Techno-Economic Analysis of Vacuum Membrane Distillation for Seawater Desalination
Abstract
1. Introduction
2. Methodology
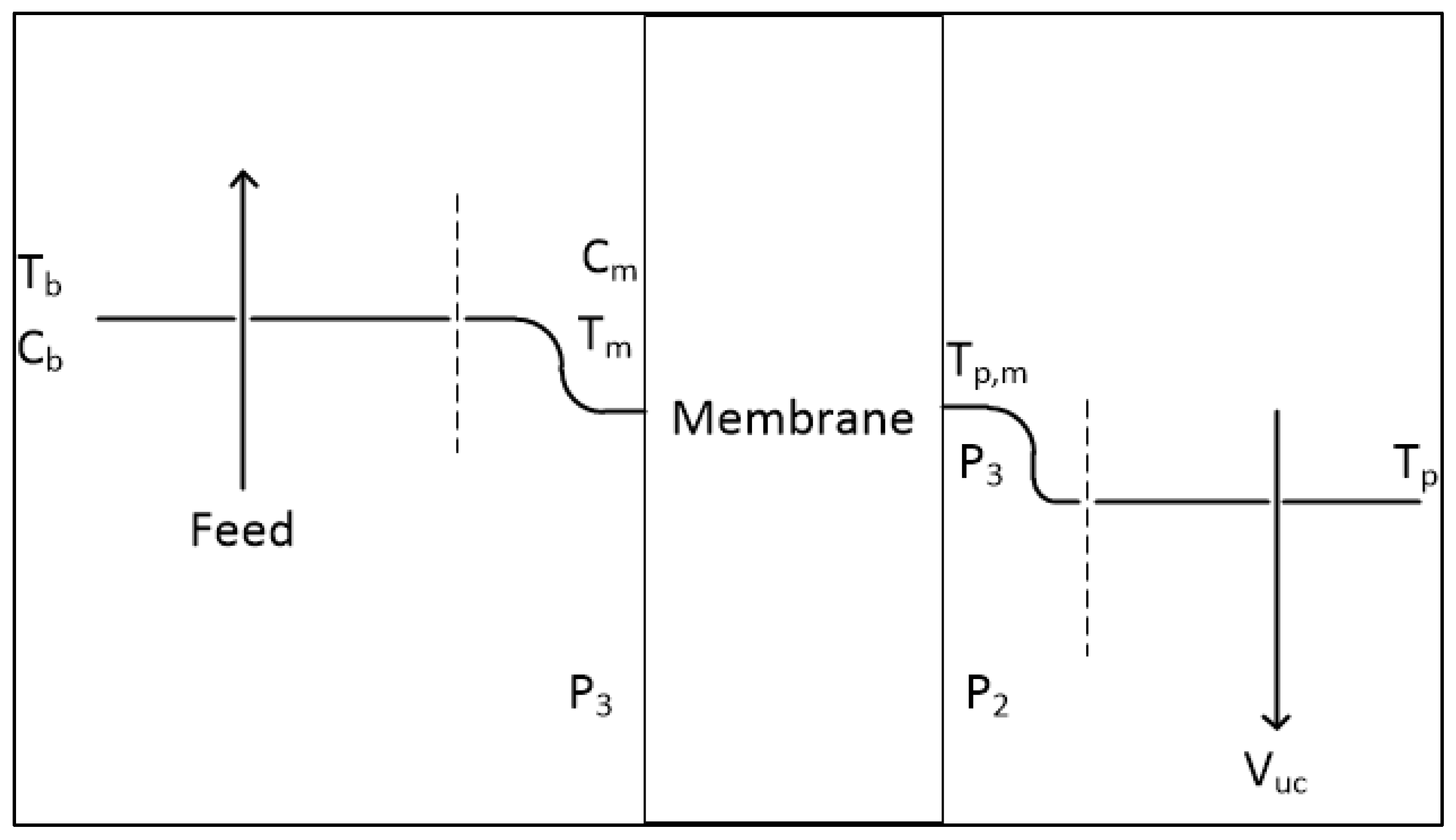
2.1. Governing Equations
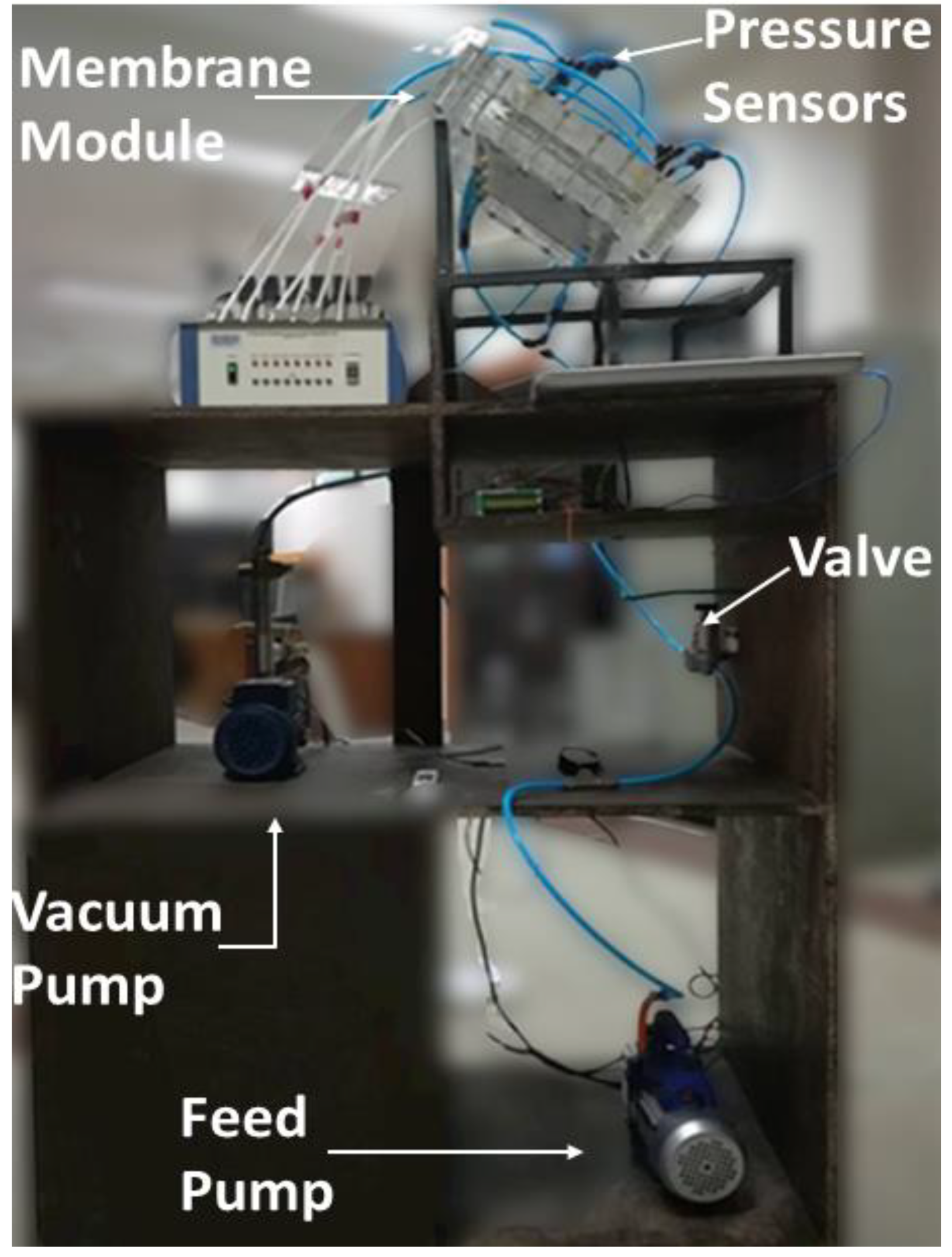
2.2. Experimental Setup
2.3. Economic Analysis
3. Results
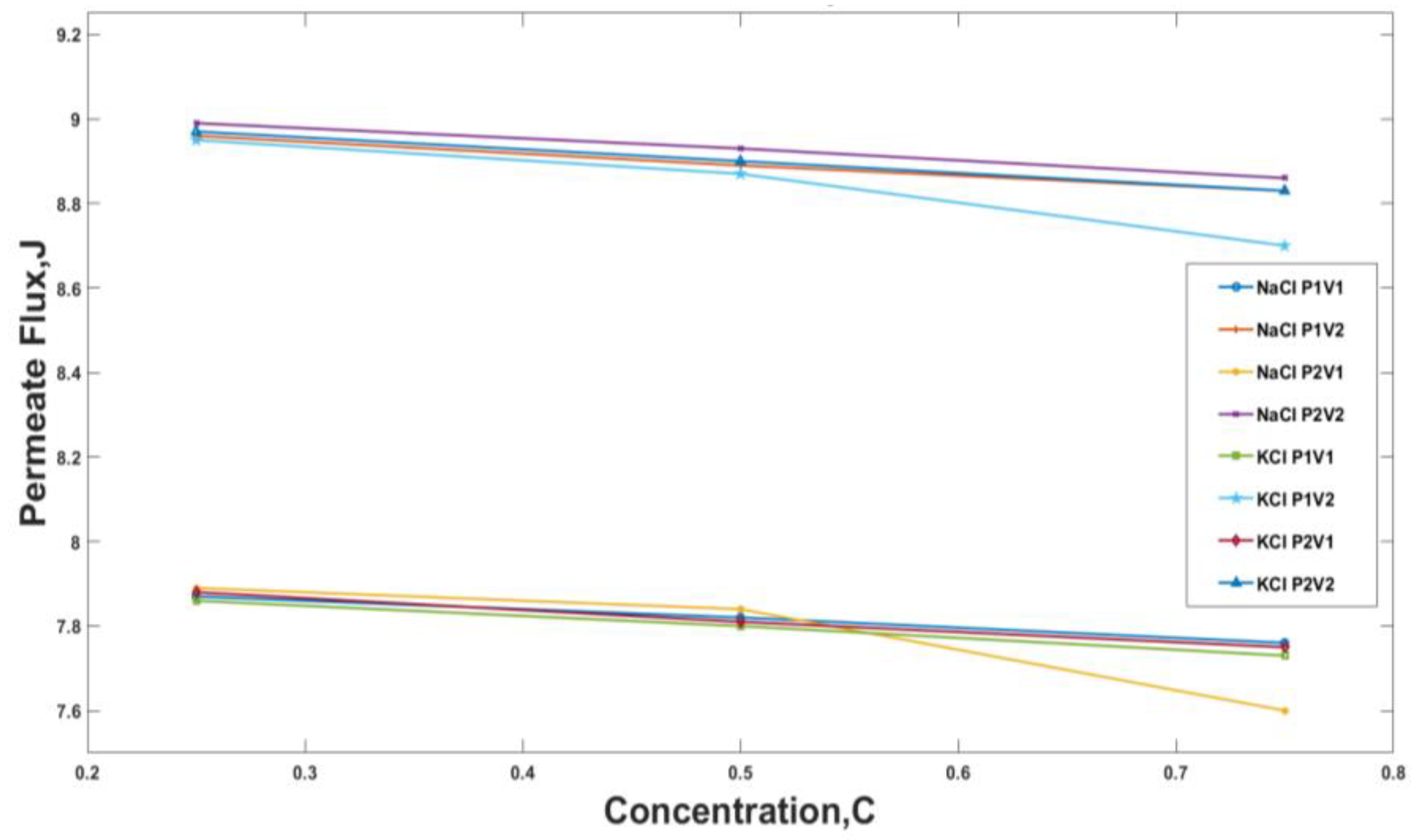
3.1. Experimental Results
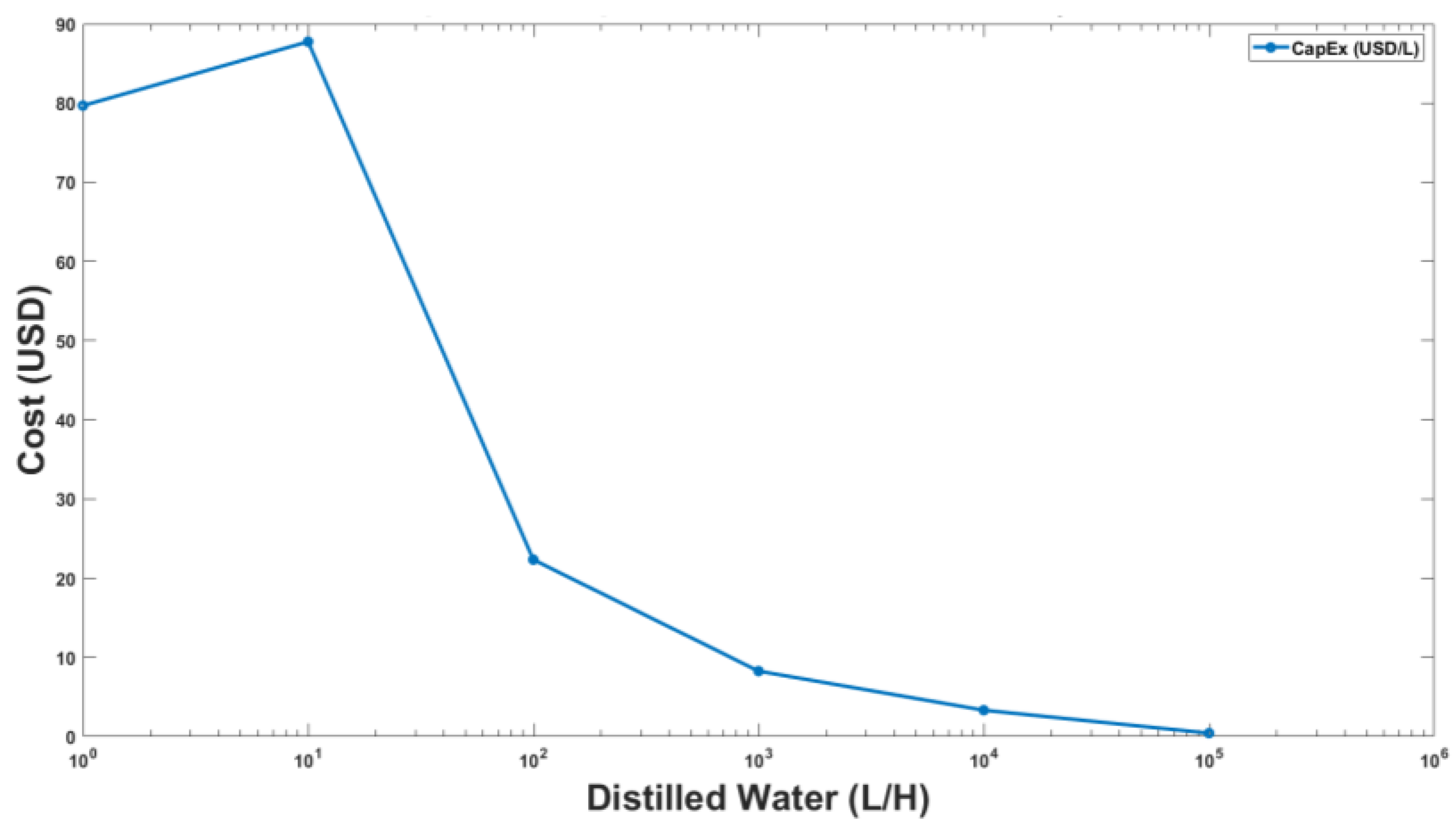
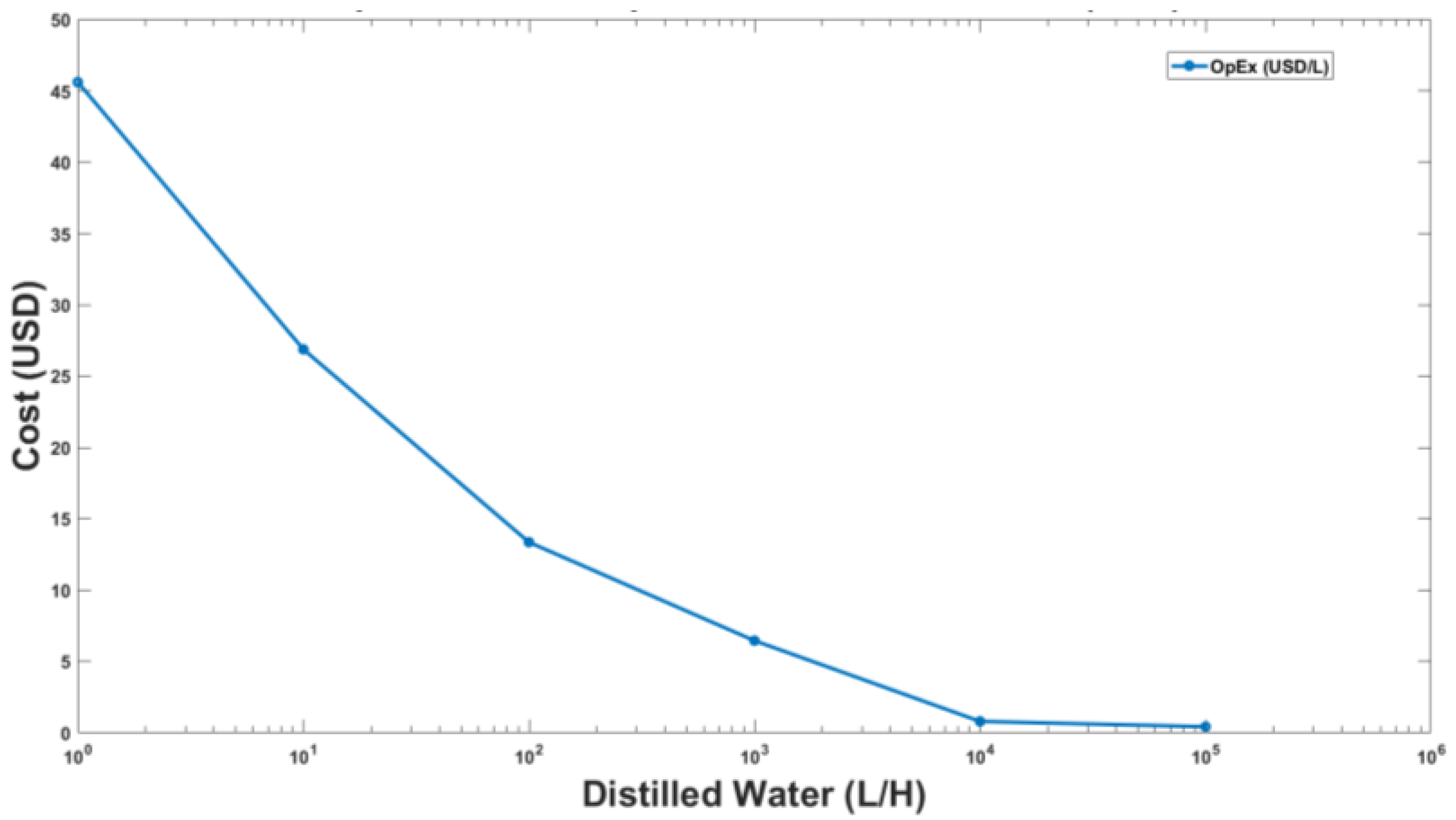
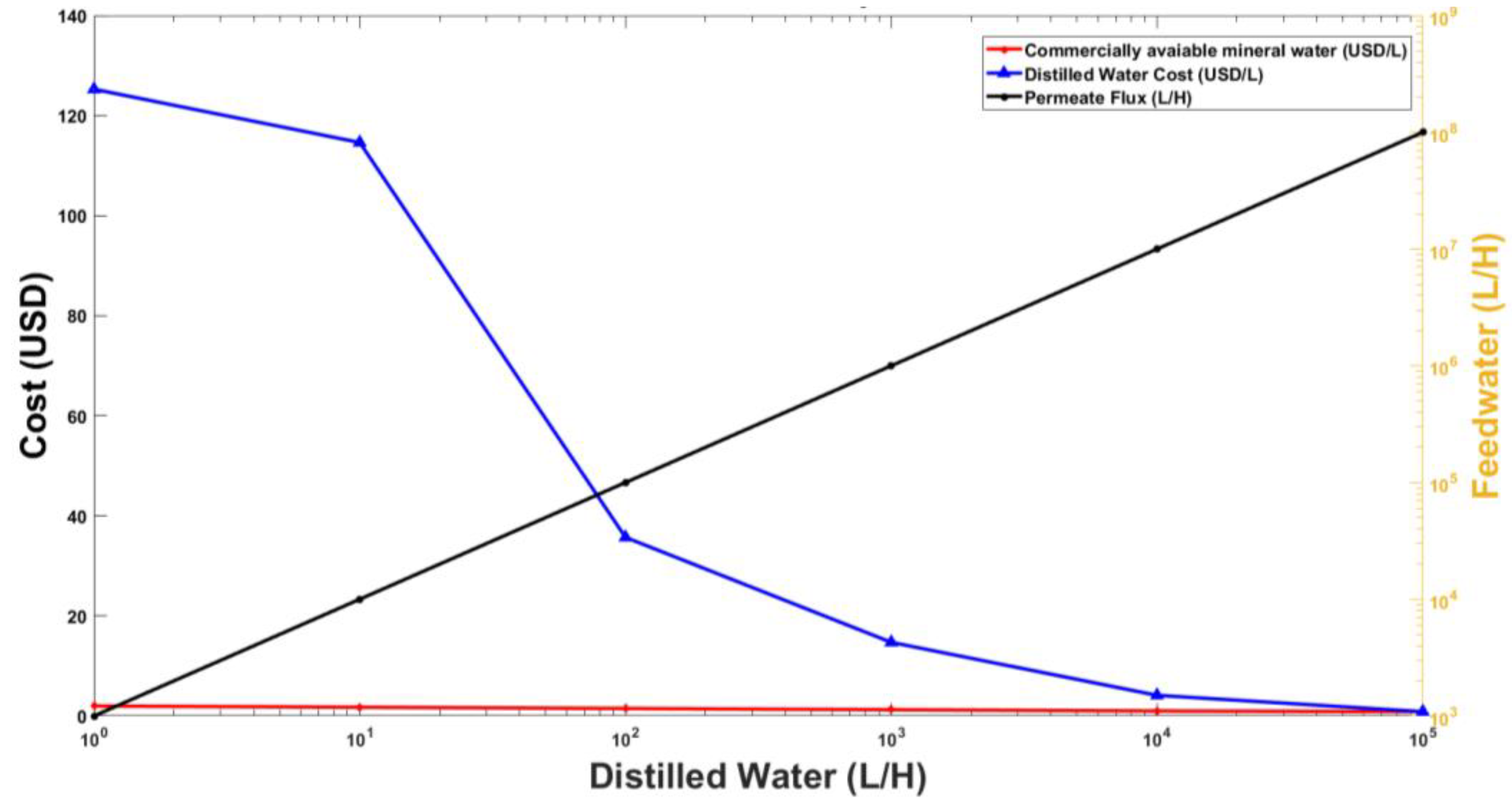
3.2. Results of Economic Analysis
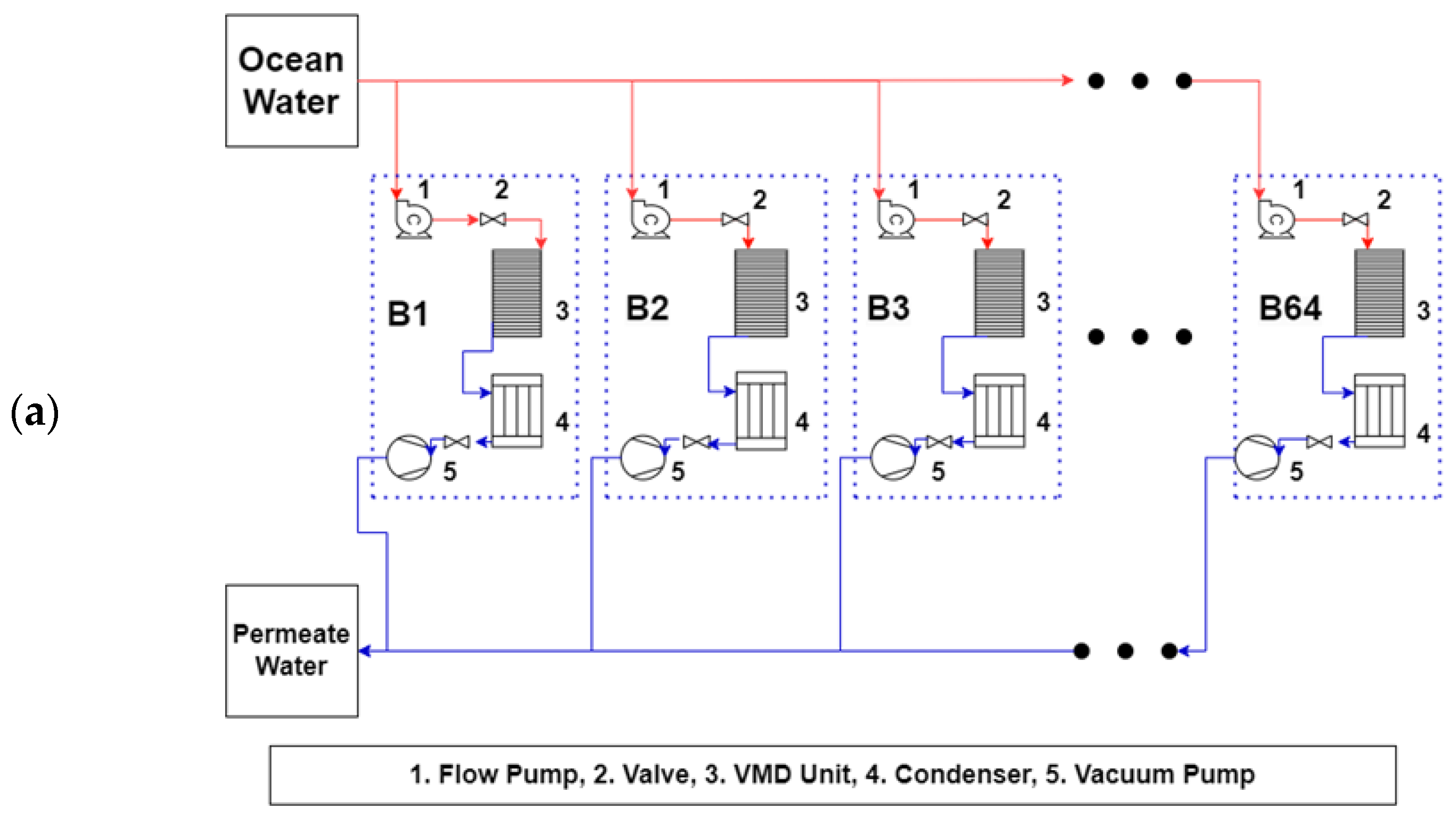
3.3. Full-Scale Plant Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malikh, A.; Qureshi, S.R.; Abbas, N.; Zaidi, A.A. Energy and exergy analyses of a solar desalination plant for Karachi Pakistan. Sustain. Energy Technol. Assess. 2020, 37, 100596. [Google Scholar] [CrossRef]
- Shoukat, G.; Idrees, H.; Sajid, M.; Ali, S.; Ayaz, Y.; Nawaz, R.; Ansari, A.R. Numerical analysis of permeate flux in reverse osmosis by varying strand geometry. Sci. Rep. 2022, 12, 16636. [Google Scholar] [CrossRef] [PubMed]
- Al-Shammiri, M.; Safar, M. Multi-effect distillation plants: State of the art. Desalination 1999, 126, 45–59. [Google Scholar] [CrossRef]
