Screening and Scale-up of Nanofiltration Membranes for Concentration of Lactose and Real Whey Permeate
Abstract
1. Introduction
- Membrane screening—Out of 10 membranes, identification of those membranes with the best performance in terms of high flux and high retention of lactose.
- Determination of process parameter settings and its extent of influence for achieving high filtration fluxes and high retention of lactose.
- Scale-up and evaluation of the influence of industrial whey permeate on the course of filtration compared to the lactose model system.
2. Materials and Methods
2.1. Feedstock
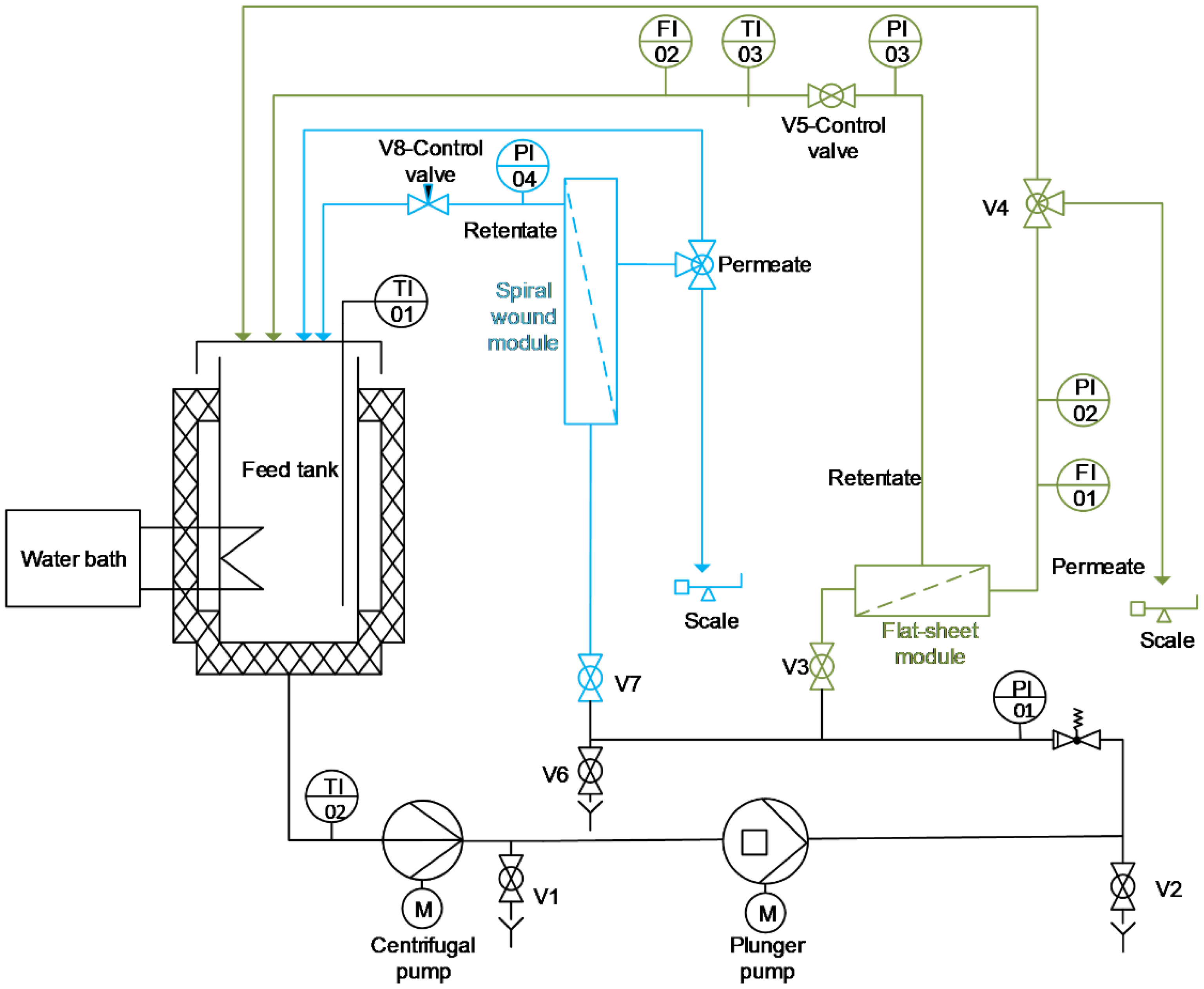
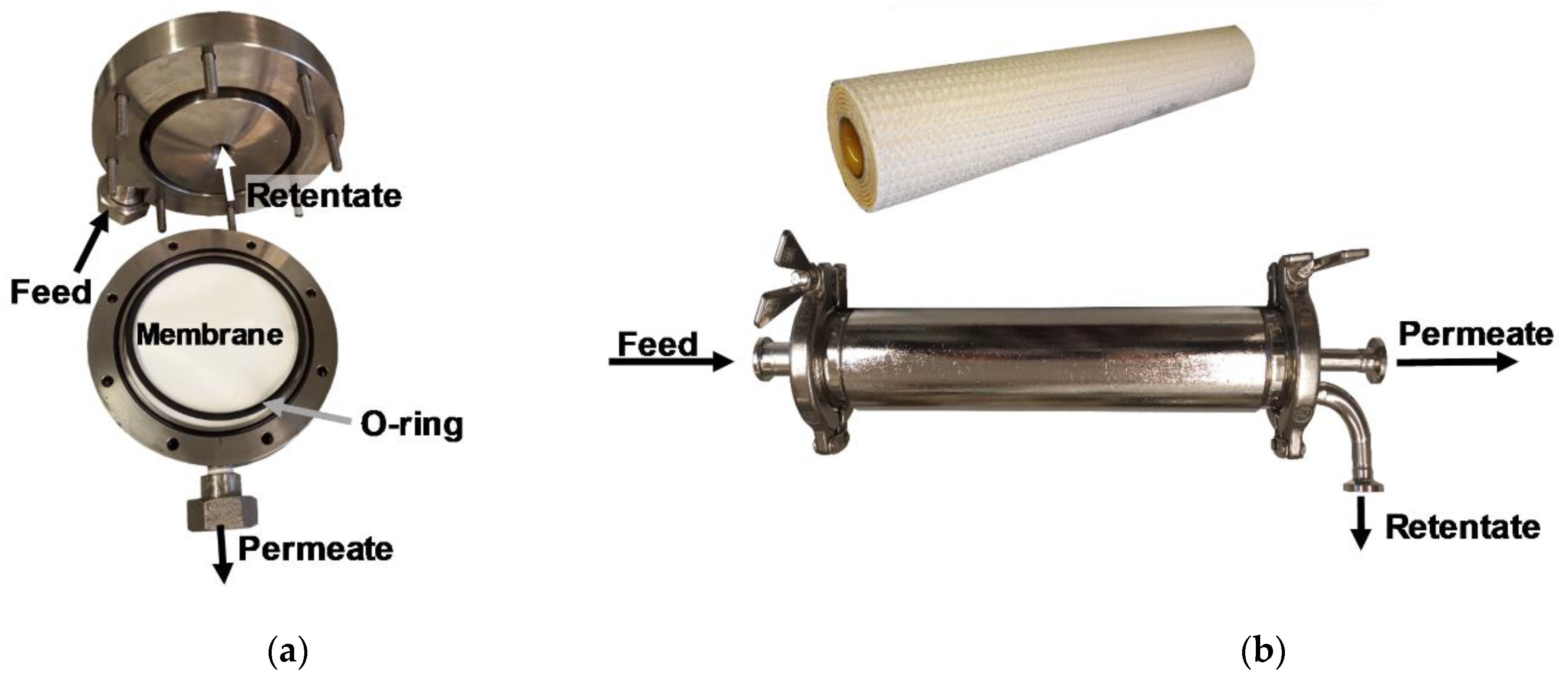
2.2. General Experimental Setup of Nanofiltration Trials
2.3. Filtration Test Scheme
2.3.1. Membranes and Experimental Matrix
2.3.2. First Stage—TMP-Screening
2.3.3. Second Stage—Parameter Studies
2.3.4. Third Stage—Scale-Up
2.4. Analysis Methods
2.4.1. Lactose Quantification
2.4.2. Quantification of Ions and Conductivity
3. Results and Discussion
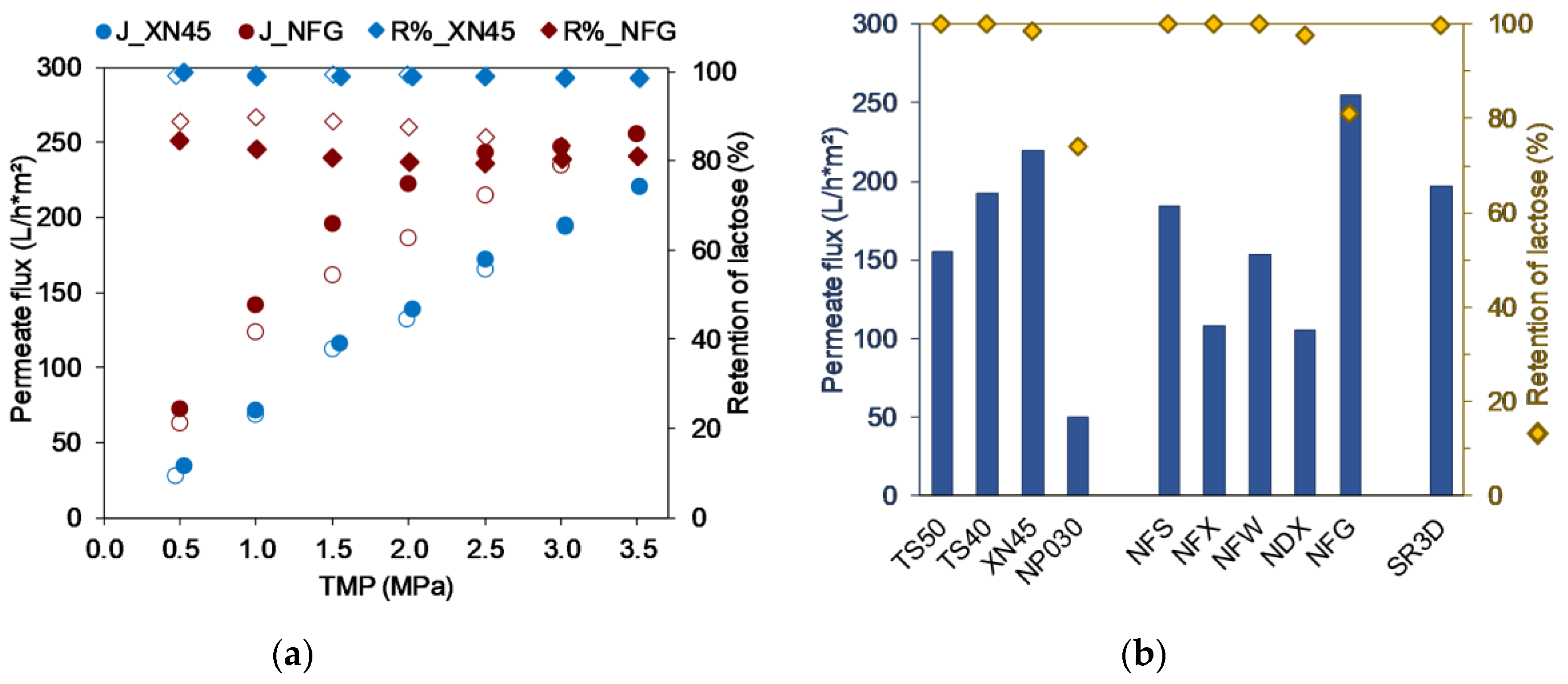
3.1. Screening and Preselection of Membranes
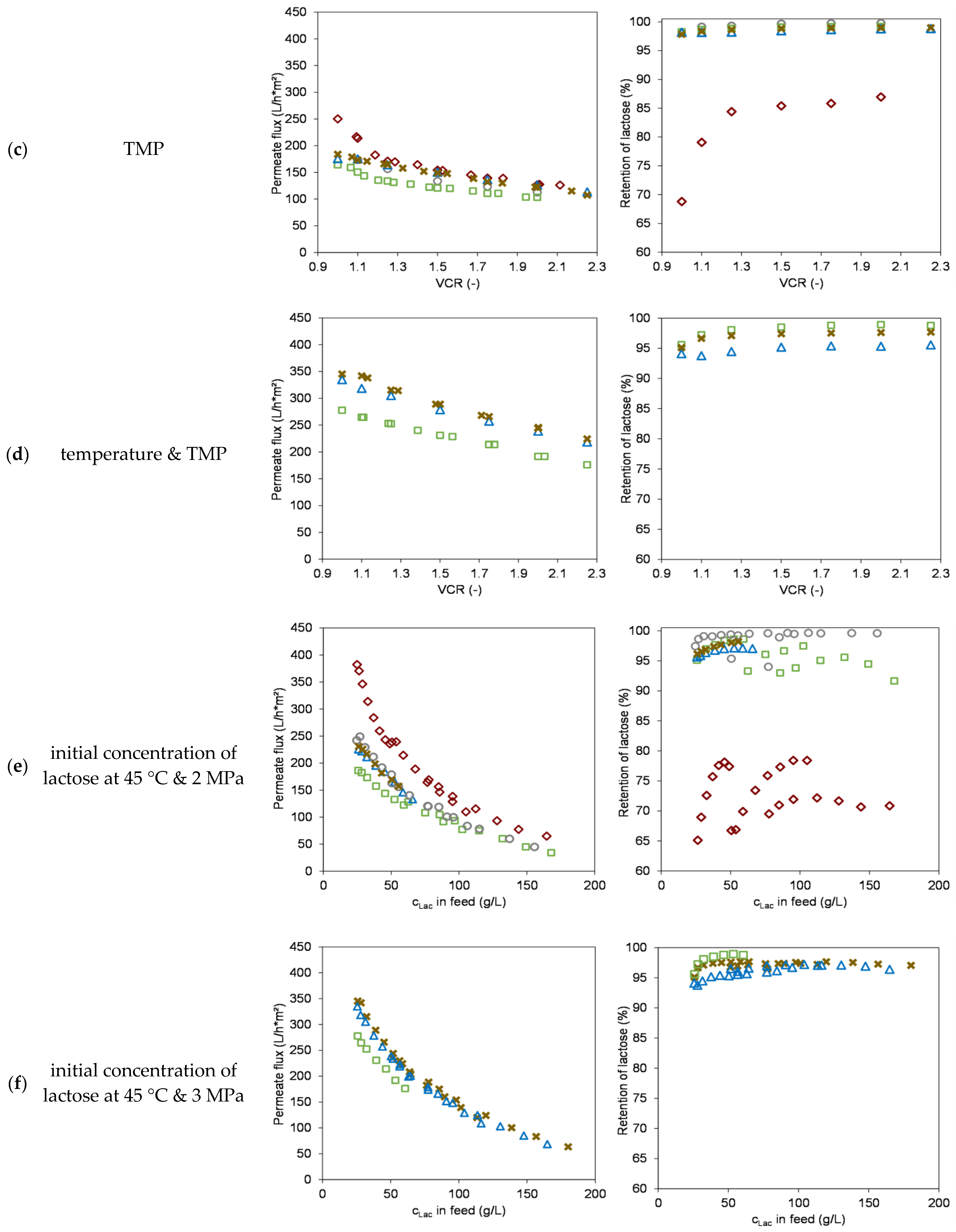
3.2. Effect of Process Parameters
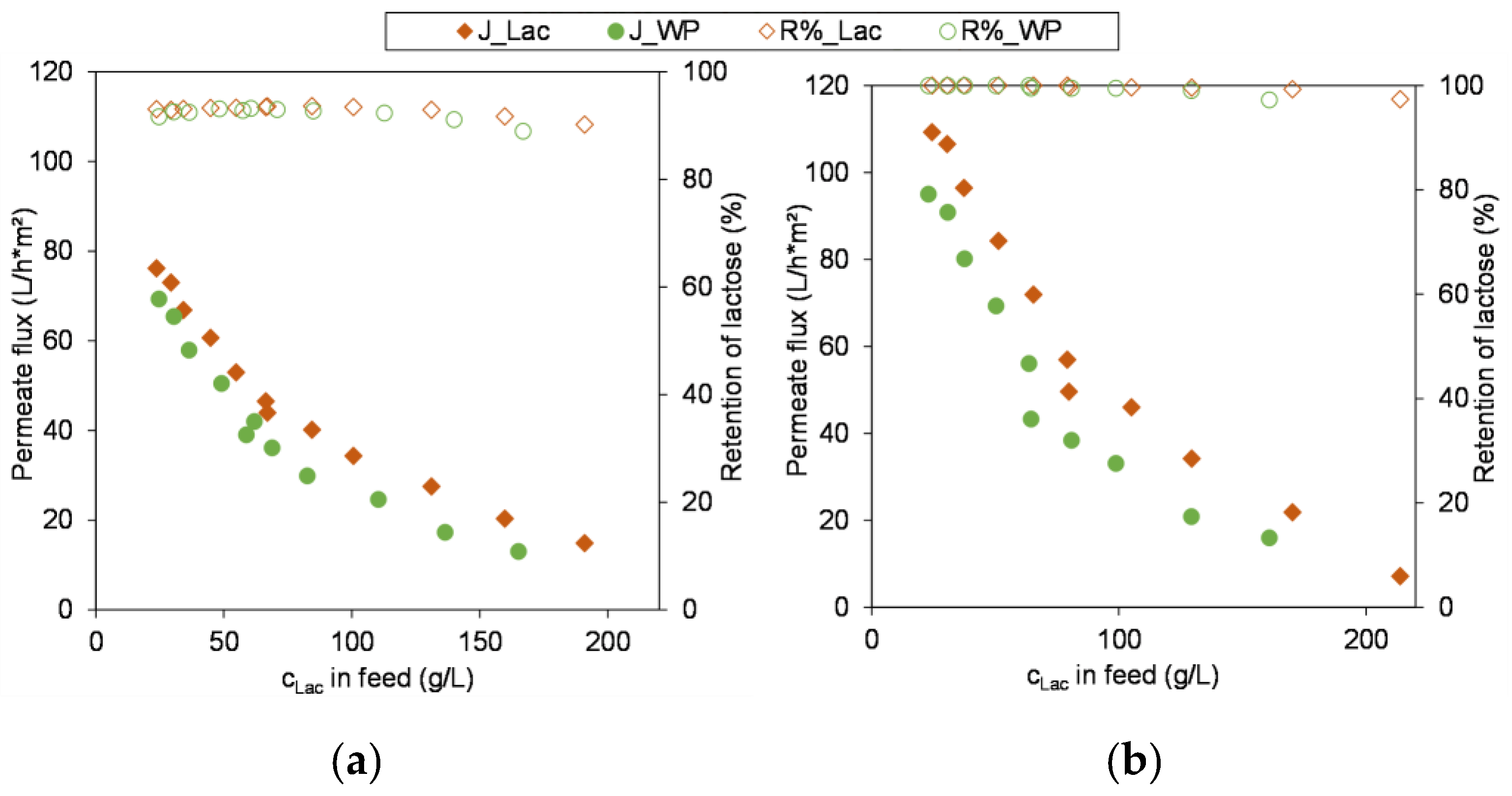
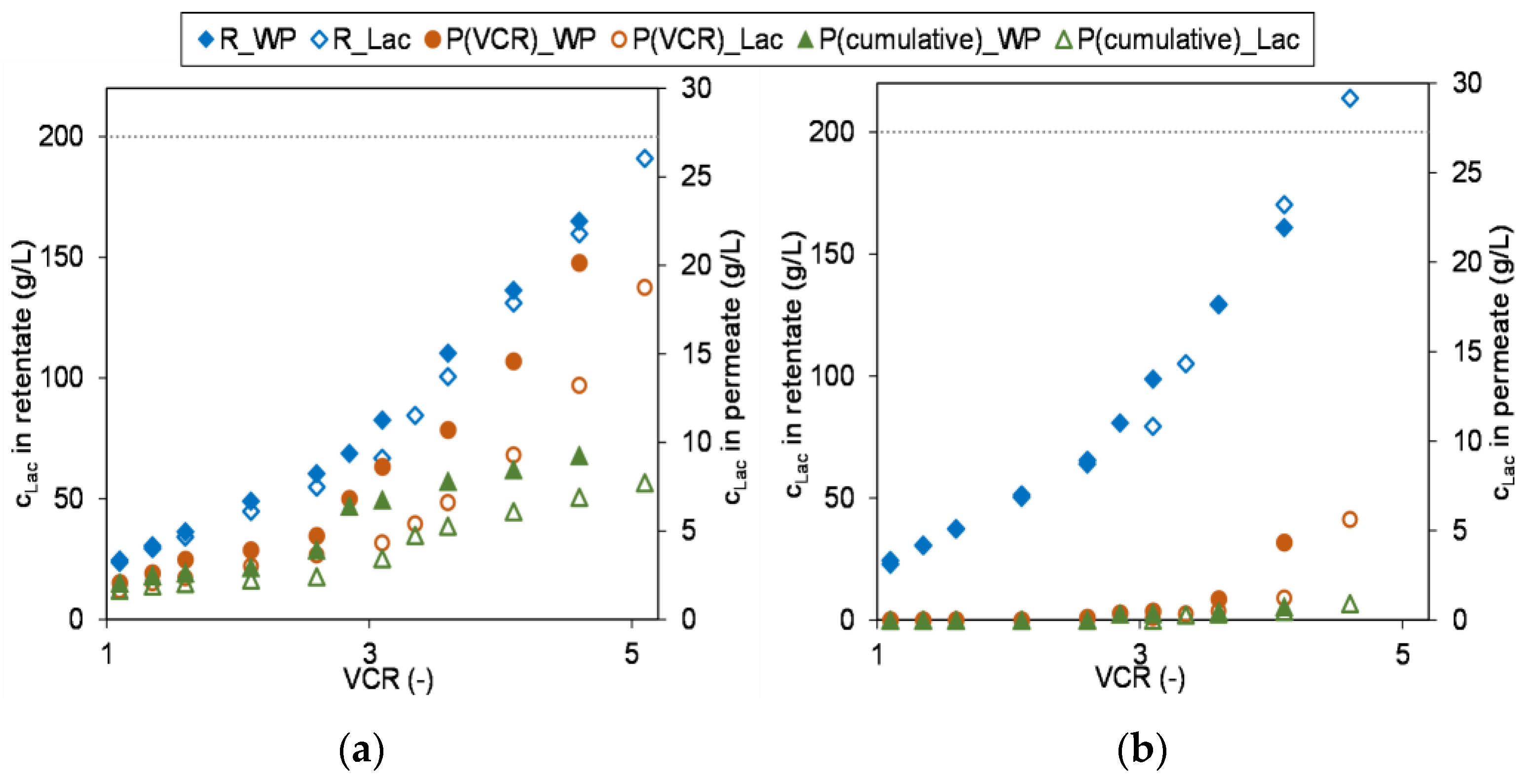
3.3. Scale-Up and Performance Using Industrial Feeds
3.3.1. Comparison of Module Performance
