Ozone Diffusion through a Hollow Fiber Membrane Contactor for Pharmaceuticals Removal and Bromate Minimization
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Matrix Studied
2.2.1. Real WWTP Effluent
2.2.2. Solution Spiked with the Targeted MP
2.2.3. Bromide Solution
2.3. Analytical Methods
2.3.1. Ozone Analysis
2.3.2. MP Analyses
2.3.3. p-CBA Analysis
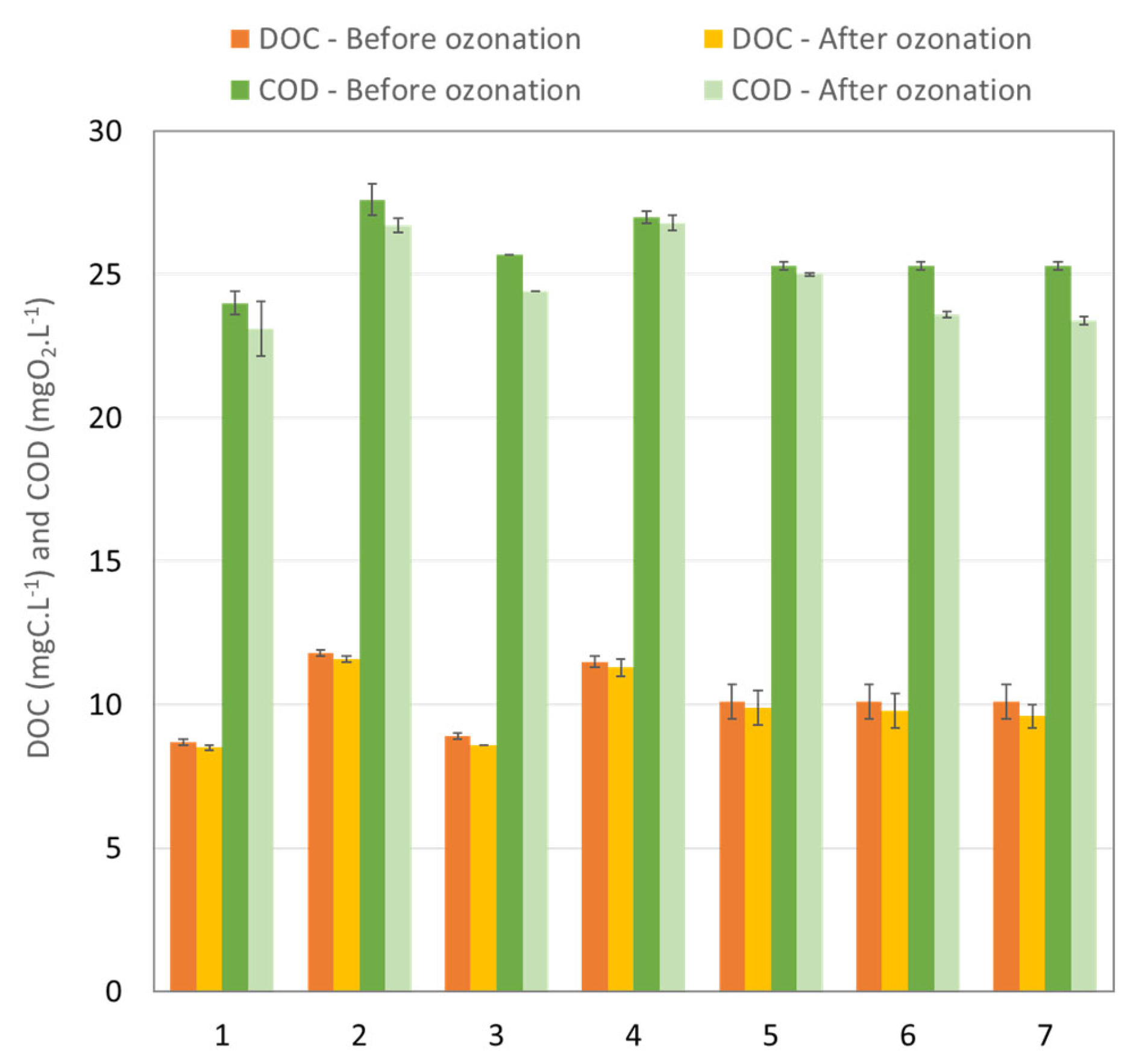
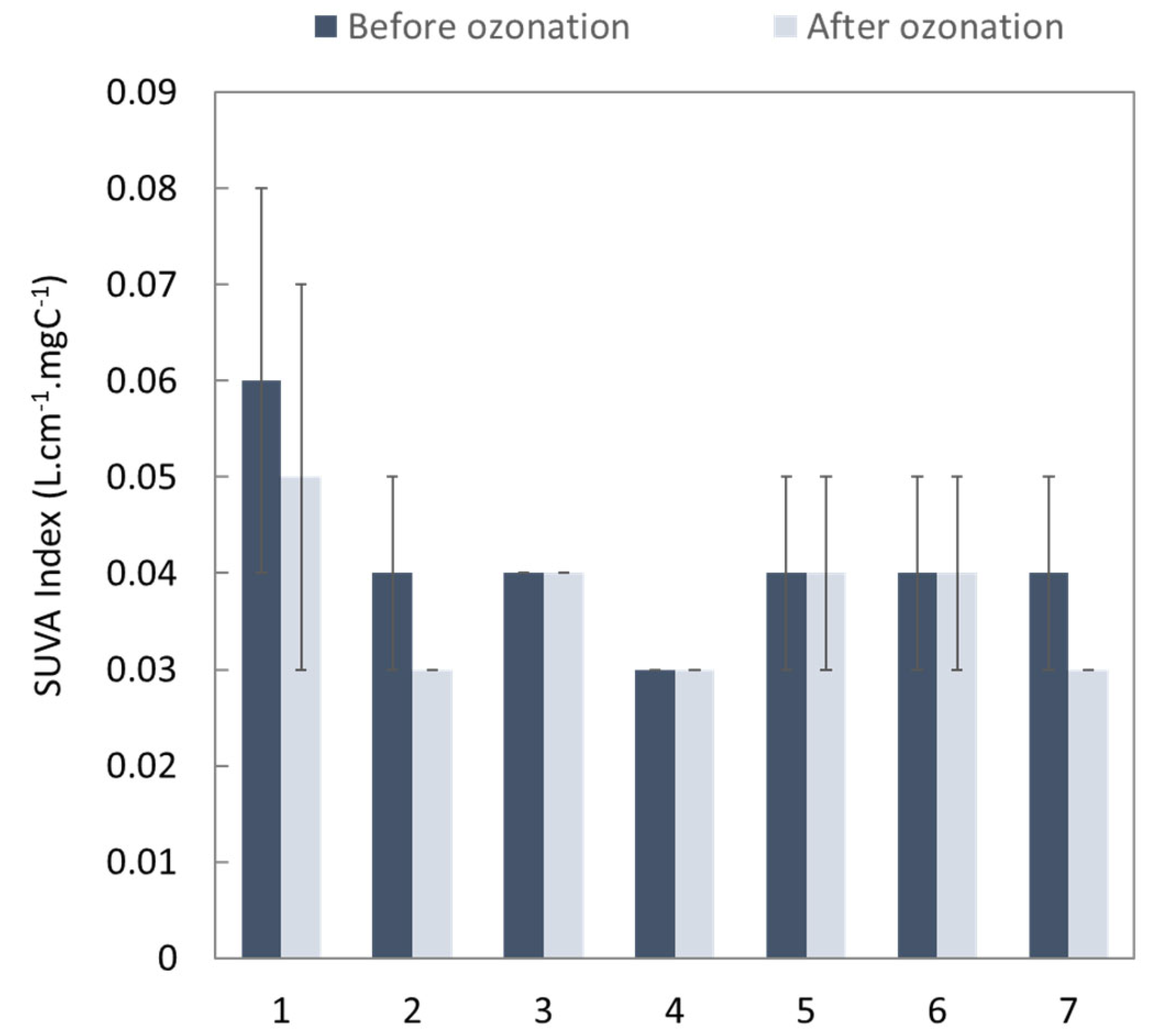
2.3.4. Characterization of Real WWTP Effluent/Global Indicator for Pollution Monitoring
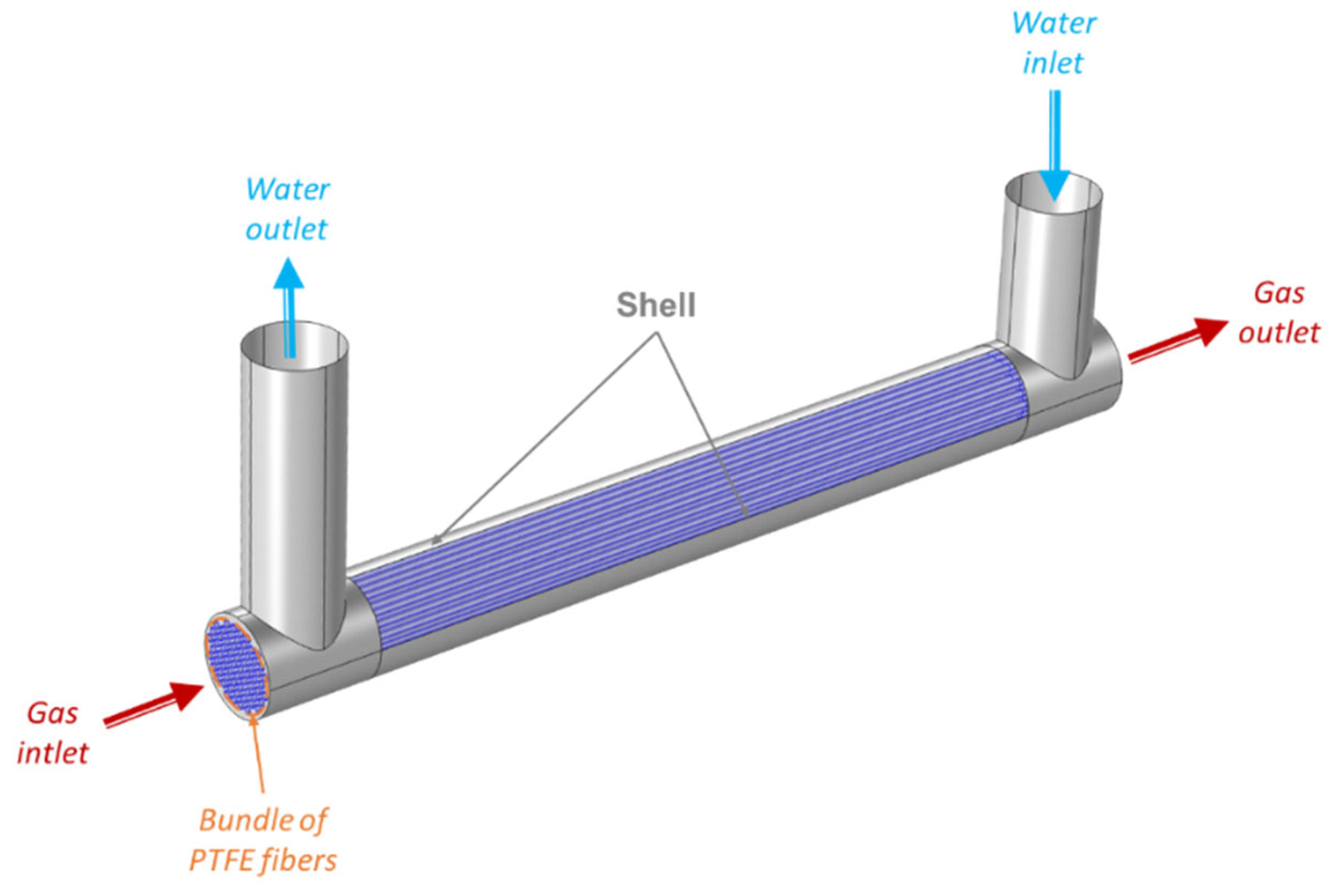
2.4. Membrane Contactor Technology
2.5. Ozonation Pilots
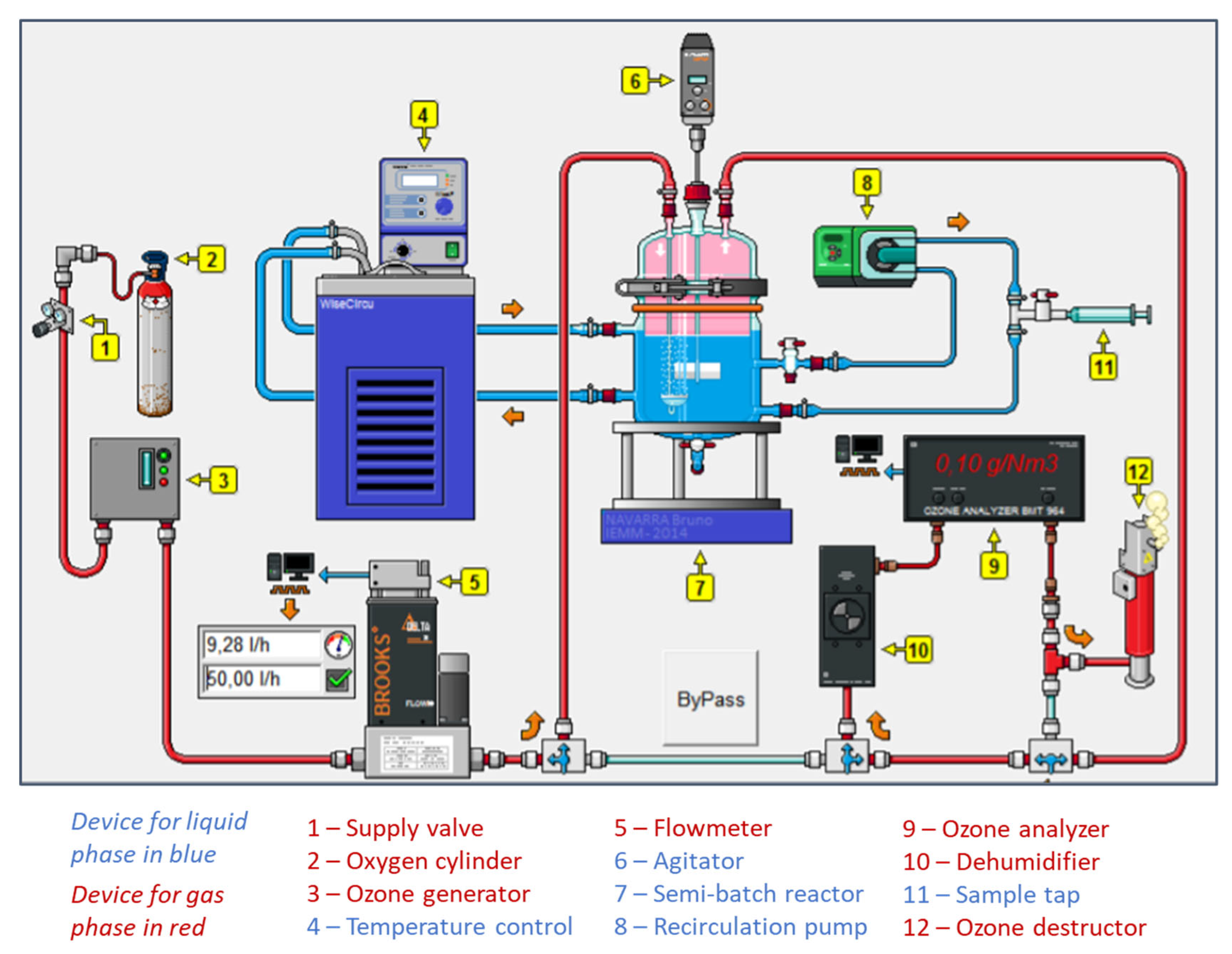
2.5.1. Description of the Pilot Ozonation System: Membrane Contactor with Liquid in Closed Loop
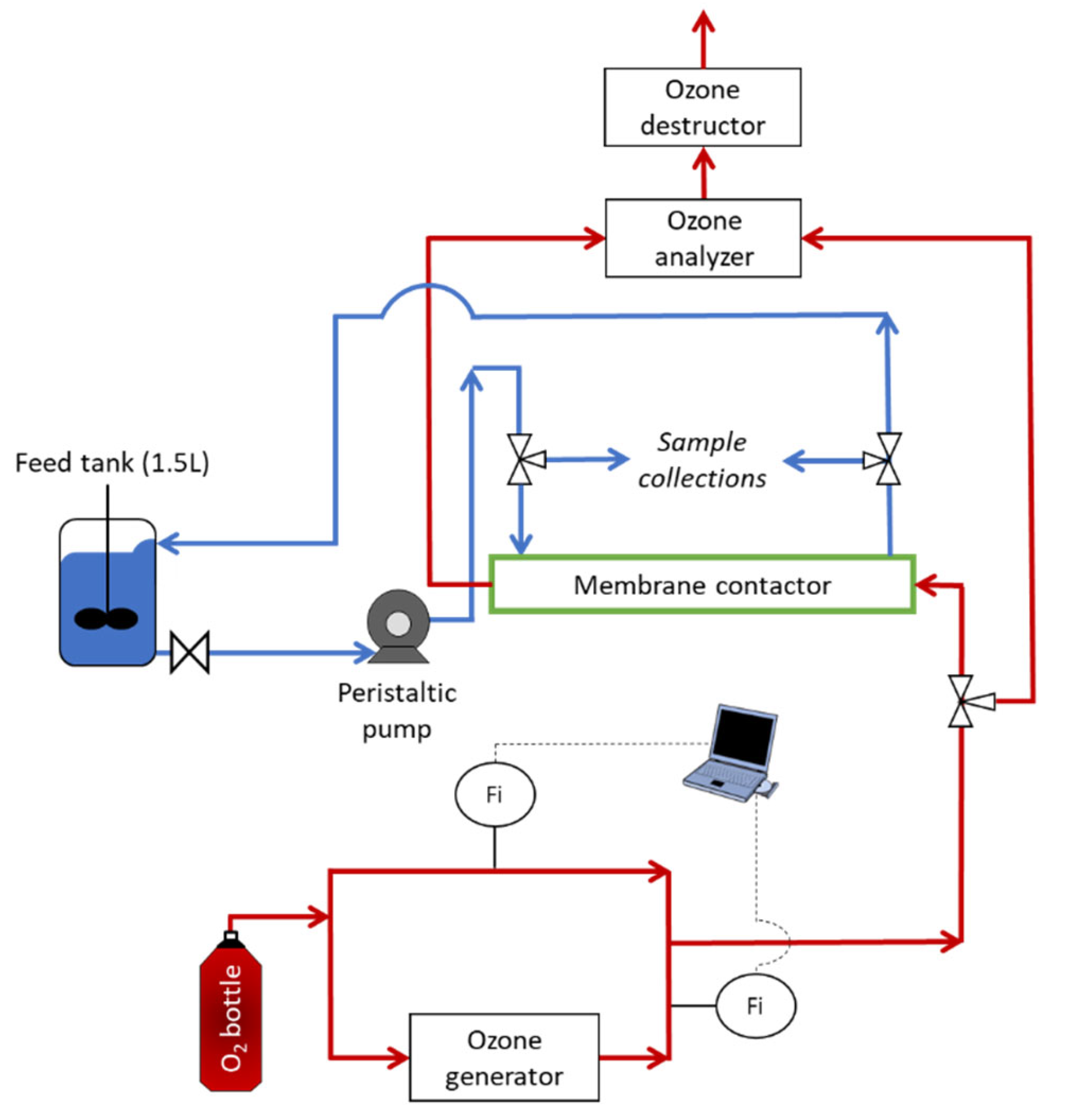
2.5.2. Description of the Pilot Ozonation System: Membrane Contactor with Liquid in Open Loop
2.5.3. Description of the Pilot Ozonation System with a Bubble Column (Semibatch Reactor)
2.6. Exposure to Hydroxyl Radicals and Molecular Ozone
2.7. Objectives, Global Parameters and Ozonation Conditions of the Experiments
2.7.1. Ozonation Experiments for the Removal of Targeted Micropollutants through a PTFE Hollow Fiber Membrane Contactor
| Experiment | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Objective | Reference | Variation in gas concentration | Variation in gas concentration | Variation in liquid flowrate | 3 successive passages of the liquid through the membrane contactor (variation in the residence time) | ||
| Qliq (L.h−1) | 46.2 | 46.2 | 46.2 | 92.3 | 47.8 | ||