- Toth, A.J. Modelling and optimisation of multi-stage flash distillation and reverse osmosis for desalination of saline process wastewater sources. Membranes 2020, 10, 265. [Google Scholar] [CrossRef]
- Bin, L.; Ling, C.; Tianyin, L.; Sajid, M. Distilled Water Production by Vacuum Heat Pump. Desalination Water Treat. 2018, 77–92. [Google Scholar] [CrossRef]
- Cui, Z.; Drioli, E.; Lee, Y.M. Recent progress in fluoropolymers for membranes. Prog. Polym. Sci. 2014, 39, 164–198. [Google Scholar] [CrossRef]
- Curcio, E.; Drioli, E. Membrane distillation and related operations—A review. Sep. Purif. Rev. 2005, 34, 35–86. [Google Scholar] [CrossRef]
- Ji, Z.; Zhao, Y.; Zhang, M.; Li, X.; Li, H. Surface Modification of ETFE Membrane and PTFE Membrane by Atmospheric DBD Plasma. Membranes 2022, 12, 510. [Google Scholar] [CrossRef]
- Piao, J.; Li, K.; Zhang, Y.; Zhang, L. Design of Laser Photothermal Conversion Membranes Based on Fluorinated Graphene. Membranes 2022, 12, 135. [Google Scholar] [CrossRef]
- Rashidi, M.M.; Mahariq, I.; Murshid, N.; Wongwises, S.; Mahian, O.; Alhuyi Nazari, M. Applying wind energy as a clean source for reverse osmosis desalination: A comprehensive review. Alex. Eng. J. 2022, 61, 12977–12989. [Google Scholar] [CrossRef]
