Carboxylated Graphene Oxide (c-GO) Embedded ThermoPlastic Polyurethane (TPU) Mixed Matrix Membrane with Improved Physicochemical Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.2.1. Preparation of Graphene Oxide
2.2.2. Synthesis of Carboxylated Graphene Oxide (c-GO)
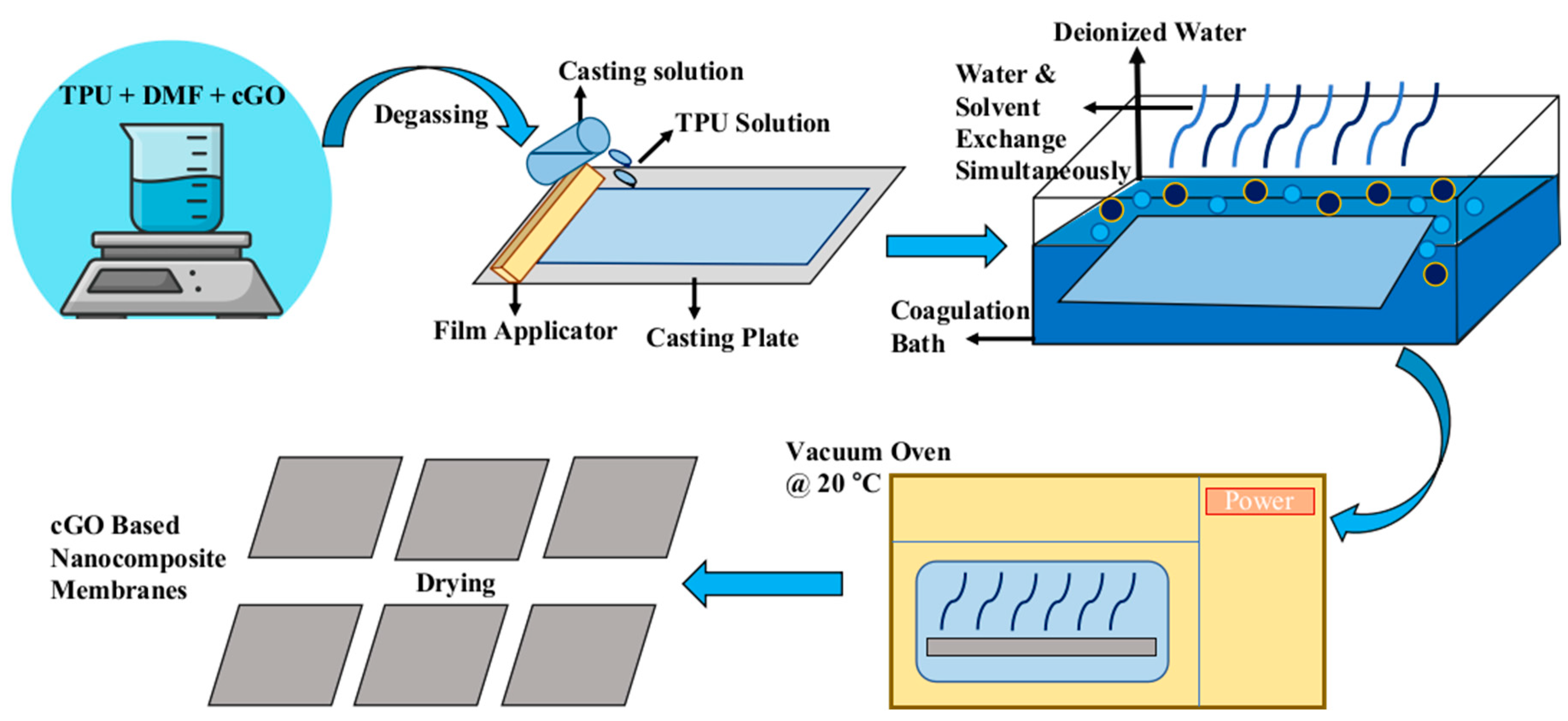
2.2.3. Fabrication of Carboxylated Graphene Oxide Based TPU Membranes
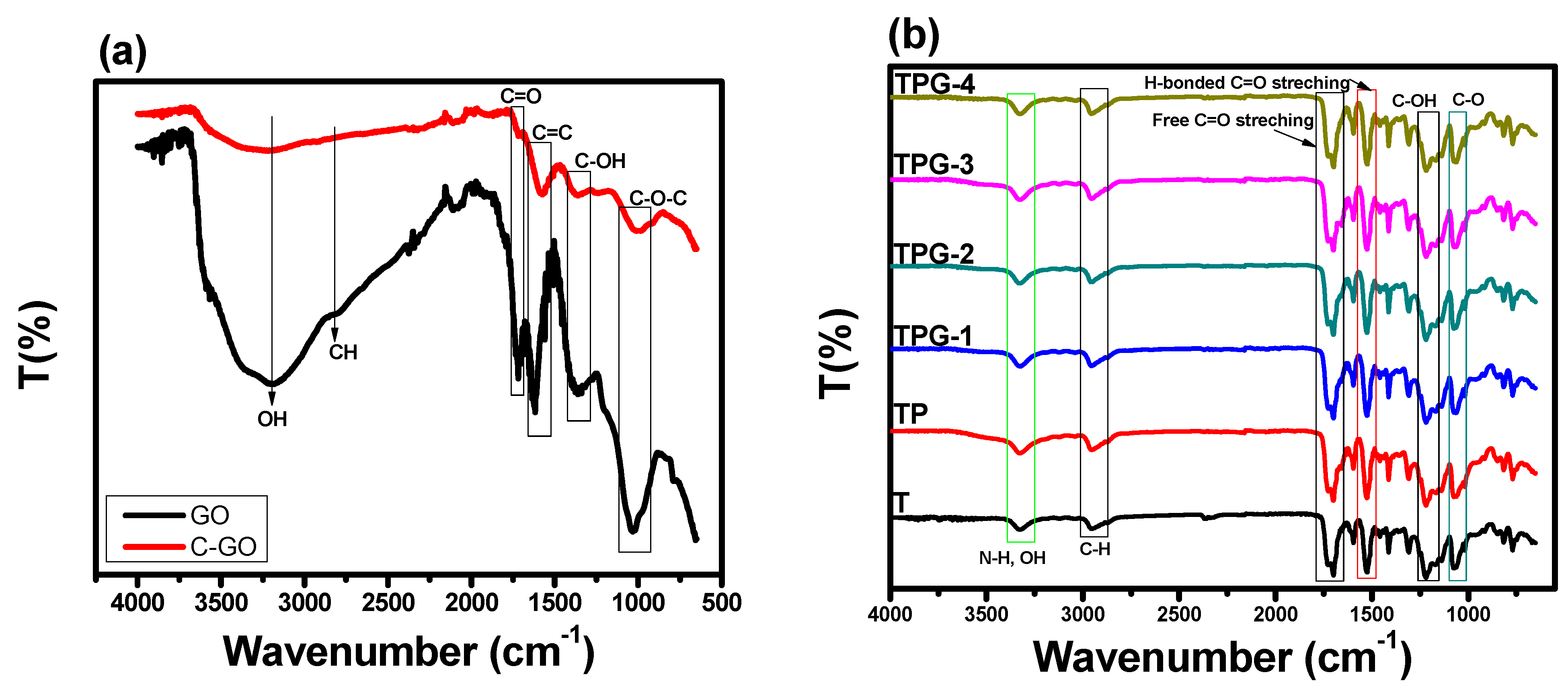
2.3. Characterization of GO and c-GO and TPU Composite Membranes
2.4. Measurement of Water Contact Angle, Pure Water Flux and Dye Rejection Rate
2.5. Membrane Porosity Determination Using Gravimetric Method
3. Result and Discussion
3.1. Characterization of Nanoparticles
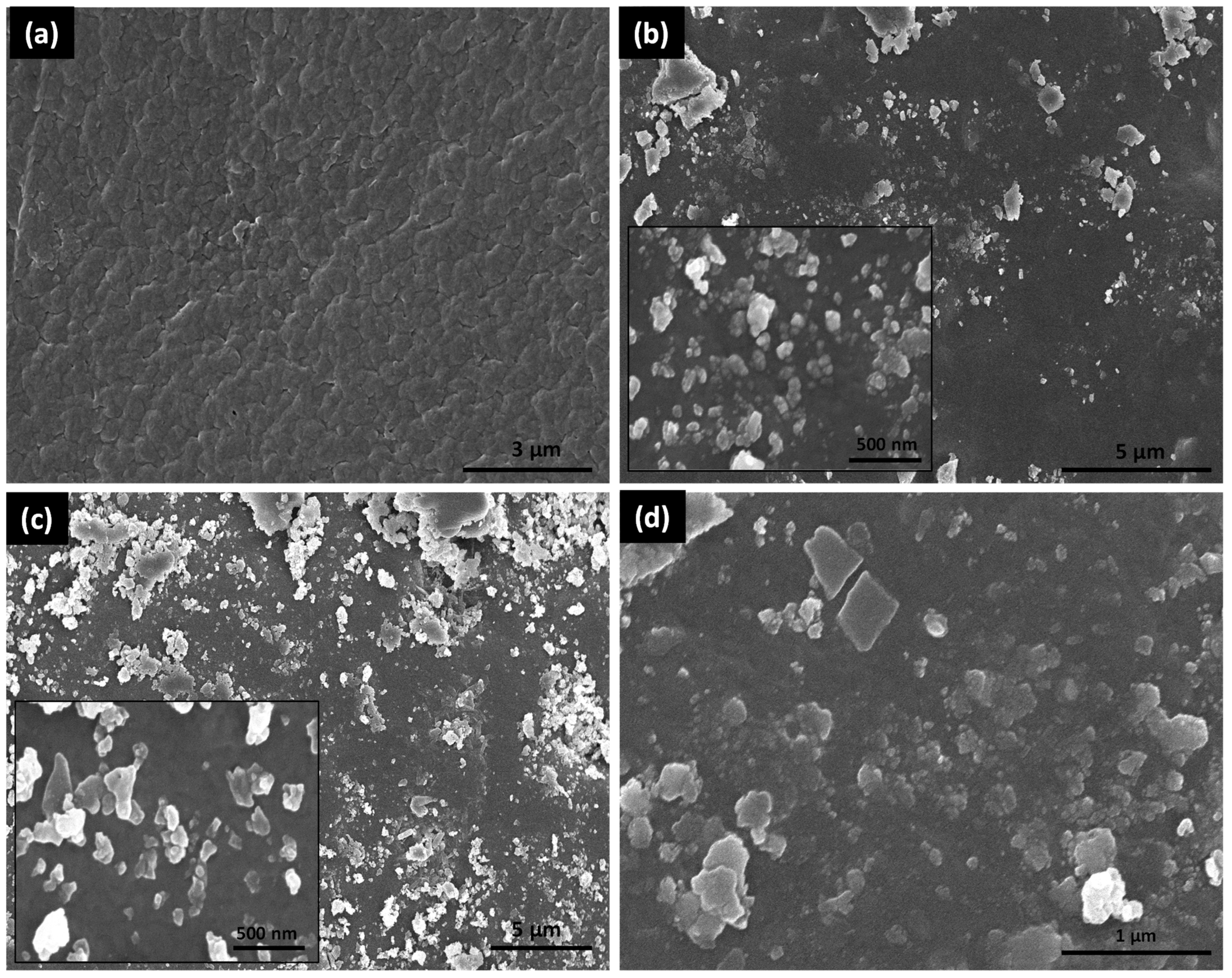
3.1.1. SEM Analysis
3.1.2. XRD
3.1.3. Contact Angle
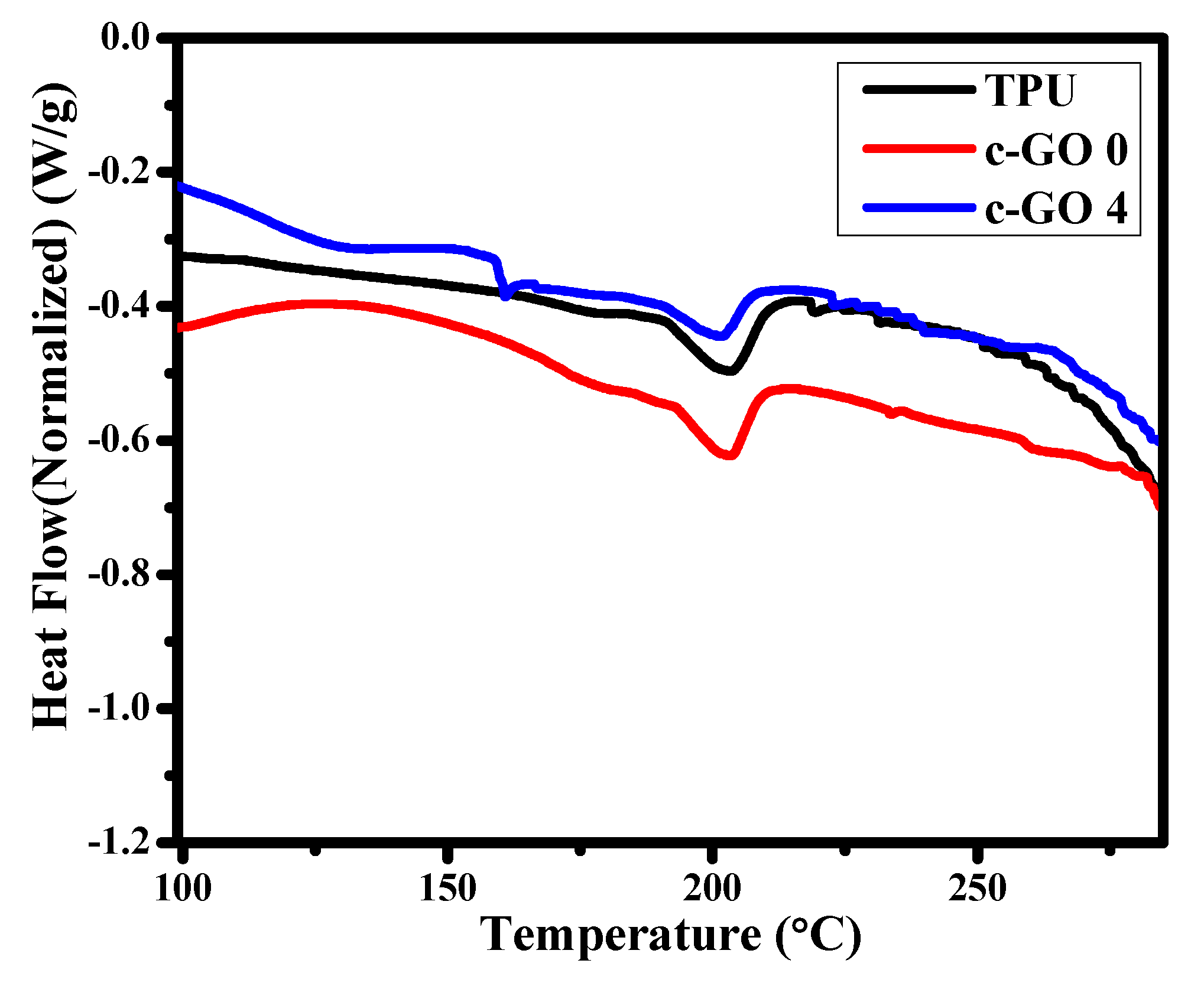
3.1.4. DSC
3.1.5. Pure Water Flux of Membranes
3.1.6. Dye Rejection by Composite Membrane
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qamar, M.; Aslam, M.; Rehan, Z.; Soomro, M.; Basahi, J.M.; Ismail, I.M.; Hameed, A. The effect of Fe3+ based visible light receptive interfacial phases on the photocatalytic activity of ZnO for the removal of 2, 4-dichlorophenoxy acetic acid in natural sunlight exposure. Sep. Purif. Technol. 2017, 172, 512–528. [Google Scholar] [CrossRef]
- Ghaee, A.; Zerafat, M.; Askari, P.; Sabbaghi, S.; Sadatnia, B. Fabrication of polyamide thin-film nanocomposite membranes with enhanced surface charge for nitrate ion removal from water resources. Environ. Technol. 2017, 38, 772–781. [Google Scholar] [CrossRef]
