Membrane Progesterone Receptors (mPRs/PAQRs) Are Going beyond Its Initial Definitions
Abstract
1. Introduction
2. Discovery of mPRs/PAQRs Enriched Our Knowledge of PRG Signaling
3. mPRs Play an Important Role in PRG Biogenesis and PRG-Mediated Signaling
3.1. mPRs Are Key Factors in the Mammalian Female Reproductive System
3.2. Different PRG Binding Mechanism between mPRs and nPRs
3.3. Different Oligomerizing Mechanism between nPRs and mPRs for Ligand-Binding
3.4. Differential Pharmacodynamics of mPRs to Ligands Shared with nPRs
4. mPR-Mediated Signaling Can Be Coupled with Other Steroid Signaling Pathways
4.1. mPRs and PGRMC Can Form Their Own Complexes
4.2. mPR-Mediated Signaling and Ionotropic Neuronal Receptor GABAAR Coupled with Their Common Ligands
4.3. Reciprocal Hormonal Regulation of mPRs and G-Protein Coupled Receptors
4.4. Crosstalk and Reciprocal Regulation between nPRs and mPRs
5. Both nPR and mPR Mediated Cellular Signaling Might Work in Parallel
5.1. mPR Subcellular Compartmentation
5.2. Cytoplasmic-Nuclear Trafficking Is the Common Theme for Steroid Hormone Receptors
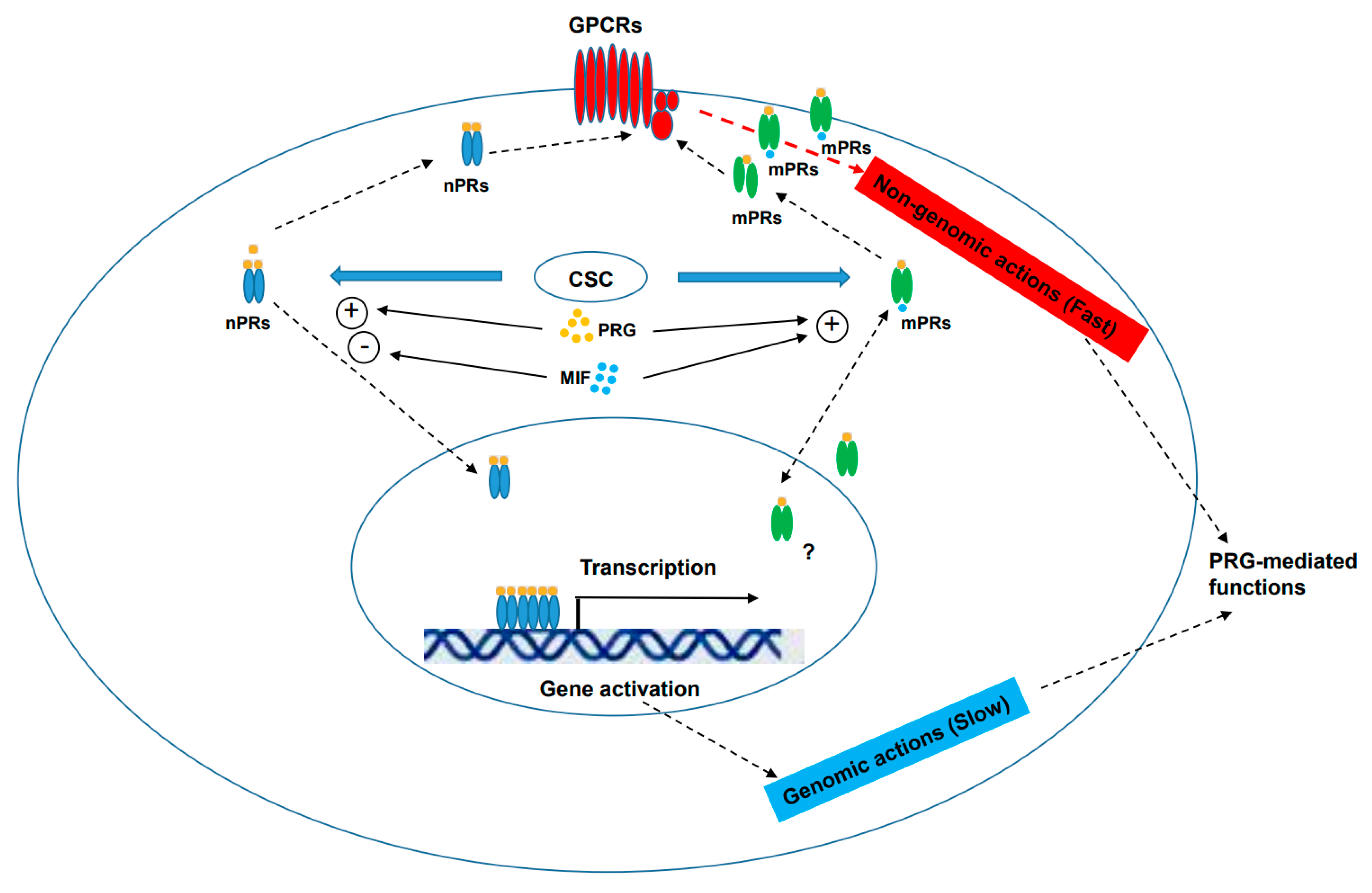
6. Key Roles of mPRs within the CmPn/CmP Signaling Network
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations:
| CCM | cerebral cavernous malformations |
| CHO | Chinese Hamster Ovary cells |
| CL | Corpus Luteum |
| CmP | CSC-mPR-PRG |
| CmPn | CSC-mPR-PRG-nPR |
| COS1 | Fibrobalst-like cell lines derived from monkeys |
| CSC | CCM signaling complex |
| EC | endothelial cell |
| ER | classic estrogen receptors |
| FSH | follicle stimulating hormone |
| GPCR | G-Protein Coupled Receptor |
| GPER | G protein-coupled estrogen receptor 1 |
| hCG | human chorionic gonadotropin |
| HEK | Human Embryonic Kidney cells |
| LH | luteinizing hormone |
| MDA-MB-231 | MD Anderson epithelial human breast cancer cell line-231 |
| MDCK | Madin-Darby Canine Kidney Tissue |
| MIF | mifepristone |
| mPR | non-classic membrane Progesterone receptor |
| NES | nuclear exit signal |
| NLS | nuclear localization signal |
| nPR | classic nuclear Progesterone receptor |
| PAQR | Progesterone and adipoQ receptor |
| PRE | PRG response element |
| PRG | Progesterone |
| PRM | Progesterone receptor modulator |
| PRGMCs | PRG receptor membrane components |
| S2R/TMEM97 | Sigma-2 Receptor/transmembrane protein 97 |
| TNBC | triple negative breast cancer |
References
- Zhang, Y.; Nadeau, M.; Faucher, F.; Lescelleur, O.; Biron, S.; Daris, M.; Rheaume, C.; Luu-The, V.; Tchernof, A. Progesterone metabolism in adipose cells. Mol. Cell Endocrinol. 2009, 298, 76–83. [Google Scholar] [CrossRef]
- Sundstrom-Poromaa, I.; Comasco, E.; Sumner, R.; Luders, E. Progesterone—Friend or foe? Front. Neuroendocrinol. 2020, 59, 100856. [Google Scholar] [CrossRef]
- Rossato, M.; Nogara, A.; Merico, M.; Ferlin, A.; Foresta, C. Identification of functional binding sites for progesterone in rat Leydig cell plasma membrane. Steroids 1999, 64, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Kalakota, N.R.; George, L.C.; Morelli, S.S.; Douglas, N.C.; Babwah, A.V. Towards an Improved Understanding of the Effects of Elevated Progesterone Levels on Human Endometrial Receptivity and Oocyte/Embryo Quality during Assisted Reproductive Technologies. Cells 2022, 11, 1405. [Google Scholar] [CrossRef] [PubMed]
- Stoffel-Wagner, B. Neurosteroid metabolism in the human brain. Eur. J. Endocrinol. 2001, 145, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Guennoun, R. Progesterone in the Brain: Hormone, Neurosteroid and Neuroprotectant. Int. J. Mol. Sci. 2020, 21, 5271. [Google Scholar] [CrossRef] [PubMed]
- DeMayo, F.J.; Lydon, J.P. 90 YEARS OF PROGESTERONE: New insights into progesterone receptor signaling in the endometrium required for embryo implantation. J. Mol. Endocrinol. 2020, 65, T1–T14. [Google Scholar] [CrossRef]
- Ali, S.; Balachandran, K.; O’Malley, B. 90 Years of progesterone: Ninety years of progesterone: The ‘other’ ovarian hormone. J. Mol. Endocrinol. 2020, 65, E1–E4. [Google Scholar] [CrossRef] [PubMed]
- Slayden, O.D.; Luo, F.; Bishop, C.V. Physiological Action of Progesterone in the Nonhuman Primate Oviduct. Cells 2022, 11, 1534. [Google Scholar] [CrossRef] [PubMed]
- Peluso, J.J. Progesterone Signaling and Mammalian Ovarian Follicle Growth Mediated by Progesterone Receptor Membrane Component Family Members. Cells 2022, 11, 1632. [Google Scholar] [CrossRef]
- Mirihagalle, S.; Hughes, J.R.; Miller, D.J. Progesterone-Induced Sperm Release from the Oviduct Sperm Reservoir. Cells 2022, 11, 1622. [Google Scholar] [CrossRef]
- Hewitt, S.C.; Wu, S.P.; Wang, T.; Young, S.L.; Spencer, T.E.; DeMayo, F.J. Progesterone Signaling in Endometrial Epithelial Organoids. Cells 2022, 11, 1760. [Google Scholar] [CrossRef] [PubMed]
- Oettel, M.; Mukhopadhyay, A.K. Progesterone: The forgotten hormone in men? Aging Male 2004, 7, 236–257. [Google Scholar] [CrossRef] [PubMed]
- Kalkhoff, R.K. Metabolic effects of progesterone. Am. J. Obstet. Gynecol. 1982, 142, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Frechou, M.; Liere, P.; Zhang, S.; Pianos, A.; Fernandez, N.; Denier, C.; Mattern, C.; Schumacher, M.; Guennoun, R. A Role of Endogenous Progesterone in Stroke Cerebroprotection Revealed by the Neural-Specific Deletion of Its Intracellular Receptors. J. Neurosci. 2017, 37, 10998–11020. [Google Scholar] [CrossRef] [PubMed]
