plants to food and feed industries. Methods Mol. Biol. 2018, 1852, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Netala, V.R.; Kotakadi, V.S.; Bobbu, P.; Gaddam, S.A.; Tartte, V. Endophytic fungal isolate mediated biosynthesis of silver nanoparticles and their free radical scavenging activity and anti microbial studies. 3 Biotech 2016, 6, 132. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.L.; Zhang, Y.J.; Gao, W.Y.; Man, S.L.; Wang, Y. In vitro and in vivo anticancer activity of steroid saponins of Paris polyphylla var. yunnanensis. Exp. Oncol. 2009, 31, 27–32. [Google Scholar]
- Yassin, A.M.; El-Deeb, N.M.; Metwaly, A.M.; Fawal, G.F.; Radwan, M.M.; Hafez, E.E. Induction of apoptosis in human cancer cells through extrinsic and intrinsic pathways by balanites Aegyptiaca furostanol saponins and saponin-coated silvernanoparticles. Der. Pharm. Lett. 2013, 182, 1675–1693. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.S.; Supan, E.M.; Ali, S.N. Toxic epidermal necrolysis associated with rifaximin. Am. J. Health Syst. Pharm. 2013, 70, 874–876. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, S. Electrostatic potentials at membrane-solution interfaces. Curr. Topics Membr. Transp. 1977, 9, 71–144. [Google Scholar]
- Liberman, E.A.; Topaly, V.P. Permeability of bimolecular phospholipid membranes for fat-soluble ions. Biofizika 1969, 14, 452–461. [Google Scholar]
- Hladky, S.B.; Haydon, D.A. Ion transfer across lipid membranes in the presence of gramicidin A. I. Studies of the unit conductance channel. Biochim. Biophys. Acta 1972, 274, 294–312. [Google Scholar] [CrossRef]
- Brockmann, H. Dipole potential of lipid membranes. Chem. Phys. Lipids 1994, 73, 57–79. [Google Scholar] [CrossRef]
- Peterson, U.; Mannock, D.A.; Lewis, R.N.; Pohl, P.; McElhaney, R.N.; Pohl, E.E. Origin of membrane dipole potential: Contribution of the phospholipid fatty acid chains. Chem. Phys. Lipids 2002, 117, 19–27. [Google Scholar] [CrossRef]
- Flewelling, R.F.; Hubbell, W.L. Hydrophobic ion interactions with membranes. Thermodynamic analysis of tetraphenylphosphonium binding to vesicles. Biophys. J. 1986, 49, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Flewelling, R.F.; Hubbell, W.L. The membrane dipole potential in a total membrane potential model. Applications to hydrophobic ion interactions with membranes. Biophys. J. 1986, 49, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Franklin, J.C.; Cafiso, D.S. Internal electrostatic potentials in bilayers: Measuring and controlling dipole potentials in lipid vesicles. Biophys. J. 1993, 65, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Cseh, R.; Benz, R. The adsorption of phloretin to lipid monolayers and bilayers cannot be explained by langmuir adsorption isotherms alone. Biophys. J. 1998, 74, 1399–1408. [Google Scholar] [CrossRef]