| Qgas (L.h−1) | 8 | ||||||
| CO3,g,inlet(g.Nm−3) | 14.7 (0.1) | 22.8 (0.4) | 30.6 (0.2) | 15.2 (0.5) | 14.9 (0.1) | ||
| pH | 7.9 ± 0.1 | 7.5 ± 0.1 | 8.0 ± 0.1 | 7.5 ± 0.1 | 7.6 ± 0.1) | ||
| CCBZ inlet (mg.L−1) | 1.92 (0.12) | 1.98 (0.02) | 2.22 (0.1) | 1.92 (0.03) | 1.98 (0.03) | 1.54 (0.07) | 1.31 (0.07) |
| CSUL inlet (mg.L−1) | 1.95 (0.11) | 1.91 (0.02) | 2.04 (0.06) | 1.85 (0.07) | 1.84 (0.03) | 1.42 (0.08) | 1.16 (0.08) |
2.7.2. Ozonation Experiments for the Study of Bromate Production: PTFE Hollow Fiber Membrane Contactor Technology versus Bubble Reactor
3. Results and Discussion
3.1. Study on Ozonation of Targeted Micropollutants through a PTFE Hollow Fiber Membrane Contactor
3.1.1. Effect of the Ozonation Process on the Global Parameters
3.1.2. Effect of Ozone Concentration
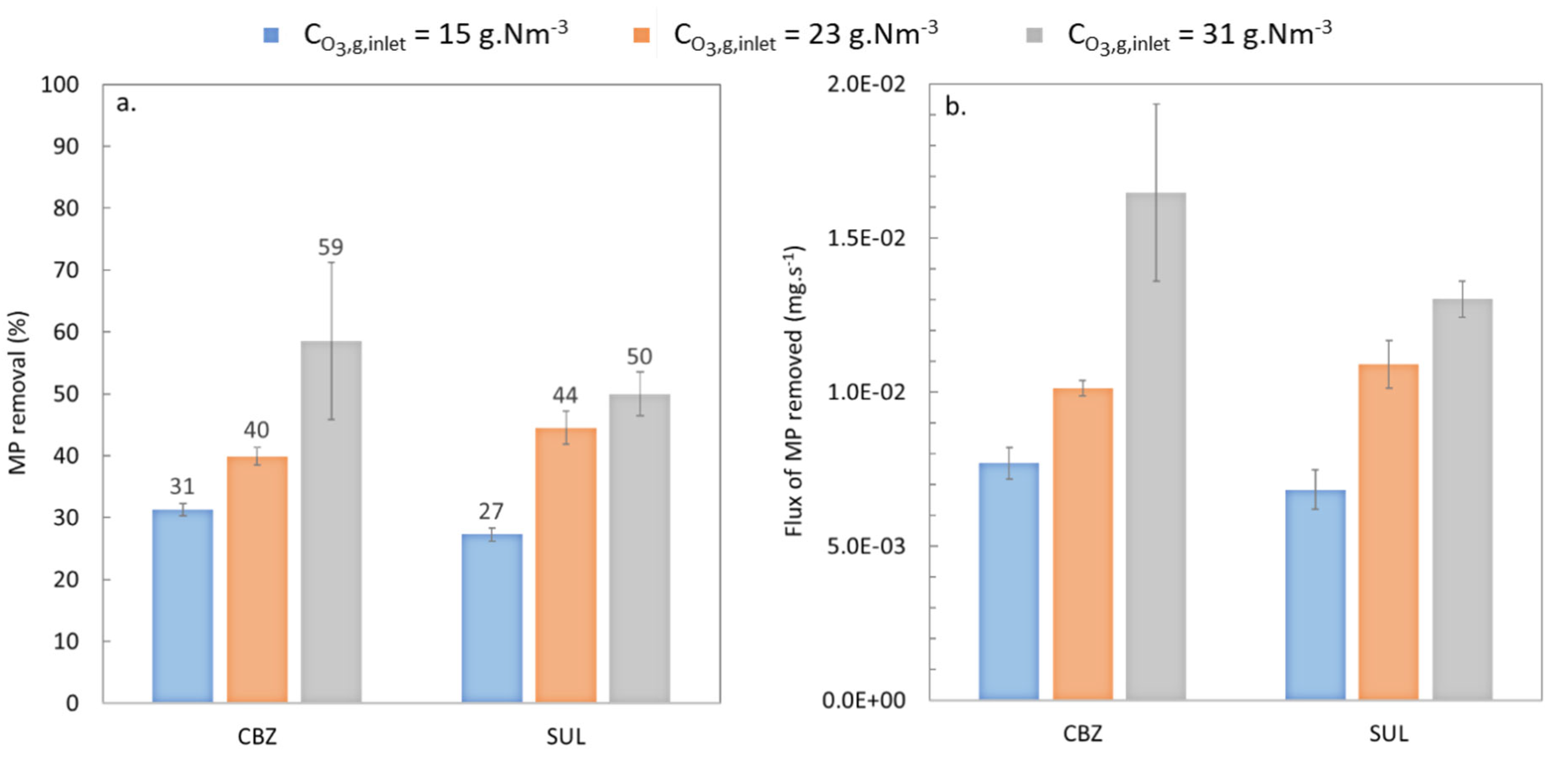
3.1.3. Effect of the Liquid Flowrate
| Liquid Flowrate | 46.2 L.h−1 | 92.3 L.h−1 | ||
|---|---|---|---|---|
| Transferred ozone (mg.min−1) | 0.92 | 1.80 | ||
| MP | CBZ | SUL | CBZ | SUL |
| Concentration of MPs at the inlet (mg.L−1) | 1.92 (0.12) | 1.95 (0.11) | 1.92 (0.04) | 1.85 (0.07) |
| Concentration of MPs at the outlet (mg.L−1) | 1.32 (0.09) | 1.42 (0.06) | 1.39 (0.03) | 1.34 (0.08) |
| Flux of MPs removed (mg.s−1) | 7.69 × 10−3 (5.13 × 10−4) | 6.79 × 10−3 (5.98 × 10−4) | 1.35 × 10−2 (1.41 × 10−3) | 1.315 × 10−2 (2.56 × 10−4) |
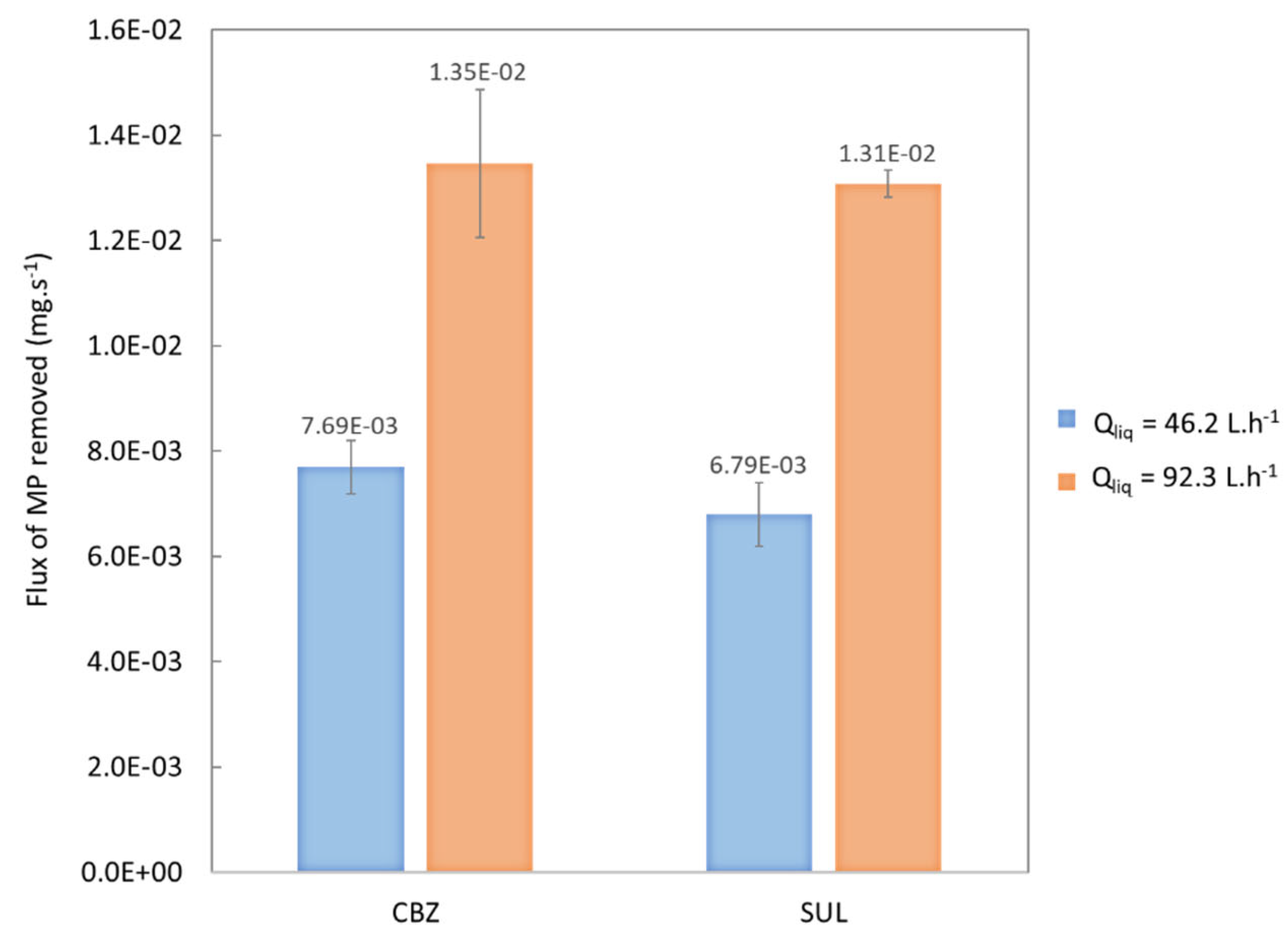
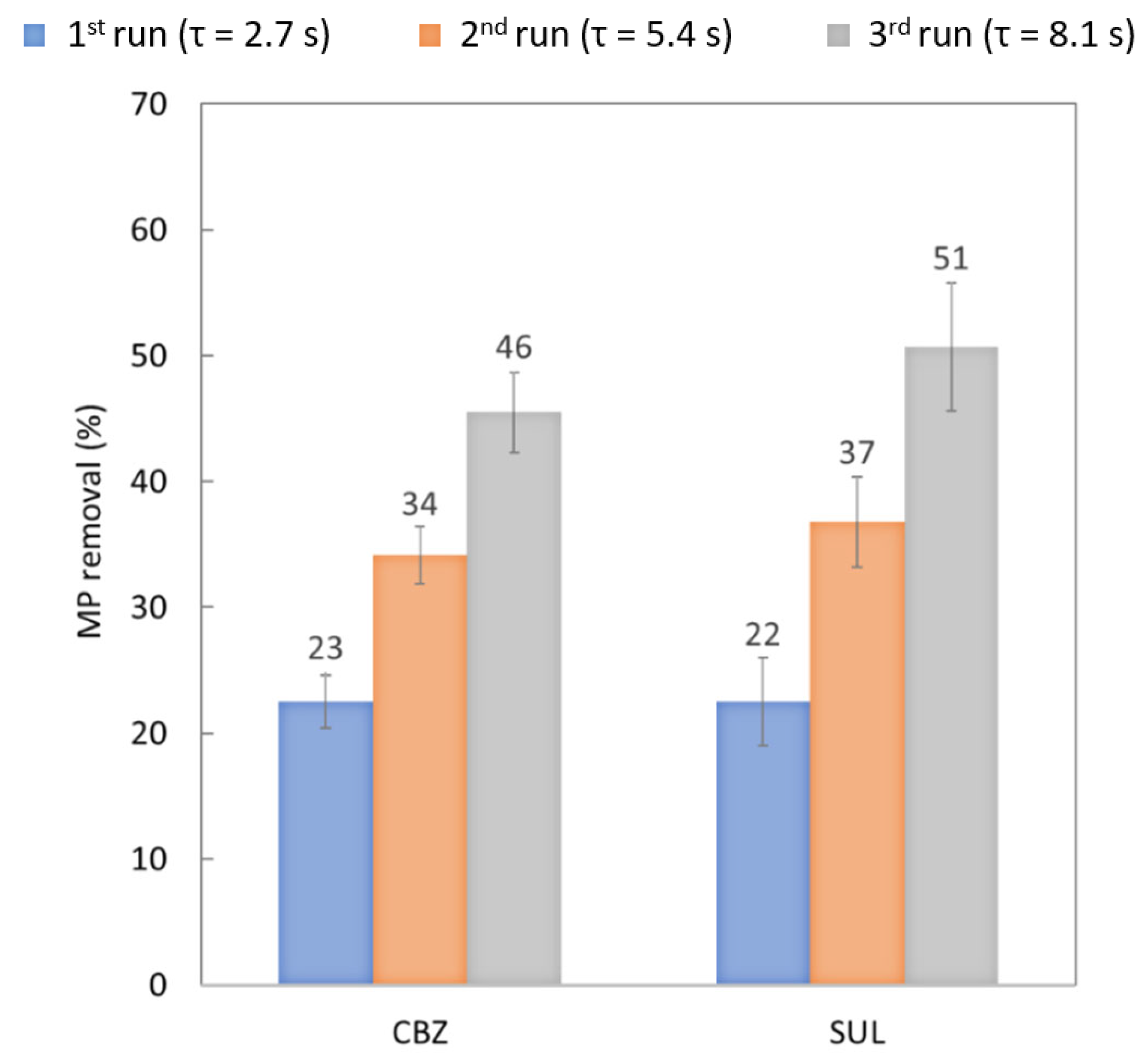
3.1.4. Effect of Residence Time: Recirculation System

3.1.5. Residual Ozone and Bromate Formation during Removal of Selected MPs