- Yang, X.; Wang, R.; Shi, L.; Fane, A.G.; Debowski, M. Performance improvement of PVDF hollow fiber-based membrane distillation process. J. Memb. Sci. 2011, 369, 437–447. [Google Scholar] [CrossRef]
- Lawson, K.W.; Lloyd, D.R. Membrane distillation. J. Memb. Sci. 1997, 124, 1–25. [Google Scholar] [CrossRef]
- Yadav, A.; Patel, R.V.; Singh, C.P.; Labhasetwar, P.K.; Shahi, V.K. Experimental study and numerical optimization for removal of methyl orange using polytetrafluoroethylene membranes in vacuum membrane distillation process. Colloids Surf. A Physicochem. Eng. Asp. 2022, 635, 128070. [Google Scholar] [CrossRef]
- Phattaranawik, J.; Jiraratananon, R.; Fane, A.G. Heat transport and membrane distillation coefficients in direct contact membrane distillation. J. Memb. Sci. 2003, 212, 177–193. [Google Scholar] [CrossRef]
- Ma, Q.; Xu, Z.; Wang, R.; Poredoš, P. Distributed vacuum membrane distillation driven by direct-solar heating at ultra-low temperature. Energy 2022, 239, 121891. [Google Scholar] [CrossRef]
- Abu-Zeid, M.A.E.R.; Zhang, Y.; Dong, H.; Zhang, L.; Chen, H.L.; Hou, L. A comprehensive review of vacuum membrane distillation technique. Desalination 2015, 356, 1–14. [Google Scholar] [CrossRef]
