Graphene Nanocomposite Membranes: Fabrication and Water Treatment Applications
Abstract
1. Introduction
2. Preparation of Graphene Nanocomposite Membranes
2.1. Graphene
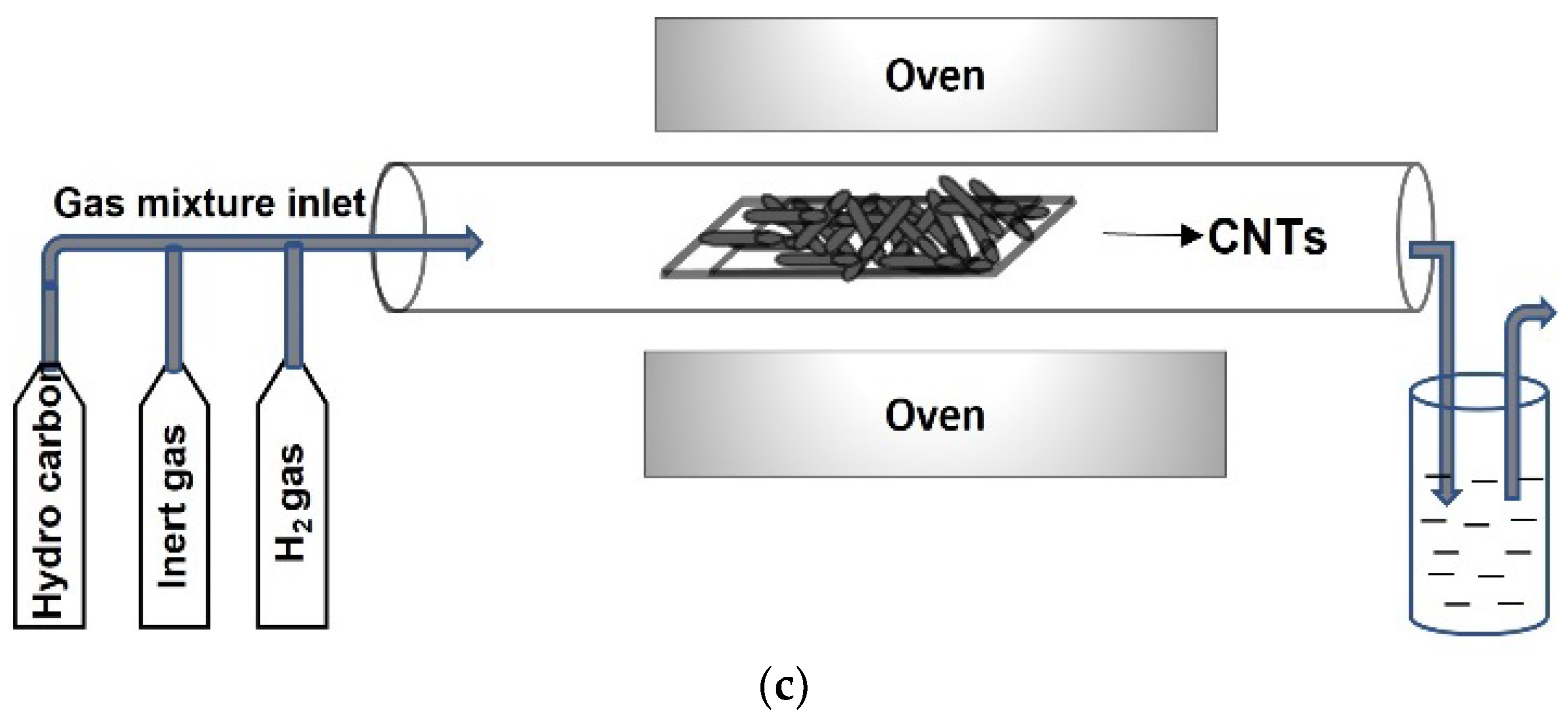
2.2. CNTs and Graphene Nanocomposites with CNTs
2.3. Graphene Nanocomposites with CNTs and Metal Oxides
2.4. The Choice of Polymers for Composite Development
2.5. Nanocomposite Membrane Fabrication Methods
2.6. Membrane Development
3. Characterizations and Characteristic Properties of Graphene Nanocomposites
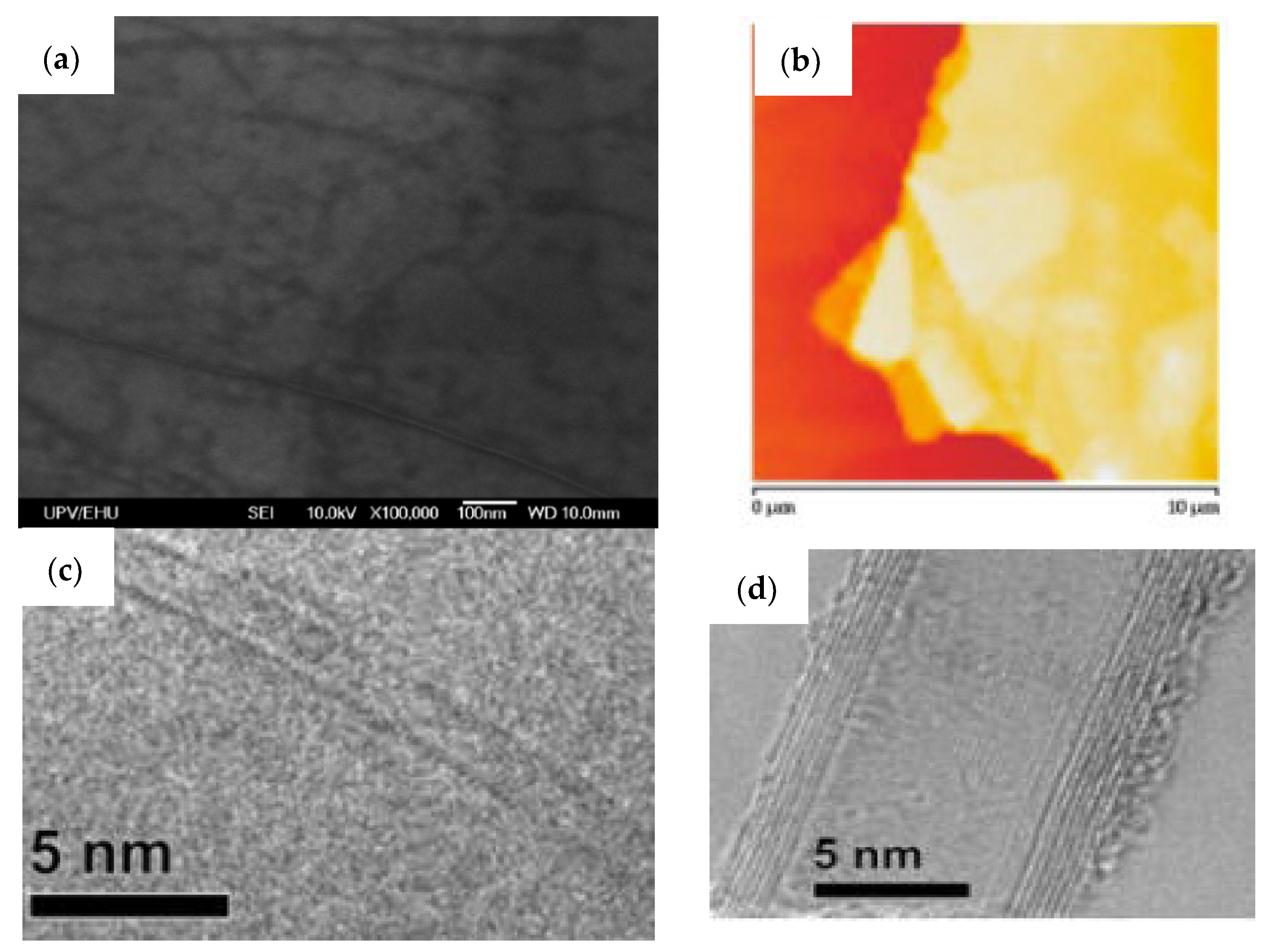
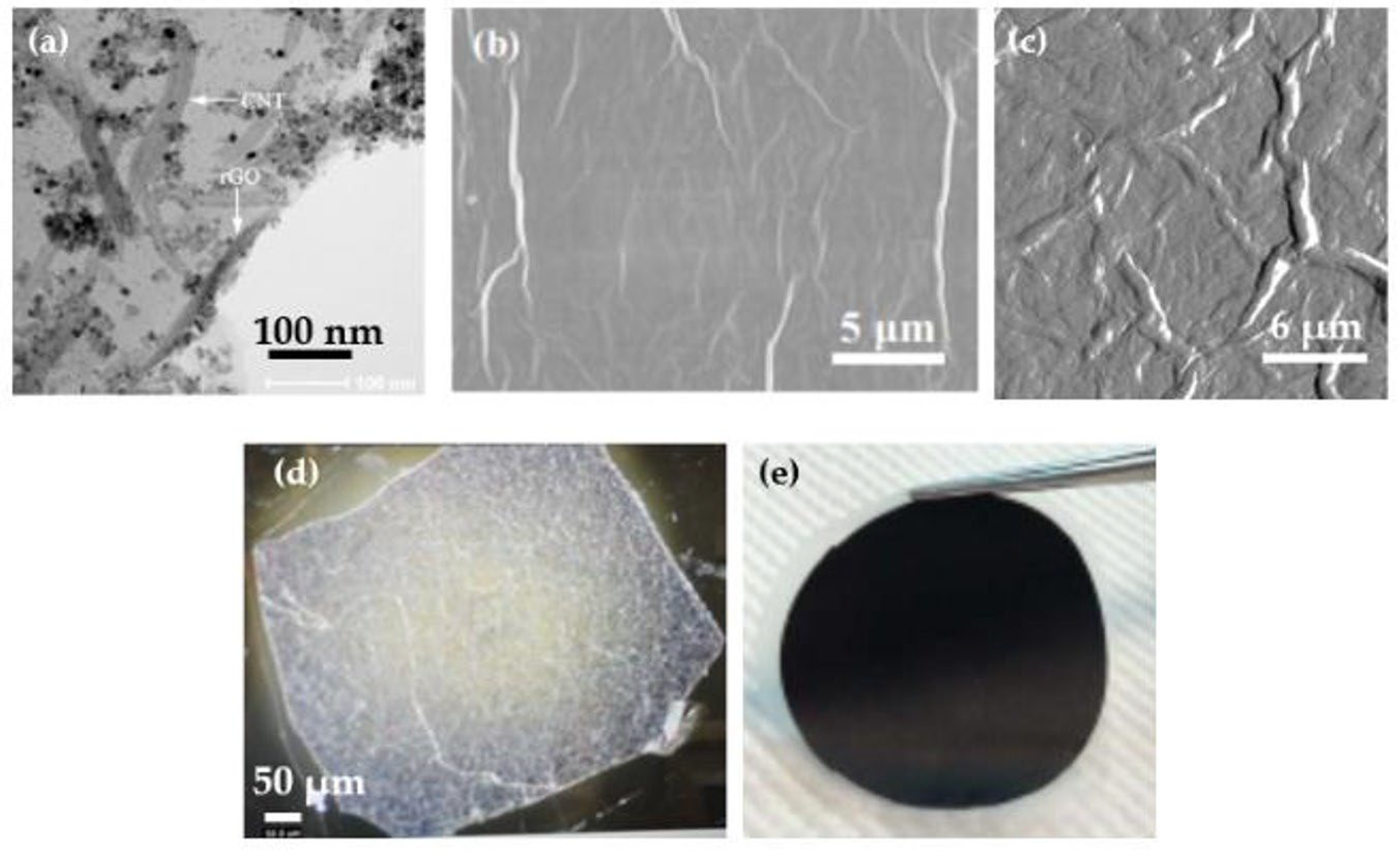