3.3.2. Concentration of Industrial Whey Permeate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Souza, R.R.; Bergamasco, R.; da Costa, S.C.; Feng, X.; Faria, S.H.B.; Gimenes, M.L. Recovery and purification of lactose from whey. Chem. Eng. Process. 2010, 49, 1137–1143. [Google Scholar] [CrossRef]
- Mawson, A.J. Bioconversions for whey utilization and waste abatement. Bioresour. Technol. 1994, 47, 195–203. [Google Scholar] [CrossRef]
- Atra, R.; Vatai, G.; Bekassy-Molnar, E.; Balint, A. Investigation of ultra- and nanofiltration for utilization of whey protein and lactose. J. Food Eng. 2005, 67, 325–332. [Google Scholar] [CrossRef]
- Gänzle, M.G.; Haase, G.; Jelen, P. Lactose: Crystallization, hydrolysis and value-added derivatives. Int. Dairy J. 2008, 18, 685–694. [Google Scholar] [CrossRef]
- Smithers, G.W. Whey and whey proteins—From ‘gutter-to-gold’. Int. Dairy J. 2008, 18, 695–704. [Google Scholar] [CrossRef]
- Silanikove, N.; Leitner, G.; Merin, U. The Interrelationships between Lactose Intolerance and the Modern Dairy Industry: Global Perspectives in Evolutional and Historical Backgrounds. Nutrients 2015, 7, 7312–7331. [Google Scholar] [CrossRef]
- Skoet, J.; Gerosa, S. Milk Availability: Trends in Production and Demand and Medium-Term Outlook; Food and Agriculture Organization: Rome, Italy, 2012. [Google Scholar]
- Mueller, I.; Kiedorf, G.; Runne, E.; Pottratz, I.; Seidel-Morgenstern, A.; Hamel, C. Process Control and Yield Enhancement of the Galacto-Oligosaccharide Formation. Chem. Ing. Tech. 2018, 90, 725–730. [Google Scholar] [CrossRef]