- Brockman, H.L.; Momsen, M.M.; Brown, R.E.; He, L.; Chun, J.; Byun, H.S.; Bittman, R. The 4,5-double bond of ceramide regulates its dipole potential, elastic properties, and packing behavior. Biophys. J. 2004, 87, 1722–1731. [Google Scholar] [CrossRef]
- Cladera, J.; Martin, I.; Ruysschaert, J.M.; O’Shea, P. Characterization of the sequence of interactions of the fusion domain of the simian immunodeficiency virus with membranes: Role of the membrane dipole potential. J. Biol. Chem. 1999, 274, 29951–29959. [Google Scholar] [CrossRef]
- Guillén, J.; Kinnunen, P.K.; Villalaín, J. Membrane insertion of the three main membranotropic sequences from SARS-CoV S2 glycoprotein. Biochim. Biophys. Acta 2008, 1778, 2765–2774. [Google Scholar] [CrossRef]
- Mojumdar, E.H.; Lyubartsev, A.P. Molecular dynamics simulations of local anesthetic articaine in a lipid bilayer. Biophys. Chem. 2010, 153, 27–35. [Google Scholar] [CrossRef]
- Efimova, S.S.; Zakharova, A.A.; Schagina, L.V.; Ostroumova, O.S. Local anesthetics affect gramicidin A channels via membrane electrostatic potentials. J. Membr. Biol. 2016, 249, 781–787. [Google Scholar] [CrossRef]
- Efimova, S.S.; Chulkov, E.G.; Ostroumova, O.S. Lipid-mediated mode of action of local anesthetics on lipid pores induced by polyenes, peptides and lipopeptides. Colloids Surf. B Biointerfaces 2018, 166, 1–8. [Google Scholar] [CrossRef]
- Latorre, R.; Donovan, J.J. Modulation of alamethicin-induced conductance by membrane composition. Acta Physiol. Scand. Suppl. 1980, 481, 37–45. [Google Scholar] [PubMed]
- Busath, D.D.; Thulin, C.D.; Hendershot, R.W.; Phillips, L.R.; Maughan, P.; Cole, C.D.; Bingham, N.C.; Morrison, S.; Baird, L.C.; Hendershot, R.J.; et al. Noncontact dipole effects on channel permeation. I. Experiments with (5F-indole)Trp13 gramicidin A channels. Biophys. J. 1998, 75, 2830–2844. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.C.; Koeppe, R.E.; Andersen, O.S. Genistein can modulate channel function by a phosphorylation-independent mechanism: Importance of hydrophobic mismatch and bilayer mechanics. Biochemistry 2003, 42, 13646–13658. [Google Scholar] [CrossRef] [PubMed]
- Luchian, T.; Mereuta, L. Phlorizin- and 6-ketocholestanol-mediated antagonistic modulation of alamethicin activity in phospholipid planar membranes. Langmuir 2006, 22, 8452–8457. [Google Scholar] [CrossRef] [PubMed]
- Ostroumova, O.S.; Kaulin, Y.A.; Gurnev, A.P.; Schagina, L.V. Effect of agents modifying the membrane dipole potential on properties of syringomycin E channels. Langmuir 2007, 23, 6889–6892. [Google Scholar] [CrossRef]
