A Comprehensive Review of Performance of Polyacrylonitrile-Based Membranes for Forward Osmosis Water Separation and Purification Process
Abstract
1. Introduction
2. Forward Osmosis Process and Polymeric Membranes
3. PAN Chemical Structure and Characteristics as Membrane Substrate
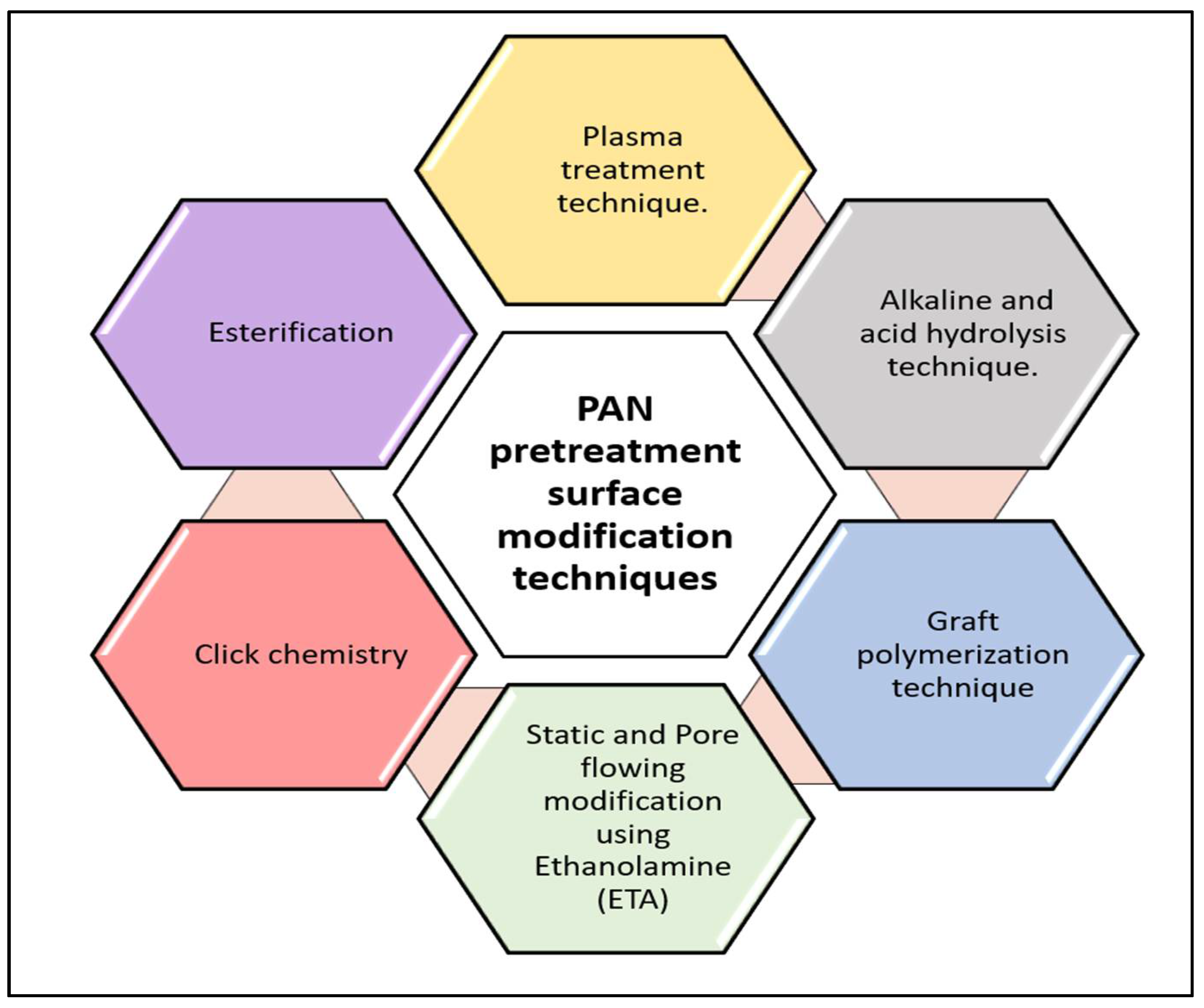
4. PAN Pretreatment Surface Modification Techniques
4.1. Plasma Treatment Technique
4.2. Graft Polymerization Technique
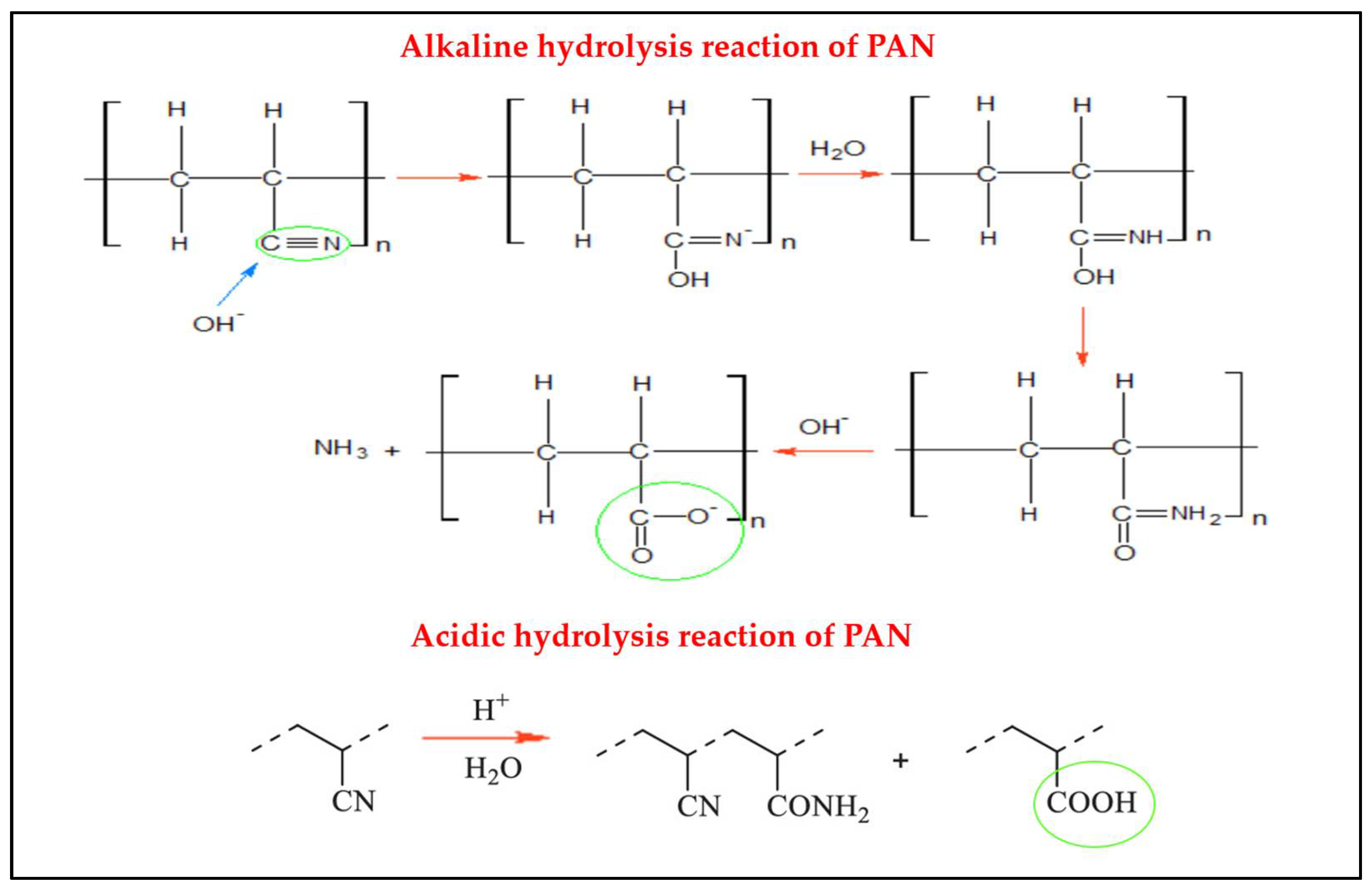
4.3. Alkaline and Acid Hydrolysis Techniques
4.4. Click Chemistry
4.5. Static and Pore-Flowing Modifications Using Ethanolamine (ETA)
4.6. Esterification
4.7. Hydrazine Cross-Linking
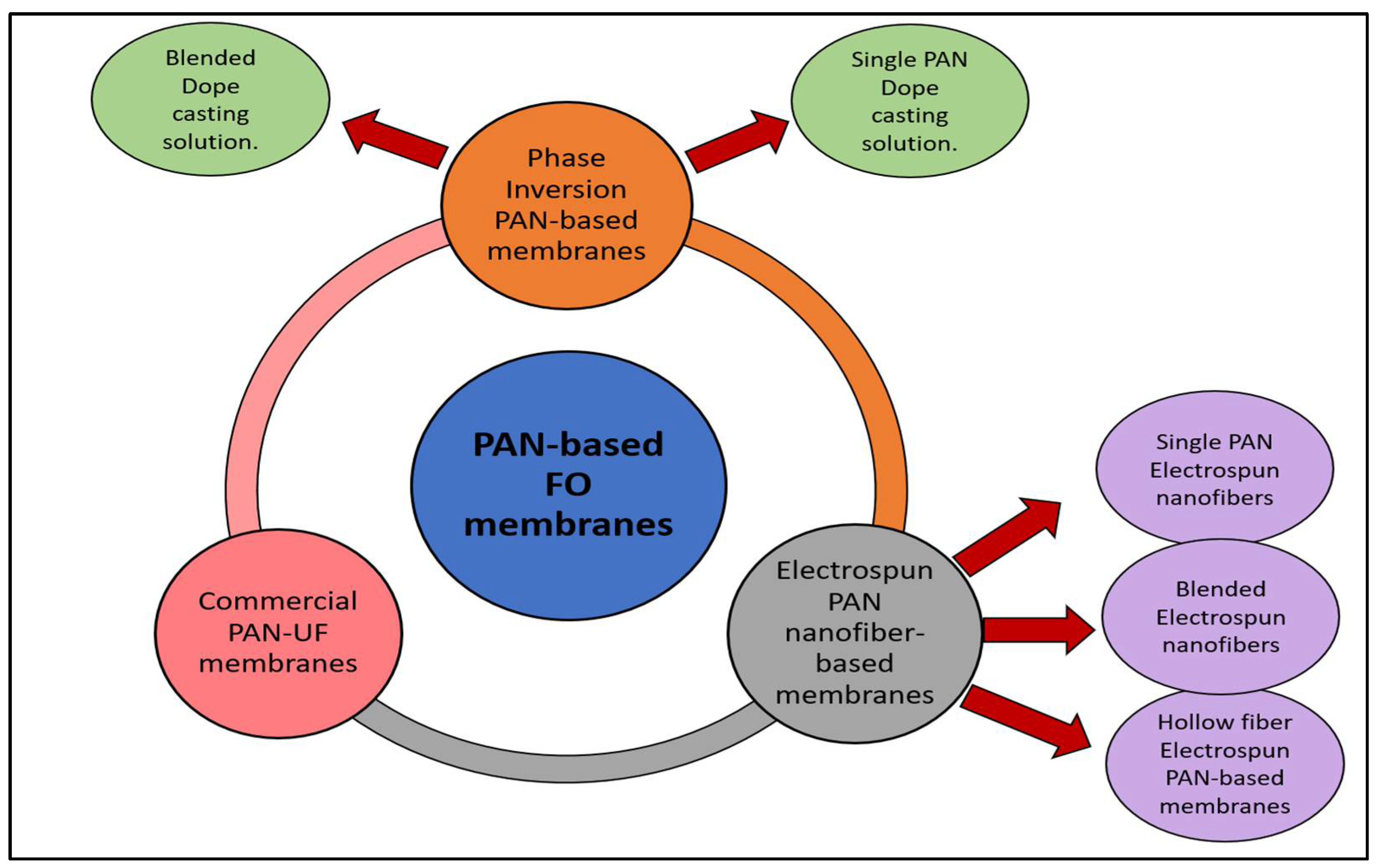
5. PAN-Based Porous FO Membranes
5.1. Casted PAN-Based Membranes in the FO Process
| Type of PAN Membrane | MWCO of PAN Polymer | Fillers-Optimal Loading wt % | Fabrication Method | Modification Techniques | Solute Type/Applications | DS and FS | Optimum Achieved Parameters under the FO Test | References |
|---|---|---|---|---|---|---|---|---|
| Casted PAN substrate | (PAN, Mw 150,000 Da) was purchased from Scientific Polymer Product (Ontario, New York) | Phase inversion (16.5 wt % PAN) | Hydrolysis IP | Salt (NaCl) | FS: DI DS: 0.5 M NaCl | Casted PAN. Jw (PRO/FO) = 11.56/9.25 LMH Js (PRO & FO) = 0.10 mole/m2h R% = 94.54% HPAN Jw (PRO/FO) = 13.88/9.25 LMH Js (PRO & FO) = 0.11 mole/m2h R% = 89.95 % | [52] | |
| Casted PAN substrate | (PAN, density = 1.15 g/cm3, molecular weight 80,000–100,000 Da) was purchased from Esfehan Polyacryl Trading Private Company (Isfahan, Iran) | - | Phase inversion (7–16 wt % PAN) | IP | Salt (NaCl) | FS: DI, NaCl (3.5 wt %) DS: 1, 2 M NaCl | PA/PAN FS: DI & DS: 1 M NaCl Jw = 31.3 LMH Js = 5.11 gMH PA/PAN FS: NaCl (3.5 wt %) & DS:2 M NaCl Jw = 26.9 LMH | [16] |
| Casted PAN substrate | (PAN, Mw = 250,000 Da) from Hubei Chushengwei Corporation (Wuhan, China) | - | Phase inversion (4 and 16 wt % PAN) | Hydrolysis IP | Salt (NaCl) | FS: DI DS: 0.5, 2 M NaCl | 4 wt % of PAN At PRO mode for DS:0.5 M NaCl Jw = 40.16 LMH, Js = 1.22 gMH 16 wt % of PAN DS:2 M NaCl Jw = 44.49 LMH, Js = 11.9 gMH | [105] |
| Casted PAN substrate | Sigma-Aldrich PAN (150,000 Da) | - | Phase inversion (12 wt % PAN) | Hydrolysis IP | Salt Simulated wastewater (Sb, Cr and aniline) | FS: DI, simulated wastewater DS: 0.5 M NaCl | TFC-PAN-1.5 wt % LiCl Jw = 16.5 LMH Js = 2.3 gMH R% of Sb (98.2%), Cr (99.9%), and aniline (92.6%). | [106] |