- Guennoun, R.; Labombarda, F.; Gonzalez Deniselle, M.C.; Liere, P.; De Nicola, A.F.; Schumacher, M. Progesterone and allopregnanolone in the central nervous system: Response to injury and implication for neuroprotection. J. Steroid Biochem. Mol. Biol. 2015, 146, 48–61. [Google Scholar] [CrossRef]
- Corner, G.W.; Allen, W.M. Physiology of the corpus luteum. II. Production of a special uterine reaction (progestational proliferation) by extracts of corpus luteum. Am. J. Obstet. Gynecol. 1970, 107, 318. [Google Scholar]
- Savouret, J.F.; Misrahi, M.; Loosfelt, H.; Atger, M.; Bailly, A.; Perrot-Applanat, M.; Vu Hai, M.T.; Guiochon-Mantel, A.; Jolivet, A.; Lorenzo, F.; et al. Molecular and cellular biology of mammalian progesterone receptors. Recent Prog. Horm. Res. 1989, 45, 65–116; discussion 116–120. [Google Scholar] [CrossRef]
- Misrahi, M.; Atger, M.; d’Auriol, L.; Loosfelt, H.; Meriel, C.; Fridlansky, F.; Guiochon-Mantel, A.; Galibert, F.; Milgrom, E. Complete amino acid sequence of the human progesterone receptor deduced from cloned cDNA. Biochem. Biophys. Res. Commun. 1987, 143, 740–748. [Google Scholar] [CrossRef]
- Loosfelt, H.; Atger, M.; Misrahi, M.; Guiochon-Mantel, A.; Meriel, C.; Logeat, F.; Benarous, R.; Milgrom, E. Cloning and sequence analysis of rabbit progesterone-receptor complementary DNA. Proc. Natl. Acad. Sci. USA 1986, 83, 9045–9049. [Google Scholar] [CrossRef]
- Conneely, O.M.; Sullivan, W.P.; Toft, D.O.; Birnbaumer, M.; Cook, R.G.; Maxwell, B.L.; Zarucki-Schulz, T.; Greene, G.L.; Schrader, W.T.; O’Malley, B.W. Molecular cloning of the chicken progesterone receptor. Science 1986, 233, 767–770. [Google Scholar] [CrossRef]
- Kastner, P.; Krust, A.; Turcotte, B.; Stropp, U.; Tora, L.; Gronemeyer, H.; Chambon, P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990, 9, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Kowalik, M.K.; Rekawiecki, R.; Kotwica, J. The putative roles of nuclear and membrane-bound progesterone receptors in the female reproductive tract. Reprod. Biol. 2013, 13, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Suzuki, T.; Inoue, T.; Tazawa, C.; Ono, K.; Moriya, T.; Saito, H.; Ishibashi, T.; Takahashi, S.; Yamada, S.; et al. Progesterone receptor subtypes in vascular smooth muscle cells of human aorta. Endocr. J. 2005, 52, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Giangrande, P.H.; McDonnell, D.P. The A and B isoforms of the human progesterone receptor: Two functionally different transcription factors encoded by a single gene. Recent Prog. Horm. Res. 1999, 54, 291–313; discussion 313–294. [Google Scholar] [PubMed]
- Younglai, E.V.; Wu, Y.J.; Kwan, T.K.; Kwan, C.Y. Non-genomic action of estradiol and progesterone on cytosolic calcium concentrations in primary cultures of human granulosa-lutein cells. Hum. Reprod. 2005, 20, 2383–2390. [Google Scholar] [CrossRef]
- Younglai, E.V.; Wu, Y.; Foster, W.G.; Lobb, D.K.; Price, T.M. Binding of progesterone to cell surfaces of human granulosa-lutein cells. J. Steroid Biochem. Mol. Biol. 2006, 101, 61–67. [Google Scholar] [CrossRef]
- Peluso, J.J.; Fernandez, G.; Pappalardo, A.; White, B.A. Membrane-initiated events account for progesterone’s ability to regulate intracellular free calcium levels and inhibit rat granulosa cell mitosis. Biol. Reprod. 2002, 67, 379–385. [Google Scholar] [CrossRef]
- Pang, Y.; Thomas, P. mPRalpha and PR co-operate in progesterone inhibition of endothelial cell focal adhesion. J. Mol. Endocrinol. 2023, 70, e220073. [Google Scholar] [CrossRef]
- Sadler, S.E.; Maller, J.L. Identification of a steroid receptor on the surface of Xenopus oocytes by photoaffinity labeling. J. Biol. Chem. 1982, 257, 355–361. [Google Scholar] [CrossRef]
- Masui, Y.; Markert, C.L. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J. Exp. Zool. 1971, 177, 129–145. [Google Scholar] [CrossRef]
- Zhu, Y.; Rice, C.D.; Pang, Y.; Pace, M.; Thomas, P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc. Natl. Acad. Sci. USA 2003, 100, 2231–2236. [Google Scholar] [CrossRef]
- Zhu, Y.; Bond, J.; Thomas, P. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc. Natl. Acad. Sci. USA 2003, 100, 2237–2242. [Google Scholar] [CrossRef] [PubMed]
- Hammes, S.R. The further redefining of steroid-mediated signaling. Proc. Natl. Acad. Sci. USA 2003, 100, 2168–2170. [Google Scholar] [CrossRef] [PubMed]
- Boonyaratanakornkit, V.; Edwards, D.P. Receptor mechanisms of rapid extranuclear signalling initiated by steroid hormones. Essays Biochem. 2004, 40, 105–120. [Google Scholar] [CrossRef]
- Masi, M.; Racchi, M.; Travelli, C.; Corsini, E.; Buoso, E. Molecular Characterization of Membrane Steroid Receptors in Hormone-Sensitive Cancers. Cells 2021, 10, 2999. [Google Scholar] [CrossRef] [PubMed]
- Kasubuchi, M.; Watanabe, K.; Hirano, K.; Inoue, D.; Li, X.; Terasawa, K.; Konishi, M.; Itoh, N.; Kimura, I. Membrane progesterone receptor beta (mPRbeta/Paqr8) promotes progesterone-dependent neurite outgrowth in PC12 neuronal cells via non-G protein-coupled receptor (GPCR) signaling. Sci. Rep. 2017, 7, 5168. [Google Scholar] [CrossRef]