- Pottratz, I.; Mueller, I.; Hamel, C. Potential and Scale-Up of Pore-Through-Flow Membrane Reactors for the Production of Prebiotic Galacto-Oligosaccharides with Immobilized β-Galactosidase. Catalysts 2022, 12, 7. [Google Scholar] [CrossRef]
- Pázmándi, M.; Maráz, A.; Ladányi, M.; Kovács, Z. The impact of membrane pretreatment on the enzymatic production of whey-derived galacto-oligosaccharides. J. Food Process Eng. 2018, 41, e12649. [Google Scholar] [CrossRef]
- Cuartas-Uribe, B.; Alcaina-Miranda, M.I.; Soriano-Costa, E.; Mendoza-Roca, J.A.; Iborra-Clar, M.I.; Lora-García, J. A study of the separation of lactose from whey ultrafiltration permeate using nanofiltration. Desalination 2009, 241, 244–255. [Google Scholar] [CrossRef]
- Räsänen, E.; Nyström, M.; Sahlstein, J.; Tossavainen, O. Comparison of commercial membranes in nanofiltration of sweet whey. Lait 2002, 82, 343–356. [Google Scholar] [CrossRef]
- Zadow, J.G. Whey and Lactose Processing; Springer: Dordrecht, The Netherlands, 1992; pp. 2–13. ISBN 9789401128940. [Google Scholar]
- Melin, T.; Rautenbach, R. Membranverfahren: Grundlagen der Modul- und Anlagenauslegung; mit 76 Tabellen, 3; Aktualisierte und erw. Aufl.; Springer: Berlin, Germany, 2007; pp. 285–306. ISBN 3-540-00071-2. [Google Scholar]
- Suárez, E.; Lobo, A.; Álvarez, S.; Riera, F.A.; Álvarez, R. Partial demineralization of whey and milk ultrafiltration permeate by nanofiltration at pilot-plant scale. Desalination 2006, 198, 274–281. [Google Scholar] [CrossRef]
