Surface-Modified Pore-Filled Anion-Exchange Membranes for Efficient Energy Harvesting via Reverse Electrodialysis
Abstract
:1. Introduction
2. Materials and Methods
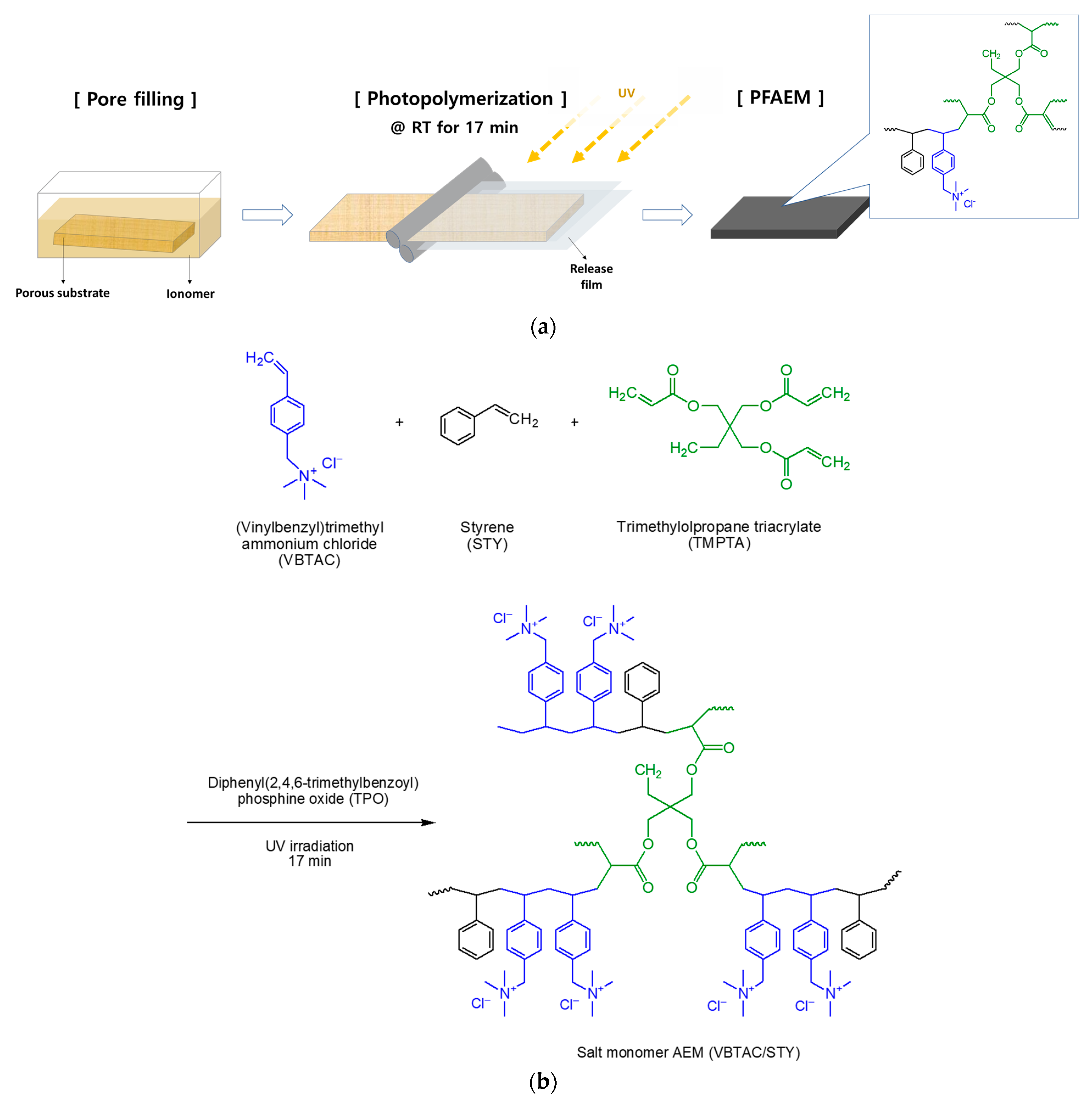
2.1. Preparation of the PFAEM
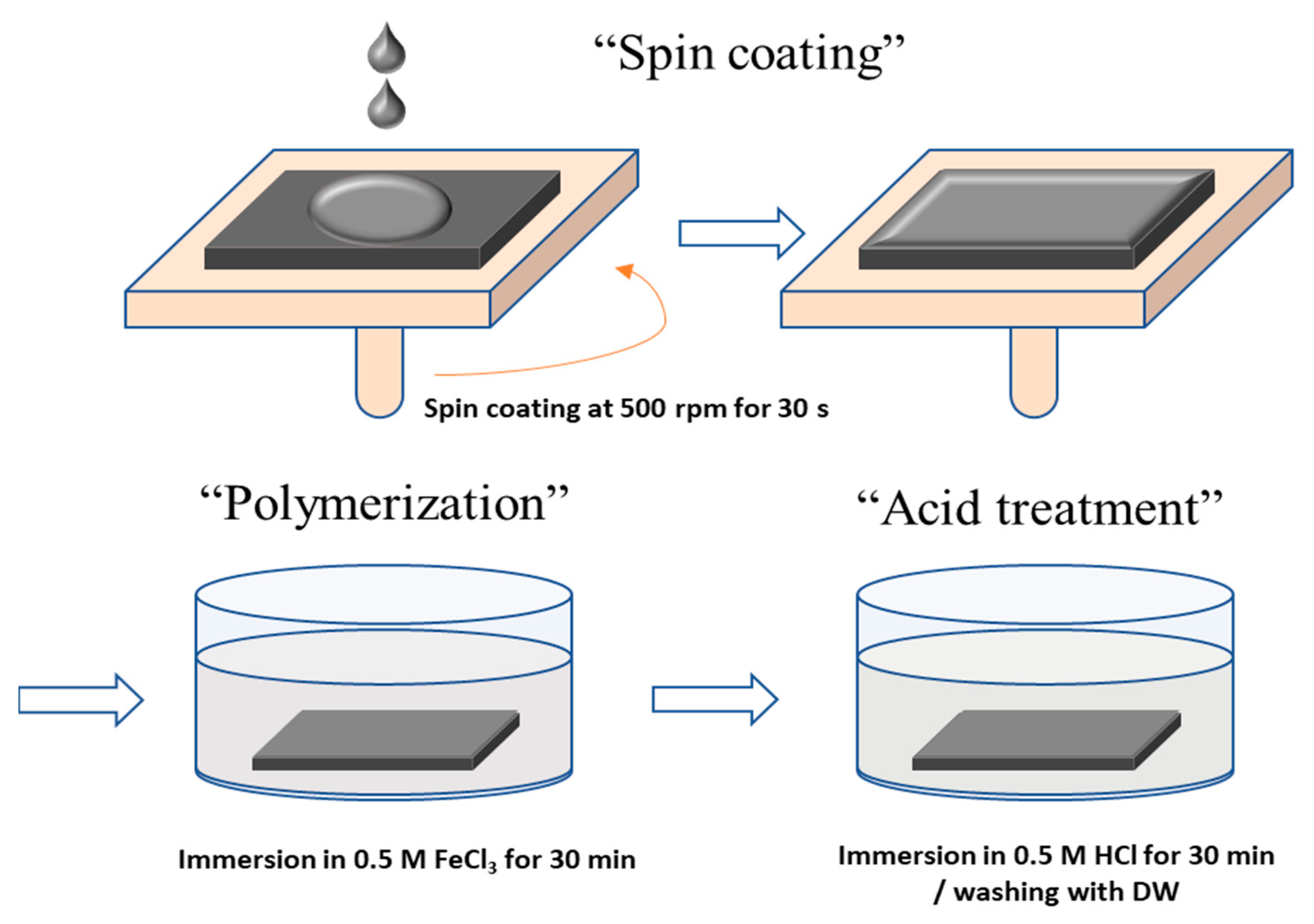
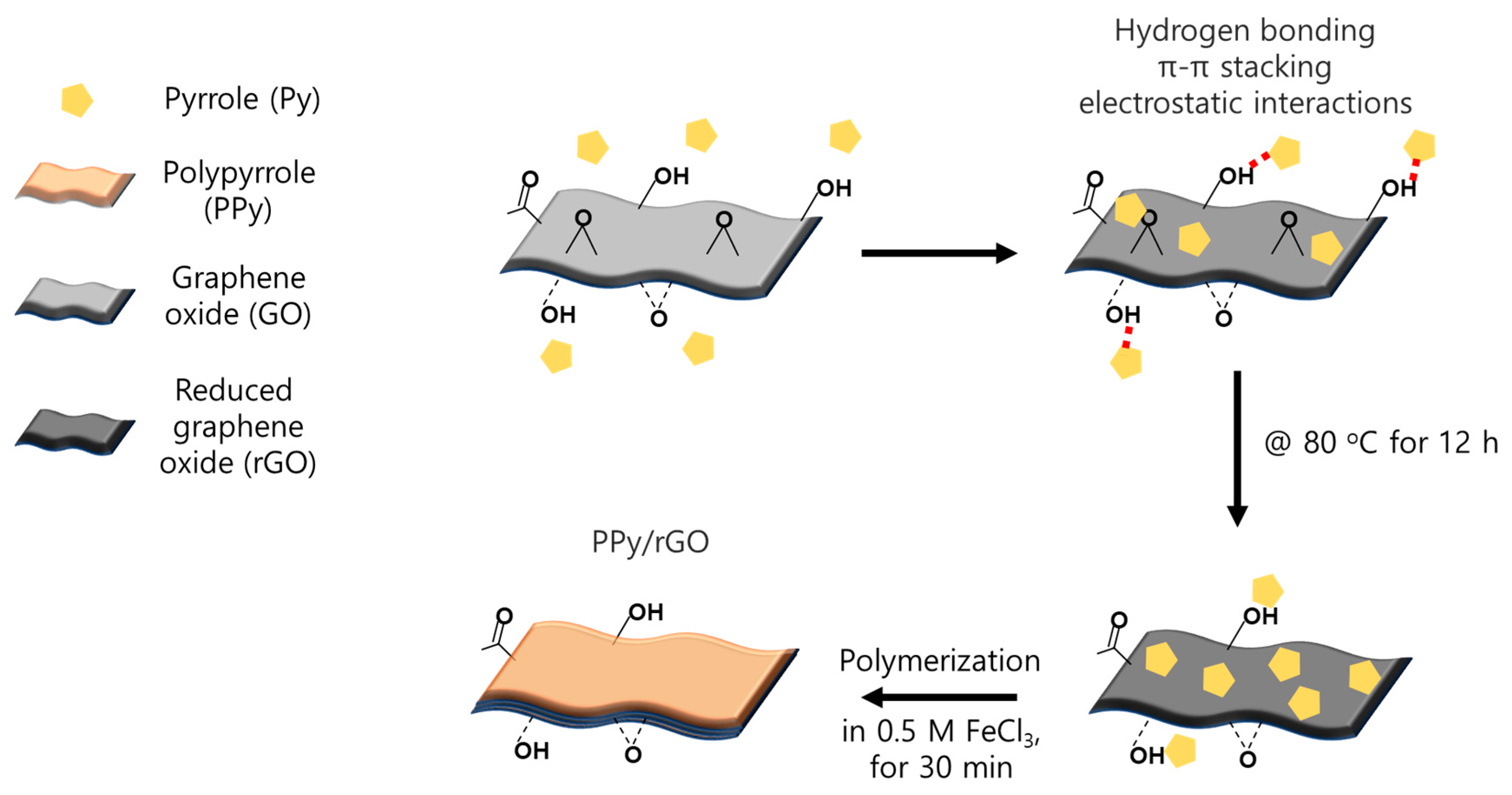
2.2. Surface Modifications of the PFAEM
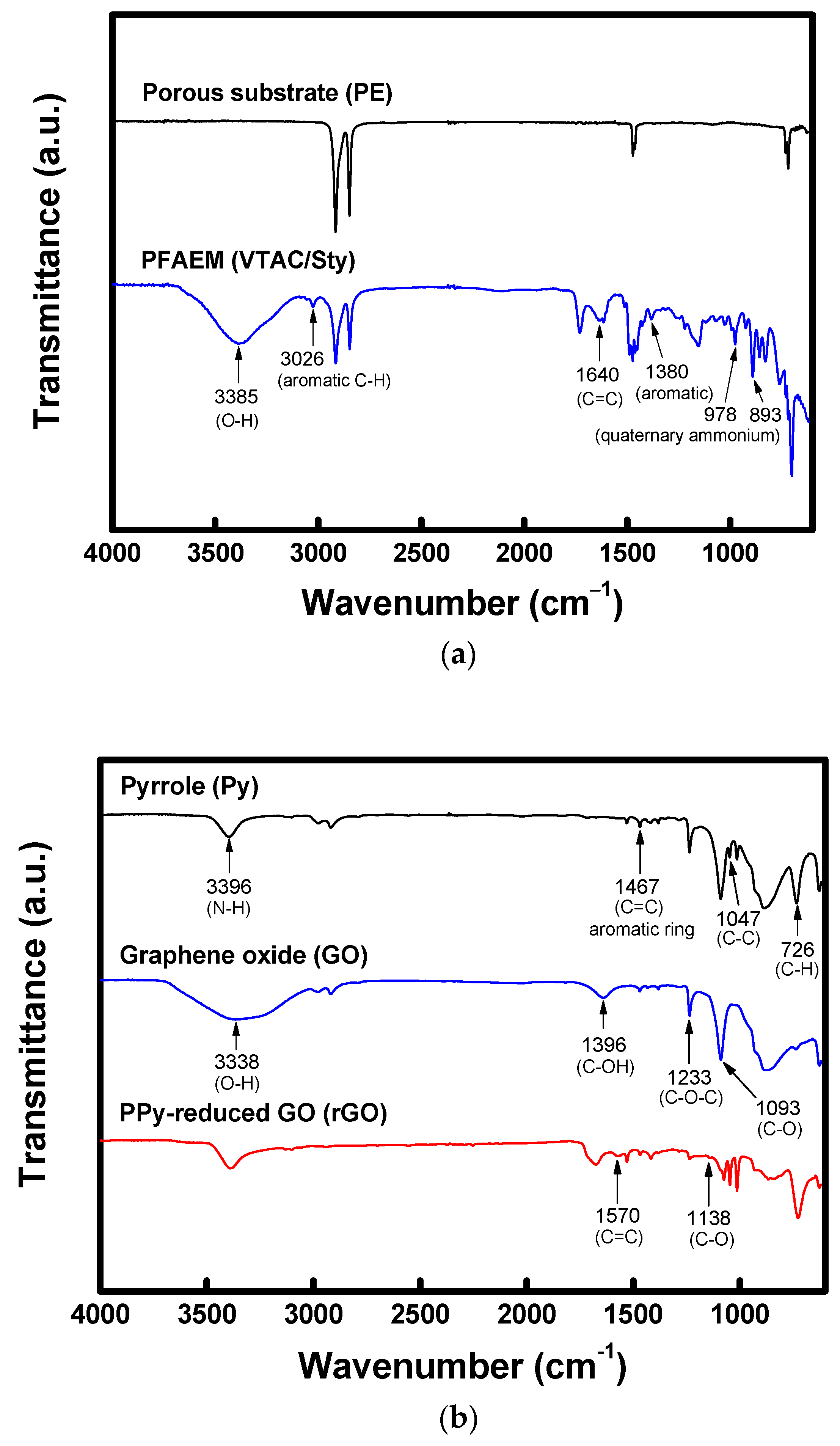
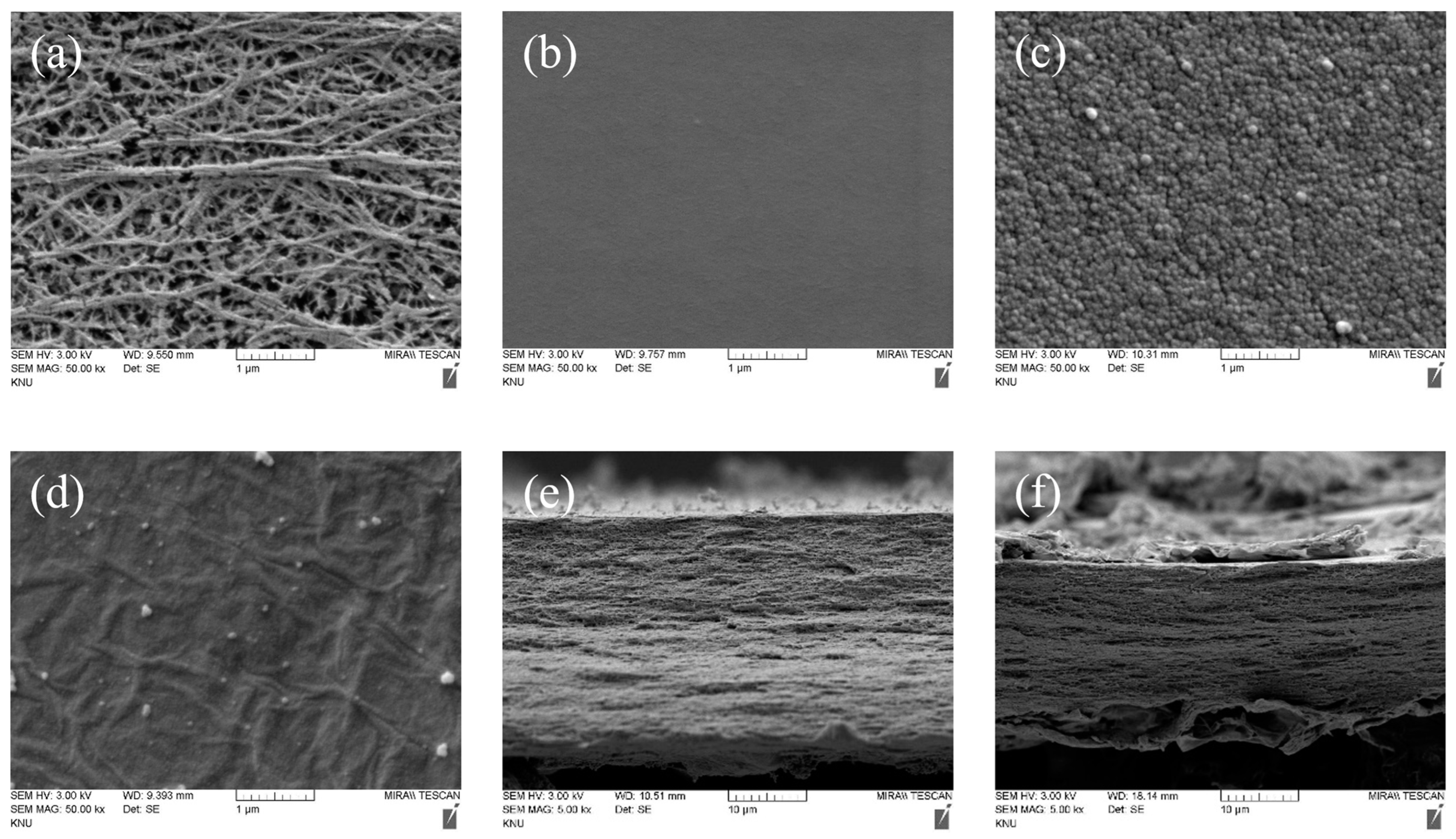
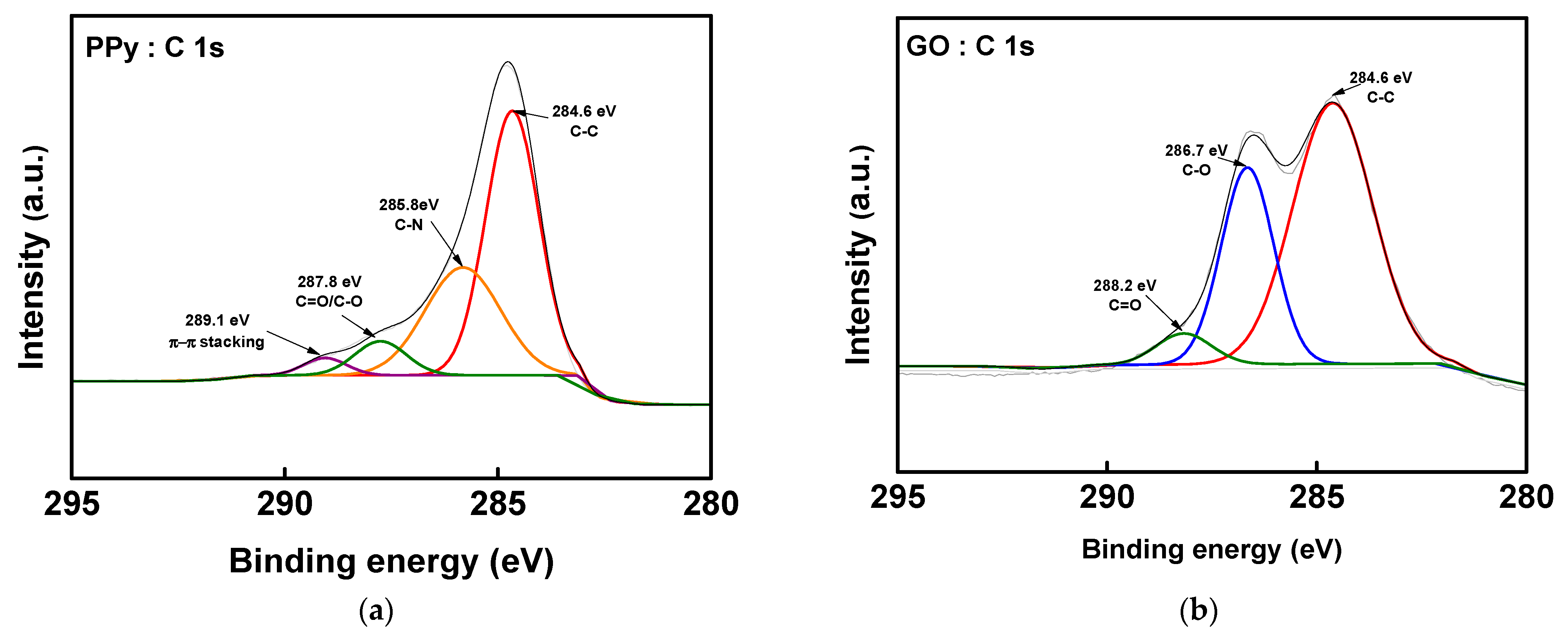
2.3. Membrane Characterization
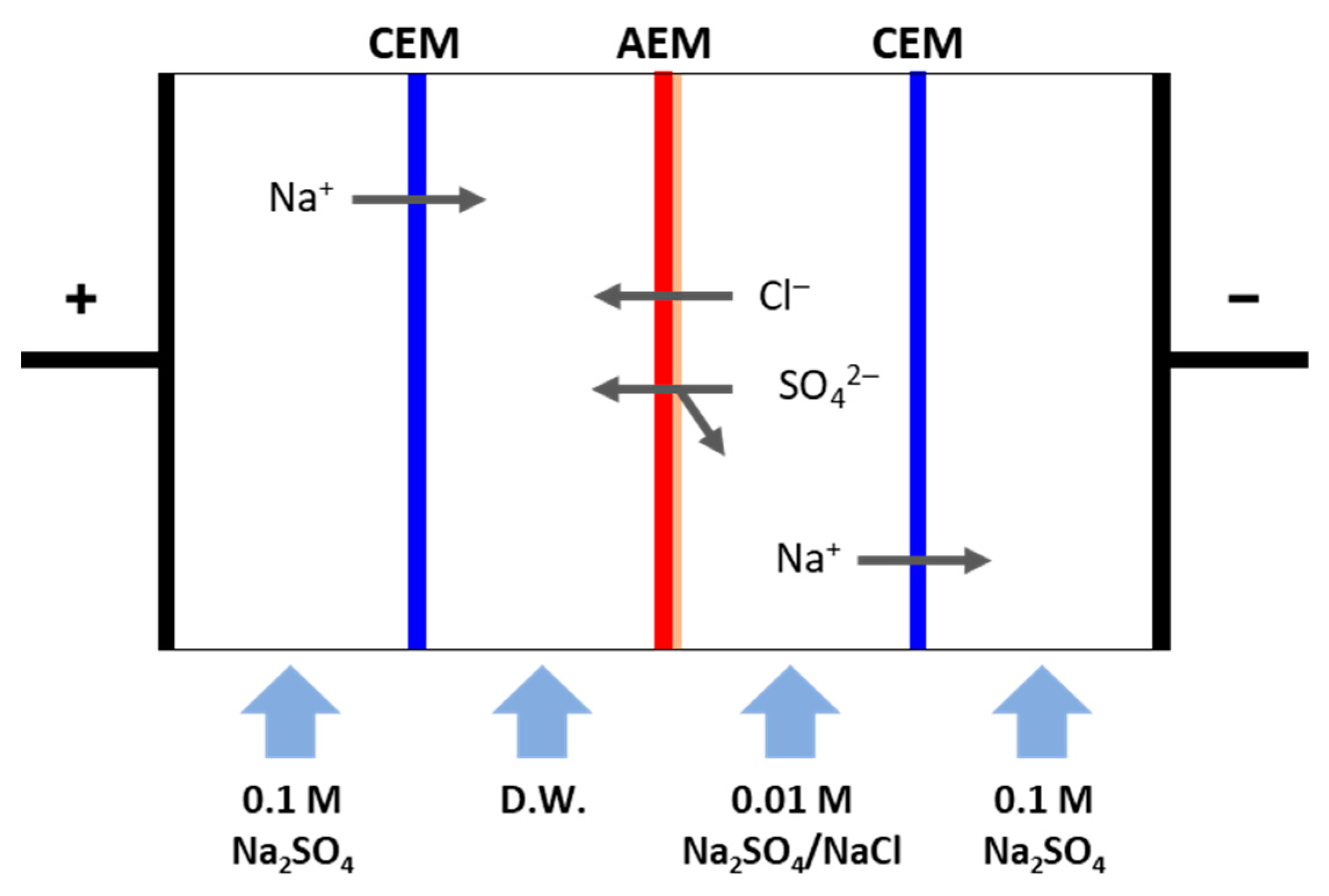
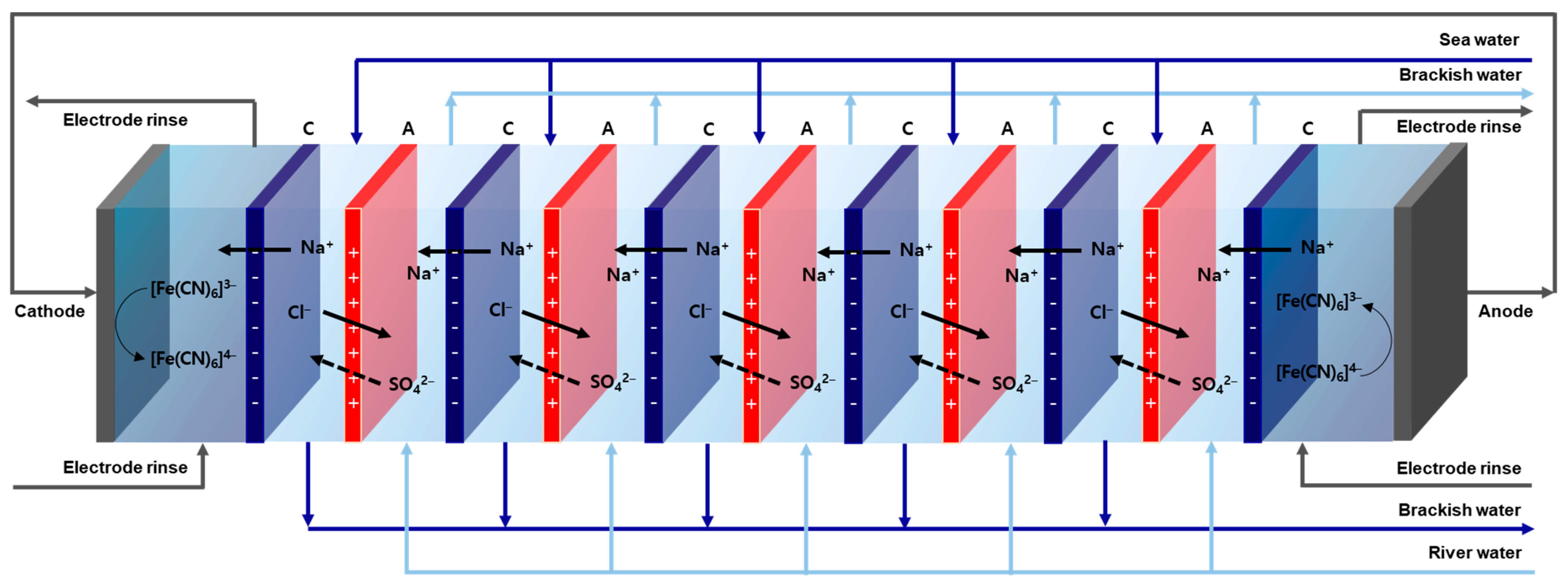
2.4. RED Performance
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Altıok, E.; Kaya, T.Z.; Güler, E.; Kabay, N.; Bryjak, M. Performance of Reverse Electrodialysis System for Salinity Gradient Energy Generation by Using a Commercial Ion Exchange Membrane Pair with Homogeneous Bulk Structure. Water 2021, 13, 814. [Google Scholar] [CrossRef]
- Seyfried, C.; Palko, H.; Dubbs, L. Potential local environmental impacts of salinity gradient energy: A review. Renew. Sust. Energ. Rev. 2019, 102, 111–120. [Google Scholar] [CrossRef]
- Post, J.W.; Veerman, J.; Hamelers, H.V.M.; Euverink, G.J.W.; Metz, S.J.; Nymeijer, K.; Buisman, C.J.N. Salinity-gradient power: Evaluation of pressure-retarded osmosis and reverse electrodialysis. J. Membr. Sci. 2007, 288, 218–230. [Google Scholar] [CrossRef]
- Jin, D.; Xi, R.; Xu, S.; Wang, P.; Wu, X. Numerical simulation of salinity gradient power generation using reverse electrodialysis. Desalination 2021, 512, 115132–115144. [Google Scholar] [CrossRef]
- Vermaas, D.A.; Guler, E.; Saakes, M.; Nijmeijer, K. Theoretical power density from salinity gradients using reverse electrodialysis. Energy Procedia 2012, 20, 170–184. [Google Scholar] [CrossRef]
- Veerman, J. Reverse Electrodialysis: Co- and Counterflow Optimization of Multistage Configurations for Maximum Energy Efficiency. Membranes 2020, 10, 206. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.G.; Zhang, B.; Glabman, S.; Uzal, N.; Dou, X.; Zhang, H.; Wei, X.; Chen, Y. Potential ion exchange membranes and system performance in reverse Electrodialysis for power generation: A review. J. Membr. Sci. 2015, 486, 71–88. [Google Scholar] [CrossRef]