- Yin, J.; Deng, B. Polymer-matrix nanocomposite membranes for water treatment. J. Membr. Sci. 2015, 479, 256–275. [Google Scholar] [CrossRef]
- Cinperi, N.C.; Ozturk, E.; Yigit, N.O.; Kitis, M. Treatment of woolen textile wastewater using membrane bioreactor, nanofiltration and reverse osmosis for reuse in production processes. J. Clean. Prod. 2019, 223, 837–848. [Google Scholar] [CrossRef]
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2018, 16, 1193–1226. [Google Scholar] [CrossRef]
- Chen, X.; Shen, Z.; Zhu, X.; Fan, Y.; Wang, W. Advanced treatment of textile wastewater for reuse using electrochemical oxidation and membrane filtration. Water Sa 2005, 31, 127–132. [Google Scholar] [CrossRef]
- Nadeem, N.; Zahid, M.; Rehan, Z.A.; Hanif, M.A.; Yaseen, M. Improved photocatalytic degradation of dye using coal fly ash-based zinc ferrite (CFA/ZnFe2O4) composite. Int. J. Environ. Sci. Technol. 2022, 19, 3045–3060. [Google Scholar] [CrossRef]
- Ashiq, H.; Nadeem, N.; Mansha, A.; Iqbal, J.; Yaseen, M.; Zahid, M.; Shahid, I. G-C3N4/Ag@CoWO4: A novel sunlight active ternary nanocomposite for potential photocatalytic degradation of rhodamine B dye. J. Phys. Chem. Solids 2021, 161, 110437. [Google Scholar] [CrossRef]
- Nadeem, N.; Abbas, Q.; Yaseen, M.; Jilani, A.; Zahid, M.; Iqbal, J.; Murtaza, A.; Janczarek, M.; Jesionowski, T. Coal fly ash-based copper ferrite nanocomposites as potential heterogeneous photocatalysts for wastewater remediation. Appl. Surf. Sci. 2021, 565, 150542. [Google Scholar] [CrossRef]