3.1. Characterizations of Materials and Nanocomposites
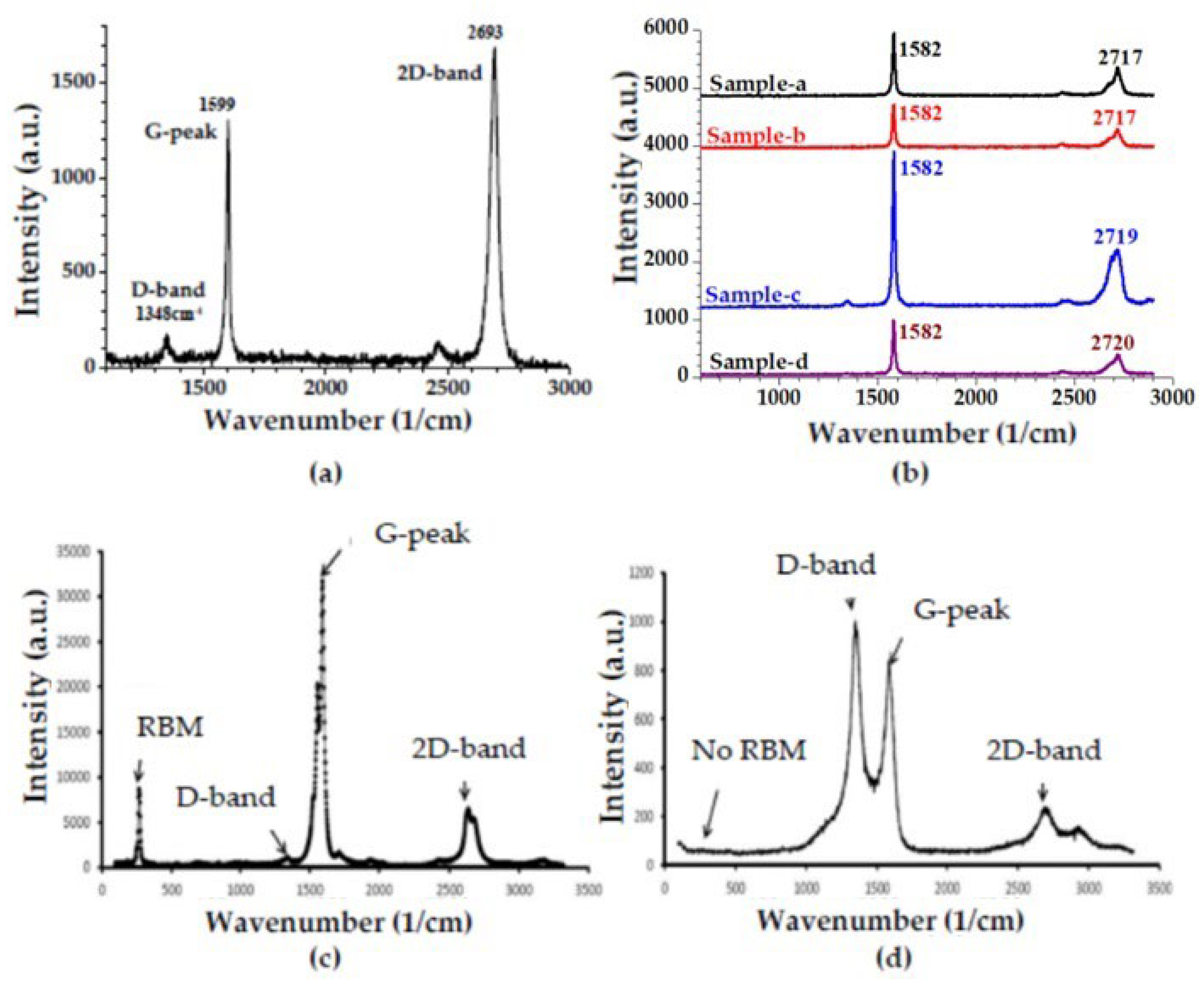
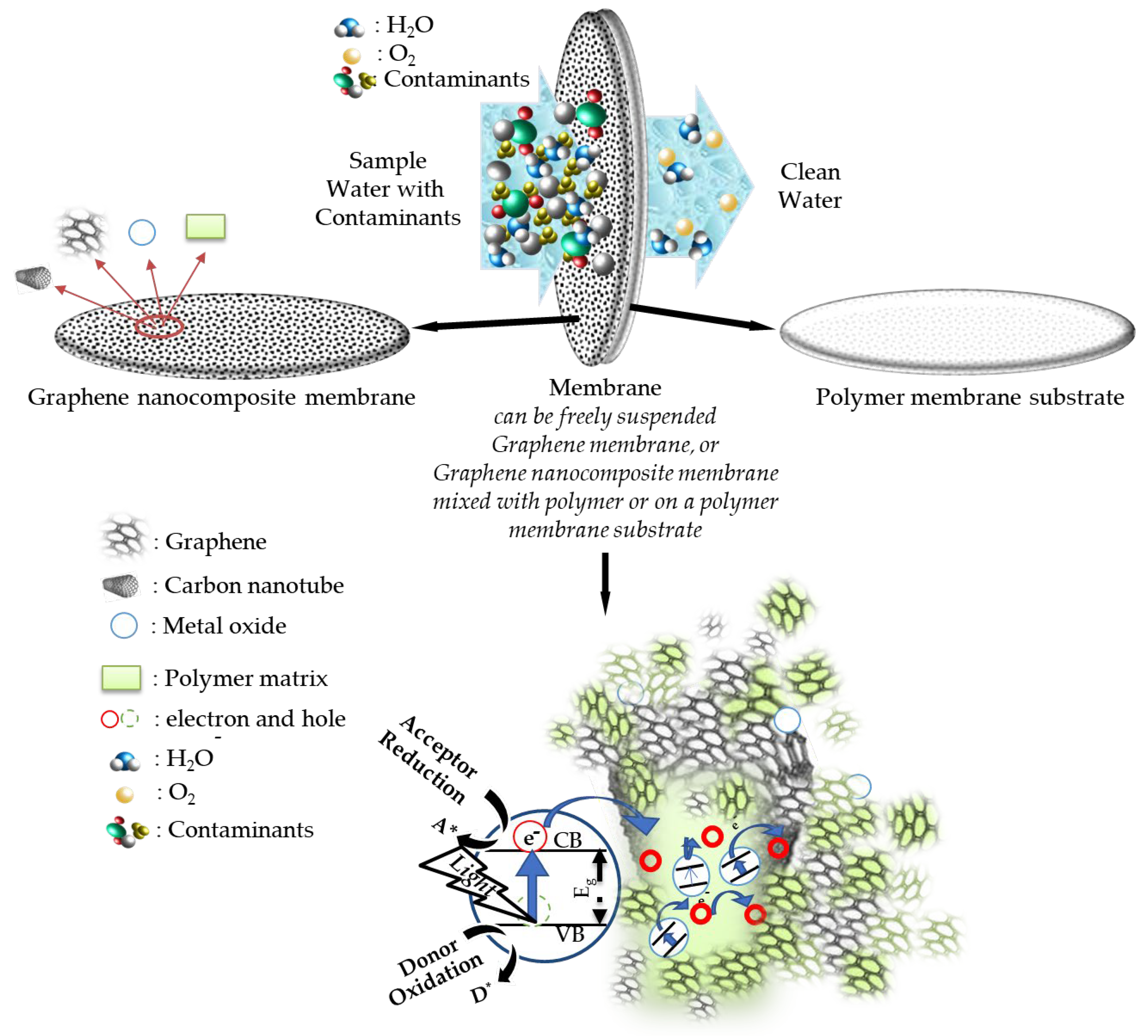
3.2. Characteristic Properties of Nanocomposite Membranes in Terms of Water Treatment
4. Water Treatment Applications of Graphene Nanocomposite Membranes
Effects of Graphene Nanocomposite Membranes Produced by Green Methods on Water Treatment Applications
5. Challenges and Future Prospects of Membrane Technology
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Health and the Environment. Available online: https://www.who.int/news/item/04-04-2022-health-and-the-environment (accessed on 19 October 2022).