| Casted PAN substrate | Sigma-Aldrich PAN (150,000 Da) | NIPS of PAN | IP | Salt, organic molecules, | FS: DI FS: NaCl solution (10 mmol/L), Na2SO4 solution (10 mmol/L), or SA solution (20 mg/L) DS: 1.17 mmol/L–47.00 mmol/L of neutralized Poly acrylic acid (PAANa) solution. | Jw = 25 LMH R% of Na2SO4 = 91.4% R% of NaCl = 21% R% of SA = 99% | [107] | |
| MOF-PAN casted substrate | Sigma-Aldrich PAN (150,000 Da) | 1 wt % of MOF particles | Phase inversion (MOF+ 18 wt % PAN) | MOF particles poured into PAN polymer matrix. PAH/PSS LBL treatment. GA crosslinking | Salt (NaCl, MgCl2) | FS: DI, 10, 100 mM NaCl DS: 0.1, 0.3, 0.5, 1, 3 M MgCl2 | Control membrane in PRO mode at DS: 0.5 M MgCl2 and FS: DI Jw = 78.1 LMH Control membrane in FO mode Jw = 28.7 LMH MOF-based membrane in PRO mode at DS: 0.5 M MgCl2 and FS: DI Jw = 107.4 LMH MOF-based membrane in PRO mode at DS: 3 M MgCl2 and FS: DI Jw = 132.7 LMH | [22] |
| Casted mixed matrix PAN+ silica gel substrate | Sigma–Aldrich, PAN Mw = 150,000 Da | 1.0 wt % of Silica gel particles | Phase inversion (18 wt % PAN+ Silica gel) | Hydrolysis PAH/PSS LBL. GA crosslinking | Salt | FS: DI or 10,100 mM NaCl. DS: 0.5 M MgCl2 | FO mode Jw = 28.6 Js = 5.8 Js/Jw = 0.20 R% by RO = 76% MgCl2 In PRO mode Jw = 77.9 Js = 6.9 Js/Jw = 0.09 | [18,108] |
| Casted PAN substrate | Sigma-Aldrich PAN (150,000 Da) | Phase inversion (Wet casting—18 wt % PAN) | LBL using polyelectrolytes. GA crosslinking | Salts (Na2SO4, MgSO4, Na3CIT (NH4)2SO4 Protein (BSA and LYS) | FS: BSA and LYS DS: 1 M Na2SO4, 1 M (NH4)2SO4 | FO mode Jw = 28 LMH for Na2SO4 DS. Jw = 40 LMH for (NH4)2SO4 DS | [116] | |
| Casted HCD-decorated PAN support layer | PAN Mw of 150,000 Da, from Aladdin Industrial Corporation (Ontario, California). | 10 wt % of hydrophobic carbon dots HCDs | Phase inversion- Nonsolvent-induced phase separation (NIPS) (HCD s + PAN) | IP | Salt (NaCl) | FS: DI DS: 1 M NaCl | TFC-0 in PRO mode Jw = 7.71 LMH Js = 4.56 gMH TFC-10% HCDs in PRO mode Jw = 15.47 LMH Js = 2.9 gMH | [109] |
| Casted PAN onto mCNT-PET membrane | Sigma-Aldrich PAN (150,000 Da) | TCNT and LCNT with a weight ratio of 3:1 | Phase inversion of 12 wt % PAN | mCNT intermediate layer by casting onto PET Hydrolysis PEI/PAA depositing. IP of PA layer by mLBL | Salt (NaCl) | FS: DI DS: 1, 2 M NaCl | PET30-mCNT-HPAN30 At DS 1 M NaCl Jw = 29.02 LMH/43.5 LMH Js = 9.4 gMH/11.1 gMH At DS 2 M NaCl Jw = 32.4 LMH/75 LMH Js = 11.9 gMH/16.4 gMH | [110] |