- Kimura, I.; Nakayama, Y.; Konishi, M.; Terasawa, K.; Ohta, M.; Itoh, N.; Fujimoto, M. Functions of MAPR (membrane-associated progesterone receptor) family members as heme/steroid-binding proteins. Curr. Protein Pept. Sci. 2012, 13, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zeng, C.; Chu, W.; Pan, F.; Rothfuss, J.M.; Zhang, F.; Tu, Z.; Zhou, D.; Zeng, D.; Vangveravong, S.; et al. Identification of the PGRMC1 protein complex as the putative sigma-2 receptor binding site. Nat. Commun. 2011, 2, 380. [Google Scholar] [CrossRef]
- Chu, U.B.; Mavlyutov, T.A.; Chu, M.L.; Yang, H.; Schulman, A.; Mesangeau, C.; McCurdy, C.R.; Guo, L.W.; Ruoho, A.E. The Sigma-2 Receptor and Progesterone Receptor Membrane Component 1 are Different Binding Sites Derived from Independent Genes. EBioMedicine 2015, 2, 1806–1813. [Google Scholar] [CrossRef]
- Zeng, C.; Riad, A.; Mach, R.H. The Biological Function of Sigma-2 Receptor/TMEM97 and Its Utility in PET Imaging Studies in Cancer. Cancers 2020, 12, 1877. [Google Scholar] [CrossRef] [PubMed]
- Riad, A.; Zeng, C.; Weng, C.C.; Winters, H.; Xu, K.; Makvandi, M.; Metz, T.; Carlin, S.; Mach, R.H. Sigma-2 Receptor/TMEM97 and PGRMC-1 Increase the Rate of Internalization of LDL by LDL Receptor through the Formation of a Ternary Complex. Sci. Rep. 2018, 8, 16845. [Google Scholar] [CrossRef]
- Zeng, C.; Weng, C.C.; Schneider, M.E., Jr.; Puentes, L.; Riad, A.; Xu, K.; Makvandi, M.; Jin, L.; Hawkins, W.G.; Mach, R.H. TMEM97 and PGRMC1 do not mediate sigma-2 ligand-induced cell death. Cell Death Discov. 2019, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Pru, J.K. Pleiotropic Actions of PGRMC Proteins in Cancer. Endocrinology 2022, 163, bqac078. [Google Scholar] [CrossRef]
- Dressing, G.E.; Alyea, R.; Pang, Y.; Thomas, P. Membrane progesterone receptors (mPRs) mediate progestin induced antimorbidity in breast cancer cells and are expressed in human breast tumors. Horm. Cancer 2012, 3, 101–112. [Google Scholar] [CrossRef]
- Karteris, E.; Zervou, S.; Pang, Y.; Dong, J.; Hillhouse, E.W.; Randeva, H.S.; Thomas, P. Progesterone signaling in human myometrium through two novel membrane G protein-coupled receptors: Potential role in functional progesterone withdrawal at term. Mol. Endocrinol. 2006, 20, 1519–1534. [Google Scholar] [CrossRef]
- Abou-Fadel, J.; Qu, Y.; Gonzalez, E.; Smith, M.; Zhang, J. Emerging roles of CCM genes during tumorigenesis with potential application as novel biomarkers across major types of cancers. Oncol. Rep. 2020, 43, 1945–1963. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Pang, Y.; Dong, J.; Groenen, P.; Kelder, J.; de Vlieg, J.; Zhu, Y.; Tubbs, C. Steroid and G protein binding characteristics of the seatrout and human progestin membrane receptor alpha subtypes and their evolutionary origins. Endocrinology 2007, 148, 705–718. [Google Scholar] [CrossRef]
- Pang, Y.; Dong, J.; Thomas, P. Progesterone increases nitric oxide synthesis in human vascular endothelial cells through activation of membrane progesterone receptor-alpha. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E899–E911. [Google Scholar] [CrossRef]
- Tokumoto, T.; Hossain, M.B.; Wang, J. Establishment of procedures for studying mPR-interacting agents and physiological roles of mPR. Steroids 2016, 111, 79–83. [Google Scholar] [CrossRef]
- Wu, X.J.; Liu, D.T.; Chen, S.; Hong, W.; Zhu, Y. Impaired oocyte maturation and ovulation in membrane progestin receptor (mPR) knockouts in zebrafish. Mol. Cell Endocrinol. 2020, 511, 110856. [Google Scholar] [CrossRef]
- Wu, X.J.; Zhu, Y. Downregulation of nuclear progestin receptor (Pgr) and subfertility in double knockouts of progestin receptor membrane component 1 (pgrmc1) and pgrmc2 in zebrafish. Gen. Comp. Endocrinol. 2020, 285, 113275. [Google Scholar] [CrossRef]
- MacLean, J.A., 2nd; Hayashi, K. Progesterone Actions and Resistance in Gynecological Disorders. Cells 2022, 11, 647. [Google Scholar] [CrossRef]
- Cope, D.I.; Monsivais, D. Progesterone Receptor Signaling in the Uterus Is Essential for Pregnancy Success. Cells 2022, 11, 1474. [Google Scholar] [CrossRef]
- Ashley, R.L.; Clay, C.M.; Farmerie, T.A.; Niswender, G.D.; Nett, T.M. Cloning and characterization of an ovine intracellular seven transmembrane receptor for progesterone that mediates calcium mobilization. Endocrinology 2006, 147, 4151–4159. [Google Scholar] [CrossRef]
- Valadez-Cosmes, P.; Vazquez-Martinez, E.R.; Cerbon, M.; Camacho-Arroyo, I. Membrane progesterone receptors in reproduction and cancer. Mol. Cell Endocrinol. 2016, 434, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Cui, W.; Dhakal, P.; Wolfe, M.W.; Rumi, M.A.; Vivian, J.L.; Roby, K.F.; Soares, M.J. Rethinking progesterone regulation of female reproductive cyclicity. Proc. Natl. Acad. Sci. USA 2016, 113, 4212–4217. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, T.; Zheng, L.; Huang, J.; Zheng, Y.; Li, J. PAQR7: An intermediary mediating nongenomic progesterone action in female reproductive tissue. Reprod. Biol. 2021, 21, 100529. [Google Scholar] [CrossRef]