- Mereuta, L.; Luchian, T.; Park, Y.; Hahm, K.S. Single-molecule investigation of the interactions between reconstituted planar lipid membranes and an analogue of the HP(2-20) antimicrobial peptide. Biochem. Biophys. Res. Commun. 2008, 373, 467–472. [Google Scholar] [CrossRef]
- Apetrei, A.; Mereuta, L.; Luchian, T. The RH 421 styryl dye induced, pore model-dependent modulation of antimicrobial peptides activity in reconstituted planar membranes. Biochim. Biophys. Acta 2009, 1790, 809–816. [Google Scholar] [CrossRef]
- Ostroumova, O.S.; Malev, V.V.; Ilin, M.G.; Schagina, L.V. Surfactin activity depends on the membrane dipole potential. Langmuir 2010, 26, 15092–15097. [Google Scholar] [CrossRef]
- Efimova, S.S.; Schagina, L.V.; Ostroumova, O.S. Channel forming activity of cecropins in lipid bilayers. Effect of agents modifying the membrane dipole potential. Langmuir 2014, 30, 7884–7892. [Google Scholar] [CrossRef]
- Efimova, S.S.; Zakharova, A.A.; Medvedev, R.Y.; Ostroumova, O.S. Ion channels induced by antimicrobial agents in model lipid membranes are modulated by plant polyphenols through surrounding lipid media. J. Membr. Biol. 2018, 251, 551–562. [Google Scholar] [CrossRef]
- Andersen, O.S.; Finkelstein, A.; Katz, I.; Cass, A. Effect of phloretin on the permeability of thin lipid membranes. J. Gen. Physiol. 1976, 67, 749–771. [Google Scholar] [CrossRef] [PubMed]
- Melnik, E.; Latorre, R.; Hall, J.E.; Tosteson, D.C. Phloretin-induced changes in ion transport across lipid bilayer membranes. J. Gen. Physiol. 1977, 69, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Bechinger, B.; Seelig, J. Interaction of electric dipoles with phospholipid head groups. A 2H and 31P NMR study of phloretin and phloretin analogues in phosphatidylcholine membranes. Biochemistry 1991, 30, 3923–3929. [Google Scholar] [CrossRef] [PubMed]
- Cseh, R.; Benz, R. Interaction of phloretin with lipid monolayers: Relationship between structural changes and dipole potential change. Biophys. J. 1999, 77, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Efimova, S.S.; Ostroumova, O.S. Effect of dipole modifiers on the magnitude of the dipole potential of sterol-containing bilayers. Langmuir 2012, 28, 9908–9914. [Google Scholar] [CrossRef]
- Ostroumova, O.S.; Efimova, S.S.; Schagina, L.V. Phloretin induced reduction in dipole potential of sterol containing bilayers. J. Membr. Biol. 2013, 246, 985–991. [Google Scholar] [CrossRef]
- Pohl, P.; Rokitskaya, T.I.; Pohl, E.E.; Saparov, S.M. Permeation of phloretin across bilayer lipid membranes monitored by dipole potential and microelectrode measurements. Biochim. Biophys. Acta 1997, 1323, 163–172. [Google Scholar] [CrossRef]