- Qi, T.; Yang, D.; Chen, X.; Qiu, M.; Fan, Y. Rapid removal of lactose for low-lactose milk by ceramic membranes. Sep. Purif. Technol. 2022, 289, 120601. [Google Scholar] [CrossRef]
- Straatsma, J.; Bargeman, G.; van der Horst, H.C.; Wesselingh, J.A. Can nanofiltration be fully predicted by a model? J. Membr. Sci. 2002, 198, 273–284. [Google Scholar] [CrossRef]
- Hiddink, J.; de Boer, R.; Nooy, P. Reverse Osmosis of Dairy Liquids. J. Dairy Sci. 1980, 63, 204–214. [Google Scholar] [CrossRef]
- Mason, E.A.; Lonsdale, H.K. Statistical-mechanical theory of membrane transport. J. Membr. Sci. 1990, 51, 1–81. [Google Scholar] [CrossRef]
- Petersen, R.J. Composite reverse osmosis and nanofiltration membranes. J. Membr. Sci. 1993, 83, 81–150. [Google Scholar] [CrossRef]
- Chen, B.-Z.; Ju, X.; Liu, N.; Chu, C.-H.; Lu, J.-P.; Wang, C.; Sun, S.-P. Pilot-scale fabrication of nanofiltration membranes and spiral-wound modules. Chem. Eng. Res. Des. 2020, 160, 395–404. [Google Scholar] [CrossRef]
- Tsuru, T.; Izumi, S.; Yoshioka, T.; Asaeda, M. Temperature effect on transport performance by inorganic nanofiltration membranes. AlChE J. 2000, 46, 565–574. [Google Scholar] [CrossRef]
- Nilsson, M.; Trägårdh, G.; Östergren, K. The influence of pH, salt and temperature on nanofiltration performance. J. Membr. Sci. 2008, 312, 97–106. [Google Scholar] [CrossRef]
- Xiao, H.-F.; Shao, D.-D.; Wu, Z.-L.; Peng, W.-B.; Akram, A.; Wang, Z.-Y.; Zheng, L.-J.; Xing, W.; Sun, S.-P. Zero liquid discharge hybrid membrane process for separation and recovery of ions with equivalent and similar molecular weights. Desalination 2020, 482, 114387. [Google Scholar] [CrossRef]
