Recent Developments in Two-Dimensional Materials-Based Membranes for Oil–Water Separation
Abstract
1. Introduction
2. MXene-Based Oil–Water Separation Membranes
2.1. MXene Materials and Their Use in Oil–Water Separation Membranes
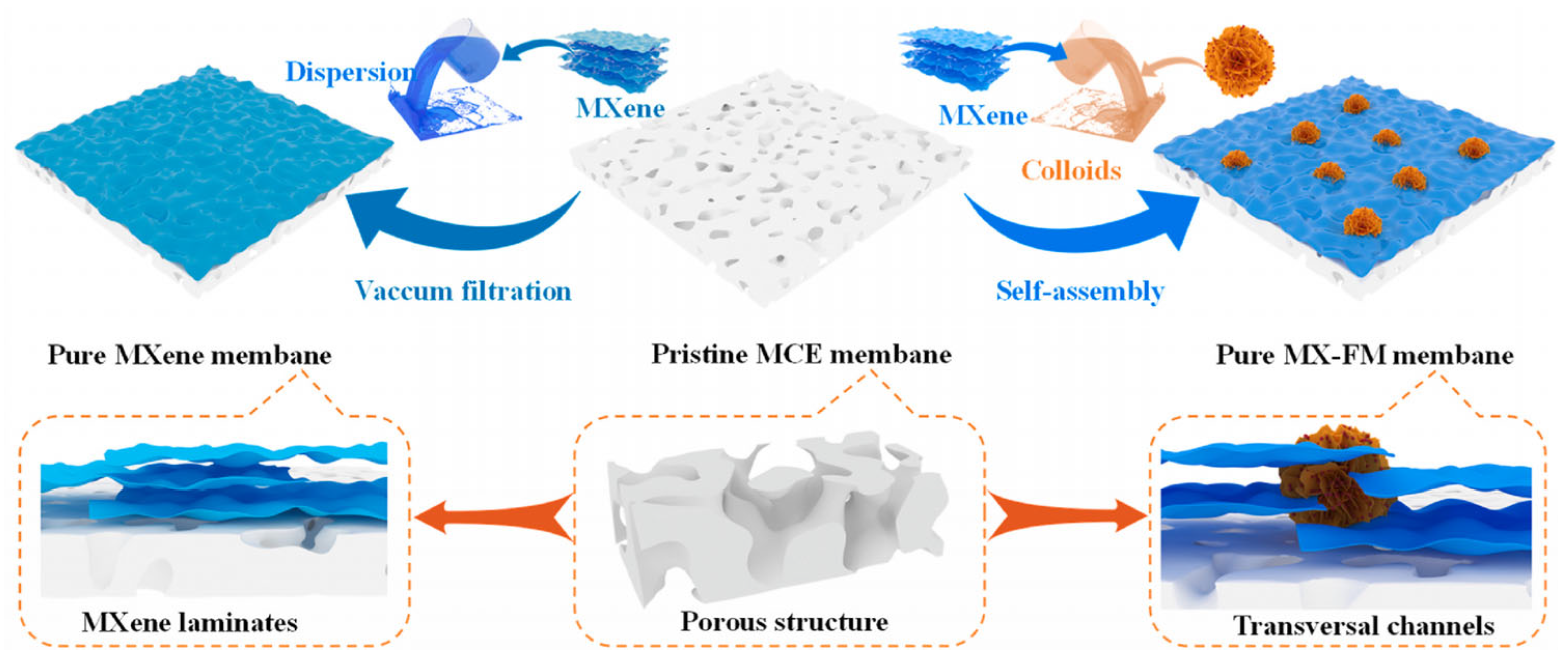
2.2. Recent Developments in MXene-Based Oil–Water Separation Membranes
3. Graphene-Based Oil–Water Separation Membranes
3.1. Graphene Materials and Their Use in Oil–Water Separation Membranes
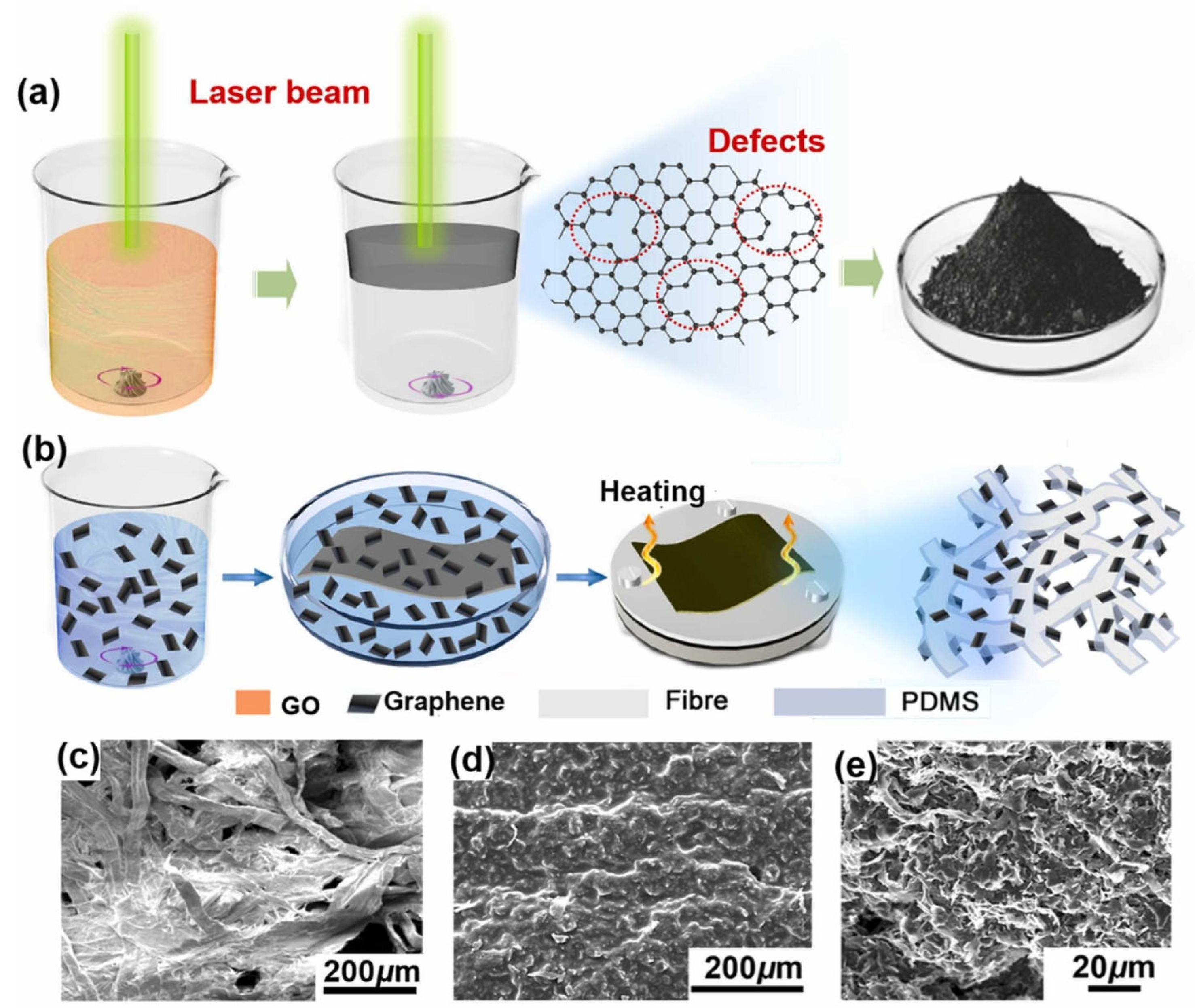
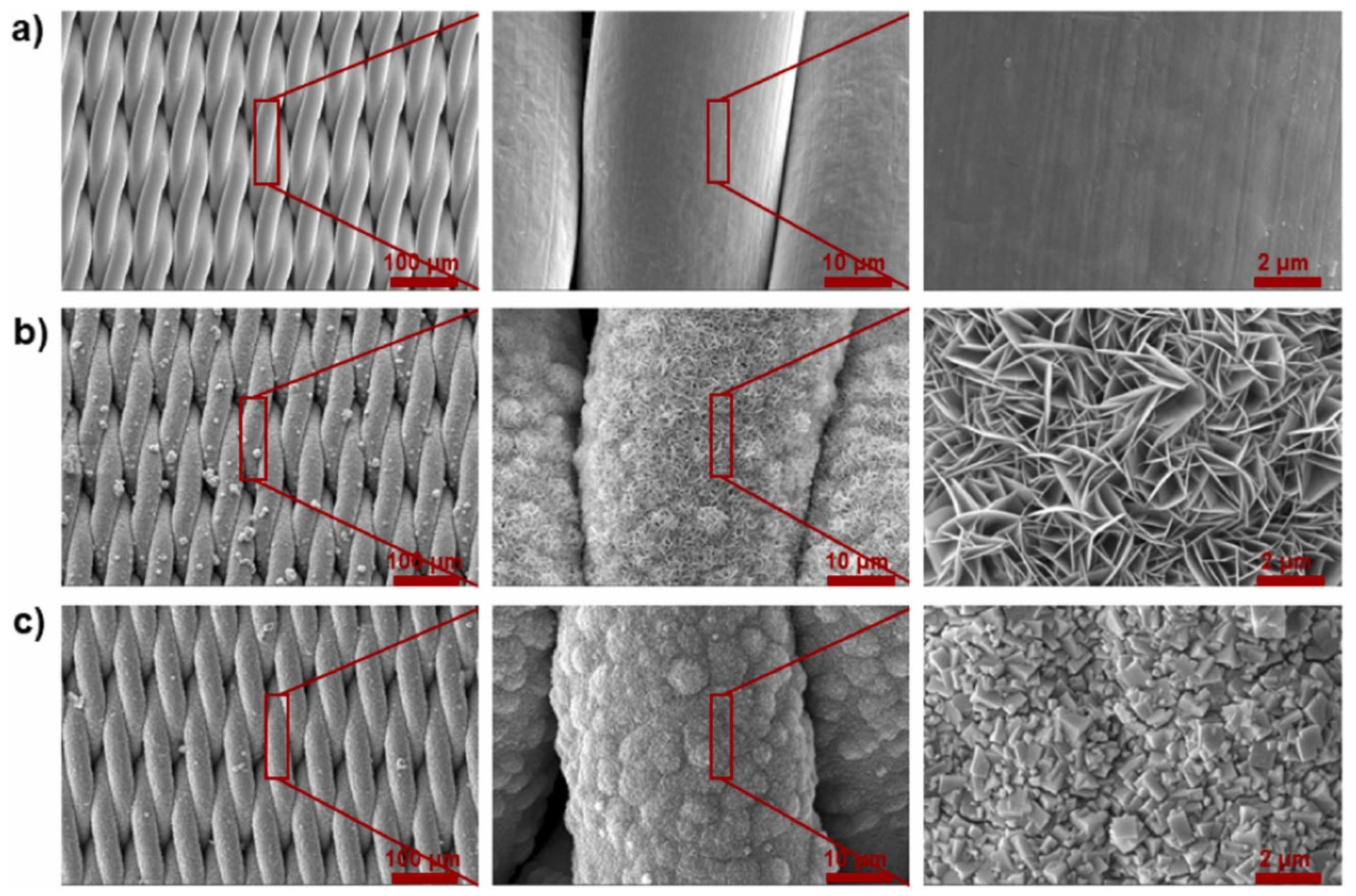
3.2. Recent Developments in Graphene-Based Oil–Water Separation Membranes
4. Metal–Organic Framework (MOF)-Based Oil–Water Separation Membranes
4.1. Metal–Organic Framework (MOF) Materials and Their Use in Oil–Water Separation Membranes
4.2. Recent Developments in MOF-Based Oil–Water Separation Membranes
5. Covalent Organic Framework (COF)-Based Oil–Water Separation Membranes
5.1. Covalent Organic Framework (COF) Materials and Their Use in Oil–Water Separation Membranes
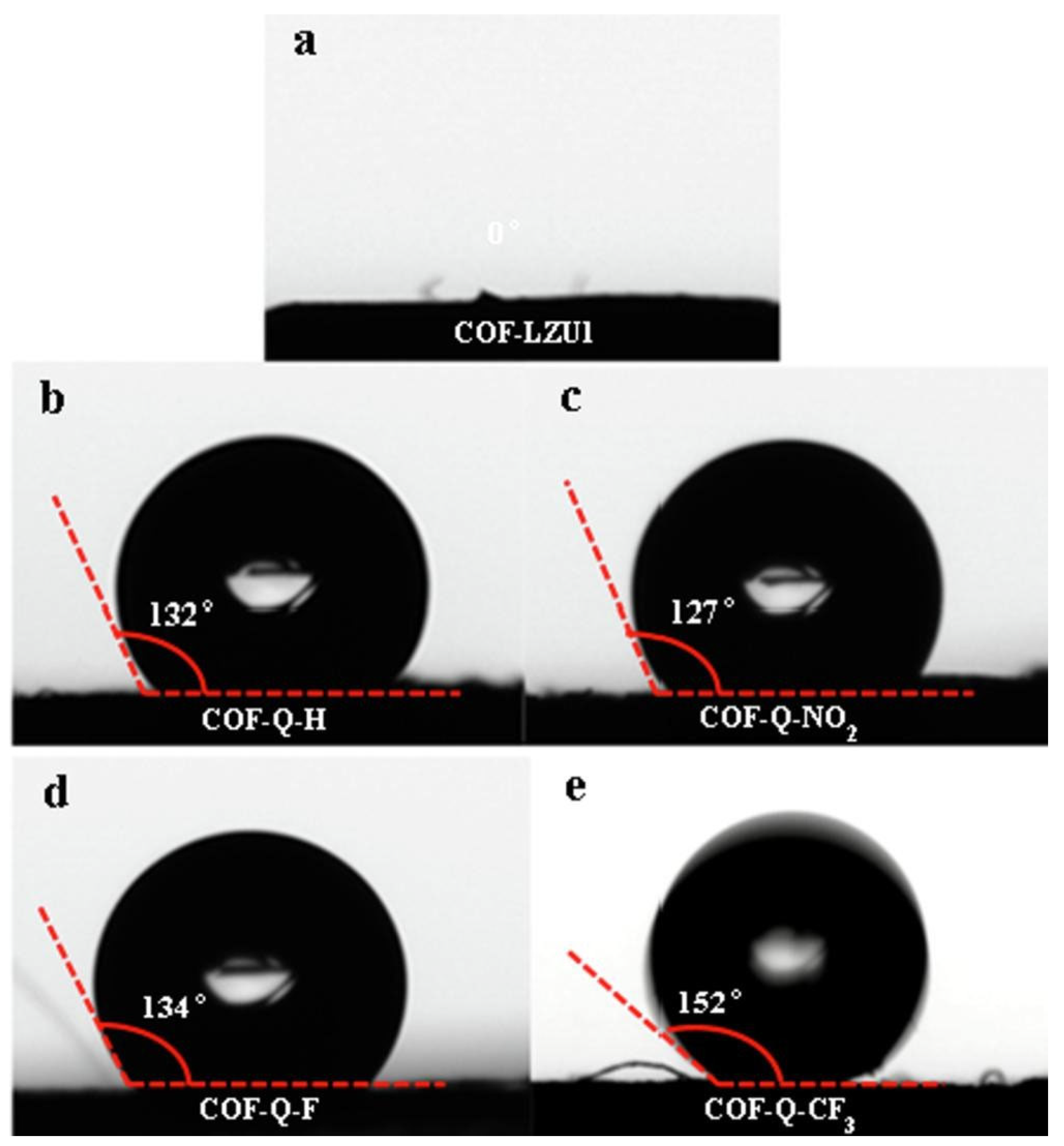
5.2. Recent Developments in COF-Based Oil–Water Separation Membranes
6. Future Directions
6.1. Membrane Thickness
6.2. Membrane Cytotoxicity and Long-Term Stability
6.3. Multifunctional Membranes
6.4. Large-Scale Fabrication
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manikandan, S.; Subbaiya, R.; Saravanan, M.; Ponraj, M.; Selvam, M.; Pugazhendhi, A. A critical review of advanced nanotechnology and hybrid membrane based water recycling, reuse, and wastewater treatment processes. Chemosphere 2022, 289, 132867. [Google Scholar] [CrossRef] [PubMed]
- Samuel, O.; Othman, M.H.D.; Kamaludin, R.; Sinsamphanh, O.; Abdullah, H.; Puteh, M.H.; Kurniawan, T.A.; Li, T.; Ismail, A.F.; Rahman, M.A.; et al. Oilfield-produced water treatment using conventional and membrane-based technologies for beneficial reuse: A critical review. J. Environ. Manag. 2022, 308, 114556. [Google Scholar] [CrossRef]
- Ismail, N.H.; Salleh, W.N.W.; Ismail, A.F.; Hasbullah, H.; Yusof, N.; Aziz, F.; Jaafar, J. Hydrophilic polymer-based membrane for oily wastewater treatment: A review. Sep. Purif. Technol. 2020, 233, 116007. [Google Scholar] [CrossRef]
- Yang, Y.; Ali, N.; Bilal, M.; Khan, A.; Ali, F.; Mao, P.; Ni, L.; Gao, X.; Hong, K.; Rasool, K.; et al. Robust membranes with tunable functionalities for sustainable oil/water separation. J. Mol. Liq. 2021, 321, 114701. [Google Scholar] [CrossRef]
- Baig, U.; Faizan, M.; Dastageer, M.A. Polyimide based super-wettable membranes/materials for high performance oil/water mixture and emulsion separation: A review. Adv. Colloid Interface Sci. 2021, 297, 102525. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Choi, W.S. 2D and 3D Bulk Materials for Environmental Remediation: Air Filtration and Oil/Water Separation. Materials 2020, 13, 5714. [Google Scholar] [CrossRef]
- Yu, H.; Wu, M.; Duan, G.; Gong, X. One-step fabrication of eco-friendly superhydrophobic fabrics for high-efficiency oil/water separation and oil spill cleanup. Nanoscale 2022, 14, 1296–1309. [Google Scholar] [CrossRef]
- Shalaby, M.S.; Sołowski, G.; Abbas, W. Recent Aspects in Membrane Separation for Oil/Water Emulsion. Adv. Mater. Interfaces 2021, 8, 2100448. [Google Scholar] [CrossRef]
- Padaki, M.; Surya Murali, R.; Abdullah, M.S.; Misdan, N.; Moslehyani, A.; Kassim, M.A.; Hilal, N.; Ismail, A.F. Membrane technology enhancement in oil–water separation. A review. Desalination 2015, 357, 197–207. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, D.; Jiang, L.; Jin, J. Recent progress in developing advanced membranes for emulsified oil/water separation. NPG Asia Mater. 2014, 6, e101. [Google Scholar] [CrossRef]
- Cheryan, M.; Rajagopalan, N. Membrane processing of oily streams. Wastewater treatment and waste reduction. J. Membr. Sci. 1998, 151, 13–28. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, X.; Wang, Y.; Qi, Y.; Zhang, Y.; Luo, J.; Cui, P.; Jiang, W. A review on oil/water emulsion separation membrane material. J. Environ. Chem. Eng. 2022, 10, 107257. [Google Scholar] [CrossRef]
- Tanudjaja, H.J.; Hejase, C.A.; Tarabara, V.V.; Fane, A.G.; Chew, J.W. Membrane-based separation for oily wastewater: A practical perspective. Water Res. 2019, 156, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Junaidi, N.F.D.; Othman, N.H.; Fuzil, N.S.; Mat Shayuti, M.S.; Alias, N.H.; Shahruddin, M.Z.; Marpani, F.; Lau, W.J.; Ismail, A.F.; Aba, N.D. Recent development of graphene oxide-based membranes for oil–water separation: A review. Sep. Purif. Technol. 2021, 258, 118000. [Google Scholar] [CrossRef]
- Diogo Januário, E.F.; de Camargo Lima Beluci, N.; Vidovix, T.B.; Vieira, M.F.; Bergamasco, R.; Salcedo Vieira, A.M. Functionalization of membrane surface by layer-by-layer self-assembly method for dyes removal. Process Saf. Environ. Prot. 2020, 134, 140–148. [Google Scholar] [CrossRef]
- Chang, X.; Wang, Z.; Quan, S.; Xu, Y.; Jiang, Z.; Shao, L. Exploring the synergetic effects of graphene oxide (GO) and polyvinylpyrrodione (PVP) on poly(vinylylidenefluoride) (PVDF) ultrafiltration membrane performance. Appl. Surf. Sci. 2014, 316, 537–548. [Google Scholar] [CrossRef]
- Nian, P.; Wang, X.; Li, S.; Wang, Z.; Wei, Y. Switchable CAU-10-H mesh membrane for on-demand separation of immiscible oil/water mixtures and emulsions. J. Environ. Chem. Eng. 2023, 11, 109656. [Google Scholar] [CrossRef]
- Rasouli, S.; Rezaei, N.; Hamedi, H.; Zendehboudi, S.; Duan, X. Superhydrophobic and superoleophilic membranes for oil-water separation application: A comprehensive review. Mater. Des. 2021, 204, 109599. [Google Scholar] [CrossRef]
- Ma, Q.; Cheng, H.; Fane, A.G.; Wang, R.; Zhang, H. Recent Development of Advanced Materials with Special Wettability for Selective Oil/Water Separation. Small 2016, 12, 2186–2202. [Google Scholar] [CrossRef]
- Shang, Y.; Si, Y.; Raza, A.; Yang, L.; Mao, X.; Ding, B.; Yu, J. An in situ polymerization approach for the synthesis of superhydrophobic and superoleophilic nanofibrous membranes for oil–water separation. Nanoscale 2012, 4, 7847–7854. [Google Scholar] [CrossRef]