- Nadeem, N.; Zahid, M.; Hanif, M.A.; Bhatti, I.A.; Shahid, I.; Rehan, Z.A.; Hussain, T.; Abbas, Q. Silver-doped metal ferrites for wastewater treatment. In Silver Nanomaterials for Agri-Food Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 599–622. [Google Scholar]
- Senusi, F.; Shahadat, M.; Ismail, S.; Abd Hamid, S. Recent advancement in membrane technology for water purification. In Modern Age Environmental Problems and their Remediation; Springer: Cham, Switzerland, 2018; pp. 147–167. [Google Scholar]
- Zahid, M.; Akram, S.; Rashid, A.; Rehan, Z.A.; Javed, T.; Shabbir, R.; Hessien, M.M.; El-Sayed, M.E. Investigating the antibacterial activity of polymeric membranes fabricated with aminated graphene oxide. Membranes 2021, 11, 510. [Google Scholar] [CrossRef]
- Khajouei, M.; Peyravi, M.; Jahanshahi, M. The potential of nanoparticles for upgrading thin film nanocomposite membranes—A review. J. Membr. Sci. Res. 2017, 3, 2–12. [Google Scholar]
- Ali, A.; Tufa, R.A.; Macedonio, F.; Curcio, E.; Drioli, E. Membrane technology in renewable-energy-driven desalination. Renew. Sustain. Energy Rev. 2018, 81, 1–21. [Google Scholar] [CrossRef]
- Ng, L.Y.; Mohammad, A.W.; Leo, C.P.; Hilal, N. Polymeric membranes incorporated with metal/metal oxide nanoparticles: A comprehensive review. Desalination 2013, 308, 15–33. [Google Scholar] [CrossRef]
- Fathizadeh, M.; Xu, W.L.; Zhou, F.; Yoon, Y.; Yu, M. Graphene oxide: A novel 2-dimensional material in membrane separation for water purification. Adv. Mater. Interfaces 2017, 4, 1600918. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Chen, J.; Peng, H.; Wang, X.; Shao, F.; Yuan, Z.; Han, H. Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale 2014, 6, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- Nisa, M.-U.; Nadeem, N.; Yaseen, M.; Iqbal, J.; Zahid, M.; Abbas, Q.; Mustafa, G.; Shahid, I. Applications of graphene-based tungsten oxide nanocomposites: A review. J. Nanostruct. Chem. 2022. [Google Scholar] [CrossRef]
- Tabasum, A.; Alghuthaymi, M.; Qazi, U.Y.; Shahid, I.; Abbas, Q.; Javaid, R.; Nadeem, N.; Zahid, M. UV-Accelerated Photocatalytic Degradation of Pesticide over Magnetite and Cobalt Ferrite Decorated Graphene Oxide Composite. Plants 2021, 10, 6. [Google Scholar] [CrossRef]
- Tabasum, A.; Bhatti, I.A.; Nadeem, N.; Zahid, M.; Rehan, Z.A.; Hussain, T.; Jilani, A. Degradation of acetamiprid using graphene-oxide-based metal (Mn and Ni) ferrites as Fenton-like photocatalysts. Water Sci. Technol. 2020, 81, 178–189. [Google Scholar] [CrossRef]
- Zahid, M.; Nadeem, N.; Hanif, M.A.; Bhatti, I.A.; Bhatti, H.N.; Mustafa, G. Metal ferrites and their graphene-based nanocomposites: Synthesis, characterization, and applications in wastewater treatment. In Magnetic Nanostructures; Springer: Cham, Switzerland, 2019; pp. 181–212. [Google Scholar]
- Zahid, M.; Nadeem, N.; Tahir, N.; Majeed, M.I.; Naqvi, S.A.R.; Hussain, T. Hybrid nanomaterials for water purification. In Multifunctional Hybrid Nanomaterials for Sustainable Agri-Food and Ecosystems; Elsevier: Amsterdam, The Netherlands, 2020; pp. 155–188. [Google Scholar]
- Lv, S.; Wei, J.; Jiang, F.; Wang, S. Adsorption-decolorization of four ionic dyes by carboxylated graphene. Chin. J. Appl. Chem. 2013, 30, 1215. [Google Scholar] [CrossRef]