- Křivčík, J.; Neděla, D.; Válek, R. Ion-exchange membrane reinforcing. Desalin. Water Treat. 2015, 56, 3214–3219. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Z.; Kong, X.-Y.; Sun, Y.; Zhu, C.; Liu, P.; Pang, J.; Jiang, L.; Wen, L. Engineered PES/SPES nanochannel membrane for salinity gradient power generation. Nano Energy 2019, 59, 354–362. [Google Scholar] [CrossRef]
- Wang, B.; Yan, J.; Wang, H.; Li, R.; Fu, R.; Jiang, C.; Nikonenko, V.; Pismenskaya, N.; Wang, Y.; Xu, T. Ionic liquid-based pore-filling anion-exchange membranes enable fast large-sized metallic anion migration in electrodialysis. J. Membr. Sci. 2023, 670, 121348–121359. [Google Scholar] [CrossRef]
- Choi, J.; Yang, S.C.; Jeong, N.-J.; Kim, H.; Kim, W.-S. Fabrication of an Anion-Exchange Membrane by Pore-Filling Using Catechol–1,4-Diazabicyclo-[2,2,2]octane Coating and Its Application to Reverse Electrodialysis. Langmuir 2018, 34, 10837–10846. [Google Scholar] [CrossRef]
- Kim, H.-K.; Lee, M.-S.; Lee, S.-Y.; Choi, Y.-W.; Jeong, N.-J.; Kim, C.-S. High power density of reverse electrodialysis with pore-filling ion exchange membranes and a high open-area spacer. J. Mater. Chem. A 2015, 3, 16302–16306. [Google Scholar] [CrossRef]
- Choi, J.; Kim, W.-S.; Kim, H.K.; Yang, S.C.; Jeong, N.J. Ultra-thin pore-filling membranes with mirror-image wave patterns for improved power density and reduced pressure drops in stacks of reverse electrodialysis. J. Membr. Sci. 2021, 620, 118885–118894. [Google Scholar] [CrossRef]
- Yang, S.C.; Choi, Y.-W.; Choi, J.; Jeong, N.; Kim, H.; Nam, J.-Y.; Jeong, H. R2R Fabrication of Pore-Filling Cation-Exchange Membranes via One-Time Impregnation and Their Application in Reverse Electrodialysis. ACS Sustain. Chem. Eng. 2019, 7, 12200–12213. [Google Scholar] [CrossRef]