| Casted PAN substrate | Sigma-Aldrich PAN (150,000 Da) | - | NIPS- Phase inversion of 12 wt % PAN | IP (Using toluene instead of hexane as a solvent for TMC) | Salt (NaCl) | FS: DI DS: 1 M NaCl | Jw of TFC-TIP = 34.2 LMH/44.5 LMH Jw of TFC-HIP = 12.9 LMH/17.0 LMH Js of TFC-TIP = 5.81 gMH/8.45 gMH Js of TFC-HIP = 6.96 gMH/9.35 gMH | [111] |
| Casted PAN substrate | Sigma-Aldrich PAN (150,000 Da) | CNTs 0.2 wt % | Phase inversion of 14 wt % PAN | Hydrolysis PEI/PAA coating IP (CNTs into MPD aqueous phase) | Salt (NaCl) | FS: DI DS: 0.5 M NaCl | Jw = 25.14 LMH Js = 8.64 gMH Js/Jw = 0.37 g/L | [112] |
| Casted PAN substrate | PAN powder (Mn: 250,000 Da) was purchased from Chushengwei Chemistry Co. Ltd. (Hubei, China). | 400 and 600 ppm are the optimal loadings of GO. | Phase inversion of 16 wt % PAN | Hydrolysis IP (GO into MPD aqueous phase | Salt (NaCl) | FS: DI DS: 2 M NaCl | At FO mode Jw = 21.6–35.4 LMH. At PRO mode Jw = 31.1–56.6 LMH Js = 2–12 gMH R% = 81–94.6% FRR% >90 % | [113] |
| Casted PAN substrate | PAN powder was obtained from Chushang Co., Ltd (Hubei, China). | 0.04 wt % of SGO@UiO-66 | Phase inversion of 15 wt % PAN | IP (SGO@UiO-66- into TMC organic phase) | Salt (NaCl) Heavy metal removal (Cu2+ and Pb2+) | FS: DI DS: 1 M NaCl | PRO mode SGO@UiO-66-TFN Membrane (M2) Jw = 15 LMH Js = 3 gMH Js/Jw = 0.2 g/L R% of NaCl (50 ppm) = 89.95% by RO test. | [114] |
| Casted Double Layer PAN | PAN powder was supplied by Chusheng Co. Ltd (Hubei, China). | 0.01 wt % of MOF-801 | Phase inversion of 15 wt % PAN | IP (PDA into MPD phase+ MOF into TMC phase) | Salt (NaCl) Heavy metal removal (Cd2+, Ni2+, Pb2+) | FS: DI DS: 1 M NaCl | FO mode R% of NaCl = 93.5%. Jw = 16.7 LMH Js = 2.8 gMH The removal rate was 94~99.2% for Ni2+, Cd2+, and Pb2+) | [115] |
| Casted PAN substrate | Sigma-Aldrich PAN (150,000 Da) | Phase inversion of 16 wt % PAN | Hydrolysis PEI/PAA electrostatic interaction IP by mLBL | Salt (NaCl) | FS: DI DS: 0.5 M NaCl | mLBL-10 Jw (FO/PRO) = 24.6 LMH/32.9 LMH Js (FO/PRO) = 2.36 gMH/3.77 gMH Js/Jw (FO/PRO) = 0.10/0.11 g/L IP-TFC: Jw (FO/PRO) = 10.9 LMH/15.6 LMH Js (FO/PRO) = 7.56 gMH/11.07 gMH Js/Jw (FO/PRO) = 0.69/0.71 g/L | [122] | |
| Casted PAN substrate | Sigma-Aldrich PAN (150,000 Da) | - | Phase inversion of 18 wt % PAN | Hydrolysis PAH/PSS layers by LBL Assembly. | Salt (MgCl2, NaCl) | DS: 1 M MgCl2 FS: DI water or 10 mM NaCl | 3# LBL FO in FO mode at FS: DI DS 1 M MgCl2 Jw = 28.7 LMH Js = 0.18 mol/m2h Js/Jw = 6.3 mM 3# LBL FO in PRO mode at FS: DI DS 1 M MgCl2 Jw = 31.7 LMH Js = 0.49 mol/m2h Js/Jw = 15.5 mM | [123] |