| Experiment | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| CBr- before ozonation (mg.L−1) | 0.30 ± 0.01 | 1.10 ± 0.02 | 0.28 ± 0.01 | 0.30 ± 0.01 | 1.56 ± 0.03 | ||
| CBr- after ozonation (mg.L−1) | 0.31 ± 0.01 | 1.10 ± 0.03 | 0.28 ± 0.01 | 0.30 ± 0.01 | 1.55 ± 0.03 | 1.55 ± 0.03 | 1.55 ± 0.03 |
| CBrO3- after ozonation (mg.L−1) | < LOD | < LOD | < LOD | < LOD | < LOD | < LOD | < LOD |
| CO3,liq (mg.L−1) | 0.05 (0.01) | 0.08 (0) | 0.08 (0.01) | 0.07 (0) | 0.04 (0.03) | 0.06 (0.03) | 0.05 (0.03) |
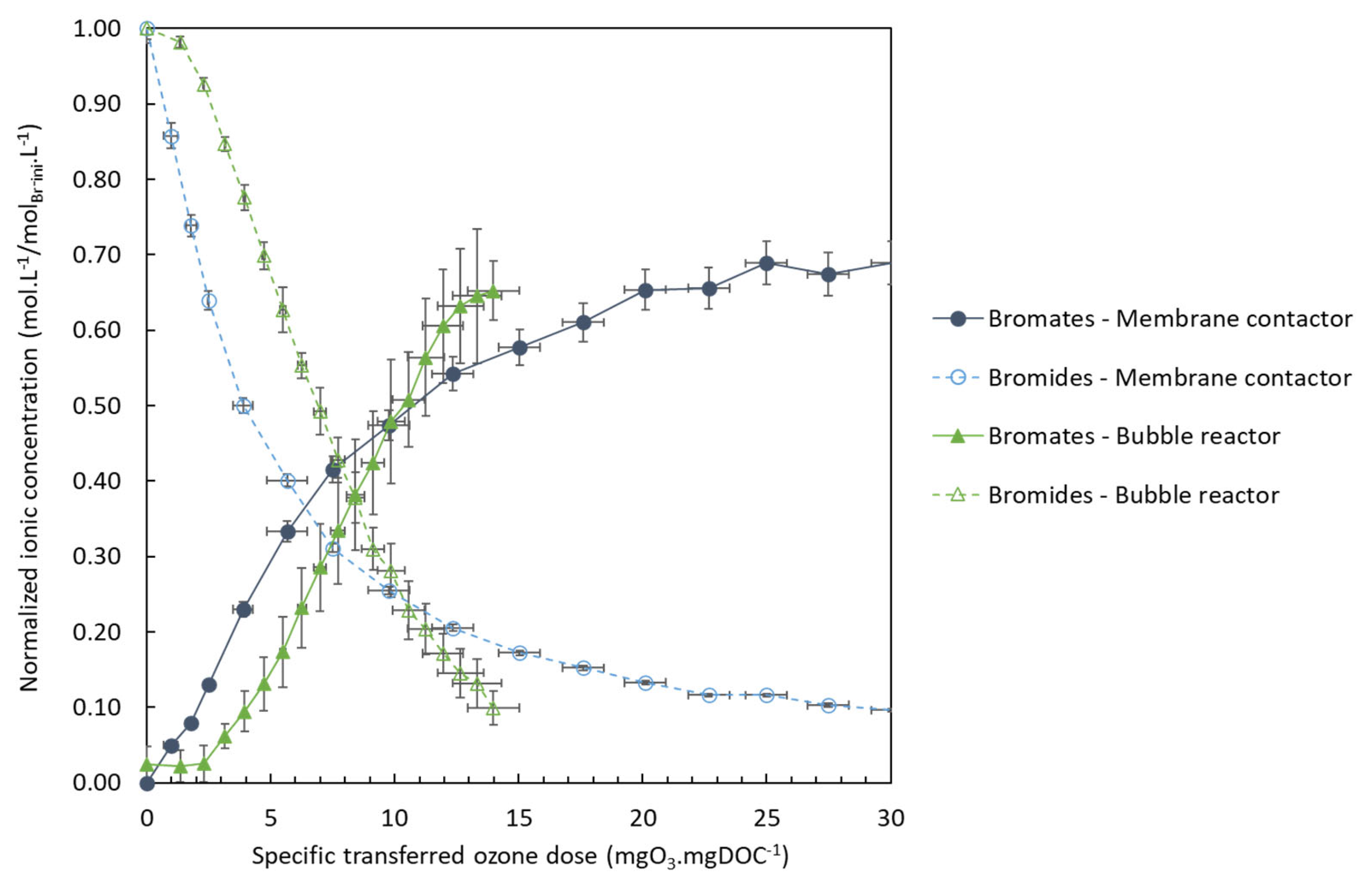
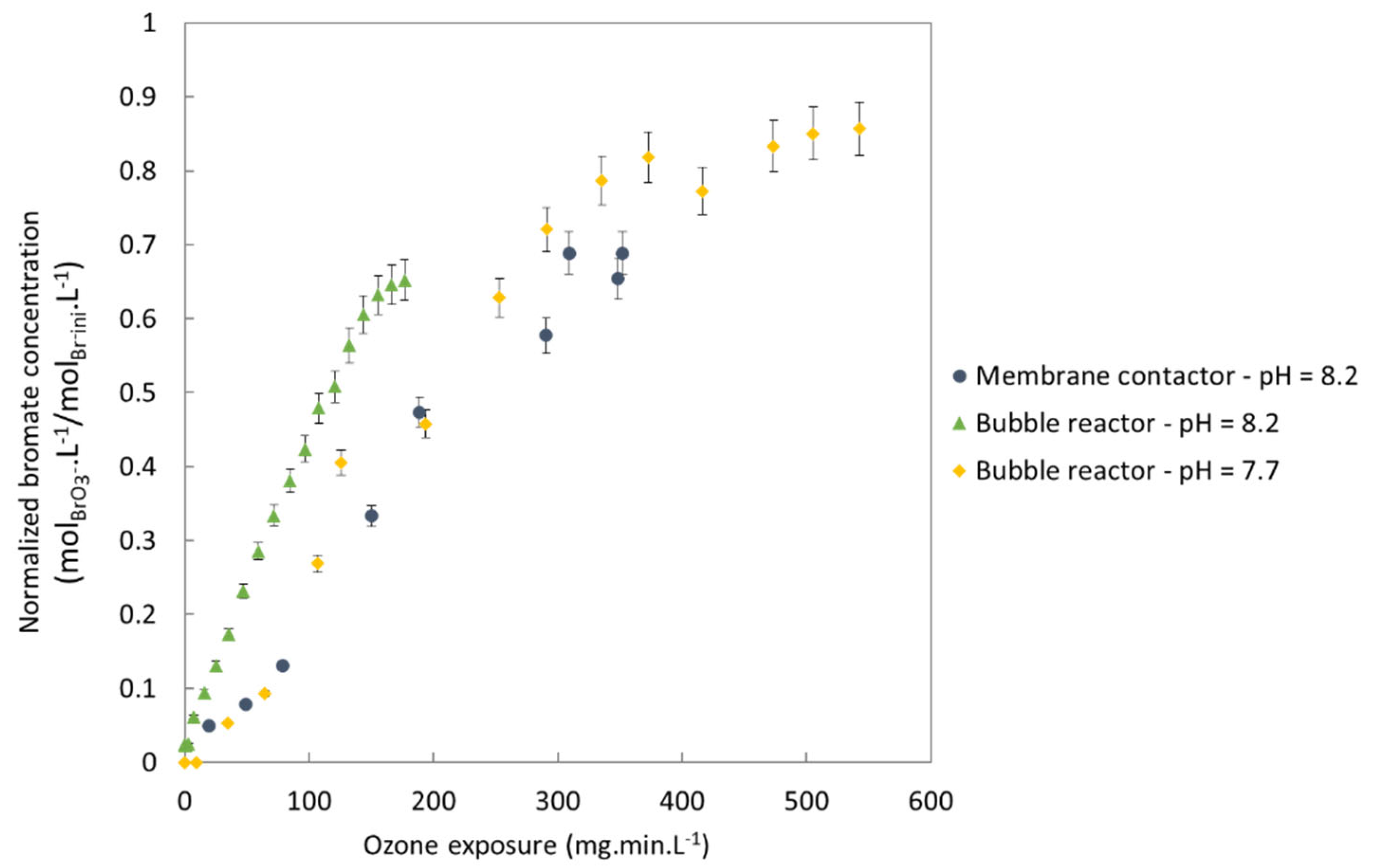
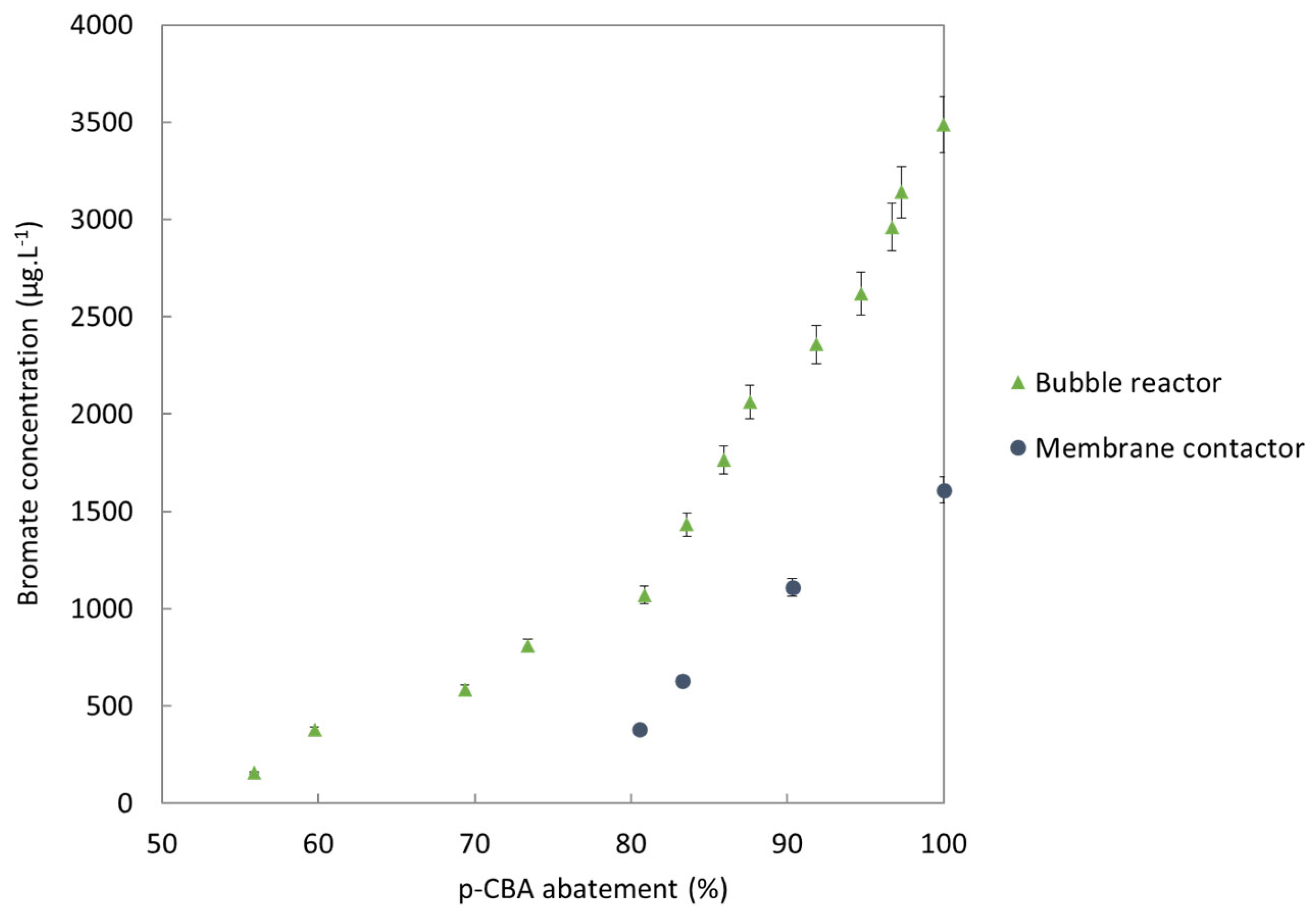
3.2. Bromate Minimization: Membrane Contactor Technology versus Bubble Reactor
3.2.1. Formation of Bromates
3.2.2. Production of Hydroxyl Radicals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Fent, K.; Weston, A.A.; Caminada, D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef] [PubMed]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Bolong, N.; Ismail, A.F.; Salim, M.R.; Matsuura, T. A review of the effects of emerging contaminants in wastewater and options for their removal. Desalination 2009, 239, 229–246. [Google Scholar] [CrossRef]
- Shojaee Nasirabadi, P.; Saljoughi, E.; Mousavi, S.M. Membrane processes used for removal of pharmaceuticals, hormones, endocrine disruptors and their metabolites from wastewaters: A review. Desalin. Water Treat. 2016, 57, 24146–24175. [Google Scholar] [CrossRef]
- Khan, N.A.; Khan, S.U.; Ahmed, S.; Farooqi, I.H.; Yousefi, M.; Mohammadi, A.A.; Changani, F. Recent trends in disposal and treatment technologies of emerging-pollutants- A critical review. TrAC—Trends Anal. Chem. 2020, 122, 115744. [Google Scholar] [CrossRef]