- Greve, P.; Kahil, T.; Mochizuki, J.; Schinko, T.; Satoh, Y.; Burek, P.; Fischer, G.; Tramberend, S.; Burtscher, R.; Langan, S.; et al. Global Assessment of Water Challenges under Uncertainty in Water Scarcity Projections. Nat. Sustain. 2018, 1, 486–494. [Google Scholar] [CrossRef]
- Rosa, L.; Chiarelli, D.D.; Rulli, M.C.; Dell’Angelo, J.; D’Odorico, P. Global Agricultural Economic Water Scarcity. Sci. Adv. 2020, 6, eaaz6031. [Google Scholar] [CrossRef] [PubMed]
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Liermann, C.R.; et al. Global Threats to Human Water Security and River Biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Michalak, A.M. Study Role of Climate Change in Extreme Threats to Water Quality. Nature 2016, 535, 349–350. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Shantha Kumar, J.; Nagar, A.; Pradeep, T. Concepts of Sustainability in Clean Water Technologies; ACS Publications: Washington, DC, USA, 2022; pp. 625–657. [Google Scholar] [CrossRef]
- Irannezhad, M.; Ahmadi, B.; Liu, J.; Chen, D.; Matthews, J.H. Global Water Security: A Shining Star in the Dark Sky of Achieving the Sustainable Development Goals. Sustain. Horiz. 2022, 1, 100005. [Google Scholar] [CrossRef]
- Roy, S.; Ragunath, S. Emerging Membrane Technologies for Water and and Challenges. Energies 2018, 11, 2997. [Google Scholar] [CrossRef]
- Nagar, A.; Pradeep, T. Clean Water through Nanotechnology: Needs, Gaps, and Fulfillment. ACS Nano 2020, 14, 6420–6435. [Google Scholar] [CrossRef]
- Raju, C.V.; Cho, C.H.; Rani, G.M.; Manju, V.; Umapathi, R.; Huh, Y.S.; Park, J.P. Emerging Insights into the Use of Carbon-Based Nanomaterials for the Electrochemical Detection of Heavy Metal Ions. Coord. Chem. Rev. 2023, 476, 214920. [Google Scholar] [CrossRef]
- Han, Y.; Xu, Z.; Gao, C. Ultrathin Graphene Nanofiltration Membrane for Water Purification. Adv. Funct. Mater. 2013, 23, 3693–3700. [Google Scholar] [CrossRef]
- Jha, P.K.; Khongnakorn, W.; Chawenjkigwanich, C.; Chowdhury, M.S.; Techato, K. Eco-Friendly Reduced Graphene Oxide Nanofilter Preparation and Application for Iron Removal. Separations 2021, 8, 68. [Google Scholar] [CrossRef]
- Cheng, M.M.; Huang, L.J.; Wang, Y.X.; Tang, J.G.; Wang, Y.; Zhao, Y.C.; Liu, G.F.; Zhang, Y.; Kipper, M.J.; Belfiore, L.A.; et al. Recent Developments in Graphene-Based/Nanometal Composite Filter Membranes. RSC Adv. 2017, 7, 47886–47897. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, H.; Xie, F.; Ma, X.; Niu, B.; Chen, M.; Zhang, H.; Zhang, Y.; Long, D. General Synthesis of Ultrafine Metal Oxide/Reduced Graphene Oxide Nanocomposites for Ultrahigh-Flux Nanofiltration Membrane. Nat. Commun. 2022, 13, 1–10. [Google Scholar] [CrossRef]
- Yuan, X.T.; Xu, C.X.; Geng, H.Z.; Ji, Q.; Wang, L.; He, B.; Jiang, Y.; Kong, J.; Li, J. Multifunctional PVDF/CNT/GO Mixed Matrix Membranes for Ultrafiltration and Fouling Detection. J. Hazard. Mater. 2020, 384, 120978. [Google Scholar] [CrossRef]
- Zhao, C.; Xu, X.; Chen, J.; Wang, G.; Yang, F. Highly Effective Antifouling Performance of PVDF/Graphene Oxide Composite Membrane in Membrane Bioreactor (MBR) System. Desalination 2014, 340, 59–66. [Google Scholar] [CrossRef]
- Khan, U.; Biccai, S.; Boland, C.S.; Coleman, J.N. Low Cost, High Performance Ultrafiltration Membranes from Glass Fiber-PTFE–Graphene Composites. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Lan, Q.; Liu, L.; Wu, Y.; Feng, C.; Ou, K.; Wang, Z.; Huang, Y.; Lv, Y.; Miao, Y.E.; Liu, T. Porous Reduced Graphene Oxide/Phenolic Nanomesh Membranes with Ternary Channels for Ultrafast Water Purification. Compos. Commun. 2022, 33, 101216. [Google Scholar] [CrossRef]
- Das, R.; Ali, M.E.; Hamid, S.B.A.; Ramakrishna, S.; Chowdhury, Z.Z. Carbon Nanotube Membranes for Water Purification: A Bright Future in Water Desalination. Desalination 2014, 336, 97–109. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Li, W.; Liu, R.; Qiu, J.; Wang, S. Water Purification Performance and Energy Consumption of Gradient Nanocomposite Membranes. Compos. Part B Eng. 2020, 202, 108426. [Google Scholar] [CrossRef]
- Gao, Y.; Yan, S.; He, Y.; Fan, Y.; Zhang, L.; Ma, J.; Hou, R.; Chen, L.; Chen, J. A Photo-Fenton Self-Cleaning Membrane Based on NH2-MIL-88B (Fe) and Graphene Oxide to Improve Dye Removal Performance. J. Membr. Sci. 2021, 626, 119192. [Google Scholar] [CrossRef]
- Wang, R.; Chen, D.; Wang, Q.; Ying, Y.; Gao, W.; Xie, L. Recent Advances in Applications of Carbon Nanotubes for Desalination: A Review. Nanomaterials 2020, 10, 1203. [Google Scholar] [CrossRef]
- Liu, H.; Wang, H.; Zhang, X. Facile Fabrication of Freestanding Ultrathin Reduced Graphene Oxide Membranes for Water Purification. Adv. Mater. 2015, 27, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Tu, Q.; Yang, Q.; Wang, H.; Li, S. Rotating Carbon Nanotube Membrane Filter for Water Desalination. Sci. Rep. 2016, 6, 26183. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.; Vasu, K.S.; Williams, C.D.; Gopinadhan, K.; Su, Y.; Cherian, C.T.; Dix, J.; Prestat, E.; Haigh, S.J.; Grigorieva, I.V.; et al. Tunable Sieving of Ions Using Graphene Oxide Membranes. Nat. Nanotechnol. 2017, 12, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Weavers, K.L.; Walker, W.H.; Petrauskas, A.; Muthukumaran, S.; Kentish, S.E.; Ashokkumar, M.; Stevens, G.W.; Ashokkumar, M.; Kinzie, K.W.; et al. Using Ultrasound to Reduce Ceramic Membrane Fouling by Silica Particles. Prepr. Ext. Abstr. 2002, 42, 106–114. [Google Scholar] [CrossRef]
- Agrawal, A.; Sharma, A.; Awasthi, K.K.; Awasthi, A. Metal Oxides Nanocomposite Membrane for Biofouling Mitigation in Wastewater Treatment. Mater. Today Chem. 2021, 21, 100532. [Google Scholar] [CrossRef]
- Al Mahri, B.B.A.; Balogun, H.A.; Yusuf, A.; Giwa, A. Electro-Osmotic Thermal Process Model for Performance Enhancement of Forward Osmosis Integrated with Membrane Distillation. Sep. Purif. Technol. 2020, 238, 116494. [Google Scholar] [CrossRef]
- Musico, Y.L.F.; Santos, C.M.; Dalida, M.L.P.; Rodrigues, D.F. Surface Modification of Membrane Filters Using Graphene and Graphene Oxide-Based Nanomaterials for Bacterial Inactivation and Removal. ACS Sustain. Chem. Eng. 2014, 2, 1559–1565. [Google Scholar] [CrossRef]
- Yalcinkaya, F.; Boyraz, E.; Maryska, J.; Kucerova, K. A Review on Membrane Technology and Chemical Surface Modification for the Oily Wastewater Treatment. Materials 2020, 13, 493. [Google Scholar] [CrossRef]
- Zuo, G.; Wang, R. Novel Membrane Surface Modification to Enhance Anti-Oil Fouling Property for Membrane Distillation Application. J. Membr. Sci. 2013, 447, 26–35. [Google Scholar] [CrossRef]
- Gryta, M.; Tomczak, W. Stability of Ar/O2 Plasma-Treated Polypropylene Membranes Applied for Membrane Distillation. Membranes 2021, 11, 531. [Google Scholar] [CrossRef]
- Vasagar, V.; Hassan, M.K.; Khraisheh, M. Membrane Surface Modification and Functionalization. Membranes 2021, 11, 877. [Google Scholar] [CrossRef]
- Liu, T.; Lyv, J.; Xu, Y.; Zheng, C.; Liu, Y.; Fu, R.; Liang, L.; Wu, J.; Zhang, Z. Graphene-Based Woven Filter Membrane with Excellent Strength and Efficiency for Water Desalination. Desalination 2022, 533, 115775. [Google Scholar] [CrossRef]
- Dumée, L.F.; Sears, K.; Schütz, J.; Finn, N.; Huynh, C.; Hawkins, S.; Duke, M.; Gray, S. Characterization and Evaluation of Carbon Nanotube Bucky-Paper Membranes for Direct Contact Membrane Distillation. J. Membr. Sci. 2010, 351, 36–43. [Google Scholar] [CrossRef]
- Akbari, A.; Sheath, P.; Martin, S.T.; Shinde, D.B.; Shaibani, M.; Banerjee, P.C.; Tkacz, R.; Bhattacharyya, D.; Majumder, M. Large-Area Graphene-Based Nanofiltration Membranes by Shear Alignment of Discotic Nematic Liquid Crystals of Graphene Oxide. Nat. Commun. 2016, 7, 1–12. [Google Scholar] [CrossRef]
- Fan, X.; Liu, Y.; Quan, X. A Novel Reduced Graphene Oxide/Carbon Nanotube Hollow Fiber Membrane with High Forward Osmosis Performance. Desalination 2019, 451, 117–124. [Google Scholar] [CrossRef]
- Goh, K.; Jiang, W.; Karahan, H.E.; Zhai, S.; Wei, L.; Yu, D.; Fane, A.G.; Wang, R.; Chen, Y. All-Carbon Nanoarchitectures as High-Performance Separation Membranes with Superior Stability. Adv. Funct. Mater. 2015, 25, 7348–7359. [Google Scholar] [CrossRef]
- Liu, Y.; Lee, J.H.D.; Xia, Q.; Ma, Y.; Yu, Y.; Yung, L.Y.L.; Xie, J.; Ong, C.N.; Vecitis, C.D.; Zhou, Z. Graphene-Based Electrochemical Filter for Water Purification. J. Mater. Chem. A 2014, 2, 16554–16562. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, Z.; Qian, B.; Chan, A.Y.H.; Wang, X.; Liu, Y.; Xin, J.H. A Facile and Scalable Method of Fabrication of Large-Area Ultrathin Graphene Oxide Nanofiltration Membrane. ACS Nano 2021, 15, 15294–15305. [Google Scholar] [CrossRef]