| Casted PAN substrate | Sigma-Aldrich PAN (150,000 Da) | - | Phase inversion of 18 wt % PAN | Hydrolysis Poly(allylamine hydrochloride) PAH/ poly(sodium 4-styrene-sulfonate PSS layers by LBL Assembly. | Salt (MgCl2, MgSO4, and Na2SO4) | FS: DI DS: MgCl2 | FO mode Jw = 20–30 LMH PRO mode Jw = 40–60 LMH | [121] |
| Casted Double-skinned PAN substrate | Sigma-Aldrich PAN (150,000 Da) | - | Phase inversion of 18 wt % PAN | Hydrolysis PAH/PSS LBL assembly and crosslinking | Salt (MgCl2) | FS: DI DS:0.5 M MgCl2 | xLBL3-0 Jw = 58.9 LMH at FS: DI, PRO mode xLBL3-0 Jw = 48.8 LMH at FS: 10 mM NaCl, PRO mode | [118] |
| Casted PAN substrate | Sigma-Aldrich PAN (150,000Da) | - | Phase inversion of 18 wt % PAN | Hydrolysis PAH/PSS LBL assembly GA crosslinking | Salt (MgCl2) | FS: DI DS: 3 M MgCl2 | XLBL-3 in PRO mode Jw = 105.4 LMH Js/Jw = 3 mM R% by RO = 95% (500 ppm MgCl2) | [124] |
| Casted PAN substrate | PAN, Mw ~50,000 Da) was supplied by the Shanghai Jingshan Petrochemical Company (China). | Phase inversion of 18 wt % PAN | Hydrolysis LBL using PEI and PSS. Liposomes and Proteoliposomes spreading. | Salt (MgCl2) | FS: DI DS:2 M MgCl2 | FO mode Jw = 13.2 LMH Js = 3.2 gMH PRO mode Jw = 15.6 LMH Js = 3.4 gMH | [117] | |
| Casted PAN substrate | Sigma-Aldrich PAN (150,000 Da) | 20 mg GO | Phase inversion of 12 wt % PAN | PDA/GO coating. PEI/PAA deposition IP of PA forming by LBL | Salt (NaCl) | FS: DI DS: 1 M NaCl | Nonwoven-PAN150-mLBL1 Jw = 10 LMH Nonwoven-PAN150-mLBL1 Js = 10.4 gMH PA forming by modified mLBL method (PAN-300 thickness) Jw = 17.6 LMH Js = 5.5 gMH | [119] |
| Casted PAN substrate | PAN, Mw: 150,000 Da Macklin | 0.5 wt % of Cyclohexylamin | NIPS- Phase inversion of 16.7 wt % PAN | IP (Cyclohexylamine into MPD aqueous phase) | Salt sodium hypochlorite NaClO | FS: DI, 0.5 NaCl DS: 2 M MgCl2 | For TMC-1 with 0.5 wt % of Cyclohexylamin in FO mode Jw = 13.2 LMH Js = 9.3 gMH Salt R% = 98.5% For TFC-0 Jw = 12.4 LMH Js = 7.1 gMH Salt R% = 98.5% The water flux of the optimal modified membrane was 10.78 LMH after chlorine exposure. | [126] |
| Casted PAN substrate | PAN was provided by Prof. Hui-An Tsai of Chung Yuan Christian University (Taiwan) and vacuum-dried at 80 °C in an oven before use. | - | Phase inversion of 18 wt % PAN | Hydrolysis IP | Salt Bacteria | FS: DI DS: 2 M NaCl | M-Ag Jw = 45 LMH in PRO mode Jw = 30 LMH in FO mode Js = 0.32 mole/m2h in PRO mode Js = 0.24 mole/m2h in FO mode | [127] |
| Casted PAN substrate | Sigma-Aldrich PAN (150,000 Da) | 0.01 wt % of AgNPs | Phase inversion of 18 wt % PAN | PAH/PSS+ AgNPs LBL GA crosslinking | Salt Bacteria | FS: DI,10 mM NaCl DS: 0.5 M MgCl2 | xLBL2.5-Ag (into PSS1) Jw = 43 LMH in PRO mode Jw = 18 LMH in FO mode Js/Jw = 0.07 g/L in PRO mode Js/Jw = 0.17 g/L in FO mode | [19] |