- Ashton, D.; Hilton, M.; Thomas, K.V. V Investigating the environmental transport of human pharmaceuticals to streams in the United Kingdom. Sci. Total Environ. 2004, 333, 167–184. [Google Scholar] [CrossRef]
- Björlenius, B.; Ripszám, M.; Haglund, P.; Lindberg, R.H.; Tysklind, M.; Fick, J. Pharmaceutical residues are widespread in Baltic Sea coastal and offshore waters—Screening for pharmaceuticals and modelling of environmental concentrations of carbamazepine. Sci. Total Environ. 2018, 633, 1496–1509. [Google Scholar] [CrossRef]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999-2000: A national reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef]
- Ternes, T.A. Occurrence of drugs in German sewage treatment plants and rivers. Water Res. 1998, 32, 3245–3260. [Google Scholar] [CrossRef]
- Valdés, M.E.; Huerta, B.; Wunderlin, D.A.; Bistoni, M.A.; Barceló, D.; Rodriguez-Mozaz, S. Bioaccumulation and bioconcentration of carbamazepine and other pharmaceuticals in fish under field and controlled laboratory experiments. Evidences of carbamazepine metabolization by fish. Sci. Total Environ. 2016, 557–558, 58–67. [Google Scholar] [CrossRef]
- UNESCO and HELCOM. Pharmaceuticals in the Aquatic Environment of the Baltic Sea Region—A Status Report UNESCO Emerging Pollutants in Water Series; Unesco Publishing: Paris, France, 2017. [Google Scholar]