- Sun, P.; Wang, K.; Wei, J.; Zhong, M.; Wu, H.Z. Effective Recovery of Acids from Iron-Based Electrolytes Using Graphene Oxide Membrane Filters. J. Mater. Chem. A 2014, 2, 7734–7737. [Google Scholar] [CrossRef]
- Joshi, R.K.; Carbone, P.; Wang, F.C.; Kravets, V.G.; Su, Y.; Grigorieva, I.V.; Wu, H.A.; Geim, A.K.; Nair, R.R. Precise and Ultrafast Molecular Sieving through Graphene Oxide Membranes. Science 2014, 343, 752–754. [Google Scholar] [CrossRef]
- Liu, G.; Jin, N.X. Graphene-Based Membranes. Chem. Soc. Rev. 2015, 44, 5016–5030. [Google Scholar] [CrossRef] [PubMed]
- Thebo, K.H.; Qian, X.; Zhang, Q.; Chen, L.; Cheng, H.M.; Ren, W. Highly Stable Graphene-Oxide-Based Membranes with Superior Permeability. Nat. Commun. 2018, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Ibrar, I.; Altaee, A.; Samal, A.K.; Karbassiyazdi, E.; Zhou, J.; Bartocci, P. High-Performance Mild Annealed CNT/GO-PVA Composite Membrane for Brackish Water Treatment. Sep. Purif. Technol. 2022, 285, 120361. [Google Scholar] [CrossRef]
- Fang, Q.; Zhou, X.; Deng, W.; Zheng, Z.; Liu, Z. Freestanding Bacterial Cellulose-Graphene Oxide Composite Membranes with High Mechanical Strength for Selective Ion Permeation. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The Rise of Graphene. In Nanoscience and Technology: A Collection of Reviews from Nature Journals; World Scientific: Bingley, UK, 2010; pp. 11–19. [Google Scholar]
- Raza, A.; Hassan, J.Z.; Mahmood, A.; Nabgan, W.; Ikram, M. Recent Advances in Membrane-Enabled Water Desalination by 2D Frameworks: Graphene and Beyond. Desalination 2022, 531, 115684. [Google Scholar] [CrossRef]
- Bhol, P.; Yadav, S.; Altaee, A.; Saxena, M.; Misra, P.K.; Samal, A.K. Graphene-Based Membranes for Water and Wastewater Treatment: A Review. ACS Appl. Nano Mater. 2021, 4, 3274–3293. [Google Scholar] [CrossRef]
- Azrague, K.; Aimar, P.; Benoit-Marquié, F.; Maurette, M.T. A New Combination of a Membrane and a Photocatalytic Reactor for the Depollution of Turbid Water. Appl. Catal. B Environ. 2007, 72, 197–204. [Google Scholar] [CrossRef]
- Lee, A.; Elam, J.W.; Darling, S.B. Membrane Materials for Water Purification: Design, Development, and Application. Environ. Sci. Water Res. Technol. 2016, 2, 17–42. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.E.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–670. [Google Scholar] [CrossRef]
- Seo, D.H.; Pineda, S.; Woo, Y.C.; Xie, M.; Murdock, A.T.; Ang, E.Y.M.; Jiao, Y.; Park, M.J.; Lim, S., II; Lawn, M.; et al. Anti-Fouling Graphene-Based Membranes for Effective Water Desalination. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Kumar, D.; Raghavan, C.M.; Sridhar, C.; Shin, J.H.; Ryu, S.H.; Jang, K.; Shin, D.S. Microwave-Assisted Synthesis, Characterization of Reduced Graphene Oxide, and Its Antibacterial Activity. Bull. Korean Chem. Soc. 2015, 36, 2034–2038. [Google Scholar] [CrossRef]
- Hu, W.; Peng, C.; Luo, W.; Lv, M.; Li, X.; Li, D.; Huang, Q.; Fan, C. Graphene-Based Antibacterial Paper. ACS Nano 2010, 4, 4317–4323. [Google Scholar] [CrossRef]
- Vecitis, C.D.; Zodrow, K.R.; Kang, S.; Elimelech, M. Electronic-Structure-Dependent Bacterial Cytotoxicity of Single-Walled Carbon Nanotubes. ACS Nano 2010, 4, 5471–5479. [Google Scholar] [CrossRef]
- Hong, S.; Constans, C.; Vinicius, M.; Martins, S.; Seow, Y.C. Scalable Graphene—Based Membrane for Ionic Sieving with Ultrahigh Charge Selectivity. Nano Lett. 2017, 17, 728–732. [Google Scholar] [CrossRef]
- Hung, W.S.; Lin, T.J.; Chiao, Y.H.; Sengupta, A.; Hsiao, Y.C.; Wickramasinghe, S.R.; Hu, C.C.; Lee, K.R.; Lai, J.Y. Graphene-Induced Tuning of the d-Spacing of Graphene Oxide Composite Nanofiltration Membranes for Frictionless Capillary Action-Induced Enhancement of Water Permeability. J. Mater. Chem. A 2018, 6, 19445–19454. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, D.; Hu, X.; Qian, Y.; Li, D. Preparation of TiO2/Carbon Nanotubes/Reduced Graphene Oxide Composites with Enhanced Photocatalytic Activity for the Degradation of Rhodamine B. Nanomaterials 2018, 8, 431. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Sanchez, V.C.; Jachak, A.; Hurt, R.H.; Kane, A.B. Biological Interactions of Graphene-Family Nanomaterials; ACS Publications: Washington, DC, USA, 2012; Volume 25, ISBN 4018639120. [Google Scholar]
- Baker, R.W. Membrane Technology and Applications; Wiley: New York, NY, USA, 2012; ISBN 9780470743720. [Google Scholar]
- Hu, Q.; Marand, E.; Dhingra, S.; Fritsch, D.; Wen, J.; Wilkes, G. Poly(Amide-Imide)/TiO2 Nano-Composite Gas Separation Membranes: Fabrication and Characterization. J. Membr. Sci. 1997, 135, 65–79. [Google Scholar] [CrossRef]
- Wang, C.; Cao, M.; Wang, P.; Ao, Y.; Hou, J.; Qian, J. Preparation of Graphene-Carbon Nanotube-TiO2 Composites with Enhanced Photocatalytic Activity for the Removal of Dye and Cr (VI). Appl. Catal. A Gen. 2014, 473, 83–89. [Google Scholar] [CrossRef]
- Oulton, R.; Haase, J.P.; Kaalberg, S.; Redmond, C.T.; Nalbandian, M.J.; Cwiertny, D.M. Hydroxyl Radical Formation during Ozonation of Multiwalled Carbon Nanotubes: Performance Optimization and Demonstration of a Reactive CNT Filter. Environ. Sci. Technol. 2015, 49, 3687–3697. [Google Scholar] [CrossRef]
- Barrejón, M.; Prato, M. Carbon Nanotube Membranes in Water Treatment Applications. Adv. Mater. Interfaces 2022, 9, 2101260. [Google Scholar] [CrossRef]
- Aslam, M.M.A.; Kuo, H.W.; Den, W.; Usman, M.; Sultan, M.; Ashraf, H. Functionalized Carbon Nanotubes (Cnts) for Water and Wastewater Treatment: Preparation to Application. Sustainability 2021, 13, 5717. [Google Scholar] [CrossRef]
- Ihsanullah Carbon Nanotube Membranes for Water Purification: Developments, Challenges, and Prospects for the Future. Sep. Purif. Technol. 2019, 209, 307–337. [CrossRef]
- Le Fevre, L.W.; Cao, J.; Kinloch, I.A.; Forsyth, A.J.; Dryfe, R.A.W. Systematic Comparison of Graphene Materials for Supercapacitor Electrodes. ChemistryOpen 2019, 8, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, T.; Panesar, D.K. A Comparison of Graphene Oxide, Reduced Graphene Oxide and Pure Graphene: Early Age Properties of Cement Composites. In Proceedings of the International Conference on Sustainable Materials, Systems and Structures (SMSS 2019) New Generation of Construction Materials, Rovinj, Croatia, 18–22 March 2019; pp. 318–325. [Google Scholar]
- Zhu, B.Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Galashev, A.E.; Rakhmanova, O.R. Mechanical and Thermal Stability of Graphene and Graphene-Based Materials. Phys. Usp. 2014, 57, 970. [Google Scholar] [CrossRef]
- Aradhana, R.; Mohanty, S.; Kumar, S. Comparison of Mechanical, Electrical and Thermal Properties in Graphene Oxide and Reduced Graphene Oxide Fi Lled Epoxy Nanocomposite Adhesives. Polymer 2018, 141, 109–123. [Google Scholar] [CrossRef]
- Bellamkonda, S.; Thangavel, N.; Hafeez, H.Y.; Neppolian, B.; Rao, G.R. Highly Active and Stable Multi-Walled Carbon Nanotubes-Graphene-TiO2 Nanohybrid: An Efficient Non-Noble Metal Photocatalyst for Water Splitting. Catal. Today 2019, 321, 120–127. [Google Scholar] [CrossRef]
- Khan, M.; Tahir, M.N.; Adil, S.F.; Khan, H.U.; Siddiqui, R.H.; Al-wartan, A.A.; Tremel, W. Graphene Based Metal and Metal Oxide Nanocomposites: Synthesis, Properties and Their Applications. J. Mater. Chem. A 2015, 37, 18753–18808. [Google Scholar] [CrossRef]
- Anpo, M. Utilization of TiO2 Photocatalysts in Green Chemistry. Pure Appl. Chem. 2000, 72, 1265–1270. [Google Scholar] [CrossRef]
- Fan, W.; Lai, Q.; Zhang, Q.; Wang, Y. Nanocomposites of TiO2 and Reduced Graphene Oxide as Efficient Photocatalysts for Hydrogen Evolution. J. Phys. Chem. C 2011, 115, 10694–10701. [Google Scholar] [CrossRef]
- Athanasekou, C.P.; Morales-Torres, S.; Likodimos, V.; Romanos, G.E.; Pastrana-Martinez, L.M.; Falaras, P.; Faria, J.L.; Figueiredo, J.L.; Silva, A.M.T. Prototype Composite Membranes of Partially Reduced Graphene Oxide/TiO2 for Photocatalytic Ultrafiltration Water Treatment under Visible Light. Appl. Catal. B Environ. 2014, 158–159, 361–372. [Google Scholar] [CrossRef]
- Jiang, G.; Lin, Z.; Chen, C.; Zhu, L.; Chang, Q.; Wang, N.; Wei, W.; Tang, H. TiO2 Nanoparticles Assembled on Graphene Oxide Nanosheets with High Photocatalytic Activity for Removal of Pollutants. Carbon 2011, 49, 2693–2701. [Google Scholar] [CrossRef]
- Gavalas, L.S. Filtering Apparatus and Method of Use. U.S. Patent 7967747B2, 28 June 2011. [Google Scholar]
- Fung, K.; Li, Y.; Fan, S.; Fajrial, A.K.; Ding, Y.; Ding, X. Acoustically Excited Microstructure for On-Demand Fouling Mitigation in a Microfluidic Membrane Filtration Device. J. Membr. Sci. Lett. 2022, 2, 100012. [Google Scholar] [CrossRef]
- NASA’s Johnson Space Center. Filtering Water with Acoustics Nanotube Technology. Available online: https://www.nasa.gov/centers/johnson/pdf/579087main_MSC-24180-1_Water-Filtering-Device.pdf (accessed on 25 October 2022).