- Al-Obaidani, S.; Curcio, E.; Macedonio, F.; di Profio, G.; Al-Hinai, H.; Drioli, E. Potential of membrane distillation in seawater desalination: Thermal efficiency, sensitivity study and cost estimation. J. Memb. Sci. 2008, 323, 85–98. [Google Scholar] [CrossRef]
- Banat, F.; Jwaied, N. Economic evaluation of desalination by small-scale autonomous solar-powered membrane distillation units. Desalination 2008, 220, 566–573. [Google Scholar] [CrossRef]
- Ali, M.I.; Summers, E.K.; Arafat, H.A.; Lienhard, V.J.H. Effects of membrane properties on water production cost in small scale membrane distillation systems. Desalination 2012, 306, 60–71. [Google Scholar] [CrossRef]
- Kullab, A.; Martin, A. Membrane distillation and applications for water purification in thermal cogeneration plants. Sep. Purif. Technol. 2011, 76, 231–237. [Google Scholar] [CrossRef]
- Meindersma, G.W.; Guijt, C.M.; de Haan, A.B. Desalination and water recycling by air gap membrane distillation. Desalination 2006, 187, 291–301. [Google Scholar] [CrossRef]
- Sarbatly, R.; Chiam, C.K. Evaluation of geothermal energy in desalination by vacuum membrane distillation. Appl. Energy 2013, 112, 737–746. [Google Scholar] [CrossRef]