- Medina-Laver, Y.; Rodriguez-Varela, C.; Salsano, S.; Labarta, E.; Dominguez, F. What Do We Know about Classical and Non-Classical Progesterone Receptors in the Human Female Reproductive Tract? A Review. Int. J. Mol. Sci. 2021, 22, 11278. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P. Membrane Progesterone Receptors (mPRs, PAQRs): Review of Structural and Signaling Characteristics. Cells 2022, 11, 1785. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Pang, Y.; Camilletti, M.A.; Castelnovo, L.F. Functions of Membrane Progesterone Receptors (mPRs, PAQRs) in Nonreproductive Tissues. Endocrinology 2022, 163, bqac147. [Google Scholar] [CrossRef]
- Thomas, P.; Pang, Y. Anti-apoptotic Actions of Allopregnanolone and Ganaxolone Mediated Through Membrane Progesterone Receptors (PAQRs) in Neuronal Cells. Front. Endocrinol. 2020, 11, 417. [Google Scholar] [CrossRef] [PubMed]
- Zuloaga, K.L.; Swift, S.N.; Gonzales, R.J.; Wu, T.J.; Handa, R.J. The androgen metabolite, 5alpha-androstane-3beta,17beta-diol, decreases cytokine-induced cyclooxygenase-2, vascular cell adhesion molecule-1 expression, and P-glycoprotein expression in male human brain microvascular endothelial cells. Endocrinology 2012, 153, 5949–5960. [Google Scholar] [CrossRef] [PubMed]
- Meffre, D.; Labombarda, F.; Delespierre, B.; Chastre, A.; De Nicola, A.F.; Stein, D.G.; Schumacher, M.; Guennoun, R. Distribution of membrane progesterone receptor alpha in the male mouse and rat brain and its regulation after traumatic brain injury. Neuroscience 2013, 231, 111–124. [Google Scholar] [CrossRef]
- Kelder, J.; Pang, Y.; Dong, J.; Schaftenaar, G.; Thomas, P. Molecular modeling, mutational analysis and steroid specificity of the ligand binding pocket of mPRalpha (PAQR7): Shared ligand binding with AdipoR1 and its structural basis. J. Steroid Biochem. Mol. Biol. 2022, 219, 106082. [Google Scholar] [CrossRef] [PubMed]
- Vasiliauskaite-Brooks, I.; Sounier, R.; Rochaix, P.; Bellot, G.; Fortier, M.; Hoh, F.; De Colibus, L.; Bechara, C.; Saied, E.M.; Arenz, C.; et al. Structural insights into adiponectin receptors suggest ceramidase activity. Nature 2017, 544, 120–123. [Google Scholar] [CrossRef]
- Polikarpova, A.V.; Maslakova, A.A.; Levina, I.S.; Kulikova, L.E.; Kuznetsov, Y.V.; Guseva, A.A.; Shchelkunova, T.A.; Zavarzin, I.V.; Smirnova, O.V. Selection of Progesterone Derivatives Specific to Membrane Progesterone Receptors. Biochemistry 2017, 82, 140–148. [Google Scholar] [CrossRef]
- Letz, M.; Bringmann, P.; Mann, M.; Mueller-Fahrnow, A.; Reipert, D.; Scholz, P.; Wurtz, J.M.; Egner, U. Investigation of the binding interactions of progesterone using muteins of the human progesterone receptor ligand binding domain designed on the basis of a three-dimensional protein model. Biochim. Biophys. Acta 1999, 1429, 391–400. [Google Scholar] [CrossRef]
- Kelder, J.; Azevedo, R.; Pang, Y.; de Vlieg, J.; Dong, J.; Thomas, P. Comparison between steroid binding to membrane progesterone receptor alpha (mPRalpha) and to nuclear progesterone receptor: Correlation with physicochemical properties assessed by comparative molecular field analysis and identification of mPRalpha-specific agonists. Steroids 2010, 75, 314–322. [Google Scholar] [CrossRef]
- Lange, C.A.; Sartorius, C.A.; Abdel-Hafiz, H.; Spillman, M.A.; Horwitz, K.B.; Jacobsen, B.M. Progesterone receptor action: Translating studies in breast cancer models to clinical insights. Adv. Exp. Med. Biol. 2008, 630, 94–111. [Google Scholar]
- Patel, B.; Elguero, S.; Thakore, S.; Dahoud, W.; Bedaiwy, M.; Mesiano, S. Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Hum. Reprod. Update 2015, 21, 155–173. [Google Scholar] [CrossRef]
- Richer, J.K.; Jacobsen, B.M.; Manning, N.G.; Abel, M.G.; Wolf, D.M.; Horwitz, K.B. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J. Biol. Chem. 2002, 277, 5209–5218. [Google Scholar] [CrossRef]
- Spitz, I.M. Progesterone antagonists and progesterone receptor modulators: An overview. Steroids 2003, 68, 981–993. [Google Scholar] [CrossRef]
- Klijn, J.G.; Setyono-Han, B.; Foekens, J.A. Progesterone antagonists and progesterone receptor modulators in the treatment of breast cancer. Steroids 2000, 65, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Baird, D.T. Antigestogens. Br. Med. Bull. 1993, 49, 73–87. [Google Scholar] [CrossRef]
- Leonhardt, S.A.; Altmann, M.; Edwards, D.P. Agonist and antagonists induce homodimerization and mixed ligand heterodimerization of human progesterone receptors in vivo by a mammalian two-hybrid assay. Mol. Endocrinol. 1998, 12, 1914–1930. [Google Scholar] [CrossRef]
- Edwards, D.P.; Leonhardt, S.A.; Gass-Handel, E. Novel mechanisms of progesterone antagonists and progesterone receptor. J. Soc. Gynecol. Investig. 2000, 7, S22–S24. [Google Scholar] [CrossRef]
- Edwards, D.P.; Altmann, M.; DeMarzo, A.; Zhang, Y.; Weigel, N.L.; Beck, C.A. Progesterone receptor and the mechanism of action of progesterone antagonists. J. Steroid Biochem. Mol. Biol. 1995, 53, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Kabe, Y.; Nakane, T.; Koike, I.; Yamamoto, T.; Sugiura, Y.; Harada, E.; Sugase, K.; Shimamura, T.; Ohmura, M.; Muraoka, K.; et al. Haem-dependent dimerization of PGRMC1/Sigma-2 receptor facilitates cancer proliferation and chemoresistance. Nat. Commun. 2016, 7, 11030. [Google Scholar] [CrossRef] [PubMed]