- Cabeza, Y.; Candela, L.; Ronen, D.; Teijon, G. Monitoring the occurrence of emerging contaminants in treated wastewater and groundwater between 2008 and 2010. The Baix Llobregat (Barcelona, Spain). J. Hazard. Mater. 2012, 239–240, 32–39. [Google Scholar] [CrossRef]
- Rosal, R.; Rodríguez, A.; Perdigón-Melón, J.A.; Petre, A.; García-Calvo, E.; Gómez, M.J.; Agüera, A.; Fernández-Alba, A.R. Occurrence of emerging pollutants in urban wastewater and their removal through biological treatment followed by ozonation. Water Res. 2010, 44, 578–588. [Google Scholar] [CrossRef]
- Yang, Y.; Ok, Y.S.; Kim, K.H.; Kwon, E.E.; Tsang, Y.F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total Environ. 2017, 596–597, 303–320. [Google Scholar] [CrossRef]
- Kråkström, M.; Saeid, S.; Tolvanen, P.; Kumar, N.; Salmi, T.; Kronberg, L.; Eklund, P. Ozonation of carbamazepine and its main transformation products: Product determination and reaction mechanisms. Environ. Sci. Pollut. Res. 2020, 27, 23258–23269. [Google Scholar] [CrossRef]
- Pistocchi, A.; Andersen, H.R.; Bertanza, G.; Brander, A.; Choubert, J.M.; Cimbritz, M.; Drewes, J.E.; Koehler, C.; Krampe, J.; Launay, M.; et al. Treatment of micropollutants in wastewater: Balancing effectiveness, costs and implications. Sci. Total Environ. 2022, 850, 157593. [Google Scholar] [CrossRef]
- Lee, Y.; von Gunten, U. Advances in predicting organic contaminant abatement during ozonation of municipal wastewater effluent: Reaction kinetics, transformation products, and changes of biological effects. Environ. Sci. Water Res. Technol. 2016, 2, 421–442. [Google Scholar] [CrossRef]