- Naidu, G.; Choi, Y.; Jeong, S.; Hwang, T.M.; Vigneswaran, S. Experiments and modeling of a vacuum membrane distillation for high saline water. J. Ind. Eng. Chem. 2014, 20, 2174–2183. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Cheng, L.H.; Xu, X.; Chen, H. Concentration of lignocellulosic hydrolyzates by solar membrane distillation. Bioresour. Technol. 2012, 123, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Cha-Umpong, W.; Hou, J.; Ji, C.; Chen, V. Open-source industrial-scale module simulation: Paving the way towards the right configuration choice for membrane distillation. Desalination 2019, 464, 48–62. [Google Scholar] [CrossRef]
- HongJin, J.; HeeYoul, K. Experimental study on the thermal performance characteristics of hollow-fiber vacuum membrane distillation module. Desalination Water Treat. 2017, 90, 1–6. [Google Scholar]
- Zhao, K.; Heinzl, W.; Wenzel, M.; Büttner, S.; Bollen, F.; Lange, G.; Heinzl, S.; Sarda, N. Experimental study of the memsys vacuum-multi-effect-membrane-distillation (V-MEMD) module. Desalination 2013, 323, 150–160. [Google Scholar] [CrossRef]
- Minier-Matar, J.; Hussain, A.; Janson, A.; Benyahia, F.; Adham, S. Field evaluation of membrane distillation technologies for desalination of highly saline brines. Desalination 2014, 351, 101–108. [Google Scholar] [CrossRef]
- Loganathan, P.; Naidu, G.; Vigneswaran, S. Mining valuable minerals from seawater: A critical review. Environ. Sci. 2017, 3, 37–53. [Google Scholar] [CrossRef]
- Bardi, U. Extracting Minerals from Seawater: An Energy Analysis. Environ. Sci. 2010, 2, 980–992. [Google Scholar] [CrossRef]
- Ding, Z.; Liu, L.; El-Bourawi, M.S.; Ma, R. Analysis of a solar-powered membrane distillation system. Desalination 2005, 172, 27–40. [Google Scholar] [CrossRef]
- Guillén-Burrieza, E.; Blanco, J.; Zaragoza, G.; Alarcón, D.-C.; Palenzuela, P.; Ibarra, M.; Gernjak, W. Experimental analysis of an air gap membrane distillation solar desalination pilot system. J. Memb. Sci. 2011, 379, 386–396. [Google Scholar] [CrossRef]
- Rotunno, P.; Lanzini, A. Leone, Energy and economic analysis of a water scrubbing based biogas upgrading process for biomethane injection into the gas grid or use as transportation fuel. Renew. Energy 2017, 102, 417–432. [Google Scholar] [CrossRef]