| Cased PAN Substrate | PAN Mw of 1,000,000 Da provided by Prof. Hui-An Tsai from Chung Yuan Christian University (Taiwan) and was vacuum-dried overnight at 60 C | - | Phase inversion of 18 wt % PAN | Hydrolysis PAH/PSS LBL assembly GA+UV crosslinking | Salt | FS: DI DS: 0.5 M MgCl2 DS: 0.5 M NaCl | At PRO mode For 3 LBL assembly Jw = 55 LMH Js = 7.5 gMH | [120] |
| Casted PAN substrate | PAN was provided by Prof. Hui-An Tsai of Chung Yuan Christian University (Taiwan) and was vacuum-dried overnight at 60 C. | - | Phase inversion of 16 wt % PAN | Hydrolysis IP | Salt Oil | FS: oily water solutions 0 ppm, 500, 5000, 50,000, 200,000 DS: 1 M NaCl. | HPAN-TFC = 11.8 LMH for FS 200,000 ppm and DS: 1 M NaCl. | [128] |
| Casted PAN substrate | PAN-MWCO 250,000 Da) was purchased from Hubei Chushengwei Corporation (Hubei, China), | - | Phase inversion of 16 wt % PAN | Hydrolysis IP (MPD+NPED (N-[3-(trimethoxysilyl) propyl] ethylenediamine) + TMC crosslinking | Salt Alginate Bovine serum albumin (BSA) polysaccharides-abundant wastewater | FS: DI DS: 0.5 and 2 M NaCl | TFC-0 at FO mode DS 0.5 M NaCl Jw = 9.67 LMH Js = 1.7 gMH R% of NaCl = 96.6% TFC-NPED 1.5 w/v% on HPAN Jw = 16.7 LMH Js = 10 gMH R% of NaCl = 94.2% | [129] |
| Casted PAN substrate | PAN was provided by Prof. Hui-An Tsai of Chung Yuan Christian University (Taiwan) and was vacuum-dried overnight at 60 C. | - | Phase inversion of 14 wt % PAN. Nexar copolymer 0.05 to 2 wt % | Nexar deposition IP | Salt | FS: DI DS: 0.5 M NaCl | In PRO mode for double-skinned (TFC and Nexar copolymer) membrane Jw = 17.2 LMH Js = 4.85 gMH In PRO mode for single-skinned (TFC) membrane Jw = 18.5 LMH Js = 5.25 gMH In FO mode for single-skinned (TFC) membrane Jw = 12.8 LMH Js = 3.43 gMH | [130] |
| Casted PAN substrate | PAN, Mw ∼150,000 Da, from Sigma Aldrich) | - | Phase inversion of 18 wt % PAN | Hydrolysis | Salt PSS | FS: DI DS: 0.1% PSS | Jw = 7.6 LMH R% of NaCl = 0 R% of PSS (70 kDa) = 97.5% | [131] |
| Casted PAN substrate | PAN Mw: 150,000 Da, from Sigma-Aldrich | - | Phase inversion of 12 wt % PAN | IP | Salt | FS: Anaerobic fluidized-bed reactor effluent DS: 0.5 or 1 M NaCl | PAN-TFC DS 0.5 M NaCl R% of NH4-N = 70% Js= 0.92 gMH Jw = 23.2 LMH | [132] |
| Casted PAN substrate | Sigma-Aldrich PAN (150,000 Da) | - | Phase inversion of 18 wt % PAN | TA/Fe coating | Salt Dye | FS: DI DS: 46.9 mM sodium polyacrylate (PAANa) | TA/Fe-PAN At FO mode R% of NaCl = 27.6% by RO test. R% of Sunset yellow = 99.5% by RO test. R% of PAANa = 96.7% by RO test. Jw = 22.5 LMH | [133] |