- Efimova, S.S.; Zakharova, A.A.; Ostroumova, O.S. Alkaloids modulate the functioning of ion channels produced by antimicrobial agents via an influence on the lipid host. Front. Cell Dev. Biol. 2020, 8, 537. [Google Scholar] [CrossRef]
- Efimova, S.S.; Ostroumova, O.S. Is the membrane lipid matrix a key target for action of pharmacologically active plant saponins? Int. J. Mol. Sci. 2021, 22, 3167. [Google Scholar] [CrossRef]
- Gawrisch, K.; Ruston, D.; Zimmerberg, J.; Parsegian, V.A.; Rand, R.P.; Fuller, N. Membrane dipole potentials, hydration forces, and the ordering of water at membrane surfaces. Biophys. J. 1992, 61, 1213–1223. [Google Scholar] [CrossRef]
- Zlodeeva, P.D.; Shekunov, E.V.; Ostroumova, O.S.; Efimova, S.S. The degree of hydroxylation of phenolic rings determines the ability of flavonoids and stilbenes to inhibit calcium-mediated membrane fusion. Nutrients 2023, 15, 1121. [Google Scholar] [CrossRef]
- Efimova, S.S.; Zakharova, A.A.; Chernyshova, D.N.; Ostroumova, O.S. The specific effect of grapefruit seed, sea-buckthorn leaves, and chaga extracts on the properties of model lipid membranes. Cell Tiss. Biol. 2023, 17, 72–80. [Google Scholar] [CrossRef]
- Rokitskaya, T.I.; Antonenko, Y.N.; Kotova, E.A. Effect of the dipole potential of a bilayer lipid membrane on gramicidin channel dissociation kinetics. Biophys. J. 1997, 73, 850–854. [Google Scholar] [CrossRef]
- Rokitskaya, T.I.; Kotova, E.A.; Antonenko, Y.N. Membrane dipole potential modulates proton conductance through gramicidin channel: Movement of negative ionic defects inside the channel. Biophys. J. 2002, 82, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Latorre, R.; Alvarez, O. Voltage-dependent channels in planar lipid bilayer membranes. Physiol. Rev. 1981, 61, 77–150. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, A.A.; Efimova, S.S.; Ostroumova, O.S. Lipid microenvironment modulates the pore-forming ability of polymyxin B. Antibiotics 2022, 11, 1445. [Google Scholar] [CrossRef]
- Chernyshova, D.N.; Tyulin, A.A.; Ostroumova, O.S.; Efimova, S.S. Discovery of the potentiator of the pore-forming ability of lantibiotic nisin: Perspectives for anticancer therapy. Membranes 2022, 12, 1166. [Google Scholar] [CrossRef]
- Ostroumova, O.S.; Efimova, S.S.; Schagina, L.V. Probing amphotericin B single channel activity by membrane dipole modifiers. PLoS ONE. 2012, 7, e30261. [Google Scholar] [CrossRef]
- Finkelstein, A.; Andersen, O.S. The gramicidin A channel: A review of its permeability characteristics with special reference to the single-file aspect of transport. J. Membr. Biol. 1981, 59, 155–171. [Google Scholar] [CrossRef]