- White, R.; Navarro-Pineda, F.S.; Cockerill, T.; Dupont, V.; Rivero, J.C.S. Techno-Economic and Life Cycle Impacts Analysis of Direct Methanation of Glycerol to Bio-Synthetic Natural Gas at a Biodiesel Refinery. Energies 2019, 12, 678. [Google Scholar] [CrossRef]
- Biniaz, P.; Ardekani, N.T.; Makarem, M.A.; Rahimpour, M.R. Water and Wastewater Treatment Systems by Novel Integrated Membrane Distillation (MD). ChemEngineering 2019, 3, 8. [Google Scholar] [CrossRef]
- Peters Max, S.; Klaus, D.T. Plant Design and Economics for Chemical Engineers; McGraw-Hill International: Columbus, OH, USA, 2018. [Google Scholar]
- Tariff Guide. Available online: https://iesco.com.pk/index.php/customer-services/tariff-guide (accessed on 8 February 2023).






| Property | Specification |
|---|---|
| Material | PTFE |
| Dimensions (Length × Width) | 250 mm × 200 mm |
| Thickness | 165 µm |
| Effective Area | 0.0336 m2 |
| Porosity | 70–75% |
| Pore size | 0.2 µm |
| Parameters | Variation | Value |
|---|---|---|
| Concentration, M | C1 | 0.25 |
| C2 | 0.5 | |
| C3 | 0.75 | |
| Pressure, kPa | P1 | 30 |
| P2 | 20 | |
| Velocity, m/s | V1 | 3.48 |
| V2 | 5.22 | |
| Temperature, K | T1 | 333 |
| T2 | 343 | |
| T3 | 353 |
| Component | Cost ($) | |
|---|---|---|
| Capital Cost | Membrane | $36/m2 |
| Feedwater Pump | 25 $ | |
| Vacuum Pump | 26 $ | |