5.2. Electrospun PAN-Based Nanofiber Membranes in the FO Process
| Type of PAN Membrane | MWCO of PAN Polymer | Fillers-Optimal Loading wt % | Fabrication Method (PAN or Blended Nanofiber). | Modification Techniques | Solute Type/Applications | DS and FS | Achieved Parameters under FO Test. | Voltage | References |
|---|---|---|---|---|---|---|---|---|---|
| Nanofiber PAN | Mw = 150,000 Da supplied by Macklin, Shanghai, China. | - | Electrospinning (PAN nanofiber) | IP | Salt (NaCl) | FS: DI DS: 1 M NaCl | At FO mode. Jw = 16 LMH Js = 4 gMH | 30 kV | [17] |
| Nanofiber PAN | PAN Mw = 70, 000 Da) supplied by Chushengwei Chemistry Co. 132 Ltd. (Hubei, China). | - | Electrospinning (14 wt % PAN nanofiber) | IP | Salt (NaCl) | FS: DI DS: 1 M NaCl | At FO mode for PAN-1500 rpm Jw = 50.7 LMH Js/Jw = 0.13 g/L At PRO mode for PAN-1500 rpm Jw = 62.9 LMH R% = 90.3% by RO test. | 20 kV | [135] |
| Nanofiber PAN | Sigma-Aldrich PAN (150,000 Da) | - | Electrospinning (9 wt % PAN nanofiber) | IP (PEI+TMC) | Salt, TOC | FS: DI, TOC DS: 10 wt % PEI | Jw (PRO/FO) =24/14 LMH Js = 0.7~1.0 gMH R% of NaCl 30–60% | 17–19 kV | [136] |
| PAN nanofiber | Sigma-Aldrich PAN (150,000 Da) | - | Electrospinning (10 and 12 wt % PAN nanofiber) | IP | Salt (NaCl) | FS: DI DS: 1 M NaCl | FO mode p-TFC membrane Jw = 31.51 LMH Js = 13.55 gMH m-TFC membrane Jw = 28.15 LMH Js = 2.53 gMH | 30 kV | [137] |
| PAN Hollow fibre membrane | Sigma-Aldrich PAN (150,000 Da) | - | Dry-jet-wet spinning (16 wt % PAN nanofiber) | IP | Salt (NaCl) | FS: DI DS: 1 M NaCl | Jw (PRO/FO) = 36.6/24.71 LMH Js (PRO/FO) = 18.75/19.20 gMH Js/Jw (PRO/FO) = 0.57/0.79 g/L | Syringe pump flow rate of 4 mL/min. | [138] |
| PAN Tubular nanofiber | Not available | Electrospinning (10% PAN nanofiber) | Hydrolysis IP | Salt (NaCl) | FS: DI DS: 0.5 M | Jw = 395.1 Js = 0.38 Js/Jw = 0.001 g/L | 20 kV | [139] | |
| Nanofiber PAN+CTA | PAN, 500,000 Da supplied by Shanghai Jinshan Petroleum Co. Ltd. (China). | - | Electrospinning (Blended nanofiber of PAN + CTA) | IP Dopamine hydrochloride DPA+ PEI coating | Salt (NaCl) chitooligosaccharide (COS) | FS: DI DS: 0.1 M chitooligosaccharide (COS), 1 M NaCl | DS: as NaCl Jw (PRO/FO) = 34.2/25.1 LMH Js (PRO/FO) = 9.6/6.1 gMH DS as COS Jw (PRO/FO) = 8.2/4.1 LMH Js = 0 gMH | 14–15 kV | [140] |