- Zubair, U.; Zahid, M.; Nadeem, N.; Ghazal, K.; AlSalem, H.S.; Binkadem, M.S.; Al-Goul, S.T.; Rehan, Z.A. The Design of Ternary Composite Polyurethane Membranes with an Enhanced Photocatalytic Degradation Potential for the Removal of Anionic Dyes. Membranes 2022, 12, 630. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, D.; Li, B.; Wang, J.; Han, S.; Liu, L.; Wei, H. A bio-inspired nacre-like layered hybrid structure of calcium carbonate under the control of carboxyl graphene. CrystEngComm 2015, 17, 520–525. [Google Scholar] [CrossRef]
- Alfalahy, H.N.; Al-Jubouri, S.M. Preparation and application of polyethersulfone ultrafiltration membrane incorporating NaX zeolite for lead ions removal from aqueous solutions. Desalin. Water Treat 2022, 3, 28–56. [Google Scholar] [CrossRef]
- Abdullah, M.; Al-Jubouri, S. Implementation of hierarchically porous zeolite-polymer membrane for Chromium ions removal. IOP Conf. Ser. Earth Environ. Sci. 2021, 779, 012099. [Google Scholar] [CrossRef]
- Verma, S.; Dutta, R.K. A facile method of synthesizing ammonia modified graphene oxide for efficient removal of uranyl ions from aqueous medium. RSC Adv 2015, 5, 77192–77203. [Google Scholar] [CrossRef]
- Song, B.; Li, L.; Lin, Z.; Wu, Z.-K.; Moon, K.-s.; Wong, C.-P. Water-dispersible graphene/polyaniline composites for flexible micro-supercapacitors with high energy densities. Nano Energy 2015, 16, 470–478. [Google Scholar] [CrossRef]
- Pruna, A.I.; Barjola, A.; Cárcel, A.C.; Alonso, B.; Giménez, E. Effect of varying amine functionalities on CO2 capture of carboxylated graphene oxide-based cryogels. Nanomaterials 2020, 10, 1446. [Google Scholar] [CrossRef]