- Lamminen, M.O.; Walker, H.W.; Weavers, L.K. Cleaning of Particle-Fouled Membranes during Cross-Flow Filtration Using an Embedded Ultrasonic Transducer System. J. Membr. Sci. 2006, 283, 225–232. [Google Scholar] [CrossRef]
- Qasim, M.; Darwish, N.N.; Mhiyo, S.; Darwish, N.A.; Hilal, N. The Use of Ultrasound to Mitigate Membrane Fouling in Desalination and Water Treatment. Desalination 2018, 443, 143–164. [Google Scholar] [CrossRef]
- Muthukumaran, S.; Kentish, S.E.; Stevens, G.W.; Ashokkumar, M. Application of Ultrasound in Membrane Separation Processes: A Review. Rev. Chem. Eng. 2006, 22, 155–194. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Lombardo, A.; Hasan, T.; Sun, Z.; Colombo, L.; Ferrari, A.C. Production and Processing of Graphene and 2d Crystals. Mater. Today 2012, 15, 63–78. [Google Scholar] [CrossRef]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.H.; Kim, P.; Choi, J.Y.; Hong, B.H. Large-Scale Pattern Growth of Graphene Films for Stretchable Transparent Electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef]
- Reina, A.; Thiele, S.; Jia, X.; Bhaviripudi, S.; Dresselhaus, M.S.; Schaefer, J.A.; Kong, J. Growth of Large-Area Single- and Bi-Layer Graphene by Controlled Carbon Precipitation on Polycrystalline Ni Surfaces. Nano Res. 2009, 2, 509–516. [Google Scholar] [CrossRef]
- Dai, B.; Fu, L.; Zou, Z.; Wang, M.; Xu, H.; Wang, S.; Liu, Z. Rational Design of a Binary Metal Alloy for Chemical Vapour Deposition Growth of Uniform Single-Layer Graphene. Nat. Commun. 2011, 2, 522. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D. Large-Area Synthesis of High-Quality and Uniform Graphene Films on Copper Foils. Mech. Eng. 2009, 2009, 1312–1314. [Google Scholar] [CrossRef] [PubMed]
- Sutter, P.W.; Flege, J.I.; Sutter, E.A. Epitaxial Graphene on Ruthenium. Nat. Mater. 2008, 7, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Berger, C.; Song, Z.; Li, X.; Wu, X.; Brown, N.; Naud, C.; Mayou, D.; Li, T.; Hass, J.; Marchenkov, A.N.; et al. Electronic Confinement and Coherence in Patterned Epitaxial Graphene. Science 2006, 312, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Emtsev, K.V.; Bostwick, A.; Horn, K.; Jobst, J.; Kellogg, G.L.; Ley, L.; McChesney, J.L.; Ohta, T.; Reshanov, S.A.; Röhrl, J.; et al. Towards Wafer-Size Graphene Layers by Atmospheric Pressure Graphitization of Silicon Carbide. Nat. Mater. 2009, 8, 203–207. [Google Scholar] [CrossRef]
- Gumfekar, S.P. Graphene-Based Materials for Clean Energy Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128137314. [Google Scholar]
- Lou, Y.; Liu, G.; Liu, S.; Shen, J.; Jin, W. A Facile Way to Prepare Ceramic-Supported Graphene Oxide Composite Membrane via Silane-Graft Modification. Appl. Surf. Sci. 2014, 307, 631–637. [Google Scholar] [CrossRef]
- Niyogi, S.; Bekyarova, E.; Itkis, M.E.; McWilliams, J.L.; Hamon, M.A.; Haddon, R.C. Solution Properties of Graphite and Graphene. J. Am. Chem. Soc. 2006, 128, 7720–7721. [Google Scholar] [CrossRef]
- Wang, X.; Zhi, L.; Müllen, K. Transparent, Conductive Graphene Electrodes for Dye-Sensitized Solar Cells. Nano Lett. 2008, 8, 323–327. [Google Scholar] [CrossRef]
- Dai, Y.; Liu, M.; Li, J.; Kang, N.; Ahmed, A.; Zong, Y.; Tu, J.; Chen, Y.; Zhang, P.; Liu, X. Graphene-Based Membranes for Water Desalination: A Literature Review and Content Analysis. Polymers 2022, 14, 4246. [Google Scholar] [CrossRef]
- Seekaew, Y.; Arayawut, O.; Timsorn, K.; Wongchoosuk, C. Synthesis, Characterization, and Applications of Graphene and Derivatives; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128132487. [Google Scholar]
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Dresselhaus, M.S.; Jing, K. Large Area, Few-Layer Graphene Films on Arbitrary Substrates by Chemical Vapor Deposition. Nano Lett. 2009, 9, 30–35. [Google Scholar] [CrossRef]
- Umapathi, R.; Venkateswara Raju, C.; Majid Ghoreishian, S.; Mohana Rani, G.; Kumar, K.; Oh, M.H.; Pil Park, J.; Suk Huh, Y. Recent Advances in the Use of Graphitic Carbon Nitride-Based Composites for the Electrochemical Detection of Hazardous Contaminants. Coord. Chem. Rev. 2022, 470, 214708. [Google Scholar] [CrossRef]
- Popov, V.N. Carbon Nanotubes: Properties and Application. Mater. Sci. Eng. R Rep. 2004, 43, 61–102. [Google Scholar] [CrossRef]
- Ma, H.; Wang, G.; Xu, Z.; Dong, X.; Zhang, X. Confining Peroxymonosulfate Activation in Carbon Nanotube Intercalated Nitrogen Doped Reduced Graphene Oxide Membrane for Enhanced Water Treatment: The Role of Nanoconfinement Effect. J. Colloid Interface Sci. 2022, 608, 2740–2751. [Google Scholar] [CrossRef] [PubMed]
- Almeida, N.A.; Martins, P.M.; Teixeira, S.; Lopes da Silva, J.A.; Sencadas, V.; Kühn, K.; Cuniberti, G.; Lanceros-Mendez, S.; Marques, P.A.A.P. TiO2/Graphene Oxide Immobilized in P(VDF-TrFE) Electrospun Membranes with Enhanced Visible-Light-Induced Photocatalytic Performance. J. Mater. Sci. 2016, 51, 6974–6986. [Google Scholar] [CrossRef]
- Mou, C.; Arif, R.; Lobach, A.S.; Khudyakov, D.V.; Spitsina, N.G.; Kazakov, V.A.; Turitsyn, S.; Rozhin, A. Poor Fluorinated Graphene Sheets Carboxymethylcellulose Polymer Composite Mode Locker for Erbium Doped Fiber Laser. Appl. Phys. Lett. 2018, 106, 061106. [Google Scholar] [CrossRef]