- Wijmans, J.G.; Baker, R.W. The solution-diffusion model: A review. J. Membr. Sci. 1995, 107, 1–21. [Google Scholar] [CrossRef]
- Hagmeyer, G.; Gimbel, R. Modelling the salt rejection of nanofiltration membranes for ternary ion mixtures and for single salts at different pH values. Desalination 1998, 117, 247–256. [Google Scholar] [CrossRef]
- Nilsson, M.; Trägårdh, G.; Östergren, K. The influence of sodium chloride on mass transfer in a polyamide nanofiltration membrane at elevated temperatures. J. Membr. Sci. 2006, 280, 928–936. [Google Scholar] [CrossRef]
- Bargeman, G.; Vollenbroek, J.M.; Straatsma, J.; Schroën, C.; Boom, R.M. Nanofiltration of multi-component feeds. Interactions between neutral and charged components and their effect on retention. J. Membr. Sci. 2005, 247, 11–20. [Google Scholar] [CrossRef]
- Koros, W.J.; Ma, Y.H.; Shimidzu, T. Terminology for membranes and membrane processes (IUPAC Recommendations 1996). Pure Appl. Chem. 1996, 68, 1479–1489. [Google Scholar] [CrossRef]
- Dutré, B.; Trägardh, G. Macrosolute-microsolute separation by ultrafiltration: A review of diafiltration processes and applications. Desalination 1994, 95, 227–267. [Google Scholar] [CrossRef]
- Yao, W.X.; Kennedy, K.J.; Tam, C.M.; Hazlett, J.D. Pre-treatment of kraft pulp bleach plant effluent by selected ultrafiltration membranes. Can. J. Chem. Eng. 1994, 72, 991–999. [Google Scholar] [CrossRef]