- Karimi, A.; Rajabi, M.; Zahedi, P. Effect of graphene oxide content on morphology and topography of polysulfone-based mixed matrix membrane for permeability and selectivity of carbon dioxide and methane. Mater. Und Werkst. 2020, 51, 1137–1147. [Google Scholar] [CrossRef]
- Xia, S.; Yao, L.; Zhao, Y.; Li, N.; Zheng, Y. Preparation of graphene oxide modified polyamide thin film composite membranes with improved hydrophilicity for natural organic matter removal. Chem. Eng. J. 2015, 280, 720–727. [Google Scholar] [CrossRef]
- Tang, L.A.; Lee, W.C.; Shi, H.; Wong, E.Y.; Sadovoy, A.; Gorelik, S.; Hobley, J.; Lim, C.T.; Loh, K.P. Highly wrinkled cross-linked graphene oxide membranes for biological and charge-storage applications. Small 2012, 8, 423–431. [Google Scholar] [CrossRef]
- Mohseniazar, M.; Barin, M.; Zarredar, H.; Alizadeh, S.; Shanehbandi, D. Potential of microalgae and lactobacilli in biosynthesis of silver nanoparticles. BioImpacts 2011, 1, 149. [Google Scholar] [PubMed]
- Ayyaru, S.; Ahn, Y.-H. Application of sulfonic acid group functionalized graphene oxide to improve hydrophilicity, permeability, and antifouling of PVDF nanocomposite ultrafiltration membranes. J. Membr. Sci. 2017, 525, 210–219. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]



| Membrane | TPU (wt %) | PVP | DMF | c-GO |
|---|---|---|---|---|
| TPU | 15% | 0% | 85% | - |
| c-GO 0 | 15% | 0% | 84.9% | 0.1% |
| c-GO 1 | 15% | 5% | 79.8% | 0.2% |
| c-GO 2 | 15% | 5% | 79.7% | 0.3% |
| c-GO 3 | 15% | 5% | 79.6% | 0.4% |
| c-GO 4 | 15% | 5% | 79.5% | 0.5% |