- Voicu, S.I.; Thakur, V.K. Graphene-Based Composite Membranes for Nanofiltration: Performances and Future Perspectives. Emergent Mater. 2022, 5, 1429–1441. [Google Scholar] [CrossRef]
- Shao, F.; Xu, C.; Ji, W.; Dong, H.; Sun, Q.; Yu, L.; Dong, L. Layer-by-Layer Self-Assembly TiO2 and Graphene Oxide on Polyamide Reverse Osmosis Membranes with Improved Membrane Durability. Desalination 2017, 423, 21–29. [Google Scholar] [CrossRef]
- Kunimatsu, M.; Nakagawa, K.; Yoshioka, T.; Shintani, T.; Yasui, T.; Kamio, E.; Tsang, S.C.E.; Li, J.; Matsuyama, H. Design of Niobate Nanosheet-Graphene Oxide Composite Nanofiltration Membranes with Improved Permeability. J. Membr. Sci. 2020, 595, 117598. [Google Scholar] [CrossRef]
- Chen, L.; Moon, J.H.; Ma, X.; Zhang, L.; Chen, Q.; Chen, L.; Peng, R.; Si, P.; Feng, J.; Li, Y.; et al. High Performance Graphene Oxide Nanofiltration Membrane Prepared by Electrospraying for Wastewater Purification. Carbon 2018, 130, 487–494. [Google Scholar] [CrossRef]
- Memisoglu, G.; Gulbahar, B.; Zubia, J.; Villatoro, J. Theoretical Modeling of Viscosity Monitoring with Vibrating Resonance Energy Transfer for Point-of-Care and Environmental Monitoring Applications. Micromachines 2018, 10, 3. [Google Scholar] [CrossRef]
- Fan, S.; Yu, Z.; Wang, X.; Tao, Y.; Shao, H.; Long, M.; Guo, X. A Facile Graphene Oxide Modified Approach towards Membrane with Prominent Improved Permeability and Antifouling Performance. Desalination 2023, 545, 116130. [Google Scholar] [CrossRef]
- Liu, T.; Liu, X.; Graham, N.; Yu, W.; Sun, K. Two-Dimensional MXene Incorporated Graphene Oxide Composite Membrane with Enhanced Water Purification Performance. J. Membr. Sci. 2020, 593, 117431. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Visible-Light Active Titanium Dioxide Nanomaterials with Bactericidal Properties. Nanomaterials 2020, 10, 124. [Google Scholar] [CrossRef]
- Athanasekou, C.P.; Moustakas, N.G.; Morales-Torres, S.; Pastrana-Martínez, L.M.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M.T.; Dona-Rodriguez, J.M.; Romanos, G.E.; Falaras, P. Ceramic Photocatalytic Membranes for Water Filtration under UV and Visible Light. Appl. Catal. B Environ. 2015, 178, 12–19. [Google Scholar] [CrossRef]
- Molinari, R.; Lavorato, C.; Argurio, P. Visible-Light Photocatalysts and Their Perspectives for Building Photocatalytic Membrane Reactors for Various Liquid Phase Chemical Conversions. Catalysts 2020, 10, 1334. [Google Scholar] [CrossRef]
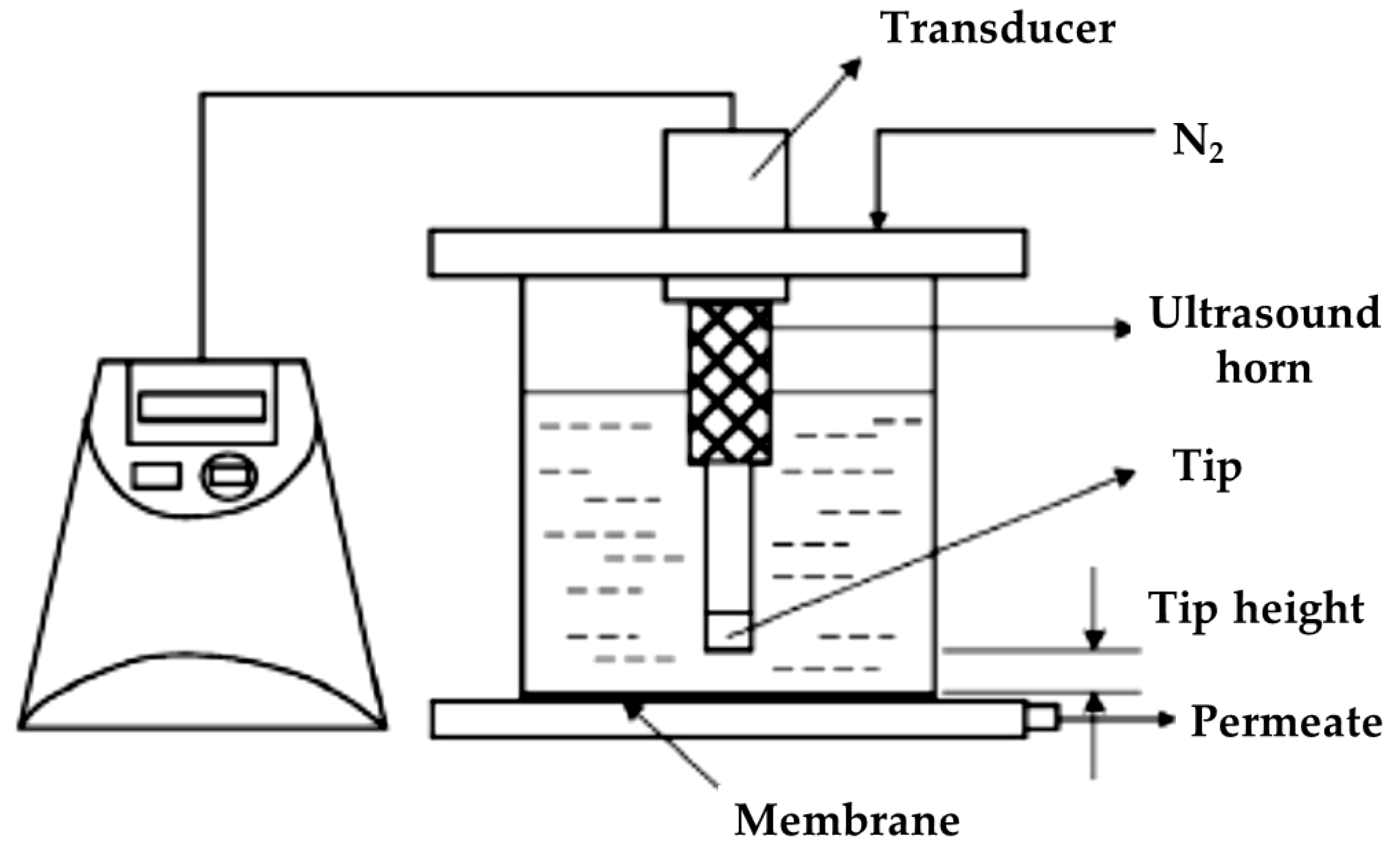
- Zhang, R.; Huang, Y.; Sun, C.; Xiaozhen, L.; Bentian, X.; Wang, Z. Study on Ultrasonic Techniques for Enhancing the Separation Process of Membrane. Ultrason. Sonochem. 2019, 55, 341–347. [Google Scholar] [CrossRef]
- Petrauskas, A. Increasing the Efficiency of Water Well Regeneration with Ultrasound by Using Acoustic Transducers Consisting Ofelements in Flexural Vibration. Ultragarsas 2009, 64, 3. [Google Scholar]
- Memisoglu, G.; Gulbahar, B.; Bello, R.F. Preparation and Characterization of Freely-Suspended Graphene Nanomechanical Membrane Devices with Quantum Dots for Point-of-Care Applications. Micromachines 2020, 11, 104. [Google Scholar] [CrossRef]
- Pettes, M.T.; Shi, L. Thermal and Structural Characterizations of Individual Single-, Double-, and Multi-Walled Carbon Nanotubes. Adv. Funct. Mater. 2009, 19, 3918–3925. [Google Scholar] [CrossRef]
- King, A.A.K.; Davies, B.R.; Noorbehesht, N.; Newman, P.; Church, T.L.; Harris, A.T.; Razal, J.M.; Minett, A.I. A New Raman Metric for the Characterisation of Graphene Oxide and Its Derivatives. Sci. Rep. 2016, 6, 1–6. [Google Scholar] [CrossRef]
- Costa, S.; Borowiak-Palen, E.; Kruszynska, M.; Bachmatiuk, A.; Kalenczuk, R.J. Characterization of Carbon Nanotubes by Raman Spectroscopy. Mater. Sci. Pol. 2008, 26, 433–441. [Google Scholar]
- Salah, N.; Abdel-Wahab, M.S.; Alshahrie, A.; Alharbi, N.D.; Khan, Z.H. Carbon Nanotubes of Oil Fly Ash as Lubricant Additives for Different Base Oils and Their Tribology Performance. RSC Adv. 2017, 7, 40295–40302. [Google Scholar] [CrossRef]
- Osler, K.; Dheda, D.; Ngoy, J.; Wagner, N.; Daramola, M.O. Synthesis and Evaluation of Carbon Nanotubes Composite Adsorbent for CO2 Capture: A Comparative Study of CO2 Adsorption Capacity of Single-Walled and Multi-Walled Carbon Nanotubes. Int. J. Coal Sci. Technol. 2017, 4, 41–49. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman Spectroscopy of Carbon Nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Hilal, N.; Ismail, A.F.; Wright, C. Membrane Fabrication; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2015. [Google Scholar]
- Goh, P.S.; Ismail, A.F. Advances in Nanocomposite Membranes. Membranes 2021, 11, 158. [Google Scholar] [CrossRef]
- Ahn, J.; Chung, W.J.; Pinnau, I.; Guiver, M.D. Polysulfone/Silica Nanoparticle Mixed-Matrix Membranes for Gas Separation. J. Membr. Sci. 2008, 314, 123–133. [Google Scholar] [CrossRef]