- Ben Amar, N.; Saidani, H.; Deratani, A.; Palmeri, J. Effect of temperature on the transport of water and neutral solutes across nanofiltration membranes. Langmuir ACS J. Surf. Colloids 2007, 23, 2937–2952. [Google Scholar] [CrossRef]
- Mänttäri, M.; Pihlajamäki, A.; Kaipainen, E.; Nyström, M. Effect of temperature and membrane pre-treatment by pressure on the filtration properties of nanofiltration membranes. Desalination 2002, 145, 81–86. [Google Scholar] [CrossRef]
- Schmidt, C.M.; Mailänder, L.K.; Hinrichs, J. Fractionation of mono- and disaccharides via nanofiltration: Influence of pressure, temperature and concentration. Sep. Purif. Technol. 2019, 211, 571–577. [Google Scholar] [CrossRef]
- Wijmans, J.G.; Nakao, S.; Smolders, C.A. Flux limitation in ultrafiltration: Osmotic pressure model and gel layer model. J. Membr. Sci. 1984, 20, 115–124. [Google Scholar] [CrossRef]
- Di Giacomo, G.; Scimia, F.; Taglieri, L. Cost-effective disposal of milk whey II: Recovery and purification of lactose and pure water from the diafiltration permeate stream. DWT 2017, 76, 339–342. [Google Scholar] [CrossRef]
- Van Gauwbergen, D.; Baeyens, J. Macroscopic fluid flow conditions in spiral-wound membrane elements. Desalination 1997, 110, 287–299. [Google Scholar] [CrossRef]
- Schipolowski, T.; Jeżowska, A.; Wozny, G. Reliability of membrane test cell measurements. Desalination 2006, 189, 71–80. [Google Scholar] [CrossRef]
- Jeżowska, A.; Schipolowski, T.; Wozny, G. Reduction of random and systematic errors of test cell measurements. Desalination 2006, 200, 160–162. [Google Scholar] [CrossRef]