| Nanofiber PAN | PAN Mw = 90,000 Da supplied by Kunshan Hongyi Plastic Co. (Suzhou, China). | - | Electrospinning (10 wt % PAN nanofiber) | IP | Salt (NaCl). Antibiotic wastewater (tetracycline hydrochloride TC wastewater). | FS: DI DS: 1 and 2 M NaCl | PA/PAN-eTFC at FO Jw = 41 LMH Js = 8.7 gMH At PRO Jw: 57 LMH at 2 M DS Js: 20 gMH at 2 M DS | 15 kV | [142] |
| Nanofiber PAN | PAN Mw = 150,000 Da supplied by Shaoxing Gimel Advanced Materials Technology Co., Ltd (China). | CS-3.5% | Electrospinning (10 and 12 wt % PAN nanofiber) | Hydrolysis CS sublayer casting. IP | Salt (NaCl). | FS: DI DS: 1.5 M NaCl | For CS-3.5 Jw in PRO/FO: 64.88/55.05 LMH Js in PRO/FO: 2.12/0.93 gMH R% of salt = 97% | 30 kV | [143] |
| Nanofiber PAN | PAN Mw = 150,000 Da supplied by Zhongna Technology Co. Ltd (China). | CS- 3.8% 0.05 wt % of OMWCNTs | Electrospinning (12 wt % PAN nanofiber) | Hydrolysis CS casting sublayer. IP (OMWCNTs into MPD aqueous phase) | Salt (NaCl) Bovine serum albumin (BSA) | FS: DI DS: 0.5 M NaCl | PA-3.8-OMWCNTs at FO mode Jw = 96.9 LMH Js = 0.73 gMH R% of NaCl = 97.4% when FS = 15 mM NaCl | 18 kV | [23] |
| Nanofiber PAN | PAN, 500,000 Da) supplied from Shanghai Jinshang Petroleum Co. Ltd. (China). | CS solution for TFC-CS-PAN-3 contains 1.75 g of CS For TFC-CS-PAN-4 contains 2 g of CS | Electrospinning (10 wt % PAN nanofiber) | CS+ GA crosslinking IP | Salt (NaCl) | DS: 2 M glucose FS; 0.1 M NaCl | TFC-CS-PAN-3 Jw = 11.9 LMH R% of NaCl = 66% TFC-CS-PAN-4 Jw = 10.7 LMH Js = 8.9 gMH salt flux R% of NaCl = 83.5% by RO test. | 15–16 kV | [144] |
| Blended Nanofiber PVDF+PAN | Sigma-Aldrich PAN (150,000 Da) | - | Electrospinning (Blended 18–20 wt % PVDF+ 0–10 wt % PAN nanofiber) | IP | Salt (NaCl) | FS: DI DS: 1 M NaCl | Optimal FO condition Js/Jw: 0.27 g/L Jw: 33.3 LMH Js: 7.8 gMH | 19–21 kV | [54] |
| Blended Nanofiber PSf/PAN | Sigma-Aldrich PAN (150,000 Da) | - | Electrospinning (Blended 20 wt % Psf + 15 wt % PAN nanofiber) | IP | Salt (NaCl, KCl, MgCl2, and MgSO4) | FS: DI DS: 1 M NaCl, 1.06 M KCl, 0.59M MgCl2, and 1.85M MgSO4 | PAN/PSf NTFC at PRO mode Jw = 38.3 LMH Js = 10.1 gMH PAN/PSf TFC at PRO mode Jw = 12.6 LMH Js = 11.6 gMH | 20 kV | [145] |
| Blended nanofiber PES/PAN | Sigma-Aldrich PAN (150,000 Da) | - | Electrospinning (Blended 18, 20, 22 wt % PES + 0–10 wt % PAN nanofiber) | IP | Salt (NaCl) | FS: DI DS: 1 M NaCl | NTFC-10 at FO mode Jw = 42.1 LMH Js/Jw = 0.27 Js = 11.4 gMH NTFC-10 at PRO mode Jw = 52.2 LMH Js/Jw = 0.24 | 21 kV | [53] |
| Blended nanofiber CA/PAN | Sigma-Aldrich PAN (150,000 Da) | - | Electrospinning (Blended CA +PAN nanofiber) Ration of PAN/CA = 0/10 to 2/8, 5/5, 8/2, and 10/0 | IP | Salt (NaCl) | FS: DI DS: 1.5 M NaCl | FO mode for PAN-20CA Jw = 44 LMH Js = 4 gMH PRO mode Jw = 55 LMH Js = 11.5 gMH | 28.5 kV | [146] |