- Sueldo, C.; Liu, X.; Peluso, J.J. Progestin and AdipoQ Receptor 7, Progesterone Membrane Receptor Component 1 (PGRMC1), and PGRMC2 and Their Role in Regulating Progesterone’s Ability to Suppress Human Granulosa/Luteal Cells from Entering into the Cell Cycle. Biol. Reprod. 2015, 93, 63. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Bixenshpaner, H.; Asraf, H.; Chakraborty, M.; Elkabets, M.; Sekler, I.; Taylor, K.M.; Hershfinkel, M. Enhanced ZnR/GPR39 Activity in Breast Cancer, an Alternative Trigger of Signaling Leading to Cell Growth. Sci. Rep. 2018, 8, 8119. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.; Park, E.Y.; Kwon, S.Y.; Shin, S.; Emerson, R.E.; Shin, Y.H.; DeMayo, F.J.; Lydon, J.P.; Coffey, D.M.; Hawkins, S.M.; et al. Targeting progesterone signaling prevents metastatic ovarian cancer. Proc. Natl. Acad. Sci. USA 2020, 117, 31993–32004. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Afrin, S.; Jones, S.I.; Segars, J. Selective Progesterone Receptor Modulators-Mechanisms and Therapeutic Utility. Endocr. Rev. 2020, 41, bnaa012. [Google Scholar] [CrossRef]
- Chen, G.; Qiu, L.; Gao, J.; Wang, J.; Dang, J.; Li, L.; Jin, Z.; Liu, X. Stress Hormones: Emerging Targets in Gynecological Cancers. Front. Cell Dev. Biol. 2021, 9, 699487. [Google Scholar] [CrossRef]
- Check, J.H.; Check, D. New Insights as to Why Progesterone Receptor Modulators, such as Mifepristone, Seem to Be More Effective in Treating Cancers that Are Devoid of the Classical Nuclear Progesterone Receptor. Anticancer Res. 2021, 41, 5873–5880. [Google Scholar] [CrossRef] [PubMed]
- Rose, F.V.; Barnea, E.R. Response of human ovarian carcinoma cell lines to antiprogestin mifepristone. Oncogene 1996, 12, 999–1003. [Google Scholar] [PubMed]
- Moe, B.G.; Vereide, A.B.; Orbo, A.; Sager, G. High concentrations of progesterone and mifepristone mutually reinforce cell cycle retardation and induction of apoptosis. Anticancer Res. 2009, 29, 1053–1058. [Google Scholar]
- Fjelldal, R.; Moe, B.T.; Orbo, A.; Sager, G. MCF-7 cell apoptosis and cell cycle arrest: Non-genomic effects of progesterone and mifepristone (RU-486). Anticancer Res. 2010, 30, 4835–4840. [Google Scholar]
- Gamarra-Luques, C.D.; Goyeneche, A.A.; Hapon, M.B.; Telleria, C.M. Mifepristone prevents repopulation of ovarian cancer cells escaping cisplatin-paclitaxel therapy. BMC Cancer 2012, 12, 200. [Google Scholar] [CrossRef]
- Iwasaki, K.; Underwood, B.; Herman, M.; Dinda, S.; Kodali, S.; Kloosterboer, H.J.; Hurd, C.; Moudgil, V.K. Effects of antiprogestins on the rate of proliferation of breast cancer cells. Mol. Cell. Biochem. 1999, 198, 141–149. [Google Scholar] [CrossRef]
- Skildum, A.; Faivre, E.; Lange, C.A. Progesterone receptors induce cell cycle progression via activation of mitogen-activated protein kinases. Mol. Endocrinol. 2005, 19, 327–339. [Google Scholar] [CrossRef]
- Bowden, R.T.; Hissom, J.R.; Moore, M.R. Growth stimulation of T47D human breast cancer cells by the anti-progestin RU486. Endocrinology 1989, 124, 2642–2644. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.E.; Pornon, A.; Ji, J.W.; Bocquel, M.T.; Chambon, P.; Gronemeyer, H. Agonistic and antagonistic activities of RU486 on the functions of the human progesterone receptor. EMBO J. 1990, 9, 3923–3932. [Google Scholar] [CrossRef]
- Zuo, L.; Li, W.; You, S. Progesterone reverses the mesenchymal phenotypes of basal phenotype breast cancer cells via a membrane progesterone receptor mediated pathway. Breast Cancer Res. 2010, 12, R34. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Hammes, S.R. Just when you thought it was safe to go into the membrane: The growing complexities of extra-nuclear progesterone signaling. Breast Cancer Res. 2010, 12, 109. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, H.; Ukena, K.; Takemori, H.; Okamoto, M.; Kawata, M.; Tsutsui, K. Expression and localization of 25-Dx, a membrane-associated putative progesterone-binding protein, in the developing Purkinje cell. Neuroscience 2004, 126, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.L.; Intlekofer, K.A.; Moura-Conlon, P.J.; Brewer, D.N.; Del Pino Sans, J.; Lopez, J.A. Nonclassical progesterone signalling molecules in the nervous system. J. Neuroendocrinol. 2013, 25, 991–1001. [Google Scholar] [CrossRef]
- Cahill, M.A. Progesterone receptor membrane component 1: An integrative review. J. Steroid Biochem. Mol. Biol. 2007, 105, 16–36. [Google Scholar] [CrossRef] [PubMed]
- Wardell, S.E.; Edwards, D.P. Mechanisms controlling agonist and antagonist potential of selective progesterone receptor modulators (SPRMs). Semin. Reprod. Med. 2005, 23, 9–21. [Google Scholar] [CrossRef]
- Wardell, S.E.; Narayanan, R.; Weigel, N.L.; Edwards, D.P. Partial agonist activity of the progesterone receptor antagonist RU486 mediated by an amino-terminal domain coactivator and phosphorylation of serine400. Mol. Endocrinol. 2010, 24, 335–345. [Google Scholar] [CrossRef]
- Tieszen, C.R.; Goyeneche, A.A.; Brandhagen, B.N.; Ortbahn, C.T.; Telleria, C.M. Antiprogestin mifepristone inhibits the growth of cancer cells of reproductive and non-reproductive origin regardless of progesterone receptor expression. BMC Cancer 2011, 11, 207. [Google Scholar] [CrossRef] [PubMed]