- Timkin, V.A.; Lazarev, V.A. Determination of the osmotic pressure of multicomponent solutions in the food industry. Pet. Chem. 2015, 55, 301–307. [Google Scholar] [CrossRef]




| Sweet Whey Permeate | |
|---|---|
| pH | 6.5 |
| conductivity (mS/cm) | 3.7 |
| Dry matter (%) | 3.2 |
| Lactose (g/L) | 26.3 (→ 25 for lactose model solution) |
| Protein (%) | 0.1 |
| Sodium (mg/L) | 169.5 |
| Potassium (mg/L) | 776.8 |
| Ammonium (mg/L) | 70.1 |
| Magnesium (mg/L) | 29.9 |
| Calcium (mg/L) | 193.0 |
| Lactic acid (mg/L) | 376.6 |
| Chloride (mg/L) | 464.5 |
| Nitrate (mg/L) | 45.6 |
| Phosphorus (mg/L) | 606.0 |
| Sulfate (mg/L) | 60.6 |
| Model | Manufacturer | MWCO 1 (g/mol) | Ø MWCO 1 (g/mol) | Membrane Chemistry 2 | Max. Operating Temperature (°C) | Max. Operating Pressure (MPa) | |
|---|---|---|---|---|---|---|---|
| TS 50 | MANN+ HUMMEL | 200–300 | 250 | Thin-Film Polypiperazine | 45 | 4.1 | |
| TS 40 | 200–300 | 250 | |||||
| XN45 | 300–500 | 400 | |||||
| NP030 | 500–600 | 550 | PES TFC | 50 | 4.0 | ||
| NFS | Synder Filtration | 100–250 | 175 | Proprietary PA TFC | 50 | if T < 35 °C: 4.1 | if T > 35 °C: 3.0 |
| NFX | 150–300 | 225 | |||||
| NFW | 300–500 | 400 | |||||
| NDX | 500–700 | 600 | |||||
| NFG | 600–800 | 700 | |||||
| SR3D | KOCH Membrane Systems | 200 | 200 | TFC PA | 45 | 4.5 | |
| Stage | Membrane Module | Filtration Mode | Tested Membranes | Feed | T (°C) | TMP 1 (MPa) | Initial Feed-c(Lac) (g/L) |
|---|---|---|---|---|---|---|---|