- Wahid, F.; Zhao, X.-J.; Duan, Y.-X.; Zhao, X.-Q.; Jia, S.-R.; Zhong, C. Designing of bacterial cellulose-based superhydrophilic/underwater superoleophobic membrane for oil/water separation. Carbohydr. Polym. 2021, 257, 117611. [Google Scholar] [CrossRef]
- Abuhasel, K.; Kchaou, M.; Alquraish, M.; Munusamy, Y.; Jeng, Y.T. Oily Wastewater Treatment: Overview of Conventional and Modern Methods, Challenges, and Future Opportunities. Water 2021, 13, 980. [Google Scholar] [CrossRef]
- Ahmad, N.A.; Goh, P.S.; Abdul Karim, Z.; Ismail, A.F. Thin Film Composite Membrane for Oily Waste Water Treatment: Recent Advances and Challenges. Membranes 2018, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Rehman, S.A.U.; Luan, H.-Y.; Farid, M.U.; Huang, H. Challenges and opportunities in functional carbon nanotubes for membrane-based water treatment and desalination. Sci. Total Environ. 2019, 646, 1126–1139. [Google Scholar] [CrossRef] [PubMed]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356, eaab0530. [Google Scholar] [CrossRef]
- Liu, Y.; Coppens, M.-O.; Jiang, Z. Mixed-dimensional membranes: Chemistry and structure–property relationships. Chem. Soc. Rev. 2021, 50, 11747–11765. [Google Scholar] [CrossRef] [PubMed]
- Ihsanullah, I.; Bilal, M. Recent advances in the development of MXene-based membranes for oil/water separation: A critical review. Appl. Mater. Today 2022, 29, 101674. [Google Scholar] [CrossRef]
- Kumar, C.; Das, S.; Jit, S. 7—Device physics and device integration of two-dimensional heterostructures. In 2D Nanoscale Heterostructured Materials; Jit, S., Das, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 195–214. [Google Scholar]
- Shanmugam, V.; Mensah, R.A.; Babu, K.; Gawusu, S.; Chanda, A.; Tu, Y.; Neisiany, R.E.; Försth, M.; Sas, G.; Das, O. A Review of the Synthesis, Properties, and Applications of 2D Materials. Part. Part. Syst. Charact. 2022, 39, 2200031. [Google Scholar] [CrossRef]
- Senapati, S.; Maiti, P. 9—Emerging bio-applications of two-dimensional nanoheterostructure materials. In 2D Nanoscale Heterostructured Materials; Jit, S., Das, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 243–255. [Google Scholar]
- Novoselov, K.S.; Mishchenko, A.; Carvalho, A.; Castro Neto, A.H. 2D materials and van der Waals heterostructures. Science 2016, 353, aac9439. [Google Scholar] [CrossRef]
- Cheng, Y.; Pu, Y.; Zhao, D. Two-Dimensional Membranes: New Paradigms for High-Performance Separation Membranes. Chem.—Asian J. 2020, 15, 2241–2270. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Jin, W.; Xu, N. Two-Dimensional-Material Membranes: A New Family of High-Performance Separation Membranes. Angew. Chem. Int. Ed. 2016, 55, 13384–13397. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Barsoum, M.W.; Gogotsi, Y. Ten Years of Progress in the Synthesis and Development of MXenes. Adv. Mater. 2021, 33, 2103393. [Google Scholar] [CrossRef] [PubMed]
- Dhamodharan, D.; Dhinakaran, V.; Byun, H.-S. MXenes: An emerging 2D material. Carbon 2022, 192, 366–383. [Google Scholar] [CrossRef]
- Ihsanullah, I. Potential of MXenes in water desalination: Current status and perspectives. Nano-Micro Lett. 2020, 12, 72. [Google Scholar] [CrossRef]
- Jiang, Q.; Lei, Y.; Liang, H.; Xi, K.; Xia, C.; Alshareef, H.N. Review of MXene electrochemical microsupercapacitors. Energy Storage Mater. 2020, 27, 78–95. [Google Scholar] [CrossRef]
- Deysher, G.; Shuck, C.E.; Hantanasirisakul, K.; Frey, N.C.; Foucher, A.C.; Maleski, K.; Sarycheva, A.; Shenoy, V.B.; Stach, E.A.; Anasori, B. Synthesis of Mo4VAlC4 MAX phase and two-dimensional Mo4VC4 MXene with five atomic layers of transition metals. ACS Nano 2019, 14, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, Y. Potential environmental applications of MXenes: A critical review. Chemosphere 2021, 271, 129578. [Google Scholar] [CrossRef]
- Dixit, F.; Zimmermann, K.; Dutta, R.; Prakash, N.J.; Barbeau, B.; Mohseni, M.; Kandasubramanian, B. Application of MXenes for water treatment and energy-efficient desalination: A review. J. Hazard. Mater. 2022, 423, 127050. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.B.; Iftekhar, S.; Maqbool, T.; Paramanik, B.K.; Tabraiz, S.; Sillanpää, M.; Zhang, Z. Two-dimensional nanoporous and lamellar membranes for water purification: Reality or a myth? Chem. Eng. J. 2021, 432, 134335. [Google Scholar] [CrossRef]
- Kumar, J.A.; Prakash, P.; Krithiga, T.; Amarnath, D.J.; Premkumar, J.; Rajamohan, N.; Vasseghian, Y.; Saravanan, P.; Rajasimman, M. Methods of synthesis, characteristics, and environmental applications of MXene: A comprehensive review. Chemosphere 2022, 286, 131607. [Google Scholar] [CrossRef] [PubMed]
- Maleski, K.; Alhabeb, M. Top-Down MXene Synthesis (Selective Etching). In 2D Metal Carbides and Nitrides (MXenes) Structure, Properties and Applications; Springer: Berlin/Heidelberg, Germany, 2019; pp. 69–87. [Google Scholar]
- Jiang, L.; Zhou, D.; Yang, J.; Zhou, S.; Wang, H.; Yuan, X.; Liang, J.; Li, X.; Chen, Y.; Li, H. 2D Single-and Few-Layered MXene: Synthesis, applications and perspectives. J. Mater. Chem. A 2022, 10, 13651–13672. [Google Scholar] [CrossRef]
- Iqbal, A.; Hong, J.; Ko, T.Y.; Koo, C.M. Improving oxidation stability of 2D MXenes: Synthesis, storage media, and conditions. Nano Converg. 2021, 8, 9. [Google Scholar] [CrossRef]
- Verger, L.; Xu, C.; Natu, V.; Cheng, H.-M.; Ren, W.; Barsoum, M.W. Overview of the synthesis of MXenes and other ultrathin 2D transition metal carbides and nitrides. Curr. Opin. Solid State Mater. Sci. 2019, 23, 149–163. [Google Scholar] [CrossRef]
- Tao, Q.; Dahlqvist, M.; Lu, J.; Kota, S.; Meshkian, R.; Halim, J.; Palisaitis, J.; Hultman, L.; Barsoum, M.W.; Persson, P.O. Two-dimensional Mo1. 33C MXene with divacancy ordering prepared from parent 3D laminate with in-plane chemical ordering. Nat. Commun. 2017, 8, 14949. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Si, C.; Zhou, J.; Sun, Z. MXene and MXene-based composites: Synthesis, properties and environment-related applications. Nanoscale Horiz. 2020, 5, 235–258. [Google Scholar] [CrossRef]
- Xiao, X.; Yu, H.; Jin, H.; Wu, M.; Fang, Y.; Sun, J.; Hu, Z.; Li, T.; Wu, J.; Huang, L. Salt-templated synthesis of 2D metallic MoN and other nitrides. ACS Nano 2017, 11, 2180–2186. [Google Scholar] [CrossRef]