- Jyothi, M.S.; Yadav, S.; Balakrishna, G. Effective Recovery of Acids from Egg Waste Incorporated PSf Membranes: A Step towards Sustainable Development. J. Membr. Sci. 2018, 549, 227–235. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marĩas, B.J.; Mayes, A.M. Science and Technology for Water Purification in the Coming Decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef]
- Dehghani, M.; Fadaei, A. Photocatalytic Oxidation of Organophosphorus Pesticides Using Zinc Oxide. Res. J. Chem. Environ. 2012, 16, 104–109. [Google Scholar]
- Sang, Y.; Liu, H.; Umar, A. Photocatalysis from UV/Vis to near-Infrared Light: Towards Full Solar-Light Spectrum Activity. ChemCatChem 2015, 7, 559–573. [Google Scholar] [CrossRef]
- Holmes, I. Sound Cleans up Water Purification. Nature 2002, 1–4. [Google Scholar] [CrossRef]
- Zou, D.; Mao, H.; Zhong, Z. Construction Strategies of Self-Cleaning Ceramic Composite Membranes for Water Treatment. Ceram. Int. 2022, 48, 7362–7373. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kobayashi, T.; Hosaka, Y.; Fujii, N. Ultrasound-Enhanced Membrane-Cleaning Processes Applied Water Treatments: Influence of Sonic Frequency on Filtration Treatments. Ultrasonics 2003, 41, 185–190. [Google Scholar] [CrossRef]
- Rout, D.R.; Jena, H.M.; Baigenzhenov, O.; Hosseini-Bandegharaei, A. Graphene-Based Materials for Effective Adsorption of Organic and Inorganic Pollutants: A Critical and Comprehensive Review. Sci. Total Environ. 2023, 863, 160871. [Google Scholar] [CrossRef]
- Zhao, R.; Li, Y.; Ji, J.; Wang, Q.; Li, G.; Wu, T.; Zhang, B. Efficient Removal of Phenol and P-Nitrophenol Using Nitrogen-Doped Reduced Graphene Oxide. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125866. [Google Scholar] [CrossRef]
- Karamipour, M.; Fathi, S.; Safari, M. Removal of Phenol from Aqueous Solution Using MOF/GO: Synthesis, Characteristic, Adsorption Performance and Mechanism. Int. J. Environ. Anal. Chem. 2021, 1–12. [Google Scholar] [CrossRef]
- Ezugbe, E.O.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Bai, X.; Zhai, Y.; Zhang, Y. Green Approach to Prepare Graphene-Based Composites with High Microwave Absorption Capacity. J. Phys. Chem. C 2011, 115, 11673–11677. [Google Scholar] [CrossRef]
- Grassi, M.; Kaykioglu, G.; Belgiorno, V.; Lofrano, G. Green Chemistry for Sustainability: Ultrasound Technology in Green Chemistry. Springer: Dordrecht, The Netherlands, 2012; ISBN 978-94-007-2408-2. [Google Scholar]
- Shaheen, S.; Saeed, Z.; Ahmad, A.; Pervaiz, M.; Younas, U.; Mahmood Khan, R.R.; Luque, R.; Rajendran, S. Green Synthesis of Graphene-Based Metal Nanocomposite for Electro and Photocatalytic Activity; Recent Advancement and Future Prospective. Chemosphere 2023, 311, 136982. [Google Scholar] [CrossRef]
- Macedonio, F.; Drioli, E. Membrane Engineering for Green Process Engineering. Engineering 2017, 3, 290–298. [Google Scholar] [CrossRef]
- Alatzoglou, C.; Patila, M.; Giannakopoulou, A.; Spyrou, K.; Yan, F.; Li, W.; Chalmpes, N.; Polydera, A.C.; Rudolf, P.; Gournis, D.; et al. Development of a Multi-Enzymatic Biocatalytic System through Immobilization on High Quality Few-Layer Bio-Graphene. Nanomaterials 2023, 13, 127. [Google Scholar] [CrossRef] [PubMed]
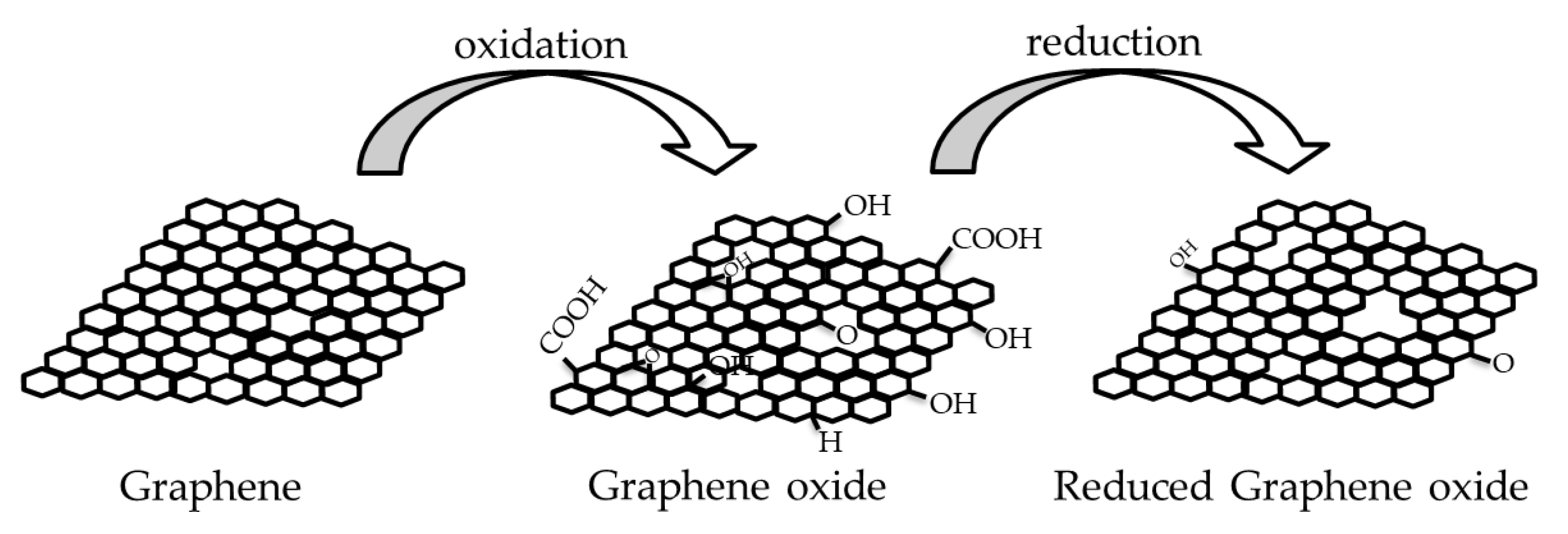

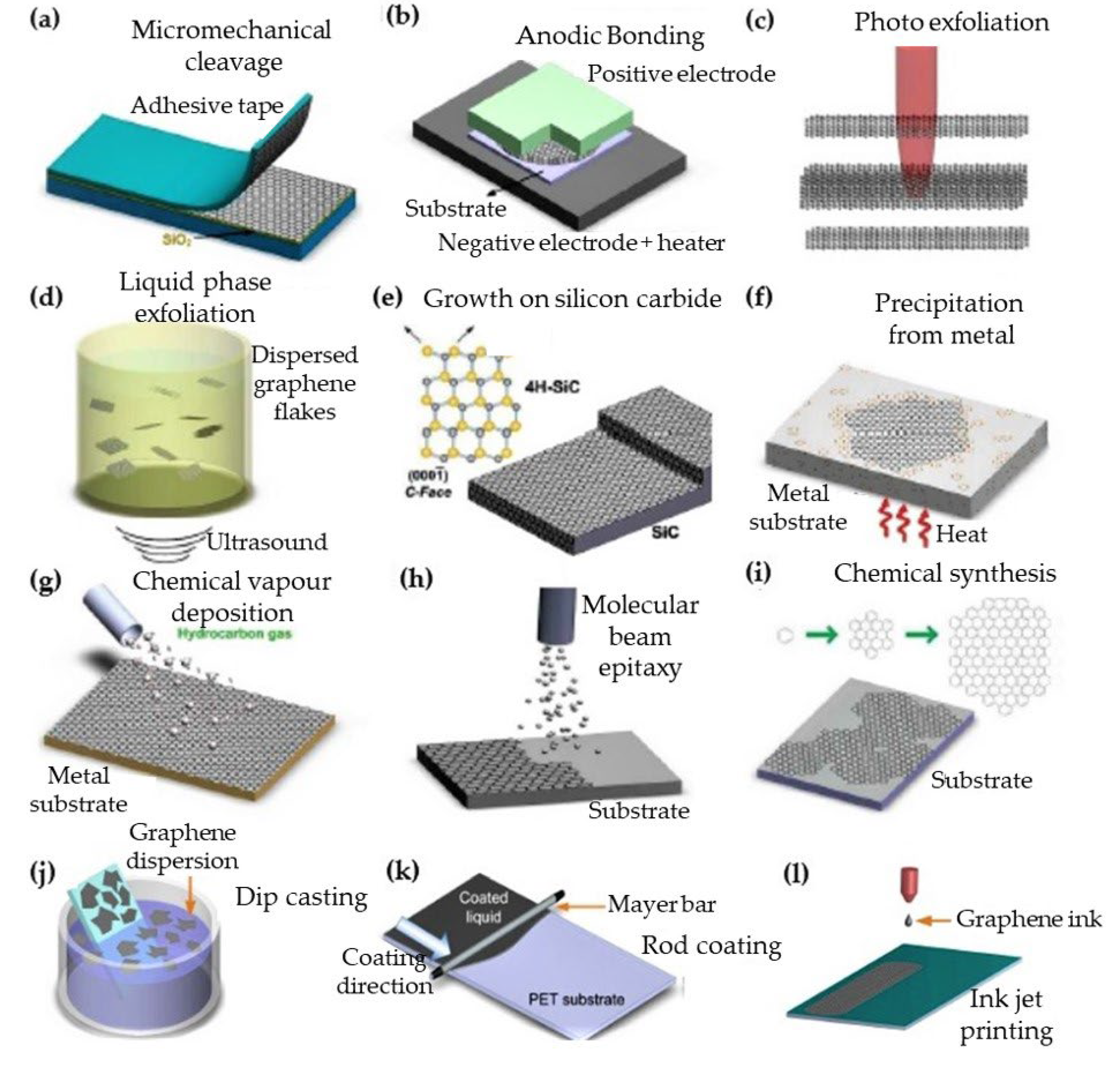

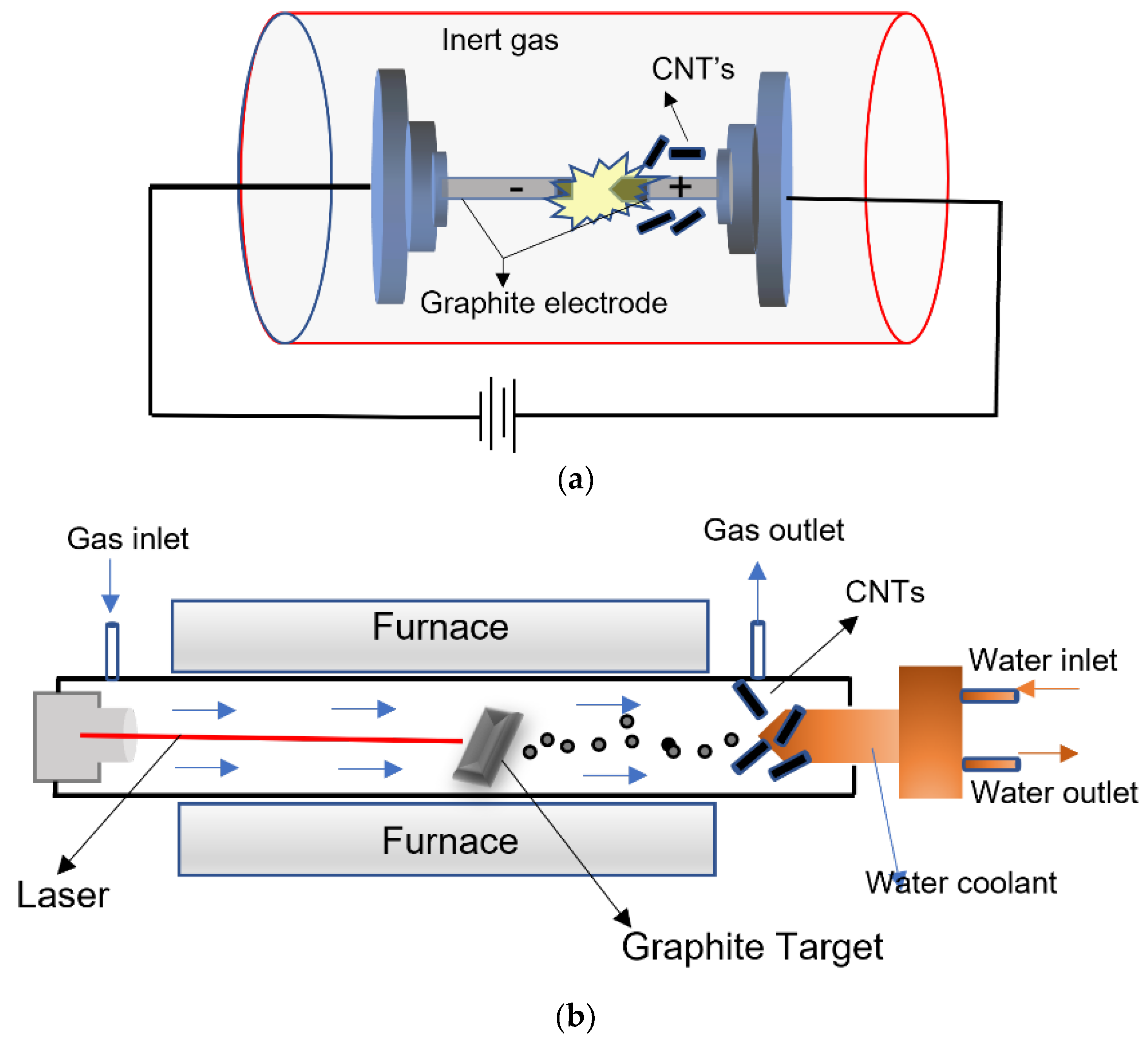

| Key Features/ Advantages | Graphene | CNTs | |||
|---|---|---|---|---|---|
| Exfoliated Graphene | Graphene Oxide | Reduced Graphene Oxide | Single Wall | Multi Wall | |
| Chemical structure | 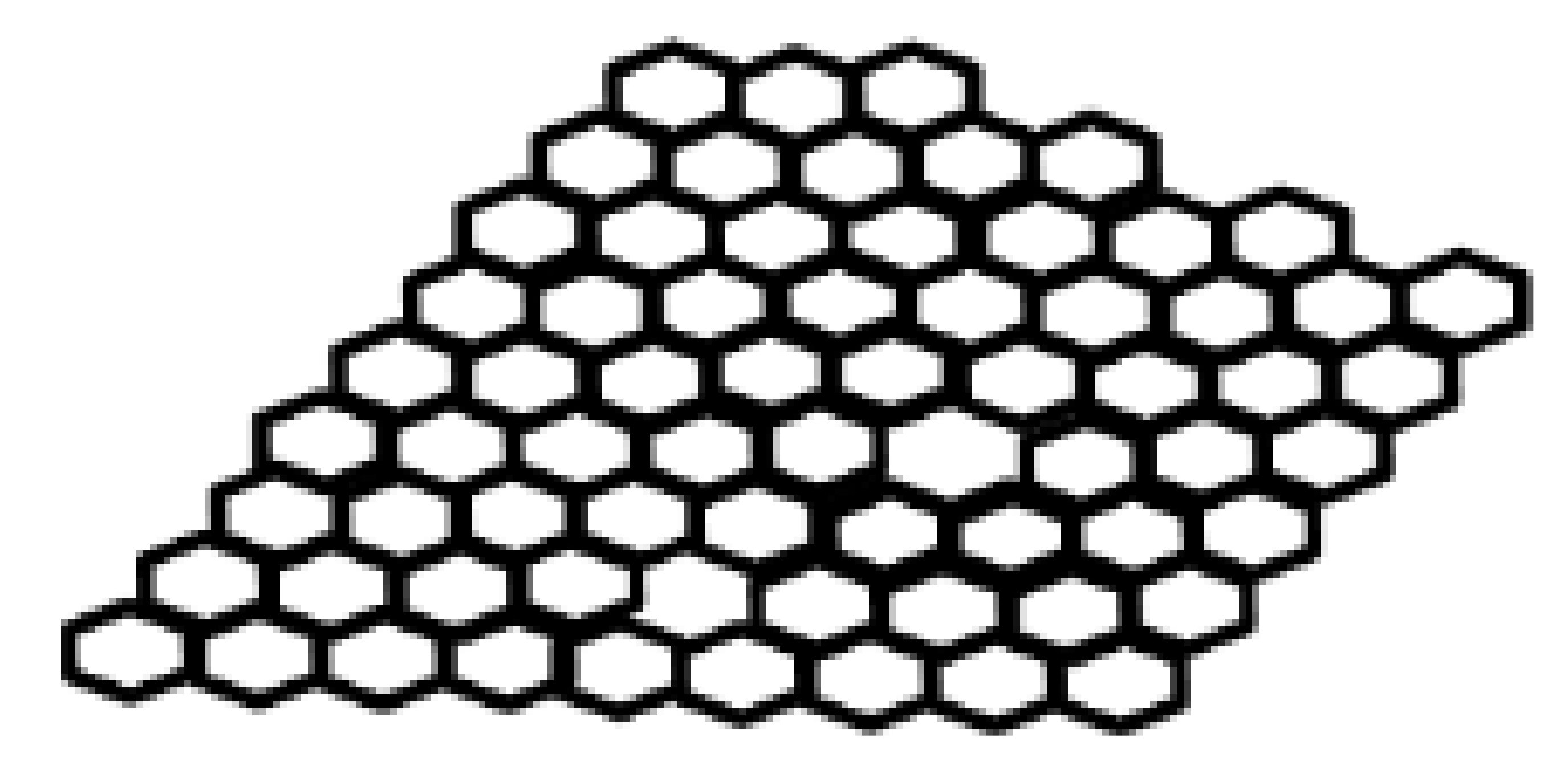 | 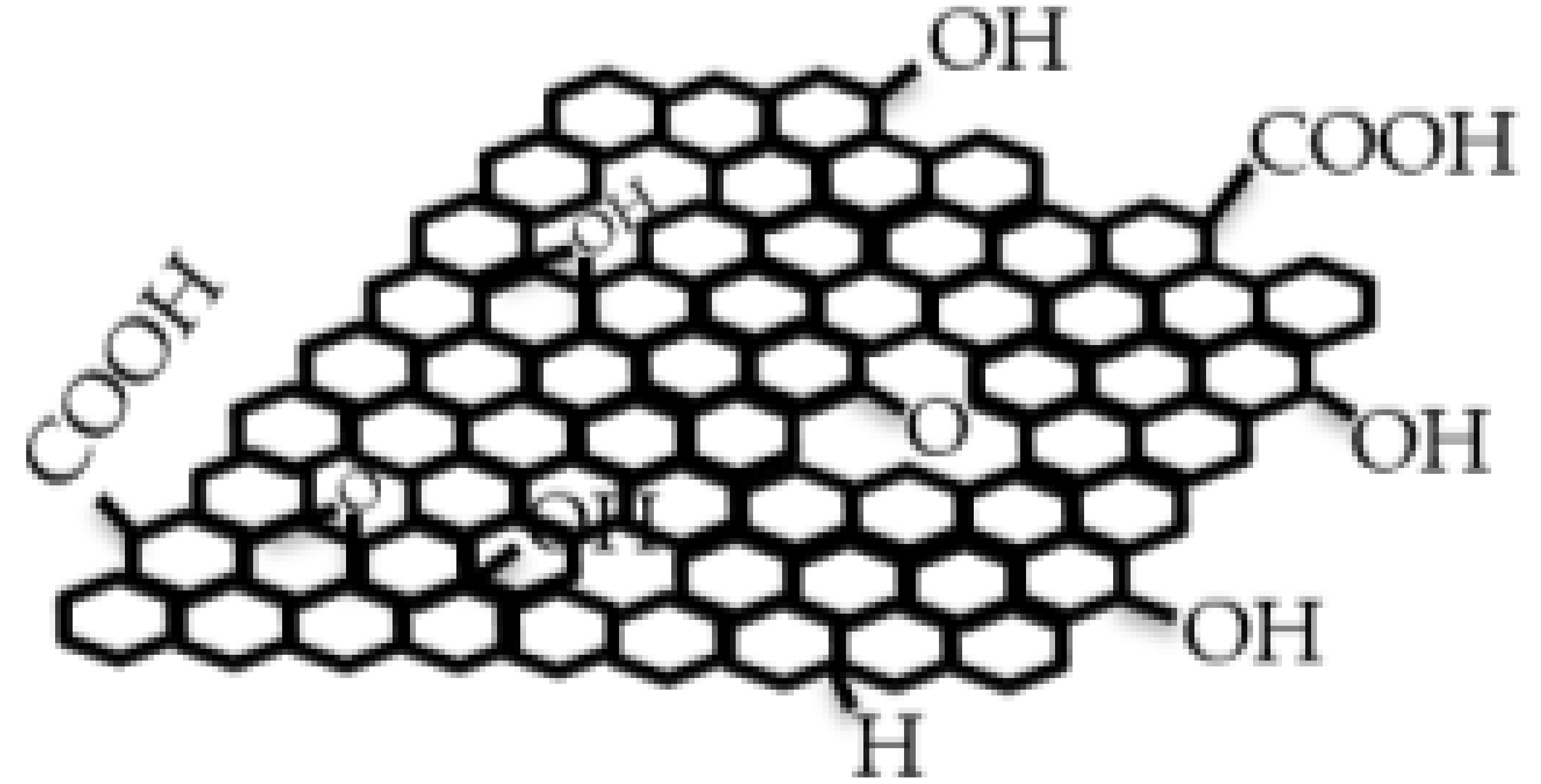 |  | 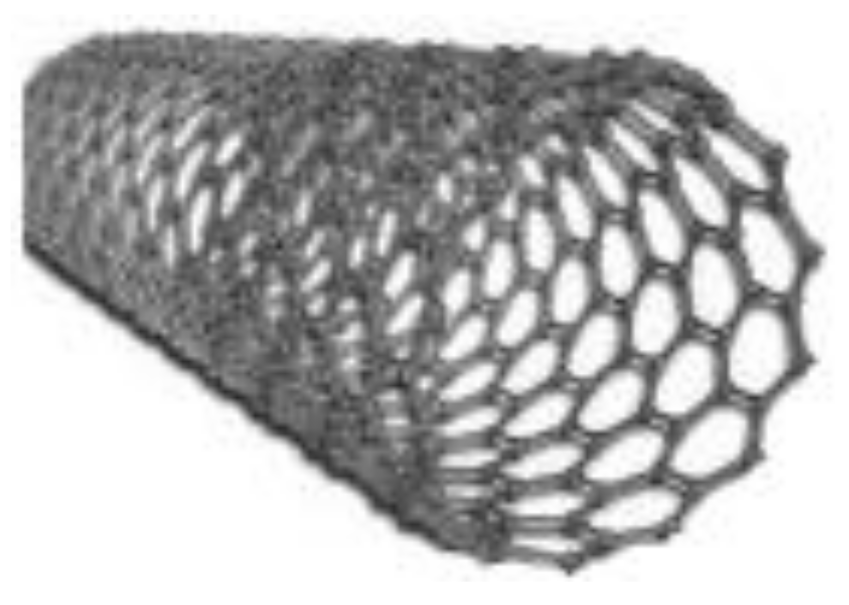 |  |
| Elastic modulus (TPa) | ~1 | >1 | >1 | ~1.4 | 0.3–1 |
| Specific surface area (m2/g) | 341–392 | 759 ± 198 | 669 ± 113 | 400–900 | 200–400 |
| Thermal stability in air (°C) | 600–800 | 600–800 | 600–800 | 600–800 | 600–800 |
| Characterization | Easy | Easy | Easy | Easy | Difficult |
| Bulk or massive production | Relatively difficult | Easy | Easy | Difficult | Easy |
| Ref. | Membrane Composition (and Treatment Type) | Membrane Preparation Method/ Physical Properties | Key Features/Advantages |
|---|---|---|---|
| [11] | Graphene (nanofiltration) | Vacuum filtration | 21.8 L m−2 h−1 bar−1 water permeability, high organic dyes and ion salts retention (>99%). Retention rate: 20–60% |
| [12] | Graphene + polymer (nanofiltration) | Casting Thickness: 95.21 μm Pore size: 0.0267 µm | High metal contaminant (iron) rejection (95.77%) |
| [14] | Graphene oxide + metal oxide (nanofiltration) | Heterogenous nucleation and diffusion-controlled growth process | 225 L m−2 h−1 bar−1 water permeability, and up to 98% selectivity in the size-exclusion separation of methyl blue |
| [15] | Graphene oxide + CNTs + (PVDF; polyvinylidene fluoride) (ultrafiltration and fouling detection) | Phase inversion | High water flux of 125.6 L m−2 h−1 Improved surface pore structure and surface roughness, hydrophilicity, and antifouling property as compared with that of pristine PVDF membranes |