- Abou-Fadel, J.; Bhalli, M.; Grajeda, B.; Zhang, J. CmP Signaling Network Leads to Identification of Prognostic Biomarkers for Triple-Negative Breast Cancer in Caucasian Women. Genet. Test. Mol. Biomark. 2022, 26, 198–219. [Google Scholar] [CrossRef]
- Abou-Fadel, J.; Grajeda, B.; Jiang, X.; Cailing-De La, O.A.; Flores, E.; Padarti, A.; Bhalli, M.; Le, A.; Zhang, J. CmP signaling network unveils novel biomarkers for triple negative breast cancer in African American women. Cancer Biomark. 2022, 34, 607–636. [Google Scholar] [CrossRef] [PubMed]
- Abou-Fadel, J.; Jiang, X.; Grajeda, B.; Padarti, A.; Ellis, C.C.; Flores, E.; Cailing-De La, O.A.; Zhang, J. CCM signaling complex (CSC) couples both classic and non-classic Progesterone receptor signaling. Cell Commun. Signal. 2022, 20, 120. [Google Scholar] [CrossRef]
- Abou-Fadel, J.; Jiang, X.; Padarti, A.; Goswami, D.; Smith, M.; Grajeda, B.; Walker, W.; Zhang, J. CCM signaling complex (CSC) is a master regulator governing homeostasis of progestins and their mediated signaling cascades. bioRxiv 2020. [Google Scholar] [CrossRef]
- Thomas, P. Characteristics of membrane progestin receptor alpha (mPRalpha) and progesterone membrane receptor component 1 (PGMRC1) and their roles in mediating rapid progestin actions. Front. Neuroendocrinol. 2008, 29, 292–312. [Google Scholar] [CrossRef]
- Thomas, P.; Pang, Y.; Dong, J. Enhancement of cell surface expression and receptor functions of membrane progestin receptor alpha (mPRalpha) by progesterone receptor membrane component 1 (PGRMC1): Evidence for a role of PGRMC1 as an adaptor protein for steroid receptors. Endocrinology 2014, 155, 1107–1119. [Google Scholar] [CrossRef]
- Wu, X.J.; Thomas, P.; Zhu, Y. Pgrmc1 Knockout Impairs Oocyte Maturation in Zebrafish. Front. Endocrinol. 2018, 9, 560. [Google Scholar] [CrossRef]
- Robel, P.; Baulieu, E.E. Neurosteroids: Biosynthesis and function. Crit. Rev. Neurobiol. 1995, 9, 383–394. [Google Scholar]
- Reddy, D.S. Pharmacology of endogenous neuroactive steroids. Crit. Rev. Neurobiol. 2003, 15, 197–234. [Google Scholar] [CrossRef]
- Mellon, S.H.; Griffin, L.D. Neurosteroids: Biochemistry and clinical significance. Trends Endocrinol. Metab. 2002, 13, 35–43. [Google Scholar] [CrossRef]
- Reddy, D.S. Neurosteroids: Endogenous role in the human brain and therapeutic potentials. Prog. Brain Res. 2010, 186, 113–137. [Google Scholar] [CrossRef]
- Rupprecht, R. Neuroactive steroids: Mechanisms of action and neuropsychopharmacological properties. Psychoneuroendocrinology 2003, 28, 139–168. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, E.A.; Herd, M.B.; Gunn, B.G.; Lambert, J.J.; Belelli, D. Neurosteroid modulation of GABAA receptors: Molecular determinants and significance in health and disease. Neurochem. Int. 2008, 52, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Majewska, M.D. Neurosteroids: Endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Prog. Neurobiol. 1992, 38, 379–395. [Google Scholar] [CrossRef]
- Thomas, P.; Pang, Y. Membrane progesterone receptors: Evidence for neuroprotective, neurosteroid signaling and neuroendocrine functions in neuronal cells. Neuroendocrinology 2012, 96, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Dong, J.; Thomas, P. Characterization, neurosteroid binding and brain distribution of human membrane progesterone receptors delta and epsilon (mPRdelta and mPRepsilon) and mPRdelta involvement in neurosteroid inhibition of apoptosis. Endocrinology 2013, 154, 283–295. [Google Scholar] [CrossRef]
- Abou-Fadel, J.; Jiang, X.; Padarti, A.; Goswami, D.G.; Smith, M.; Grajeda, B.; Bhalli, M.; Le, A.; Walker, W.E.; Zhang, J. mPR-Specific Actions Influence Maintenance of the Blood-Brain Barrier (BBB). Int. J. Mol. Sci. 2022, 23, 9684. [Google Scholar] [CrossRef]
- Kapur, J.; Joshi, S. Progesterone modulates neuronal excitability bidirectionally. Neurosci. Lett. 2021, 744, 135619. [Google Scholar] [CrossRef]
- Castelnovo, L.F.; Thomas, P. Membrane Progesterone Receptor alpha (mPRalpha/PAQR7) Promotes Survival and Neurite Outgrowth of Human Neuronal Cells by a Direct Action and Through Schwann Cell-like Stem Cells. J. Mol. Neurosci. 2022, 72, 2067–2080. [Google Scholar] [CrossRef]
- Krietsch, T.; Fernandes, M.S.; Kero, J.; Losel, R.; Heyens, M.; Lam, E.W.; Huhtaniemi, I.; Brosens, J.J.; Gellersen, B. Human homologs of the putative G protein-coupled membrane progestin receptors (mPRalpha, beta, and gamma) localize to the endoplasmic reticulum and are not activated by progesterone. Mol. Endocrinol. 2006, 20, 3146–3164. [Google Scholar] [CrossRef]
- Thomas, P.; Zhu, Y.; Pace, M. Progestin membrane receptors involved in the meiotic maturation of teleost oocytes: A review with some new findings. Steroids 2002, 67, 511–517. [Google Scholar] [CrossRef]
- Pace, M.C.; Thomas, P. Activation of a pertussis toxin-sensitive, inhibitory G-protein is necessary for steroid-mediated oocyte maturation in spotted seatrout. Dev. Biol. 2005, 285, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.T.; Hu, T.; Arterburn, M.; Boyle, B.; Bright, J.M.; Emtage, P.C.; Funk, W.D. PAQR proteins: A novel membrane receptor family defined by an ancient 7-transmembrane pass motif. J. Mol. Evol. 2005, 61, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L.; Kupchak, B.R.; Garitaonandia, I.; Hoang, L.K.; Maina, A.S.; Regalla, L.M.; Lyons, T.J. Heterologous expression of human mPRalpha, mPRbeta and mPRgamma in yeast confirms their ability to function as membrane progesterone receptors. Steroids 2008, 73, 1160–1173. [Google Scholar] [CrossRef] [PubMed]