- Jia, J.; Xiong, T.; Zhao, L.; Wang, F.; Liu, H.; Hu, R.; Zhou, J.; Zhou, W.; Chen, S. Ultrathin N-doped Mo2C nanosheets with exposed active sites as efficient electrocatalyst for hydrogen evolution reactions. ACS Nano 2017, 11, 12509–12518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, Z.; Wang, H.; Chan, C.H.; Chan, N.Y.; Chen, X.X.; Dai, J.-Y. Plasma-enhanced pulsed-laser deposition of single-crystalline M o 2 C ultrathin superconducting films. Phys. Rev. Mater. 2017, 1, 034002. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, F.; Wang, H.; Chan, C.H.; Lu, W.; Dai, J.-y. Substrate orientation-induced epitaxial growth of face centered cubic Mo 2 C superconductive thin film. J. Mater. Chem. C 2017, 5, 10822–10827. [Google Scholar] [CrossRef]
- Li, Z.-K.; Liu, Y.; Li, L.; Wei, Y.; Caro, J.; Wang, H. Ultra-thin titanium carbide (MXene) sheet membranes for high-efficient oil/water emulsions separation. J. Membr. Sci. 2019, 592, 117361. [Google Scholar] [CrossRef]
- Lin, Q.; Zeng, G.; Yan, G.; Luo, J.; Cheng, X.; Zhao, Z.; Li, H. Self-cleaning photocatalytic MXene composite membrane for synergistically enhanced water treatment: Oil/water separation and dyes removal. Chem. Eng. J. 2022, 427, 131668. [Google Scholar] [CrossRef]
- Khatami, M.; Iravani, S. MXenes and MXene-based materials for the removal of water pollutants: Challenges and opportunities. Comments Inorg. Chem. 2021, 41, 213–248. [Google Scholar] [CrossRef]
- Huang, Z.; Shen, L.; Lin, H.; Li, B.; Chen, C.; Xu, Y.; Li, R.; Zhang, M.; Zhao, D. Fabrication of fibrous MXene nanoribbons (MNRs) membrane with efficient performance for oil-water separation. J. Membr. Sci. 2022, 661, 120949. [Google Scholar] [CrossRef]
- Hu, J.; Zhan, Y.; Zhang, G.; Feng, Q.; Yang, W.; Chiao, Y.-H.; Zhang, S.; Sun, A. Durable and super-hydrophilic/underwater super-oleophobic two-dimensional MXene composite lamellar membrane with photocatalytic self-cleaning property for efficient oil/water separation in harsh environments. J. Membr. Sci. 2021, 637, 119627. [Google Scholar] [CrossRef]
- Kong, N.; Shen, L.; Zeng, Q.; Chen, C.; Teng, J.; Xu, Y.; Zhao, L.; Lin, H. Transversal nanochannel-enabled MXene laminated membranes for superior oil-water separation: A fluid mosaic cytomembrane inspired approach. J. Membr. Sci. 2023, 680, 121735. [Google Scholar] [CrossRef]
- Foller, T.; Wang, H.; Joshi, R. Rise of 2D materials-based membranes for desalination. Desalination 2022, 536, 115851. [Google Scholar] [CrossRef]
- Lin, Q.; Zeng, G.; Pu, S.; Yan, G.; Luo, J.; Wan, Y.; Zhao, Z. A dual regulation strategy for MXene-based composite membrane to achieve photocatalytic self-cleaning properties and multi-functional applications. Chem. Eng. J. 2022, 443, 136335. [Google Scholar] [CrossRef]
- Sun, A.; Zhan, Y.; Feng, Q.; Yang, W.; Dong, H.; Liu, Y.; Chen, X.; Chen, Y. Assembly of MXene/ZnO heterojunction onto electrospun poly(arylene ether nitrile) fibrous membrane for favorable oil/water separation with high permeability and synergetic antifouling performance. J. Membr. Sci. 2022, 663, 120933. [Google Scholar] [CrossRef]
- Lin, Q.; Liu, Y.; Yang, Z.; He, Z.; Wang, H.; Zhang, L.; Belle Marie Yap Ang, M.; Zeng, G. Construction and application of two-dimensional MXene-based membranes for water treatment: A mini-review. Results Eng. 2022, 15, 100494. [Google Scholar] [CrossRef]
- Hou, K.; Zhou, H.; Zhu, K.; Xie, C.; Liu, S.; He, Y.; Zhu, X.; Wu, M.; Huang, T. Superwetting Ti3C2TX MXene membranes intercalated with sodium alginate for oil/water separation. Carbohydr. Polym. Technol. Appl. 2023, 5, 100278. [Google Scholar] [CrossRef]
- Liu, J.; Cui, L.; Losic, D. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 2013, 9, 9243–9257. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Z.; Wang, J.; Li, J.; Lin, Y. Graphene and graphene oxide: Biofunctionalization and applications in biotechnology. Trends Biotechnol. 2011, 29, 205–212. [Google Scholar] [CrossRef]
- Yu, W.; Sisi, L.; Haiyan, Y.; Jie, L. Progress in the functional modification of graphene/graphene oxide: A review. RSC Adv. 2020, 10, 15328–15345. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Sahoo, S.; Wang, N.; Huczko, A. Graphene research and their outputs: Status and prospect. J. Sci. Adv. Mater. Devices 2020, 5, 10–29. [Google Scholar] [CrossRef]
- Sanchez, V.C.; Jachak, A.; Hurt, R.H.; Kane, A.B. Biological Interactions of Graphene-Family Nanomaterials: An Interdisciplinary Review. Chem. Res. Toxicol. 2012, 25, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Seabra, A.B.; Paula, A.J.; de Lima, R.; Alves, O.L.; Durán, N. Nanotoxicity of Graphene and Graphene Oxide. Chem. Res. Toxicol. 2014, 27, 159–168. [Google Scholar] [CrossRef]
- Pan, X.; Ji, J.; Zhang, N.; Xing, M. Research progress of graphene-based nanomaterials for the environmental remediation. Chin. Chem. Lett. 2020, 31, 1462–1473. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Graphene/elastomer nanocomposites. Carbon 2015, 95, 460–484. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Mohanty, S.; Mukherjee, S.; Basu, S.; Mishra, M. Graphene and graphene oxide as nanomaterials for medicine and biology application. J. Nanostruct. Chem. 2018, 8, 123–137. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior Thermal Conductivity of Single-Layer Graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- McAllister, M.J.; Li, J.-L.; Adamson, D.H.; Schniepp, H.C.; Abdala, A.A.; Liu, J.; Herrera-Alonso, M.; Milius, D.L.; Car, R.; Prud’homme, R.K.; et al. Single Sheet Functionalized Graphene by Oxidation and Thermal Expansion of Graphite. Chem. Mater. 2007, 19, 4396–4404. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Soin, N.; Roy, S.S. Role of graphene/metal oxide composites as photocatalysts, adsorbents and disinfectants in water treatment: A review. RSC Adv. 2014, 4, 3823–3851. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.-e.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef]
- Whitener, K.E.; Sheehan, P.E. Graphene synthesis. Diam. Relat. Mater. 2014, 46, 25–34. [Google Scholar] [CrossRef]