- Kelkar, D.A.; Chattopadhyay, A. The gramicidin ion channel: A model membrane protein. Biochim. Biophys. Acta 2007, 1768, 2011–2025. [Google Scholar] [CrossRef]
- Andersen, O.S.; Koeppe, R.E., 2nd. Molecular determinants of channel function. Physiol. Rev. 1992, 72 (Suppl. 4), S89–S158. [Google Scholar] [CrossRef] [PubMed]
- Malev, V.V.; Schagina, L.V.; Gurnev, P.A.; Takemoto, J.Y.; Nestorovich, E.M.; Bezrukov, S.M. Syringomycin E channel: A lipidic pore stabilized by lipopeptide? Biophys. J. 2002, 82, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- Ostroumova, O.S.; Gurnev, P.A.; Schagina, L.V.; Bezrukov, S.M. Asymmetry of syringomycin E channel studied by polymer partitioning. FEBS Lett. 2007, 581, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, T. The structure and function of amphotericin B cholesterol pores in lipid bilyer membranes. Ann. N. Y. Acad. Sci. 1974, 235, 448–468. [Google Scholar] [CrossRef] [PubMed]
- Marty, A.; Finkelstein, A. Pores formed in lipid bilayer membranes by nystatin, Differences in its one-sided and two-sided action. J. Gen. Physiol. 1975, 65, 515–526. [Google Scholar] [CrossRef]
- Borisova, M.P.; Brutyan, R.A.; Ermishkin, L.N. Mechanism of anion-cation selectivity of amphotericin B channels. J. Membr. Biol. 1986, 90, 13–20. [Google Scholar] [CrossRef]
- Jordan, P.C. Electrostatic modeling of ion pores. II. Effects attributable to the membrane dipole potential. Biophys. J. 1983, 41, 189–195. [Google Scholar] [CrossRef]
- Ostroumova, O.S.; Shchagina, L.V.; Malev, V.V. The effect of dipole potential of lipid bilayers on the properties of ion channels formed by cyclic lipodepsipeptide syringomycin E. Biochem. (Moscow) Suppl. Ser. A Membr. Cell Biol. 2008, 2, 259–270. [Google Scholar] [CrossRef]





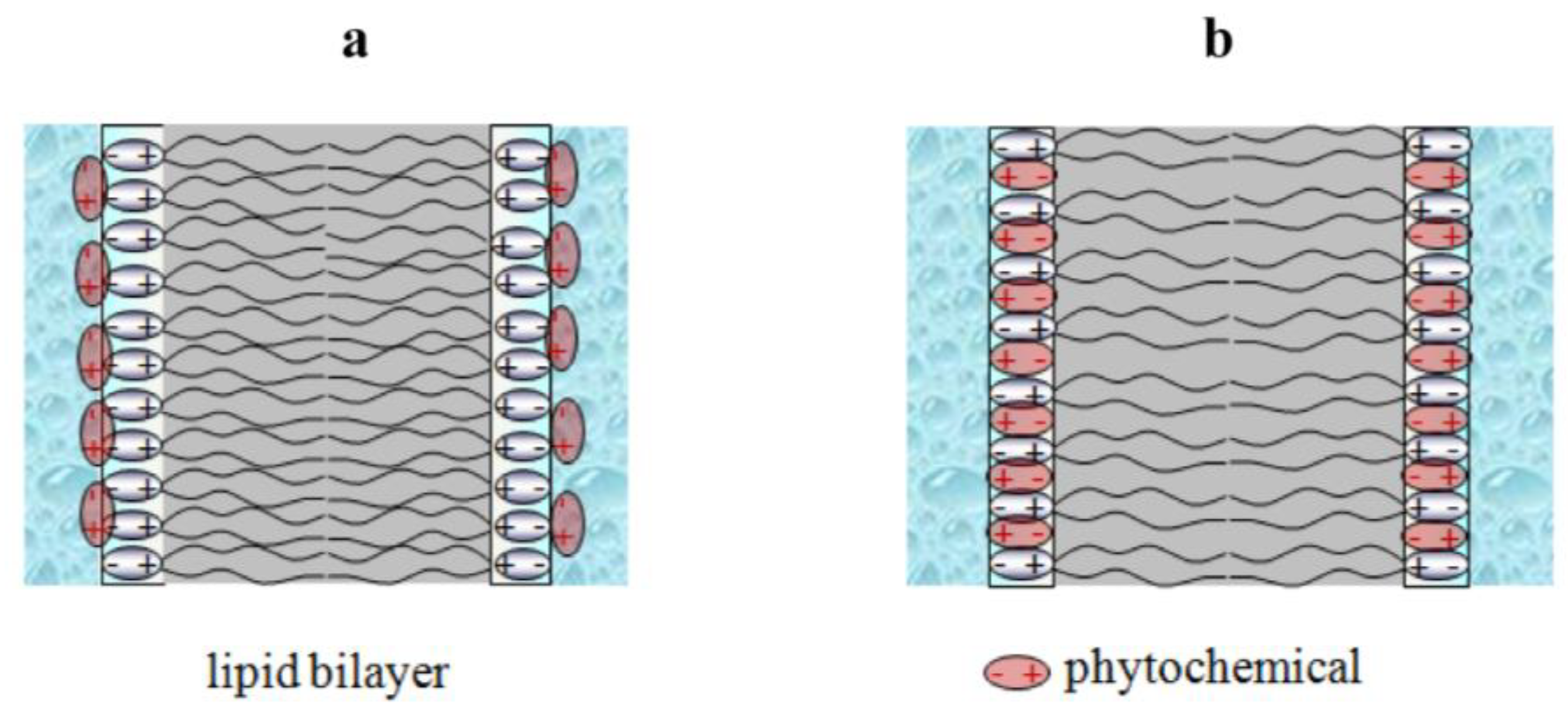

| Class | Phytochemical | Charge * | LogD * | µ $, D | −Δφb(max), mV | −Δφd(max), mV | References |
|---|---|---|---|---|---|---|---|
| polyphenols # | phloretin | −0.23 | 3.79 | 3.22 | 147 ± 7 ~220 ~190 | – | [122] [100] @ [124] Ω |
| phlorizin | −0.27 | 0.85 | 1.63 | 92 ± 4 | nd | [122] | |
| 4′-hydroxychalcone | −0.25 | 3.46 | 2.43 | 38 ± 7 | 35 ± 10 | [117] | |
| butein | −0.76 | 2.84 | 5.90 | 120 ± 19 | 150 ± 12 | ||
| cardamonin | −0.66 | 3.36 | 1.83 | 59 ± 12 | 38 ± 9 | ||
| licochalcone A | −0.30 | 4.67 | 3.79 | 66 ± 12 | 43 ± 11 | ||
| isoliquiritigenin | −0.75 | 3.15 | 2.69 | 41 ± 12 | 31 ± 11 | unpublished data | |
| liquiritigenin | −0.30 | 2.34 | 0.34 | 66 ± 25 | 30 ± 13 | [117] | |
| naringenin | −0.28 | 2.70 | 1.30 | 72 ± 11 | 73 ± 14 | ||