- Kim, H.; Choi, J.; Jeong, N.; Jung, Y.-G.; Kim, H.; Kim, D.; Yang, S.C. Correlations between Properties of Pore-Filling Ion Exchange Membranes and Performance of a Reverse Electrodialysis Stack for High Power Density. Membranes 2021, 11, 609. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Kang, Y.; Han, J.-H.; Jang, K.; Kim, C.-M.; Kim, I.S. Developments and future prospects of reverse electrodialysis for salinity gradient power generation: Influence of ion exchange membranes and electrodes. Desalination 2020, 491, 114540–114554. [Google Scholar] [CrossRef]
- Nazif, A.; Karkhanechi, H.; Saljoughi, E.; Mousavi, S.M.; Matsuyama, H. Recent progress in membrane development, affecting parameters, and applications of reverse electrodialysis: A review. J. Water Process Eng. 2022, 47, 102706–102736. [Google Scholar] [CrossRef]
- Besha, A.T.; Tsehaye, M.T.; Aili, D.; Zhang, W.; Tufa, R.A. Design of Monovalent Ion Selective Membranes for Reducing the Impacts of Multivalent Ions in Reverse Electrodialysis. Membranes 2020, 10, 7. [Google Scholar] [CrossRef]
- Pan, J.; Ding, J.; Tan, R.; Chen, G.; Zhao, Y.; Gao, C.; Van der Bruggen, B.; Shen, J. Preparation of a monovalent selective anion exchange membrane through constructing a covalently crosslinked interface by electro-deposition of polyethyleneimine. J. Membr. Sci. 2017, 539, 263–272. [Google Scholar] [CrossRef]
- Liu, H.; Ruan, H.; Zhao, Y.; Pan, J.; Sotto, A.; Gao, C.; Van der Bruggen, B.; Shen, J. A facile avenue to modify polyelectrolyte multilayers on anion exchange membranes to enhance monovalent selectivity and durability simultaneously. J. Membr. Sci. 2017, 543, 310–318. [Google Scholar] [CrossRef]
- Post, J.W.; Hamelers, H.V.M.; Buisman, C.J.N. Influence of multivalent ions on power production from mixing salt and fresh water with a reverse electrodialysis system. J. Membr. Sci. 2009, 330, 65–72. [Google Scholar] [CrossRef]
- Ran, J.; Wu, L.; He, Y.; Yang, Z.; Wang, Y.; Jiang, C.; Ge, L.; Bakangura, E.; Xu, T. Ion exchange membranes: New developments and applications. J. Membr. Sci. 2017, 522, 267–291. [Google Scholar] [CrossRef]