- Moussatche, P.; Lyons, T.J. Non-genomic progesterone signalling and its non-canonical receptor. Biochem. Soc. Trans. 2012, 40, 200–204. [Google Scholar] [CrossRef]
- Thomas, P. Reprint of “Role of G protein-coupled estrogen receptor (GPER/GPR30) in maintenance of meiotic arrest in fish oocytes”. J. Steroid Biochem. Mol. Biol. 2018, 176, 23–30. [Google Scholar] [CrossRef]
- Pang, Y.; Thomas, P. Role of G protein-coupled estrogen receptor 1, GPER, in inhibition of oocyte maturation by endogenous estrogens in zebrafish. Dev. Biol. 2010, 342, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.H.; Lange, C.A. Deciphering Steroid Receptor Crosstalk in Hormone-Driven Cancers. Endocrinology 2018, 159, 3897–3907. [Google Scholar] [CrossRef]
- Thomas, C.; Gustafsson, J.A. Progesterone receptor-estrogen receptor crosstalk: A novel insight. Trends Endocrinol. Metab. 2015, 26, 453–454. [Google Scholar] [CrossRef]
- Salazar, M.; Lerma-Ortiz, A.; Hooks, G.M.; Ashley, A.K.; Ashley, R.L. Progestin-mediated activation of MAPK and AKT in nuclear progesterone receptor negative breast epithelial cells: The role of membrane progesterone receptors. Gene 2016, 591, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Pedroza, D.A.; Subramani, R.; Tiula, K.; Do, A.; Rashiraj, N.; Galvez, A.; Chatterjee, A.; Bencomo, A.; Rivera, S.; Lakshmanaswamy, R. Crosstalk between progesterone receptor membrane component 1 and estrogen receptor alpha promotes breast cancer cell proliferation. Lab. Investig. 2021, 101, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Pecci, A.; Ogara, M.F.; Sanz, R.T.; Vicent, G.P. Choosing the right partner in hormone-dependent gene regulation: Glucocorticoid and progesterone receptors crosstalk in breast cancer cells. Front. Endocrinol. 2022, 13, 1037177. [Google Scholar] [CrossRef]
- Giulianelli, S.; Vaque, J.P.; Soldati, R.; Wargon, V.; Vanzulli, S.I.; Martins, R.; Zeitlin, E.; Molinolo, A.A.; Helguero, L.A.; Lamb, C.A.; et al. Estrogen receptor alpha mediates progestin-induced mammary tumor growth by interacting with progesterone receptors at the cyclin D1/MYC promoters. Cancer Res. 2012, 72, 2416–2427. [Google Scholar] [CrossRef] [PubMed]
- Dosiou, C.; Hamilton, A.E.; Pang, Y.; Overgaard, M.T.; Tulac, S.; Dong, J.; Thomas, P.; Giudice, L.C. Expression of membrane progesterone receptors on human T lymphocytes and Jurkat cells and activation of G-proteins by progesterone. J. Endocrinol. 2008, 196, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Sleiter, N.; Pang, Y.; Park, C.; Horton, T.H.; Dong, J.; Thomas, P.; Levine, J.E. Progesterone receptor A (PRA) and PRB-independent effects of progesterone on gonadotropin-releasing hormone release. Endocrinology 2009, 150, 3833–3844. [Google Scholar] [CrossRef]
- Boonyaratanakornkit, V.; Hamilton, N.; Marquez-Garban, D.C.; Pateetin, P.; McGowan, E.M.; Pietras, R.J. Extranuclear signaling by sex steroid receptors and clinical implications in breast cancer. Mol. Cell Endocrinol. 2018, 466, 51–72. [Google Scholar] [CrossRef]
- Padarti, A.; Zhang, J. Recent advances in cerebral cavernous malformation research. Vessel Plus 2018, 2, 21. [Google Scholar] [CrossRef]
- Abou-Fadel, J.; Smith, M.; Falahati, K.; Zhang, J. Comparative omics of CCM signaling complex (CSC). Chin. Neurosurg. J. 2020, 6, 4. [Google Scholar] [CrossRef]
- Abou-Fadel, J.; Vasquez, M.; Grajeda, B.; Ellis, C.; Zhang, J. Systems-wide analysis unravels the new roles of CCM signal complex (CSC). Heliyon 2019, 5, e02899. [Google Scholar] [CrossRef]
- Renteria, M.; Belkin, O.; Jang, D.; Aickareth, J.; Bhalli, M.; Zhang, J. CmPn signaling networks in the tumorigenesis of breast cancer. Front. Endocrinol. 2022, 13, 1013892. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Abou-Fadel, J.S. Calm the raging hormone—A new therapeutic strategy involving progesterone-signaling for hemorrhagic CCMs. Vessel Plus 2021, 5, 48. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Pang, Y.; Tubbs, C.; Thomas, P. Induction of sperm hypermotility through membrane progestin receptor alpha (mPRalpha): A teleost model of rapid, multifaceted, nongenomic progestin signaling. Gen. Comp. Endocrinol. 2019, 279, 60–66. [Google Scholar] [CrossRef]
- Salhi, A.; Lemale, J.; Paris, N.; Bloch-Faure, M.; Crambert, G. Membrane progestin receptors: Beyond the controversy, can we move forward? Biomol. Concepts 2010, 1, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Zhou, L.; Chen, X.; Gainey, L.O.; Xiao, J.; Nanes, M.S.; Hou, A.; You, S.; Chen, Q. Progesterone and Src family inhibitor PP1 synergistically inhibit cell migration and invasion of human basal phenotype breast cancer cells. Biomed. Res. Int. 2015, 2015, 426429. [Google Scholar] [CrossRef] [PubMed]
- Tischkau, S.A.; Ramirez, V.D. A specific membrane binding protein for progesterone in rat brain: Sex differences and induction by estrogen. Proc. Natl. Acad. Sci. USA 1993, 90, 1285–1289. [Google Scholar] [CrossRef]