- Bourgin, M.; Borowska, E.; Helbing, J.; Hollender, J.; Kaiser, H.-P.; Kienle, C.; McArdell, C.S.; Simon, E.; von Gunten, U. Effect of operational and water quality parameters on conventional ozonation and the advanced oxidation process O3/H2O2: Kinetics of micropollutant abatement, transformation product and bromate formation in a surface water. Water Res. 2017, 122, 234–245. [Google Scholar] [CrossRef]
- Yao, W.; Rehman, S.W.U.; Wang, H.; Yang, H.; Yu, G.; Wang, Y. Pilot-scale evaluation of micropollutant abatements by conventional ozonation, UV/O3, and an electro-peroxone process. Water Res. 2018, 138, 106–117. [Google Scholar] [CrossRef]
- Lee, Y.; Kovalova, L.; McArdell, C.S.; von Gunten, U. Prediction of micropollutant elimination during ozonation of a hospital wastewater effluent. Water Res. 2014, 64, 134–148. [Google Scholar] [CrossRef]
- Reungoat, J.; Macova, M.; Escher, B.I.I.; Carswell, S.; Mueller, J.F.F.; Keller, J. Removal of micropollutants and reduction of biological activity in a full scale reclamation plant using ozonation and activated carbon filtration. Water Res. 2010, 44, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.; Costa, R.; Quinta-Ferreira, R.M.; Martins, R.C. Application of ozonation for pharmaceuticals and personal care products removal from water. Sci. Total Environ. 2017, 586, 265–283. [Google Scholar] [CrossRef] [PubMed]
- Vittenet, J.; Aboussaoud, W.; Mendret, J.; Pic, J.S.; Debellefontaine, H.; Lesage, N.; Faucher, K.; Manero, M.H.; Thibault-Starzyk, F.; Leclerc, H.; et al. Catalytic ozonation with γ-Al2O3 to enhance the degradation of refractory organics in water. Appl. Catal. A Gen. 2015, 504, 519–532. [Google Scholar] [CrossRef]