| [17] | Graphene + polymer on glass fiber (ultrafiltration) | Dip-coating (commercial glass fibre membrane was soaked in the mixture of Graphene (prepared by liquid phase exfoliation) and soluble polymer binder solution) | Improved selectivity (by ×103 compared to the neat glass fibre membrane) |
| [18] | Graphene + polymer (purification) | Vacuum filtration | Ultrafast water permeability while remaining high rejections |
| [20] | Cellulose ester/ graphene oxide on cellulose ester support (filtration) | Pumping/casting and hot pressed | 21.34 L m−2 h−1 bar−1 water permeability, with 96.08% salt rejection rate, 35.8% energy-saving in the membrane filtration process |
| [21] | Graphene + polymer (purification) | Vacuum filtration | 68.21 L m−2 h−1 bar−1 water permeability, high rejection (over 97%) for dyes (like methylene blue, Congo red) |
| [23] | Reduced graphene oxide (purification) | Vacuum filtration Thickness: 0.02–0.200 μm, Diameter: 4 cm | Freestanding ultrathin graphene-based membranes |
| [29] | Graphene + polymer + graphene oxide on cellulose nitrate support (filtration) | Vacuum filtration Pore size: 8 µm | Better dispersion of graphene and graphene oxide (thanks to the polymer), greater bacteria cell damage. |
| [35] | CNTs/PTFE on poly-ethylene grid support (distillation) | Vacuum filtration Pore size: 0.2 μm | 12 kg m−2 h−1 water permeability (flux rate) and 99.9% salt rejection |
| [36] | Graphene oxide on nylon substrate (nanofiltration) | Dr-Blade (5 × 5 cm2), Gravure printing (13 × 14 cm2), and Vacuum filtration Thickness range: 0.15 ± 15 µm Substrate pore size: 0.2 μm | 71 L m−2 h−1 bar−1 water permeability, high rejection (over 95%) for various dyes |
| [37] | Graphene + CNTs | Electrophoretic deposition and chemical reduction | Improved water flux, high rejection (~94.0%) |
| [38] | Graphene + CNTs + graphene oxide | Vacuum filtration Thickness: 1.23 µm | 52.7 L m−2 h−1 bar−1 water permeability, high rejection (over 98%) for dyes (such as methylene blue) |
| [39] | Graphene + CNT on polymer (PTFE; polytetrafluoroethylene) (purification) | Vacuum filtration Thickness: 15–20 µm Pore size: 5 µm | 0.010 mol h−1 m−2 oxidation rate with 88% tetracycline removal |
| [40] | Graphene oxide (purification and molecular separation) | Rod-coating | 60.0 kg m−2 h−1 water permeability and a high separation efficiency (~96.0%) for a sodium sulfate |
| [42] | Graphene oxide (filtration) | Vacuum filtration Thickness: 1 μm Pore size: 0.2 μm | 0.2 L m−2 h−1 bar−1 water permeability |
| [44] | Graphene oxide (purification) | Vacuum filtration | 10,000 L m−2 h−1 bar−1 water permeability, high rejection (~100%) for dyes (like methylene blue, rhodamine B) |
| [45] | Graphene + Polymer (PVA; polyvinyl alcohol)/ CNT on cellulose ester support (water treatment) | Vacuum filtration Pore size: 0.22 µm | Nanocomposite improved the separation performance (94.2% sodium sulphate and 85.86% sodium chloride rejections with high permeate rate (14.2–13.45 L m−2 h−1 at 5 bar)) |
| [46] | Graphene oxide: bacterial cellulose (molecular separation) | Vacuum filtration | Freestanding graphene-based membranes |
| [53] | Graphene/PTFE (desalination) | Ambient-air CVD and wet-transfer | 99.9% salt rejection, antifouling, long-term flux stable membranes |
| [57] | Graphene oxide/ silicon nitride/silicone (ionic sieving) | Thickness: 3 µm 200 × 200 nm2 membrane | ~10−4 mol cm−2 h−1 ion permeation rate, 96% ion selectivity |
| [108] | Graphene oxide/ niobate nanosheet (nanofiltration) | Vacuum filtration 7.07 × 10−4 m2 membrane | 20 L m−2 h−1 bar−1 water permeability |
| [109] | Graphene oxide/ nylon microfiltration membrane (nanofiltration) | Electro spraying 100 mm diameter membrane | 11.13–20.23 L m−2 h−1 bar−1 water permeability, more than 98.88% organic dye rejection |
| [111] | Graphene oxide + silicon dioxide: PTFE | Layer by layer self-assembly, Dip-coating (commercial PTFE immersion/soaking in solution) | 560.2 L m−2 h−1 water flux 50% fouling inhibition |
| [112] | Graphene oxide/ MXene on mixed cellulose ester | Vacuum filtration Thickness: 550 nm | 71.9 L m−2 h−1 bar−1 water permeability High dye rejection (100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Memisoglu, G.; Murugesan, R.C.; Zubia, J.; Rozhin, A.G. Graphene Nanocomposite Membranes: Fabrication and Water Treatment Applications. Membranes 2023, 13, 145. https://doi.org/10.3390/membranes13020145
Memisoglu G, Murugesan RC, Zubia J, Rozhin AG. Graphene Nanocomposite Membranes: Fabrication and Water Treatment Applications. Membranes. 2023; 13(2):145. https://doi.org/10.3390/membranes13020145
Chicago/Turabian StyleMemisoglu, Gorkem, Raghavan Chinnambedu Murugesan, Joseba Zubia, and Aleksey G. Rozhin. 2023. "Graphene Nanocomposite Membranes: Fabrication and Water Treatment Applications" Membranes 13, no. 2: 145. https://doi.org/10.3390/membranes13020145
APA StyleMemisoglu, G., Murugesan, R. C., Zubia, J., & Rozhin, A. G. (2023). Graphene Nanocomposite Membranes: Fabrication and Water Treatment Applications. Membranes, 13(2), 145. https://doi.org/10.3390/membranes13020145