- Josefsberg Ben-Yehoshua, L.; Lewellyn, A.L.; Thomas, P.; Maller, J.L. The role of Xenopus membrane progesterone receptor beta in mediating the effect of progesterone on oocyte maturation. Mol. Endocrinol. 2007, 21, 664–673. [Google Scholar] [CrossRef]
- Zhu, Y.; Hanna, R.N.; Schaaf, M.J.; Spaink, H.P.; Thomas, P. Candidates for membrane progestin receptors--past approaches and future challenges. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2008, 148, 381–389. [Google Scholar] [CrossRef]
- Foster, H.; Reynolds, A.; Stenbeck, G.; Dong, J.; Thomas, P.; Karteris, E. Internalisation of membrane progesterone receptor-alpha after treatment with progesterone: Potential involvement of a clathrin-dependent pathway. Mol. Med. Rep. 2010, 3, 27–35. [Google Scholar] [CrossRef]
- Fernandes, M.S.; Brosens, J.J.; Gellersen, B. Honey, we need to talk about the membrane progestin receptors. Steroids 2008, 73, 942–952. [Google Scholar] [CrossRef]
- Lemale, J.; Bloch-Faure, M.; Grimont, A.; El Abida, B.; Imbert-Teboul, M.; Crambert, G. Membrane progestin receptors alpha and gamma in renal epithelium. Biochim. Biophys. Acta 2008, 1783, 2234–2240. [Google Scholar] [CrossRef]
- Guiochon-Mantel, A.; Milgrom, E. Cytoplasmic-nuclear trafficking of steroid hormone receptors. Trends Endocrinol. Metab. 1993, 4, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, R.K.; Lavrovsky, Y.; Ahn, S.C.; Song, C.S.; Chatterjee, B.; Roy, A.K. Dynamics of intracellular movement and nucleocytoplasmic recycling of the ligand-activated androgen receptor in living cells. Mol. Endocrinol. 2000, 14, 1162–1174. [Google Scholar] [CrossRef]
- Ozanne, D.M.; Brady, M.E.; Cook, S.; Gaughan, L.; Neal, D.E.; Robson, C.N. Androgen receptor nuclear translocation is facilitated by the f-actin cross-linking protein filamin. Mol. Endocrinol. 2000, 14, 1618–1626. [Google Scholar] [CrossRef] [PubMed]
- Mazaira, G.I.; Echeverria, P.C.; Galigniana, M.D. Nucleocytoplasmic shuttling of the glucocorticoid receptor is influenced by tetratricopeptide repeat-containing proteins. J. Cell Sci. 2020, 133, jcs238873. [Google Scholar] [CrossRef]
- Moriyama, T.; Yoneda, Y.; Oka, M.; Yamada, M. Transportin-2 plays a critical role in nucleocytoplasmic shuttling of oestrogen receptor-alpha. Sci. Rep. 2020, 10, 18640. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, R.K.; Amazit, L.; Lescop, P.; Milgrom, E.; Guiochon-Mantel, A. Mechanisms of progesterone receptor export from nuclei: Role of nuclear localization signal, nuclear export signal, and ran guanosine triphosphate. Mol. Endocrinol. 1998, 12, 1684–1695. [Google Scholar] [CrossRef]
- Peluso, J.J.; Pappalardo, A.; Losel, R.; Wehling, M. Progesterone membrane receptor component 1 expression in the immature rat ovary and its role in mediating progesterone’s antiapoptotic action. Endocrinology 2006, 147, 3133–3140. [Google Scholar] [CrossRef]
- Peluso, J.J.; Liu, X.; Gawkowska, A.; Lodde, V.; Wu, C.A. Progesterone inhibits apoptosis in part by PGRMC1-regulated gene expression. Mol. Cell Endocrinol. 2010, 320, 153–161. [Google Scholar] [CrossRef]
- Peluso, J.J.; Liu, X.; Gawkowska, A.; Johnston-MacAnanny, E. Progesterone activates a progesterone receptor membrane component 1-dependent mechanism that promotes human granulosa/luteal cell survival but not progesterone secretion. J. Clin. Endocrinol. Metab. 2009, 94, 2644–2649. [Google Scholar] [CrossRef]
- Peluso, J.J.; Liu, X.; Saunders, M.M.; Claffey, K.P.; Phoenix, K. Regulation of ovarian cancer cell viability and sensitivity to cisplatin by progesterone receptor membrane component-1. J. Clin. Endocrinol. Metab. 2008, 93, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P. Rapid steroid hormone actions initiated at the cell surface and the receptors that mediate them with an emphasis on recent progress in fish models. Gen. Comp. Endocrinol. 2012, 175, 367–383. [Google Scholar] [CrossRef]
- Abou-Fadel, J.; Zhang, J. Systems Wide Analysis of CCM Signaling Complex Alterations in CCM-Deficient Models Using Omics Approaches. Methods Mol. Biol. 2020, 2152, 325–344. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Padarti, A.; Qu, Y.; Sheng, S.; Abou-Fadel, J.; Badr, A.; Zhang, J. Alternatively spliced isoforms reveal a novel type of PTB domain in CCM2 protein. Sci. Rep. 2019, 9, 15808. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aickareth, J.; Hawwar, M.; Sanchez, N.; Gnanasekaran, R.; Zhang, J. Membrane Progesterone Receptors (mPRs/PAQRs) Are Going beyond Its Initial Definitions. Membranes 2023, 13, 260. https://doi.org/10.3390/membranes13030260
Aickareth J, Hawwar M, Sanchez N, Gnanasekaran R, Zhang J. Membrane Progesterone Receptors (mPRs/PAQRs) Are Going beyond Its Initial Definitions. Membranes. 2023; 13(3):260. https://doi.org/10.3390/membranes13030260
Chicago/Turabian StyleAickareth, Justin, Majd Hawwar, Nickolas Sanchez, Revathi Gnanasekaran, and Jun Zhang. 2023. "Membrane Progesterone Receptors (mPRs/PAQRs) Are Going beyond Its Initial Definitions" Membranes 13, no. 3: 260. https://doi.org/10.3390/membranes13030260
APA StyleAickareth, J., Hawwar, M., Sanchez, N., Gnanasekaran, R., & Zhang, J. (2023). Membrane Progesterone Receptors (mPRs/PAQRs) Are Going beyond Its Initial Definitions. Membranes, 13(3), 260. https://doi.org/10.3390/membranes13030260