- Salmeron-Sanchez, I.; Asenjo-Pascual, J.; Aviles-Moreno, J.R.; Ocón, P. Microstructural description of ion exchange membranes: The effect of PPy-based modification. J. Membr. Sci. 2022, 659, 120771–120782. [Google Scholar] [CrossRef]
- Salmeron-Sanchez, I.; Asenjo-Pascual, J.; Aviles-Moreno, J.R.; Perez-Flores, J.C.; Mauleón, P.; Ocón, P. Chemical physics insight of PPy-based modified ion exchange membranes: A fundamental approach. J. Membr. Sci. 2022, 643, 120020–120032. [Google Scholar] [CrossRef]
- Tufa, R.A.; Piallat, T.; Hnát, J.; Fontananova, E.; Paidar, M.; Chanda, D.; Curcio, E.; di Profio, G.; Bouzek, K. Salinity gradient power reverse electrodialysis: Cation exchange membrane design based on polypyrrole-chitosan composites for enhanced monovalent selectivity. Chem. Eng. J. 2020, 380, 122461–122474. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, K.; Ruan, H.; Xue, L.; Van der Bruggen, B.; Gao, C.; Shen, J. Sulfonated reduced graphene oxide modification layers to improve monovalent anions selectivity and controllable resistance of anion exchange membrane. J. Membr. Sci. 2017, 536, 167–175. [Google Scholar] [CrossRef]
- Amarnath, C.A.; Hong, C.E.; Kim, N.H.; Ku, B.-C.; Kuila, T.; Lee, J.H. Efficient synthesis of graphene sheets using pyrrole as a reducing agent. Carbon 2011, 49, 3497–3502. [Google Scholar] [CrossRef]
- Hofmann, U.; Frenzel, A. Die Reduktion von Graphitoxyd mit Schwefelwasserstoff. Kolloid-Z. Carbon 1934, 68, 149–151. [Google Scholar]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Si, Y.; Samulski, E.T. Synthesis of water soluble graphene. Nano Lett. 2008, 8, 1679–1682. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-Based Composite Materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yang, J.; Park, J.; Gou, X.; Wang, B.; Liu, H.; Yao, J. Facile synthesis and characterization of graphene nanosheets. J. Phys. Chem. C 2008, 112, 8192–8195. [Google Scholar] [CrossRef]