- von Gunten, U. Ozonation of drinking water: Part I. Oxidation kinetics and product formation. Water Res. 2003, 37, 1443–1467. [Google Scholar] [CrossRef]
- Von Sonntag, C.; Von Gunten, U. Chemistry of Ozone in Water and Wastewater Treatment; IWA Publishing: London, UK, 2012; ISBN 1843393131. [Google Scholar]
- Stylianou, S.K.; Katsoyiannis, I.A.; Ernst, M.; Zouboulis, A.I. Impact of O3 or O3/H2O2 treatment via a membrane contacting system on the composition and characteristics of the natural organic matter of surface waters. Environ. Sci. Pollut. Res. 2017, 25, 12246–12255. [Google Scholar] [CrossRef]
- Richardson, S.D.; Plewa, M.J.; Wagner, E.D.; Schoeny, R.; Demarini, D.M. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research. Mutat. Res. 2007, 636, 178–242. [Google Scholar] [CrossRef]
- Soltermann, F.; Abegglen, C.; Tschui, M.; Stahel, S.; von Gunten, U. Options and limitations for bromate control during ozonation of wastewater. Water Res. 2017, 116, 76–85. [Google Scholar] [CrossRef]
- von Gunten, U. Ozonation of drinking water: Part II. Disinfection and by-product formation in presence of bromide, iodide or chlorine. Water Res. 2003, 37, 1469–1487. [Google Scholar] [CrossRef]
- USEPA. Disinfectants and disinfection byproducts : Proposed rule. Fed. Reg. 1998, 63, 69389–69476. [Google Scholar]
- AIDA Aida Directive N 98/83/Ce du 03/11/98 Relative à la Qualité Des Eaux Destinées à la Consommation Humaine. 1998. Available online: https://aida.ineris.fr/consultation_document/1017 (accessed on 1 September 2021).