| quercetin | −1.25 | 1.00 | 4.42 | 104 ± 7 | nd | [122] | |
| myricetin | −1.42 | 0.65 | 4.95 | 111 ± 11 | nd | ||
| rutin | −1.20 | −2.02 | 2.51 | 42 ± 6 | nd | ||
| biochanin A | −1.02 | 2.27 | 3.29 | 109 ± 11 | nd | ||
| genistein | −1.06 | 2.12 | 3.81 | 70 ± 10 | nd | ||
| genistin | −0.60 | 0.44 | 3.52 | 7 ± 2 | nd | ||
| daidzein | −0.92 | 1.77 | 2.57 | 20 ± 6 | nd | ||
| catechin | −0.03 | 1.78 | 3.67 | 6 ± 2 | nd | ||
| taxifolin | −0.34 | 1.65 | 2.52 | 2 ± 1 | nd | ||
| resveratrol | −0.11 | 3.37 | 0.53 | 11 ± 4 | 9 ± 5 | [117] | |
| piceatannol | −0.12 | 3.06 | 1.59 | 15 ± 4 | 10 ± 6 | unpublished data | |
| alkaloids § | caffeine | 0 | −0.55 | 3.29 | 2 ± 2 | nd | [125] |
| pentoxifylline | 0 | 0.23 | 5.44 | 4 ± 2 | nd | ||
| 1,7–dimethylxanthine | 0 | 0.24 | 3.59 | 23 ± 5 | 21 ± 6 | ||
| 3,9–dimethylxanthine | −0.02 | −0.82 | 7.29 | 4 ± 3 | nd | ||
| theophylline | −0.28 | −0.89 | 6.53 | 41 ± 16 | 40 ± 5 | ||
| 3–isobutyl–1–methylxanthine | −0.09 | 0.40 | 6.99 | 22 ± 3 | 20 ± 9 | ||
| 7–(β–hydroxyethyl) theophylline | 0 | −1.24 | 2.26 | 6 ± 2 | nd | ||
| lupinine | 1.00 | −1.52 | 1.24 | 3 ± 3 | nd | ||
| cotinine | 0 | 0.21 | 4.95 | 6 ± 2 | nd | ||
| atropine | 0.99 | −0.41 | 3.58 | 4 ± 4 | nd | ||
| quinine | 0.98 | 0.86 | 2.39 | 26 ± 9 | 16 ± 6 | ||
| berberine | 1.00 | −1.28 | nd | 3 ± 2 | nd | ||
| piperine | 0 | 2.78 | 5.37 | 51 ± 8 | 40 ± 13 | ||
| melatonin | 0 | 1.15 | 4.93 | 26 ± 9 | 15 ± 8 | ||
| tabersonine | 0.98 | 0.90 | 1.28 | 6 ± 2 | nd | ||
| colchicine | 0 | 1.46 | 6.53 | 27 ± 5 | nd | ||
| capsaicin | 0 | 3.75 | 4.66 | 118 ± 11 | 92 ± 11 | ||
| dihydrocapsaicin | 0 | 4.11 | 4.95 | 119 ± 12 | 92 ± 15 | ||
| hordenine | 0.98 | 0.06 | 0.99 | 29 ± 8 | 23 ± 11 | ||
| synephrine | 0.97 | −1.39 | 2.32 | 41 ± 12 | 24 ± 9 | ||
| conessine | nd | −1.45 | 1.81 | 19 ± 6 | 12 ± 7 | ||
| solasodine | 0.99 | 2.50 | nd | 5 ± 2 | nd | ||
| solanidine | 0.98 | 1.39 | 1.24 | 2 ± 2 | nd | [126] | |
| saponins and related compounds & | digitonin | 0 | −4.96 | 3.79 | 36 ± 4 | na | |
| tribulosin | nd | nd | 5.96 | 47 ± 6 | nd | ||
| dioscin | 0 | 1.71 | 4.27 | 39 ± 8 | nd | ||
| diosgenin | 0 | 4.93 | 1.38 | 6 ± 2 | nd | ||
| escin | −1.00 | −4.29 | 7.71 | 20 ± 5 | nd | ||
| uvaol | 0 | 6.11 | 1.41 | 1 ± 1 | nd | ||
| lupeol | 0 | 7.45 | 1.23 | 1 ± 1 | nd | ||
| betulin | 0 | 6.17 | 0.99 | 1 ± 1 | nd |
| Agent | Phytochemical * | Parameters | References | |
|---|---|---|---|---|
| gsc/gosc | τsc/τosc | |||
| GrA | phloretin | 1.4 ± 0.2 | 12.7 ± 4.4 | [110,130] |
| genistein | 1.1 ± 0.1 | 4.3 ± 1.5 | [110] | |
| daidzein | 1.0 ± 0.1 | 2.4 ± 0.3 | ||
| pentoxifylline | 1.0 ± 0.1 | 1.2 ± 0.6 | [125] | |