- Yang, S.; Shen, C.; Liang, Y.; Tong, H.; He, W.; Shi, X.; Zhang, X.; Gao, H.-J. Graphene nanosheets-polypyrrole hybrid material as a highly active catalyst support for formic acid electro-oxidation. Nanoscale 2011, 3, 3277–3284. [Google Scholar] [CrossRef]
- Choi, M.-J.; Chae, K.-J.; Ajayi, F.F.; Kim, K.-Y.; Yu, H.-W.; Kim, C.-W.; Kim, I.S. Effects of biofouling on ion transport through cation exchange membranes and microbial fuel cell performance. Bioresour. Technol. 2011, 102, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.-M.; Jia, Y.-X.; Guo, R.-Q.; Wang, M. Heterogeneous anion-exchange membrane: Influences of charged binders with crosslinking structure on electrodialytic performance. J. Membr. Sci. 2018, 557, 67–75. [Google Scholar] [CrossRef]
- Choi, J.-H.; Kim, S.-H.; Moon, S.-H. Heterogeneity of ion-exchange membranes: The effects of membrane heterogeneity on transport properties. J. Colloid Interface Sci. 2001, 241, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Liao, J.; Jiang, Y.; Zhu, J.; Li, J.; Zhao, Y.; Van der Bruggen, B.; Sotto, A.; Shen, J. “Sandwich”-like structure modified anion exchange membrane with enhanced monovalent selectivity and fouling resistant. J. Membr. Sci. 2018, 556, 98–106. [Google Scholar] [CrossRef]
- Leharazu-Larrañaga, A.; Zhao, Y.; Molina, S.; Garcia-Calvo, E.; Van der Bruggen, B. Alternating current enhanced deposition of a monovalent selective coating for anion exchange membranes with antifouling properties. Sep. Purif. Technol. 2019, 229, 115807–115814. [Google Scholar] [CrossRef]
- Irfan, M.; Ge, L.; Wang, Y.; Yang, Z.; Xu, T. Hydrophobic Side Chains Impart Anion Exchange Membranes with High Monovalent−Divalent Anion Selectivity in Electrodialysis. ACS Sustain. Chem. Eng. 2019, 7, 4429–4442. [Google Scholar] [CrossRef]
- Mulyati, S.; Takagi, R.; Fujii, A.; Ohmukai, Y.; Matsuyama, H. Simultaneous improvement of the monovalent anion selectivity and antifouling properties of an anion exchange membrane in an electrodialysis process, using polyelectrolyte multilayer deposition. J. Membr. Sci. 2013, 431, 113–120. [Google Scholar] [CrossRef]