- Pinkernell, U.; Von Gunten, U. Bromate minimization during ozonation: Mechanistic considerations. Environ. Sci. Technol. 2001, 35, 2525–2531. [Google Scholar] [CrossRef]
- Merle, T.; Pronk, W.; Von Gunten, U. MEMBRO3X, a Novel Combination of a Membrane Contactor with Advanced Oxidation (O3 /H2O2) for Simultaneous Micropollutant Abatement and Bromate Minimization. Environ. Sci. Technol. Lett 2017, 4, 13. [Google Scholar] [CrossRef]
- Stylianou, S.K.; Katsoyiannis, I.A.; Mitrakas, M.; Zouboulis, A.I. Application of a ceramic membrane contacting process for ozone and peroxone treatment of micropollutant contaminated surface water. J. Hazard. Mater. 2018, 358, 129–135. [Google Scholar] [CrossRef]
- Schmitt, A.; Mendret, J.; Roustan, M.; Brosillon, S. Ozonation using hollow fiber contactor technology and its perspectives for micropollutants removal in water: A review. Sci. Total Environ. 2020, 729, 138664. [Google Scholar] [CrossRef]
- Wenten, I.G.; Julian, H.; Panjaitan, N.T. Ozonation through ceramic membrane contactor for iodide oxidation during iodine recovery from brine water. Desalination 2012, 306, 29–34. [Google Scholar] [CrossRef]
- Berry, M.J.; Taylor, C.M.; King, W.; Chew, Y.M.J.; Wenk, J. Modelling of Ozone Mass-Transfer through Non-Porous Membranes for Water Treatment. Water 2017, 9, 452. [Google Scholar] [CrossRef]
- Schmitt, A.; Mendret, J.; Brosillon, S. Evaluation of an ozone diffusion process using a hollow fiber membrane contactor. Chem. Eng. Res. Des. 2021, 177, 291–303. [Google Scholar] [CrossRef]
- Huber, M.M.; Canonica, S.; Park, G.-Y.; von Gunten, U. Oxidation of Pharmaceuticals during Ozonation and Advanced Oxidation Processes. Environ. Sci. Technol. 2003, 37, 1016–1024. [Google Scholar] [CrossRef]
- Mathon, B.; Coquery, M.; Liu, Z.; Penru, Y.; Guillon, A.; Esperanza, M.; Miège, C.; Choubert, J.M. Ozonation of 47 organic micropollutants in secondary treated municipal effluents: Direct and indirect kinetic reaction rates and modelling. Chemosphere 2021, 262, 127969. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Rey, A. Free Radical and Direct Ozone Reaction Competition to Remove Priority and Pharmaceutical Water Contaminants with Single and Hydrogen Peroxide Ozonation Systems. Ozone Sci. Eng. 2018, 40, 251–265. [Google Scholar] [CrossRef]
- Bader, H.; Hoigné, J. Determination of Ozone In Water By The Indigo Method: A Submitted Standard Method. Ozone Sci. Eng. J. Int. Ozone Assoc. 1982, 4, 169–176. [Google Scholar] [CrossRef]
- Weishaar, J.L.; Fram, M.S.; Fujii, R.; Mopper, K. Evaluation of Specific Ultraviolet Absorbance as an Indicator of the Chemical Composition and Reactivity of Dissolved Organic Carbon. Environ. Sci. Technol. 2003, 37, 4702–4708. [Google Scholar] [CrossRef] [PubMed]
- Bamperng, S.; Suwannachart, T.; Atchariyawut, S.; Jiraratananon, R. Ozonation of dye wastewater by membrane contactor using PVDF and PTFE membranes. Sep. Purif. Technol. 2010, 72, 186–193. [Google Scholar] [CrossRef]
- Wang, R.; Shi, L.; Tang, C.Y.; Chou, S.; Qiu, C.; Fane, A.G. Characterization of novel forward osmosis hollow fiber membranes. J. Memb. Sci. 2010, 355, 158–167. [Google Scholar] [CrossRef]
- Iversen, S.B.; Bhatia, V.K.; Dam-Johansen, K.; Jonsson, G. Characterization of microporous membranes for use in membrane contactors. J. Memb. Sci. 1997, 130, 205–217. [Google Scholar] [CrossRef]
- Neta, P.; Dorfman, L.M. Pulse radiolysis studies. XIII. Rate constants for the reaction of hydroxyl radicals with aromatic compounds in aqueous solutions. In Radiation Chemistry; ACS Publications: Washington, DC, USA, 1968. [Google Scholar]
- Pi, Y.; Schumacher, J.; Jekel, M. The use of para-chlorobenzoic acid (pCBA) as an ozone/hydroxyl radical probe compound. Ozone Sci. Eng. 2005, 27, 431–436. [Google Scholar] [CrossRef]
- Penru, Y.; Miege, C.; Daval, A.; Guillon, A.; Esperanza, M.; Crétollier, C.; Masson, M.; Le Goaziou, Y.; Baig, S.; Ruel, S.M.; et al. Traitement des micropolluants émergents par ozonation tertiaire. Performances de la station d’épuration des Bouillides, Sophia-Antipolis, France. Aqua Gas 2017, 2017, 8. [Google Scholar]
- Von Gunten, U.; Hoigne, J. Bromate Formation during Ozonation of Bromide-Containing Waters: Interaction of Ozone and Hydroxyl Radical Reactions. Environ. Sci. Technol. 1994, 28, 1234–1242. [Google Scholar] [CrossRef]
- Haag, W.R.; Hoigné, J. Ozonation of Bromide-Containing Waters: Kinetics of Formation of Hypobromous Acid and Brómate. Environ. Sci. Technol. 1983, 17, 261–267. [Google Scholar] [CrossRef]
- Chao, P.-F. Role of Hydroxyl Radicals and Hypobromous Acid Reactions on Bromate Formation during Ozonation; Arizona State University: Tempe, AZ, USA, 2002. [Google Scholar]
- Roustan, M. Transferts Gaz-Liquide dans les Procédés de Traitement des Eaux et des Effluents Gazeux; Tec & doc: Paris, France, 2003; ISBN 2743006056. [Google Scholar]
- Von Gunten, U.; Hoigne, J.; Bruchet, A. Bromate formation during ozonation of bromide-containing waters. Water Supply 1995, 13, 45–50. [Google Scholar]
| Compound | Formula | MW (g.mol−1) | kO3 (M−1.s−1) | kOH (M−1.s−1) | Log Kow | Solubility in Water (25 °C, mg.L−1) | Semi-Developed Formula |
|---|---|---|---|---|---|---|---|
| Carbamazepine (CBZ) | C15H12N2O | 236 | 3.0 × 105 a | 8.8 × 109 b | 2.45 b | 18 b | 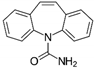 |
| Sulfamethoxazole (SUL) | C10H11N3O3S | 253 | 4.2 × 105 c | 3.2 × 109 b | 0.89 b | 610 b | 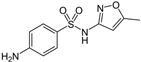 |
| Parameters | Mean Value | Standard Deviation |
|---|---|---|
| TOC (mgC.L−1) | 6.8 | 0.7 |
| COD (mgO2.L−1) | 18.0 | 1.0 |
| pH | 7.7 | 0.2 |
| SUVA254 (L.cm−1.mgC−1) | 0.02 | 0.01 |
| Average Ionic Composition (mg/L) | |||
|---|---|---|---|
| Sodium (Na+) | 88.1 ± 0.8 | Bromate (BrO3−) | < LOD |
| Ammonium (NH4+) | < LOD | Chloride (Cl−) | 155 ± 6 |
| Potassium (K+) | 22.6 ± 0.3 | Nitrite (NO2−) | 0.2 ± 0.2 |
| Magnesium (Mg+) | 10.1 ± 0.3 | Chlorate (ClO3−) | 0.5 ± 0.3 |
| Calcium (Ca2+) | 92.0 ± 0.7 | Bromide (Br−) | 0.29 ± 0.03 |
| Sulphate (SO42−) | 84 ± 10 | Phosphate (PO43−) | 0.3 ± 0.3 |
| PTFE Fibers | |||
|---|---|---|---|
| Number * | 65 | Effective Contact Length (cm) * | 60 |
| Inner/outer diameter (mm) * | 0.45/0.87 | Effective contact surface (m²) | 0.107 |
| Specific exchange surface a (m²/m3) c | 2948 | N2 permeance (GPU) * | 33,904 |
| Porosity a | 0.58 | Tortuosity b | 3.47 |
| Stainless steel shell | |||
| Inside diameter (mm) * | 9.5 | Filling rate * | 54.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmitt, A.; Mendret, J.; Cheikho, H.; Brosillon, S. Ozone Diffusion through a Hollow Fiber Membrane Contactor for Pharmaceuticals Removal and Bromate Minimization. Membranes 2023, 13, 171. https://doi.org/10.3390/membranes13020171
Schmitt A, Mendret J, Cheikho H, Brosillon S. Ozone Diffusion through a Hollow Fiber Membrane Contactor for Pharmaceuticals Removal and Bromate Minimization. Membranes. 2023; 13(2):171. https://doi.org/10.3390/membranes13020171
Chicago/Turabian StyleSchmitt, Alice, Julie Mendret, Hani Cheikho, and Stephan Brosillon. 2023. "Ozone Diffusion through a Hollow Fiber Membrane Contactor for Pharmaceuticals Removal and Bromate Minimization" Membranes 13, no. 2: 171. https://doi.org/10.3390/membranes13020171
APA StyleSchmitt, A., Mendret, J., Cheikho, H., & Brosillon, S. (2023). Ozone Diffusion through a Hollow Fiber Membrane Contactor for Pharmaceuticals Removal and Bromate Minimization. Membranes, 13(2), 171. https://doi.org/10.3390/membranes13020171