- Charrier, A.; Coati, A.; Argunova, T.; Thibaudau, F.; Garreau, Y.; Pinchaux, R.; Forbeaux, I.; Debever, J.-M.; Sauvage-Simkin, M.; Themlin, J.-M. Solid-state decomposition of silicon carbide for growing ultra-thin heteroepitaxial graphite films. J. Appl. Phys. 2002, 92, 2479–2484. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.-S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Ri Kim, H.; Song, Y.I. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; He, Z.; Gong, H.; He, L. Recent advances in oil-water separation materials with special wettability modified by graphene and its derivatives: A review. Chem. Eng. Process.—Process Intensif. 2022, 170, 108678. [Google Scholar] [CrossRef]
- Guo, F.; Zhang, C.; Wang, Q.; Hu, W.; Cao, J.; Yao, J.; Jiang, L.; Wu, Z. Modification of poly (vinylidene fluoride) membranes with aluminum oxide nanowires and graphene oxide nanosheets for oil–water separation. J. Appl. Polym. Sci. 2019, 136, 47493. [Google Scholar] [CrossRef]
- Yuliwati, E.; Ismail, A.F. Effect of additives concentration on the surface properties and performance of PVDF ultrafiltration membranes for refinery produced wastewater treatment. Desalination 2011, 273, 226–234. [Google Scholar] [CrossRef]
- Nishigochi, S.; Ishigami, T.; Maruyama, T.; Hao, Y.; Ohmukai, Y.; Iwasaki, Y.; Matsuyama, H. Improvement of antifouling properties of polyvinylidene fluoride hollow fiber membranes by simple dip coating of phosphorylcholine copolymer via hydrophobic interactions. Ind. Eng. Chem. Res. 2014, 53, 2491–2497. [Google Scholar] [CrossRef]
- Sun, J.; Jia, H.; Bi, H.; Que, M.; Chen, L.; Zhang, Q.; Xiong, Y.; Xie, X.; Sun, Y. Laser-assisted synthesis of graphene-based paper for both oil/water mixtures and emulsions separation. Process Saf. Environ. Prot. 2022, 159, 674–684. [Google Scholar] [CrossRef]
- Liu, Y.; Coppens, M.-O. Cell Membrane-Inspired Graphene Nanomesh Membrane for Fast Separation of Oil-in-Water Emulsions. Adv. Funct. Mater. 2022, 32, 2200199. [Google Scholar] [CrossRef]
- Yang, C.; Long, M.; Ding, C.; Zhang, R.; Zhang, S.; Yuan, J.; Zhi, K.; Yin, Z.; Zheng, Y.; Liu, Y. Antifouling graphene oxide membranes for oil-water separation via hydrophobic chain engineering. Nat. Commun. 2022, 13, 7334. [Google Scholar] [CrossRef] [PubMed]
- Galli, G.; Martinelli, E. Amphiphilic polymer platforms: Surface engineering of films for marine antibiofouling. Macromol. Rapid Commun. 2017, 38, 1600704. [Google Scholar] [CrossRef]
- Chen, X.; Zhan, Y.; Sun, A.; Feng, Q.; Yang, W.; Dong, H.; Chen, Y.; Zhang, Y. Anchoring the TiO2@crumpled graphene oxide core–shell sphere onto electrospun polymer fibrous membrane for the fast separation of multi-component pollutant-oil–water emulsion. Sep. Purif. Technol. 2022, 298, 121605. [Google Scholar] [CrossRef]
- Yang, J.; Sun, J.; Wang, Z.; Wang, L. Surfactant-modified graphene oxide complex-coating functionalized material with robust switchable oil/water wettability for high-performance on-demand emulsion separation. Surf. Coat. Technol. 2022, 439, 128431. [Google Scholar] [CrossRef]
- Ma, L.; Wang, J.; Li, J.; Pang, Y.; He, J.; Peng, L.; Li, Y.; Li, K.; Qu, M. Intelligent composite foam with reversible tunable superwettability for efficient and sustainable oil/water separation and high-concentration organic wastewater purification. Process Saf. Environ. Prot. 2021, 149, 144–157. [Google Scholar] [CrossRef]
- Yan, T.; Zhang, T.; Zhao, G.; Zhang, C.; Li, C.; Jiao, F. Magnetic textile with pH-responsive wettability for controllable oil/water separation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 575, 155–165. [Google Scholar] [CrossRef]
- Awwad, M.; Bilal, M.; Sajid, M.; Nawaz, M.S.; Ihsanullah, I. MOF-based membranes for oil/water separation: Status, challenges, and prospects. J. Environ. Chem. Eng. 2023, 11, 109073. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Yuan, X.; Zhang, J.; Chew, J.W. Metal-organic framework membranes for wastewater treatment and water regeneration. Coord. Chem. Rev. 2020, 404, 213116. [Google Scholar] [CrossRef]
- Gangu, K.K.; Maddila, S.; Mukkamala, S.B.; Jonnalagadda, S.B. A review on contemporary Metal–Organic Framework materials. Inorg. Chim. Acta 2016, 446, 61–74. [Google Scholar] [CrossRef]
- Kuppler, R.J.; Timmons, D.J.; Fang, Q.-R.; Li, J.-R.; Makal, T.A.; Young, M.D.; Yuan, D.; Zhao, D.; Zhuang, W.; Zhou, H.-C. Potential applications of metal-organic frameworks. Coord. Chem. Rev. 2009, 253, 3042–3066. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Safaei, M.; Foroughi, M.M.; Ebrahimpoor, N.; Jahani, S.; Omidi, A.; Khatami, M. A review on metal-organic frameworks: Synthesis and applications. TrAC Trends Anal. Chem. 2019, 118, 401–425. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Xue, H.; Pang, H.; Xu, Q. Metal–organic frameworks as a platform for clean energy applications. EnergyChem 2020, 2, 100027. [Google Scholar] [CrossRef]
- Xuan, W.; Zhu, C.; Liu, Y.; Cui, Y. Mesoporous metal–organic framework materials. Chem. Soc. Rev. 2012, 41, 1677–1695. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Xue, M.; Zhu, G. Metal–organic framework membranes: From synthesis to separation application. Chem. Soc. Rev. 2014, 43, 6116–6140. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Liu, Y.; Chen, M.; Sadrzadeh, M.; Xu, Z.; Gan, D.; Huang, Z.; Ma, L.; Yang, B.; Zhou, Y. Robust superhydrophilic and underwater superoleophobic membrane optimized by Cu doping modified metal-organic frameworks for oil-water separation and water purification. J. Membr. Sci. 2021, 640, 119755. [Google Scholar] [CrossRef]
- Lu, W.; Duan, C.; Zhang, Y.; Gao, K.; Dai, L.; Shen, M.; Wang, W.; Wang, J.; Ni, Y. Cellulose-based electrospun nanofiber membrane with core-sheath structure and robust photocatalytic activity for simultaneous and efficient oil emulsions separation, dye degradation and Cr (VI) reduction. Carbohydr. Polym. 2021, 258, 117676. [Google Scholar] [CrossRef] [PubMed]
- Geng, Q.; Dong, S.; Li, Y.; Wu, H.; Yang, X.; Ning, X.; Yuan, D. High-Performance photoinduced antimicrobial membrane toward efficient PM2.5-0.3 capture and Oil-Water separation. Sep. Purif. Technol. 2022, 284, 120267. [Google Scholar] [CrossRef]