- Na, C.-K.; Park, H.-J. Photoinduced grafting of vinyl benzyl trimethyl ammonium chloride on polyester nonwoven fabric with surfactant coating and its anion-exchange properties. J. Appl. Polym. Sci. 2015, 132, 41674–41681. [Google Scholar] [CrossRef]
- Morales, D.V.; Rivas, B.L.; Gonzalez, M. Poly(4-vinylbenzyl)trimethylammonium chloride) resin with removal properties for vanadium(V) and molybdenum(VI). A thermodynamic and kinetic study. J. Chil. Chem. Soc. 2021, 66, 5118–5124. [Google Scholar] [CrossRef]
- Hermán, V.; Takacs, H.; Duclairoir, F.; Renault, O.; Tortai, J.H.; Viala, B. Core double–shell cobalt/graphene/polystyrene magnetic nanocomposites synthesized by in situ sonochemical polymerization. RSC Adv. 2015, 5, 51371–51381. [Google Scholar] [CrossRef]
- Tang, R.; Zhang, Y.; Zhang, Y.; Yu, Z. Synthesis and characterization of chitosan based dye containing quaternary ammonium group. Carbohydr. Polym. 2016, 139, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, H.; Yang, T.-H.; Bae, B.; Tran, N.A.T.; Cho, Y.; Jung, N.; Shin, D. Quaternary ammonium-bearing perfluorinated polymers for anion exchange membrane applications. Membranes 2020, 10, 306. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Parrondo, J.; Sankarasubramanian, S.; Bhattacharyya, K.; Ghosh, M.; Ramani, V. Alkaline stability of pure aliphatic based anion exchange membranes containing cycloaliphatic quaternary ammonium cations. J. Electrochem. Soc. 2020, 167, 124504–124512. [Google Scholar] [CrossRef]
- Chuah, R.; Gopinath, S.C.B.; Anbu, P.; Salimi, M.N.; Yaakub, A.R.W.; Lakshmipriya, T. Synthesis and characterization of reduced graphene oxide using the aqueous extract of Eclipta prostrata. 3 Biotech 2020, 10, 364–373. [Google Scholar] [CrossRef]
- Fan, X.; Yang, Z.; He, N. Hierarchical nanostructured polypyrrole/graphene composites as supercapacitor electrode. RSC Adv. 2015, 5, 15096–15102. [Google Scholar] [CrossRef]
- Naikoo, R.A.; Tomar, R. Fabrication of a novel Zeolite-X/Reduced graphene oxide/Polypyrrole nanocomposite and its role in sensitive detection of CO. Mater. Chem. Phys. 2018, 211, 225–233. [Google Scholar] [CrossRef]