| Sr# | Membranes | Porosity (%) |
|---|---|---|
| 1 | TPU | 72.48 |
| 2 | c-GO 0 | 74.70 |
| 3 | c-GO 1 | 87.54 |
| 4 | c-GO 2 | 88.53 |
| 5 | c-GO 3 | 90.42 |
| 6 | c-GO 4 | 91.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahid, M.; Saeeda, M.; Nadeem, N.; Shakir, H.M.F.; El-Saoud, W.A.; Attala, O.A.; Attia, K.A.; Rehan, Z.A. Carboxylated Graphene Oxide (c-GO) Embedded ThermoPlastic Polyurethane (TPU) Mixed Matrix Membrane with Improved Physicochemical Characteristics. Membranes 2023, 13, 144. https://doi.org/10.3390/membranes13020144
Zahid M, Saeeda M, Nadeem N, Shakir HMF, El-Saoud WA, Attala OA, Attia KA, Rehan ZA. Carboxylated Graphene Oxide (c-GO) Embedded ThermoPlastic Polyurethane (TPU) Mixed Matrix Membrane with Improved Physicochemical Characteristics. Membranes. 2023; 13(2):144. https://doi.org/10.3390/membranes13020144
Chicago/Turabian StyleZahid, Muhammad, Maryam Saeeda, Nimra Nadeem, Hafiz Muhammad Fayzan Shakir, Waleed A. El-Saoud, Osama A. Attala, Kamal A. Attia, and Zulfiqar Ahmad Rehan. 2023. "Carboxylated Graphene Oxide (c-GO) Embedded ThermoPlastic Polyurethane (TPU) Mixed Matrix Membrane with Improved Physicochemical Characteristics" Membranes 13, no. 2: 144. https://doi.org/10.3390/membranes13020144
APA StyleZahid, M., Saeeda, M., Nadeem, N., Shakir, H. M. F., El-Saoud, W. A., Attala, O. A., Attia, K. A., & Rehan, Z. A. (2023). Carboxylated Graphene Oxide (c-GO) Embedded ThermoPlastic Polyurethane (TPU) Mixed Matrix Membrane with Improved Physicochemical Characteristics. Membranes, 13(2), 144. https://doi.org/10.3390/membranes13020144