- Lu, W.; Duan, C.; Liu, C.; Zhang, Y.; Meng, X.; Dai, L.; Wang, W.; Yu, H.; Ni, Y. A self-cleaning and photocatalytic cellulose-fiber- supported “Ag@AgCl@MOF- cloth’’ membrane for complex wastewater remediation. Carbohydr. Polym. 2020, 247, 116691. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wei, W.; Yin, Y.; Liu, M.; Zheng, C.; Zhang, Y.; Deng, P. Continuous ultrathin UiO-66-NH2 coatings on a polymeric substrate synthesized by a layer-by-layer method: A kind of promising membrane for oil–water separation. Nanoscale 2020, 12, 6658–6663. [Google Scholar] [CrossRef] [PubMed]
- Gholami, F.; Zinadini, S.; Zinatizadeh, A.A. Preparation of high performance CuBTC/PES ultrafiltration membrane for oily wastewater separation; A good strategy for advanced separation. J. Environ. Chem. Eng. 2020, 8, 104482. [Google Scholar] [CrossRef]
- Zhang, R.; Cao, J.; Liu, Y.-N.; Guan, J.; He, M.; Jiang, Z. Metal–organic framework-intercalated graphene oxide membranes for highly efficient oil/water separation. Ind. Eng. Chem. Res. 2020, 59, 16762–16771. [Google Scholar] [CrossRef]
- Zhu, X.; Yu, Z.; Liu, Y.; Li, X.; Long, R.; Wang, P.; Wang, J. NH2-MIL-125@ PAA composite membrane for separation of oil/water emulsions and dyes. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 127542. [Google Scholar] [CrossRef]
- Xie, A.; Wu, Y.; Liu, Y.; Xue, C.; Ding, G.; Cheng, G.; Cui, J.; Pan, J. Robust antifouling NH2-MIL-88B coated quartz fibrous membrane for efficient gravity-driven oil-water emulsion separation. J. Membr. Sci. 2022, 644, 120093. [Google Scholar] [CrossRef]
- Xiang, X.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Mil-53 (Fe)-loaded polyacrylonitrile membrane with superamphiphilicity and double hydrophobicity for effective emulsion separation and photocatalytic dye degradation. Sep. Purif. Technol. 2022, 282, 119910. [Google Scholar] [CrossRef]
- Song, P.; Lu, Q. Porous clusters of metal-organic framework coated stainless steel mesh for highly efficient oil/water separation. Sep. Purif. Technol. 2020, 238, 116454. [Google Scholar] [CrossRef]
- Baker, R.W. Future Directions of Membrane Gas Separation Technology. Ind. Eng. Chem. Res. 2002, 41, 1393–1411. [Google Scholar] [CrossRef]
- Deng, Y.; Wu, Y.; Chen, G.; Zheng, X.; Dai, M.; Peng, C. Metal-organic framework membranes: Recent development in the synthesis strategies and their application in oil-water separation. Chem. Eng. J. 2021, 405, 127004. [Google Scholar] [CrossRef]
- Yin, X.; He, Y.; He, T.; Li, H.; Wu, J.; Zhou, L.; Li, S.; Li, C. A durable MOF-303-coated stainless steel mesh with robust anti-oil-fouling performance for multifunctional oil/water separation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 657, 130515. [Google Scholar] [CrossRef]
- Ezazi, M.; Shrestha, B.; Kim, S.-I.; Jeong, B.; Gorney, J.; Hutchison, K.; Lee, D.H.; Kwon, G. Selective Wettability Membrane for Continuous Oil−Water Separation and In Situ Visible Light-Driven Photocatalytic Purification of Water. Glob. Chall. 2020, 4, 2000009. [Google Scholar] [CrossRef]
- Shrestha, B.; Ezazi, M.; Kwon, G. Engineered Nanoparticles with Decoupled Photocatalysis and Wettability for Membrane-Based Desalination and Separation of Oil-Saline Water Mixtures. Nanomaterials 2021, 11, 1397. [Google Scholar] [CrossRef]
- Xie, A.; Cui, J.; Yang, J.; Chen, Y.; Lang, J.; Li, C.; Yan, Y.; Dai, J. Photo-Fenton self-cleaning PVDF/NH2-MIL-88B(Fe) membranes towards highly-efficient oil/water emulsion separation. J. Membr. Sci. 2020, 595, 117499. [Google Scholar] [CrossRef]
- Yang, B.; Ding, L.; Yao, H.; Chen, Y.; Shi, J. A Metal-Organic Framework (MOF) Fenton Nanoagent-Enabled Nanocatalytic Cancer Therapy in Synergy with Autophagy Inhibition. Adv. Mater. 2020, 32, 1907152. [Google Scholar] [CrossRef] [PubMed]
- Guerin, T.F. Long-term performance of a land treatment facility for the bioremediation of non-volatile oily wastes. Resour. Conserv. Recycl. 2000, 28, 105–120. [Google Scholar] [CrossRef]
- He, X.-T.; Li, B.-Y.; Liu, J.-X.; Tao, W.-Q.; Li, Z. Facile fabrication of 2D MOF-Based membrane with hierarchical structures for ultrafast Oil-Water separation. Sep. Purif. Technol. 2022, 297, 121488. [Google Scholar] [CrossRef]
- Jin, L.; Wang, Y.; Xue, T.; Xie, J.; Xu, Y.; Yao, Y.; Li, X. Smart amphiphilic random copolymer-coated sponge with pH-switchable wettability for on-demand oil/water separation. Langmuir 2019, 35, 14473–14480. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Jin, B.; Zhang, Q.; Zhan, X.; Chen, F. pH-induced switchable superwettability of efficient antibacterial fabrics for durable selective oil/water separation. ACS Appl. Mater. Interfaces 2017, 9, 30161–30170. [Google Scholar] [CrossRef]
- Zong, C.; Hu, M.; Azhar, U.; Chen, X.; Zhang, Y.; Zhang, S.; Lu, C. Smart copolymer-functionalized flexible surfaces with photoswitchable wettability: From superhydrophobicity with “rose petal” effect to superhydrophilicity. ACS Appl. Mater. Interfaces 2019, 11, 25436–25444. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Yang, S.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. A robust absorbent material based on light-responsive superhydrophobic melamine sponge for oil recovery. Adv. Mater. Interfaces 2016, 3, 1500683. [Google Scholar] [CrossRef]
- Fan, S.; Li, Z.; Fan, C.; Chen, J.; Huang, H.; Chen, G.; Liu, S.; Zhou, H.; Liu, R.; Feng, Z. Fast-thermoresponsive carboxylated carbon nanotube/chitosan aerogels with switchable wettability for oil/water separation. J. Hazard. Mater. 2022, 433, 128808. [Google Scholar] [CrossRef] [PubMed]
- Kung, C.H.; Zahiri, B.; Sow, P.K.; Mérida, W. On-demand oil-water separation via low-voltage wettability switching of core-shell structures on copper substrates. Appl. Surf. Sci. 2018, 444, 15–27. [Google Scholar] [CrossRef]
- Nadar, S.S.; Vaidya, L.B.; Maurya, S.S.; Rathod, V.K. Polysaccharide based metal organic frameworks (polysaccharide–MOF): A review. Coord. Chem. Rev. 2019, 396, 1–21. [Google Scholar] [CrossRef]
- Mohamed, M.; Abd-El-Nabey, B. Fabrication of a biological metal–organic framework based superhydrophobic textile fabric for efficient oil/water separation. Sci. Rep. 2022, 12, 15483. [Google Scholar] [CrossRef] [PubMed]