- Yang, X.; Cao, L.; Wang, J.; Chen, L. Sandwich-like Polypyrrole/Reduced Oxide Graphene Nano-sheets Integrated Gelatin Hydrogel as Mechanically and Thermally Sensitive Skin-like Bio-Electronics. ACS Sustain. Chem. Eng. 2020, 8, 10726–10739. [Google Scholar]
- Xu, S.; Hao, H.; Chen, Y.; Li, W.; Shen, W.; Shearing, P.R.; Brett, D.J.L.; He, G. Flexible all-solid-state supercapacitors based on PPy/rGO nanocomposite on cotton fabric. Nanotechnology 2021, 32, 305401. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Zhou, J.; Wu, J.; Li, H.; Zhao, W.; He, C.; Liu, Y.; Chen, Y.; Fu, Y.; Duan, H. High Performance Acoustic Wave Nitrogen Dioxide Sensor with Ultraviolet Activated 3D Porous Architecture of Ag-Decorated Reduced Graphene Oxide and Polypyrrole Aerogel. Interfaces 2021, 13, 42094–42103. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Y.; Chen, J.; Li, X.; Walsh, F.C.; Ouyang, J.-H.; Jia, D.; Zhou, Y. Three-dimensional graphene oxide/polypyrrole composite electrodes fabricated by one-step electrodeposition for high performance supercapacitors. J. Mater. Chem. A 2015, 3, 14445–14457. [Google Scholar] [CrossRef]
- Liu, W.; Fang, Y.; Xu, P.; Lin, Y.; Yin, X.; Tang, G.; He, M. Two-Step Electrochemical Synthesis of Polypyrrole/Reduced Graphene Oxide Composites as Efficient Pt-Free Counter Electrode for Plastic Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2014, 6, 16249–16256. [Google Scholar] [CrossRef] [PubMed]
- Purkait, T.; Singh, G.; Kamboj, N.; Das, M.; Dey, R.S. All-porous heterostructure of reduced graphene oxide–polypyrrole–nanoporous gold for a planar flexible supercapacitor showing outstanding volumetric capacitance and energy density. J. Mater. Chem. A 2018, 6, 22858–22869. [Google Scholar] [CrossRef]
- Tansel, B. Significance of thermodynamic and physical characteristics on permeation of ions during membrane separation: Hydrated radius, hydration free energy and viscous effects. Sep. Purif. Technol. 2012, 86, 119–126. [Google Scholar] [CrossRef]