| piperine | 0.9 ± 0.1 | 0.9 ± 0.6 | ||
| capsaicin | 1.1 ± 0.1 | 2.0 ± 1.3 | ||
| dihydrocapsaicin | 1.2 ± 0.1 | 1.6 ± 0.8 | ||
| synephrine | 1.1 ± 0.1 | 0.9 ± 0.6 | ||
| tribulosin | 1.1 ± 0.1 | 1.6 ± 0.6 | [126] | |
| lupeol | 1.0 ± 0.1 | 1.0 ± 0.5 | ||
| SrE | phloretin | 0.6 ± 0.1 | 0.01 ± 0.01 | [112] |
| myricetin | 0.6 ± 0.2 | 0.05 ± 0.01 | unpublished data & | |
| 4′-hydroxychalcone | 0.9 ± 0.2 | nd | [117] | |
| butein | 0.6 ± 0.1 | nd | ||
| cardamonin | 1.0 ± 0.2 | nd | ||
| liquiritigenin | 0.9 ± 0.2 | nd | ||
| naringenin | 0.7 ± 0.1 | nd | ||
| licochalcone A | 1.0 ± 0.2 | nd | ||
| resveratrol | 0.9 ± 0.2 | nd | ||
| pentoxifylline | 1.0 ± 0.1 | 1.2 ± 0.1 | [125] | |
| piperine | 1.0 ± 0.1 | 0.04 ± 0.01 | ||
| capsaicin | 0.9 ± 0.1 | 0.01 ± 0.01 | ||
| dihydrocapsaicin | 0.9 ± 0.1 | 0.01 ± 0.01 | ||
| synephrine | 1.0 ± 0.1 | 0.6 ± 0.1 | ||
| AmB | phloretin | 0.3 ± 0.1 | nd | [135] |
| quercetin | 0.6 ± 0.1 | nd | ||
| Agent | Phytochemical * | Imc/Iomc | References |
|---|---|---|---|
| SrE | phloretin | ~20,000 | [145] |
| SuF | phloretin | 0.02 ÷ 0.2 | [115] |
| CeC | phloretin | 0.3 ± 0.2 | [116,125] |
| myricetin | 1.1 ± 0.1 | ||
| pentoxifylline | 0.9 ± 0.1 | ||
| piperine | 0.3 ± 0.1 | ||
| capsaicin | 0.1 ± 0.1 | ||
| dihydrocapsaicin | 0.2 ± 0.1 | ||
| synephrine | 1.1 ± 0.4 | ||
| PmB | phloretin | 28 ± 4 | [133] |
| NiS | phloretin | 5.3 ± 1.3 | [134] |
| capsaicin | 11.3 ± 1.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efimova, S.S.; Ostroumova, O.S. Modulation of the Dipole Potential of Model Lipid Membranes with Phytochemicals: Molecular Mechanisms, Structure–Activity Relationships, and Implications in Reconstituted Ion Channels. Membranes 2023, 13, 453. https://doi.org/10.3390/membranes13040453
Efimova SS, Ostroumova OS. Modulation of the Dipole Potential of Model Lipid Membranes with Phytochemicals: Molecular Mechanisms, Structure–Activity Relationships, and Implications in Reconstituted Ion Channels. Membranes. 2023; 13(4):453. https://doi.org/10.3390/membranes13040453
Chicago/Turabian StyleEfimova, Svetlana S., and Olga S. Ostroumova. 2023. "Modulation of the Dipole Potential of Model Lipid Membranes with Phytochemicals: Molecular Mechanisms, Structure–Activity Relationships, and Implications in Reconstituted Ion Channels" Membranes 13, no. 4: 453. https://doi.org/10.3390/membranes13040453
APA StyleEfimova, S. S., & Ostroumova, O. S. (2023). Modulation of the Dipole Potential of Model Lipid Membranes with Phytochemicals: Molecular Mechanisms, Structure–Activity Relationships, and Implications in Reconstituted Ion Channels. Membranes, 13(4), 453. https://doi.org/10.3390/membranes13040453