| Installation cost of VMD | Installation Cost | 25% of Total Equipment Cost |
| Instrumentation and Control Cost | 25% of Total Equipment Cost |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idrees, H.; Ali, S.; Sajid, M.; Rashid, M.; Khawaja, F.I.; Ali, Z.; Anwar, M.N. Techno-Economic Analysis of Vacuum Membrane Distillation for Seawater Desalination. Membranes 2023, 13, 339. https://doi.org/10.3390/membranes13030339
Idrees H, Ali S, Sajid M, Rashid M, Khawaja FI, Ali Z, Anwar MN. Techno-Economic Analysis of Vacuum Membrane Distillation for Seawater Desalination. Membranes. 2023; 13(3):339. https://doi.org/10.3390/membranes13030339
Chicago/Turabian StyleIdrees, Hassaan, Sara Ali, Muhammad Sajid, Muhammad Rashid, Fahad Iqbal Khawaja, Zaib Ali, and Muhammad Nabeel Anwar. 2023. "Techno-Economic Analysis of Vacuum Membrane Distillation for Seawater Desalination" Membranes 13, no. 3: 339. https://doi.org/10.3390/membranes13030339
APA StyleIdrees, H., Ali, S., Sajid, M., Rashid, M., Khawaja, F. I., Ali, Z., & Anwar, M. N. (2023). Techno-Economic Analysis of Vacuum Membrane Distillation for Seawater Desalination. Membranes, 13(3), 339. https://doi.org/10.3390/membranes13030339