- Nightingale, E.R. Phenomenological Theory of Ion Solvation. Effective Radii of Hydrated Ions. J. Phys. Chem. A 1959, 63, 1381–1387. [Google Scholar] [CrossRef]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef]
- Rijnaarts, T.; Shenkute, N.; Wood, J.A.; De Vos, W.M.; Nijmeijer, K. Divalent cation removal by Donnan dialysis for improved reverse electrodialysis. ACS Sustain. Chem. Eng. 2018, 6, 7035–7041. [Google Scholar] [CrossRef]


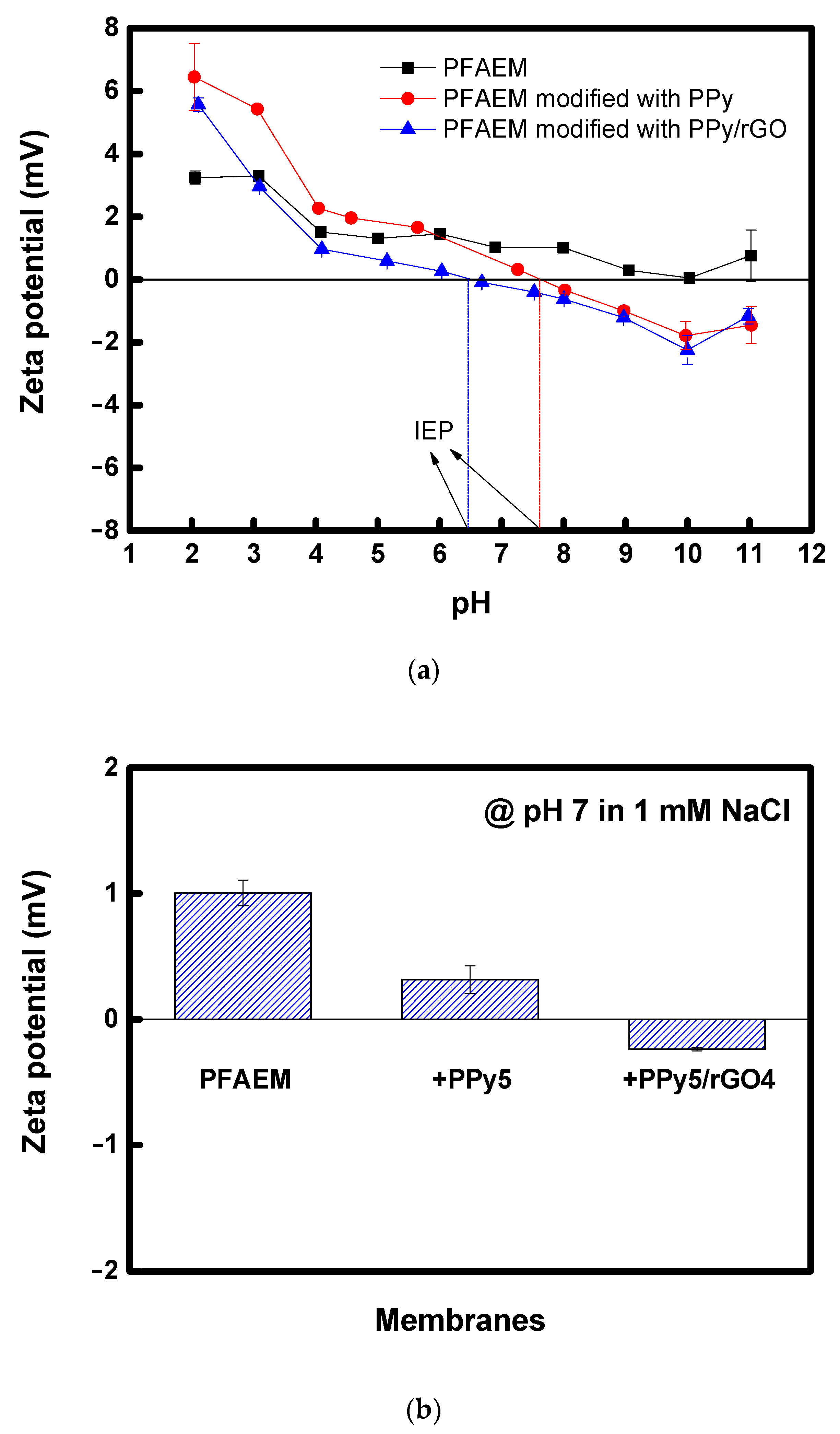
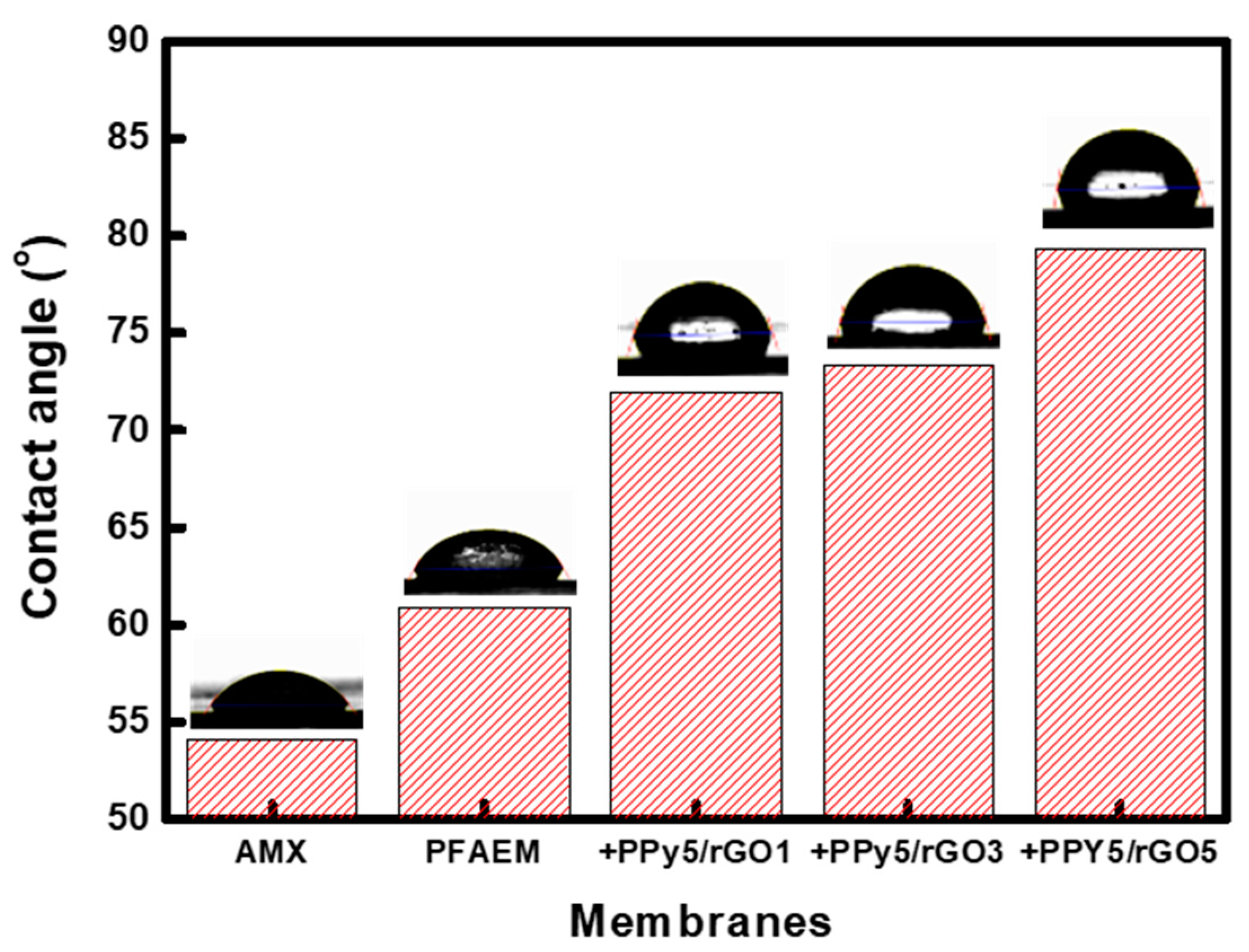
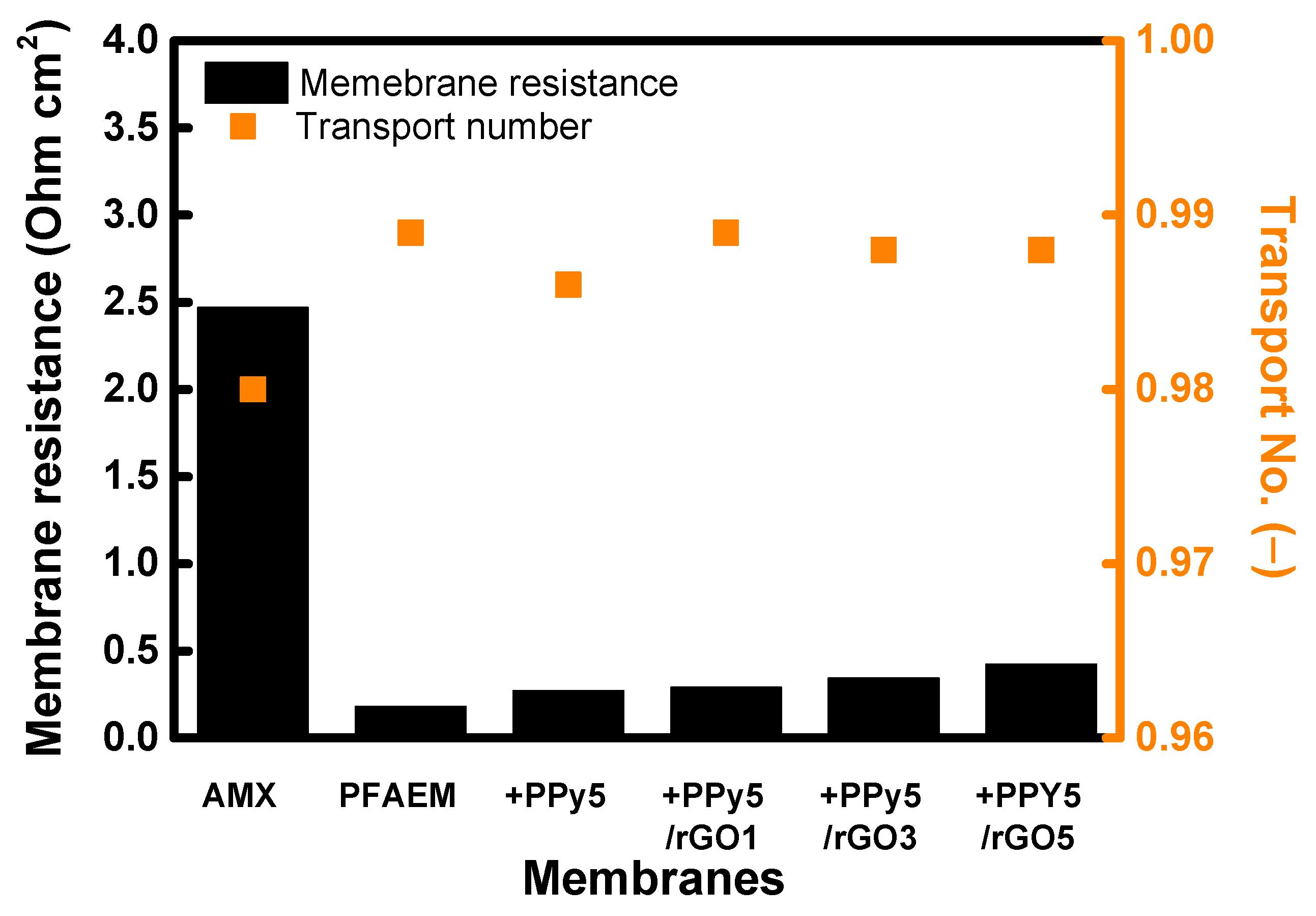
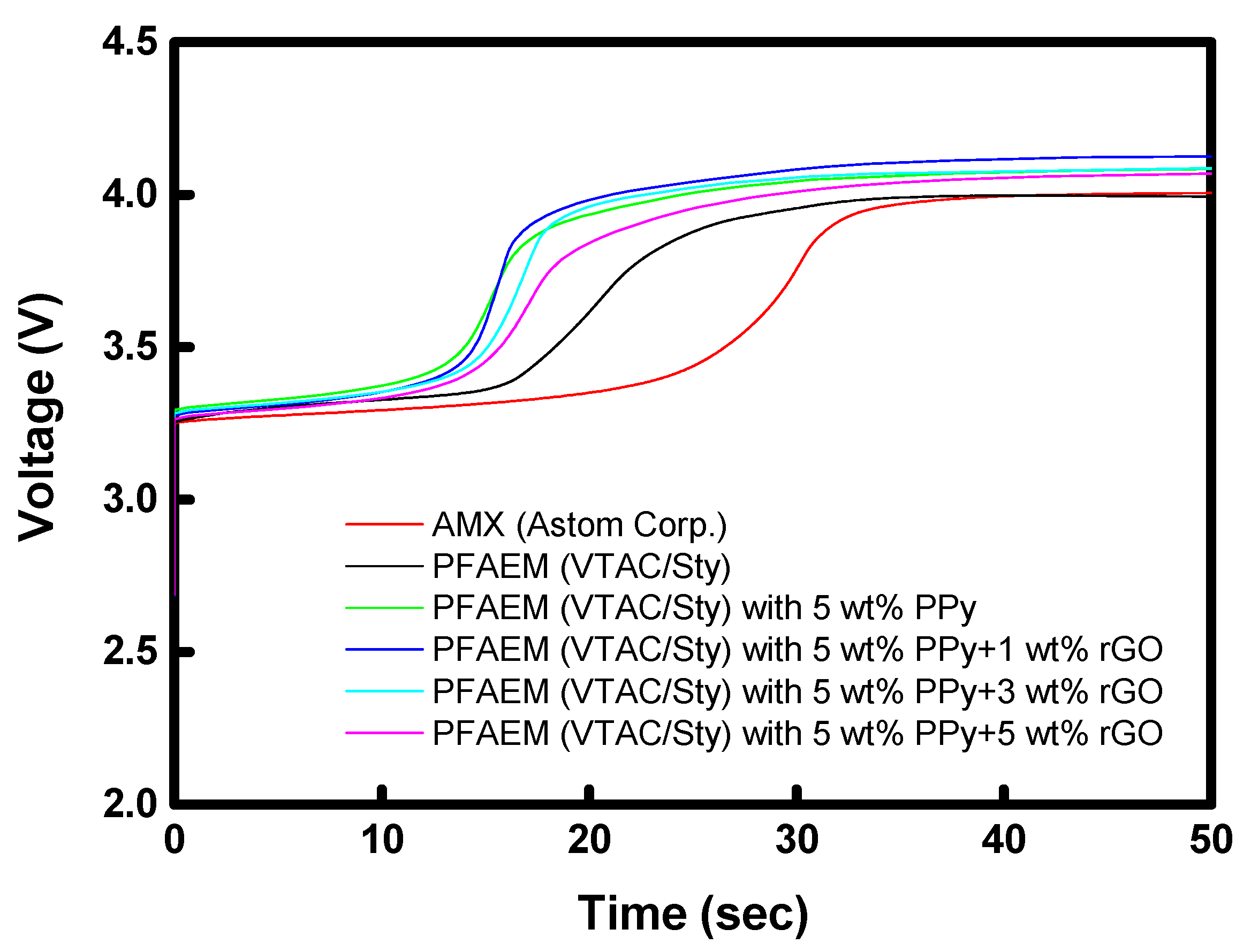

| Membrane | ε (−) |
|---|---|
| AMX (Astom Corp.) | 0.993 |
| PFAEM | 0.802 |
| PFAEM + 5 wt% PPy | 0.719 |
| PFAEM + 5 wt% PPy/1 wt% rGO | 0.741 |
| PFAEM + 5 wt% PPy/3 wt% rGO | 0.750 |
| PFAEM + 5 wt% PPy/5 wt% rGO | 0.754 |
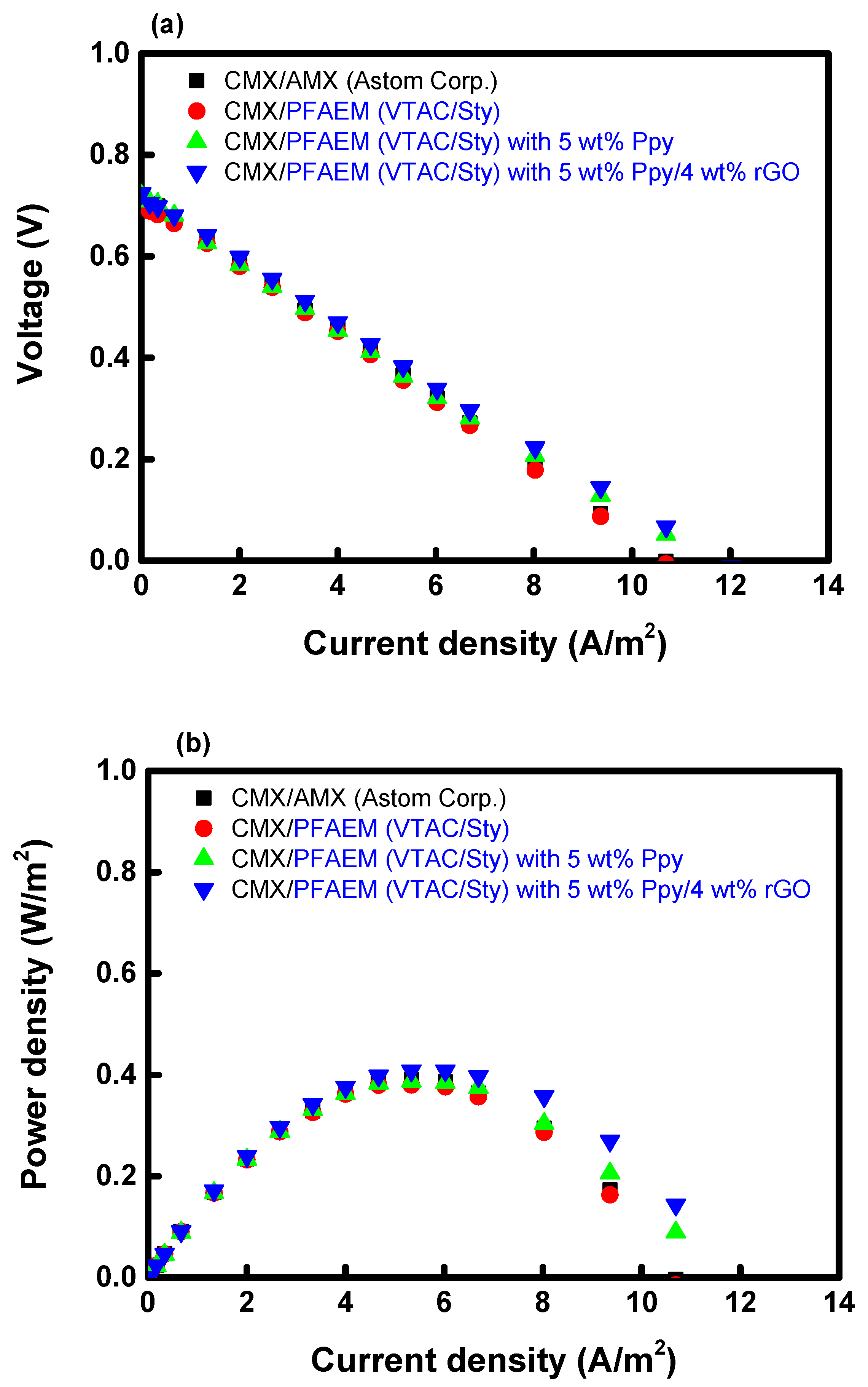
| Membranes | OCV (V) | Power Density (W/m2/Cell Pair) |
|---|---|---|
| CMX/AMX | 0.712 | 0.387 |
| CMX/PFAEM | 0.708 | 0.379 |
| CMX/PFAEM + 5 wt% PPy | 0.720 | 0.391 |
| CMX/PFAEM + 5 wt% PPy/4 wt% rGO | 0.724 | 0.408 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Kim, D.-H.; Kang, M.-S. Surface-Modified Pore-Filled Anion-Exchange Membranes for Efficient Energy Harvesting via Reverse Electrodialysis. Membranes 2023, 13, 894. https://doi.org/10.3390/membranes13120894
Lee J-H, Kim D-H, Kang M-S. Surface-Modified Pore-Filled Anion-Exchange Membranes for Efficient Energy Harvesting via Reverse Electrodialysis. Membranes. 2023; 13(12):894. https://doi.org/10.3390/membranes13120894
Chicago/Turabian StyleLee, Ji-Hyeon, Do-Hyeong Kim, and Moon-Sung Kang. 2023. "Surface-Modified Pore-Filled Anion-Exchange Membranes for Efficient Energy Harvesting via Reverse Electrodialysis" Membranes 13, no. 12: 894. https://doi.org/10.3390/membranes13120894
APA StyleLee, J.-H., Kim, D.-H., & Kang, M.-S. (2023). Surface-Modified Pore-Filled Anion-Exchange Membranes for Efficient Energy Harvesting via Reverse Electrodialysis. Membranes, 13(12), 894. https://doi.org/10.3390/membranes13120894