- Waller, P.J.; Gándara, F.; Yaghi, O.M. Chemistry of Covalent Organic Frameworks. Acc. Chem. Res. 2015, 48, 3053–3063. [Google Scholar] [CrossRef]
- Feng, X.; Ding, X.; Jiang, D. Covalent organic frameworks. Chem. Soc. Rev. 2012, 41, 6010–6022. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.-Y.; Wang, W. Covalent organic frameworks (COFs): From design to applications. Chem. Soc. Rev. 2013, 42, 548–568. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Chen, F.; Fang, Q.; Qiu, S. Design and applications of three dimensional covalent organic frameworks. Chem. Soc. Rev. 2020, 49, 1357–1384. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, S.; Chen, Y.; Zhang, Z.; Ma, S. Covalent organic frameworks for separation applications. Chem. Soc. Rev. 2020, 49, 708–735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, B.-H.; Ma, M.-Q.; Wang, Z.; Xu, Z.-K. Ultrathin metal/covalent–organic framework membranes towards ultimate separation. Chem. Soc. Rev. 2019, 48, 3811–3841. [Google Scholar] [CrossRef]
- Khayum, M.A.; Kandambeth, S.; Mitra, S.; Nair, S.B.; Das, A.; Nagane, S.S.; Mukherjee, R.; Banerjee, R. Chemically Delaminated Free-Standing Ultrathin Covalent Organic Nanosheets. Angew. Chem. Int. Ed. 2016, 55, 15604–15608. [Google Scholar] [CrossRef]
- Kandambeth, S.; Biswal, B.P.; Chaudhari, H.D.; Rout, K.C.; Kunjattu, H.S.; Mitra, S.; Karak, S.; Das, A.; Mukherjee, R.; Kharul, U.K.; et al. Selective Molecular Sieving in Self-Standing Porous Covalent-Organic-Framework Membranes. Adv. Mater. 2017, 29, 1603945. [Google Scholar] [CrossRef]
- Yang, H.; Wu, H.; Yao, Z.; Shi, B.; Xu, Z.; Cheng, X.; Pan, F.; Liu, G.; Jiang, Z.; Cao, X. Functionally graded membranes from nanoporous covalent organic frameworks for highly selective water permeation. J. Mater. Chem. A 2018, 6, 583–591. [Google Scholar] [CrossRef]
- Yuan, S.; Li, X.; Zhu, J.; Zhang, G.; Van Puyvelde, P.; Van der Bruggen, B. Covalent organic frameworks for membrane separation. Chem. Soc. Rev. 2019, 48, 2665–2681. [Google Scholar] [CrossRef]
- Lei, R.; Zha, Z.; Hao, Z.; Wang, J.; Wang, Z.; Zhao, S. Ultrathin and high-performance covalent organic frameworks composite membranes generated by oligomer triggered interfacial polymerization. J. Membr. Sci. 2022, 650, 120431. [Google Scholar] [CrossRef]
- Dey, K.; Pal, M.; Rout, K.C.; Kunjattu, H.S.; Das, A.; Mukherjee, R.; Kharul, U.K.; Banerjee, R. Selective molecular separation by interfacially crystallized covalent organic framework thin films. J. Am. Chem. Soc. 2017, 139, 13083–13091. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shi, B.; Yang, H.; Guan, J.; Liang, X.; Fan, C.; You, X.; Wang, Y.; Zhang, Z.; Wu, H.; et al. Assembling covalent organic framework membranes with superior ion exchange capacity. Nat. Commun. 2022, 13, 1020. [Google Scholar] [CrossRef]
- Zhang, K.; He, Z.; Gupta, K.M.; Jiang, J. Computational design of 2D functional covalent–organic framework membranes for water desalination. Environ. Sci. Water Res. Technol. 2017, 3, 735–743. [Google Scholar] [CrossRef]
- Shen, J.; Yuan, J.; Shi, B.; You, X.; Ding, R.; Zhang, T.; Zhang, Y.; Deng, Y.; Guan, J.; Long, M.; et al. Homointerface covalent organic framework membranes for efficient desalination. J. Mater. Chem. A 2021, 9, 23178–23187. [Google Scholar] [CrossRef]
- Huang, N.; Zhai, L.; Xu, H.; Jiang, D. Stable Covalent Organic Frameworks for Exceptional Mercury Removal from Aqueous Solutions. J. Am. Chem. Soc. 2017, 139, 2428–2434. [Google Scholar] [CrossRef]
- Ding, S.-Y.; Dong, M.; Wang, Y.-W.; Chen, Y.-T.; Wang, H.-Z.; Su, C.-Y.; Wang, W. Thioether-Based Fluorescent Covalent Organic Framework for Selective Detection and Facile Removal of Mercury(II). J. Am. Chem. Soc. 2016, 138, 3031–3037. [Google Scholar] [CrossRef]
- Ning, G.-H.; Chen, Z.; Gao, Q.; Tang, W.; Chen, Z.; Liu, C.; Tian, B.; Li, X.; Loh, K.P. Salicylideneanilines-Based Covalent Organic Frameworks as Chemoselective Molecular Sieves. J. Am. Chem. Soc. 2017, 139, 8897–8904. [Google Scholar] [CrossRef] [PubMed]
- Shinde, D.B.; Sheng, G.; Li, X.; Ostwal, M.; Emwas, A.-H.; Huang, K.-W.; Lai, Z. Crystalline 2D Covalent Organic Framework Membranes for High-Flux Organic Solvent Nanofiltration. J. Am. Chem. Soc. 2018, 140, 14342–14349. [Google Scholar] [CrossRef] [PubMed]
- Qahtan, T.F.; Gondal, M.A.; Dastageer, M.A.; Kwon, G.; Ezazi, M.; Al-Kuban, M.Z. Thermally sensitized membranes for crude oil–water remediation under visible light. ACS Appl. Mater. Interfaces 2020, 12, 48572–48579. [Google Scholar] [CrossRef]
- Shrestha, B.; Ezazi, M.; Rad, S.V.; Kwon, G. Predicting kinetics of water-rich permeate flux through photocatalytic mesh under visible light illumination. Sci. Rep. 2021, 11, 21065. [Google Scholar] [CrossRef]
- Chen, A.; Guo, H.; Zhou, J.; Li, Y.; He, X.; Chen, L.; Zhang, Y. Polyacrylonitrile nanofibers coated with covalent organic frameworks for oil/water separation. ACS Appl. Nano Mater. 2022, 5, 3925–3936. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, J.; Ren, Z.; Guan, Z.-H. Postsynthetically modified hydrophobic covalent organic frameworks for enhanced oil/water and CH4/C2H2 separation. Chem. Eng. J. 2022, 448, 137687. [Google Scholar] [CrossRef]
- Wu, X.; Hong, Y.-L.; Xu, B.; Nishiyama, Y.; Jiang, W.; Zhu, J.; Zhang, G.; Kitagawa, S.; Horike, S. Perfluoroalkyl-Functionalized Covalent Organic Frameworks with Superhydrophobicity for Anhydrous Proton Conduction. J. Am. Chem. Soc. 2020, 142, 14357–14364. [Google Scholar] [CrossRef]
- Liu, Y.; Li, W.; Yuan, C.; Jia, L.; Liu, Y.; Huang, A.; Cui, Y. Two-Dimensional Fluorinated Covalent Organic Frameworks with Tunable Hydrophobicity for Ultrafast Oil–Water Separation. Angew. Chem. Int. Ed. 2022, 61, e202113348. [Google Scholar] [CrossRef]
- Das, G.; Skorjanc, T.; Prakasam, T.; Garai, B.; Abubakar, S.; Zalch, C.S.; Gándara, F.; Pasricha, R.; Sharma, S.K.; Varghese, S.; et al. Hydrophobicity Tuning in Isostructural Urchin-Shaped Covalent Organic Framework Nanoparticles by Pore Surface Engineering for Oil–Water Separation. ACS Appl. Nano Mater. 2022, 5, 13745–13751. [Google Scholar] [CrossRef]