| TMP-Screening | Flat-sheet | recirculation | all | lactose model solution | 20 | 0.5→3.5→0.5 | 25 |
| Parameter studies | Flat-sheet | concentration | TS40; XN45; NFS; NFG; SR3D | lactose model solution | 20 | 2 | 25 |
| 3 | |||||||
| 45 | 2 | ||||||
| XN45; SR3D; TS40 | 3 | 25 | |||||
| XN45; SR3D | 50 | ||||||
| 75 | |||||||
| NFS; NFG; TS40 | 2 | 50 | |||||
| 75 | |||||||
| Scale-up | 1812’’ spiral wound | concentration | NFS | lactose model solution | 45 | 2 | 25–75 |
| whey permeate | |||||||
| XN45 | lactose model solution | 45 | 3 | ||||
| lactose model solution | 20 | 2 | |||||
| whey permeate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hofmann, K.; Hamel, C. Screening and Scale-up of Nanofiltration Membranes for Concentration of Lactose and Real Whey Permeate. Membranes 2023, 13, 173. https://doi.org/10.3390/membranes13020173
Hofmann K, Hamel C. Screening and Scale-up of Nanofiltration Membranes for Concentration of Lactose and Real Whey Permeate. Membranes. 2023; 13(2):173. https://doi.org/10.3390/membranes13020173
Chicago/Turabian StyleHofmann, Katrin, and Christof Hamel. 2023. "Screening and Scale-up of Nanofiltration Membranes for Concentration of Lactose and Real Whey Permeate" Membranes 13, no. 2: 173. https://doi.org/10.3390/membranes13020173
APA StyleHofmann, K., & Hamel, C. (2023). Screening and Scale-up of Nanofiltration Membranes for Concentration of Lactose and Real Whey Permeate. Membranes, 13(2), 173. https://doi.org/10.3390/membranes13020173