- Liang, Q.; Jiang, B.; Yang, N.; Zhang, L.; Sun, Y.; Zhang, L. Superhydrophilic Modification of Polyvinylidene Fluoride Membrane via a Highly Compatible Covalent Organic Framework–COOH/Dopamine-Integrated Hierarchical Assembly Strategy for Oil–Water Separation. ACS Appl. Mater. Interfaces 2022, 14, 45880–45892. [Google Scholar] [CrossRef]
- Zhang, S.; Bilal, M.; Adeel, M.; Barceló, D.; Iqbal, H.M. MXene-based designer nanomaterials and their exploitation to mitigate hazardous pollutants from environmental matrices. Chemosphere 2021, 283, 131293. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Lu, Y.; Wang, Y.; Guo, Y.; Shi, W. Preparation of 2D Materials and Their Application in Oil—Water Separation. Biomimetics 2023, 8, 35. [Google Scholar] [CrossRef] [PubMed]


| Membrane Materials | Synthesis/Fabrication Methods | Wettability | Permeate Flux | Type of Oils Separated | Separation Efficiency/Rejection Rate | Characteristics, Strengths, and Shortcomings |
|---|---|---|---|---|---|---|
| (i) MXene | ||||||
| [56] Fibrous MXene nanoribbons (MNRs) and mixed cellulose (MCE) | Vacuum filtration | Superhydrophilic + Underwater oleophobic (UOCA of 125°) | 15,860.24 L m−2 h−1 bar−1 | Oil–water mixtures n-hexane, edible oil, gasoline, diesel | >99% | By using ultraviolet light irradiation, photocatalytic self-cleaning can effectively increase the flux recovery rate (FRR). |
| [57] MXene nanosheets and porous polyvinylidene fluoride (PVDF) substrate and photocatalyst β-FeOOH | Vacuum filtration and in-site mineralization Intercalating the positively charged colloidal nanoparticles of Fe(OH)3 | Superhydrophilic (WCA = 0°) + Underwater superoleophobic (UOCA ~ 151–160°) | ~500.3–1022.7 L m−2 h−1 | Oil-in-water emulsions Crude oil, isooctane, n-hexane, toluene, petroleum ether, 1,3,5-trimethyl benzene, dichloromethane | >99% | Self-cleaning capabilities due to photo-Fenton property, and low oil adhesion. Chemically stable under conditions of high temperature (90 °C), high salinity, and severe corrosion. |
| [58] MXene laminates embedded with ferro-ferric oxide doped molybdenum disulfide flower-like composites | Facile fabrication strategy with vacuum filtration and self-assembly | Hydrophilic (WCA ~ 64.4–74.5°) | Oil mixtures 3.75 × 103 L m−2 h−1 Emulsions 4.25 × 102 L m−2 h−1 | Oil–water mixtures and emulsions n-hexane and edible oil | >99% | Under visible light, the fouled membranes permeability could be recovered by 99%. |
| [54] MXene nanosheets and N-doped Bi2O2CO3 nanoparticles (N-BOC) | Chemical etching, ultrasonic-aided exfoliation and vacuum filtration | Hydrophilic | Up to ~ 815.3 L m−2 h−1 | SDS/lubricating oil/H2O and SDS/vegetable oil/H2O emulsions | >99% | In situ fouling removal via photocatalysis mechanism. The excellent photocatalytic capability of N-BOC was enhanced by the 2D/2D heterojunction structure between MXene and N-BOC. |
| [63] Ti3C2TX MXene nanosheets membranes intercalated with sodium alginate | Pre-crosslinking and drop coating | Hydrophilic (WCA = 36°) + Underwater superoleophobic (UOCA ~ 151°) | >42.98 L m−2 h−1 bar−1 | Surfactants-stable oil-in-water emulsions n-hexane, octane, toluene | 97.22–99.62% - | Extremely low affinity for oil droplets. Significantly lower rejection to anionic dye compared to cationic dye. |
| Membrane Materials | Synthesis/Fabrication Methods | Wettability | Permeate Flux | Type of Oils Separated | Separation Efficiency/Rejection Rate | Characteristics, Strengths, and Shortcomings |
|---|---|---|---|---|---|---|
| (ii) Graphene | ||||||
| [89] Graphene and hydrophobic polydimethylsiloxane (PDMS) | Coating on an inexpensive paper tissue | Superhydrophobic (WCA = 153.26°) + Superoleophilic | 4421 L m−2 h−1 | Oil/water Mixtures and emulsions Heptane, gasoline, engine oil, soybean oil | 99.99% and up to 99.85% | Resistance to acid/alkali, impact, and friction resistance have been greatly improved. |
| [90] Cell membrane-inspired graphene nanomesh modified with chitosan | Vacuum-aided self-assembly method and synthesized via etching of nanopores on graphene oxide | Hydrophilic + Superoleophobic (UOCA up to 159.8°) | 3989 L m−2 h−1 bar−1 | Surfactant-stabilized oil-in-water emulsions Silicone oil, sunflower oil, octane, pump oil | 98.7% | Membrane is modified with the hydrophilic polymer chitosan to provide a hydration layer that prevents foulants from contacting. |
| [91] Graphene oxide with phytic acid (PA) and perfluorocarboxylic acids | Sequentially assembled | Hydrophilic + Superoleophobic (UOCA ~ 165°) | ~620 L m−2 h−1 bar−1 | n-Hexane, hexadecane, vacuum pump oil, corn oil | >98% | An important variable impacting the anti-fouling performance is the perfluoroalkyl chain length because it can adjust the surface hydration structure. Potential toxicity of perfluorocarboxylic acids. |
| [93] TiO2@crumpled graphene oxide core–shell spheres onto electrospun poly (arylene ether nitrile) to obtain fibrous composite membrane | Simple spraying technique | Superhydrophilic + Underwater superoleophobic (UOCA ~ 152–162°) | 4830–5160 L m−2 h−1 (SFE) 3062–3514 L m−2 h−1 (SSE) | Oil-in-water surfactant-free (SFE) and surfactant-stabilized (SSE) emulsions 1,3,5-trimethyl benzene, isooctane, n-hexane, n-heptane, petroleum ether | >99% | Molecular structure of poly (arylene ether nitrile) was rich in ether bond, benzene ring, and cyano-groups, which gave the membrane a high degree of temperature and corrosion resistance. Structure stability could be efficiently ensured by chemical crosslinking mediated by polydopamine and interactions with TA via hydrogen bonds. |
| [94] Surfactant-modified graphene oxide (GO-CTAB) on metal meshes | Simple one-step electrodeposition technique | Hydrophilic/ underwater- superoleophobic + Superhydrophobic/oleophilic (switchable) | 1800 L m−2 h−1 (Oil-in-water emulsion) 850 L m−2 h−1 (Water-in-oil emulsion) | Petroleum ether, n-hexane, n-hexadecane, diesel oil, soybean oil, dichloromethane | ~99% for hydrophilic membrane and superhydrophobic membrane (seawater/dichloromethane mixture) | Due to the reduced conjugation and negative electron cloud scattering, GO demonstrated exceptional corrosion resistance. |
| Membrane Materials | Synthesis/Fabrication Methods | Wettability | Permeate Flux | Type of Oils Separated | Separation Efficiency/Rejection Rate | Characteristics, Strengths, and Shortcomings |
|---|---|---|---|---|---|---|
| (iii) Metal–Organic Framework (MOF) | ||||||
| [119] MOF-303-coated stainless steel mesh | Simple hydrothermal method to form well-ordered MOF-303 crystals on layered double-hydroxide-modified mesh surface | Superhydrophilic (WCA = 5°) + Superoleophobic (UOCA ~ 151–166°) | 12,308–13,300 L m−2 h−1 (Oil/water mixtures) >2037 L m−2 h−1 (Emulsions) | Crude oil, cyclohexane, petroleum ether, diesel | >99.35% (Oil/water mixtures) Up to 99.74% (Emulsions) | Remarkable anti-oil-fouling and self-cleaning capabilities. Adsorbed water acted as a cushion to keep oils off the membrane surface. |
| [114] NH2-MIL-88B-coated quartz fibrous membrane | One-step solvothermal method | Superhydrophilic (WCA = 0°) + Superoleophobic (UOCA = 161.3°) | Above 350 L m−2 h−1 (oil-in-water emulsions) | n-hexane, dichloroethane, petroleum ether, toluene | Up to 99.4% | Good anti-fouling self-cleaning ability. The foulant on the membrane surface can be degraded by Fenton-like catalytic NM88B. |
| [125] 2D Cu triphenylene catecholate MOF with unique 2D hierarchical structures grown on copper mesh | One-step electrochemical deposition | Superhydrophilic (WCA = 0°) + Underwater superoleophobic (UOCA = 163.1°) | 146.3–329.6 k L m−2 h−1 | Cyclohexane, n-hexane, n-pentane, crude oil, petroleum ether, mineral oil, xylene | Less than 24.6 mg L−1 of oil residue | Membrane exhibits good recyclability and stability in corrosive environments. As more oil accumulated on the membrane’s surface and impeded the passage of water, the permeability slightly decreased. |
| [17] Hierarchical micro-/nanostructures prepared by interpenetrating CAU-10-H crystals grown on the mesh | Solvothermal synthesis | Under-liquid dual superlyophobic UW superoleophobic (~147–155°) + UO superhydrophobic (~145–154°) (Switchable) | 1.85 × 105 L m−2 h−1 | Immiscible oil/water mixtures and emulsions n-hexane, cyclohexane, methylbenzene, petroleum ether, dichloromethan, chloroform, tetrachloromethane | >99.92% | In diverse hostile environments with alkaline, acidic, and high concentration salt solutions, membrane exhibits exceptional heat and corrosion resistance. |
| [133] Copper cores and aspartic acid as a ligand + Stearic acid | Electrochemical process | Superhydrophobic textile fabric WCA of 158° ± 1.3, and a water sliding angle of 2° ± 0.2 | 15,400–15,700 L m−2 h−1 | Oil–water mixtures and emulsions Silicone oil, petroleum ether, n-hexane | 95–99.4% | Bio-MOF is a renewable material. Fabric retains its super hydrophobicity after 55 cycles of abrasion, and it also does so after immersion in water solutions with pH range of 5 to 9. |
| Membrane Materials | Synthesis/Fabrication Methods | Wettability | Permeate Flux | Type of Oils Separated | Separation Efficiency/Rejection Rate | Characteristics, Strengths, and Shortcomings |
|---|---|---|---|---|---|---|
| (iv) Covalent Organic Framework (COF) | ||||||
| [155] Schiff base COFs on electrospun polyacrylonitrile (PAN) nanofibers + Alkyl (Lauryl) | Dip coating | Superhydrophobic (WCA of ~167°) + Oleophilic | Up to ~350 L m−2 h−1 | Suspension of water droplets in oil Paraffin oil, soybean oil, vacuum pump oil, octane | >95% | Stable separation ability after 10 cycles of filtration. Limited information on the fouling resistance of the membrane. |
| [158] 2D COFs on stainless steel net substrates | Condensation reaction of fluorine and/or isopropyl functional groups and perfluorodialdehyde with triamines | Superhydrophobic (WCA = 150.1°) | ~2.84 × 105 L m−2 h−1 | Oil/water mixing systems Petroleum ether, CH2Cl2, CHCl3, n-heptane, kerosene, toluene, cyclohexane | >99.5% | Excellent resistance to water, acid, and base, and self-cleaning properties. |
| [156] Aromatic quinoline-linked COFs | Transforming the dynamic imine linkages into quinoline-linked aromatic rings via aza-Diels–Alder process | Superhydrophobic (WCA up to ~152°) | 1.10 × 104–2.20 × 104 L m−2 h−1 | Emulsions Toluene, cyclohexane, chlorobenzene, chloroform | Toluene 89.3%, cyclohexane 86.4%, chlorobenzene 95.3%, chloroform 99.6% | COFs maintained good crystallinity after exposure to acid, base, oxidizing agent, reducing agent, and boiling water. |
| [159] Imine-linked COFs via condensation of 1,3,5-tris(4-aminophenyl) benzene (TAB) with pyridine-based aldehyde linkers | Microwave-aided synthesis | Superhydrophobic (WCA = 155 ± 2°) + Oleophilic | - | Oil/water separation Pump oil, engine oil, vegetable oil | Removal capacity: Vegetable oil 530 ± 90 wt%, pump oil 518 ± 99 wt%, engine oil 550 ± 80 wt% | Retention of chemical functionalities after immersion in boiling water. Regeneration ability by oleophilic solvents. |
| [160] Carboxylated COF (COF-COOH) integrated with polydopamine (PDA) assembled on PVDF microfiltration membrane | One-step dip coating method | Superhydrophilic + Underwater superoleophobic (160–165°) | Up to 1843.48 L m−2 h−1 bar−1 | Emulsions Diesel, kerosene, soybean oil, n-hexane, petroleum ether | >98% | Good anti-fouling due to the strong electrostatic repulsion induced by carboxyl groups and the robust hydration layer formed by hierarchical nanostructures. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ezazi, M.; Quazi, M.M. Recent Developments in Two-Dimensional Materials-Based Membranes for Oil–Water Separation. Membranes 2023, 13, 677. https://doi.org/10.3390/membranes13070677
Ezazi M, Quazi MM. Recent Developments in Two-Dimensional Materials-Based Membranes for Oil–Water Separation. Membranes. 2023; 13(7):677. https://doi.org/10.3390/membranes13070677
Chicago/Turabian StyleEzazi, Mohammadamin, and M. M. Quazi. 2023. "Recent Developments in Two-Dimensional Materials-Based Membranes for Oil–Water Separation" Membranes 13, no. 7: 677. https://doi.org/10.3390/membranes13070677
APA StyleEzazi, M., & Quazi, M. M. (2023). Recent Developments in Two-Dimensional Materials-Based Membranes for Oil–Water Separation. Membranes, 13(7), 677. https://doi.org/10.3390/membranes13070677