| SiO2/PAN nanofibrous | Sigma-Aldrich PAN (150,000 Da) | 15 wt/wt % of SiO2 NPs | Electrospinning (SiO2 NPs +12 wt % PAN nanofiber) | IP | Salt (NaCl) | FS: DI DS: 1 M NaCl | At FO mode. Jw: 58 LMH Js: 8.7 gMH Js/Jw: 0.15 g/L At PRO mode. Jw: 82 LMH Js: 11.5 gMH | 28.5 kV | [21] |
| AgNO3 /PAN nanofibrous | PAN, Mw = 90,000Da) was purchased from Kunshan Hongyu Plastic Co., Ltd. (China). | 2 wt % of AgNO3 | Electrospinning (AgNO3+ 10 wt % PAN nanofiber) | IP | Salt (NaCl) | FS: DI DS: 0.5 M NaCl | Jw:(PRO/FO) = 29.21/21.58 LMH Js:(PRO/FO) = 17.5/7.5 gMH | 15 kV | [51] |
| PAN nanofiber | PAN, Mw = 250,000 Da was purchased from DuPont Co., Ltd. | 0.2 wt % Dopamine modified HNTs | Electrospinning (14 wt % PAN nanofiber) | Dopamine coating Vacuum filtrating modified HNTs. IP | Salt (NaCl) | FS: DI DS: 0.5 M–2 M NaCl | At FO mode and DS 1 M Jw = 28 LMH Js = 2.8 gMH At PRO mode and DS 1 M Jw = 45 LMH Js = 4.2 gMH | 17 kV | [147] |
| PAN nanofiber | PAN, Mw = 150,000 Da) was purchased from Kunshan Hongyi Plastic Co., Ltd. (China). | 2 wt % of CNTs | Electrospinning (12 wt % PAN nanofiber) | CNTs interlayer IP | Salt (NaCl) | FS: DI DS: 1 M NaCl | PAN-CNTs-2 In PRO- Jw = 61.6 LMH In PRO- Js = 7.7 gMH In FO- Jw = 49.2 LMH In FO- Js = 7.2 gMH | 15 kV | [20] |
| PAN nanofiber | PAN, Mw = 250,000 Da) were obtained from China National Petroleum Corporation | 6 mL of PDA NPs | Electrospinning (17 wt % PAN nanofiber) | PDA NPs vacuum filtered as an interlayer. IP | Salt (NaCl) Heavy metal removal (Cu+2) | FS: DI DS: 1 M NaCl | TFC-6 mL PDA NPs Jw = 28.5 LMH R% of Cu+2 = 97% | 16 kV | [148] |
| PAN nanofiber | PAN powder from Sigma-Aldrich | 28 μg/cm2 of GO | Electrospinning (10 wt % PAN nanofiber) | GO vacuum filtered as an interlayer. IP | Salt (NaCl) | FS: DI DS: 1 M NaCl | At FO mode SRSF: 0.26 g/L Jw: 32.7 LMH Js: 8.5 gMH | 21 kV | [149] |
| As spun-PBI–POSS/PAN nanofiltration hollow fibre membranes, | PAN copolymer was provided by Prof. Hui-An Tsai from Chung Yuan Christian University, Taiwan | 0.5 wt % of POSS | Spinning (As-spun PBI–POSS/PAN) | (PAN for inner substrate layer) (PBI and POSS for outer selective layer) | Salt (MgCl2, NaCl) | FS: DI DS: 2 M MgCl2 | FO mode for As-spun PBI–POSS/PAN Jw = 17.7 LMH Js = 27.6 gMH Js //Jw = 1.6 g/L FO mode for Annealed PBI–POSS/PAN Jw = 12.6 LMH Js = 8.8 gMH Js //Jw = 0.7 g/L | Outer dope flow rate = 6 m/min | [150] |
| As spun-PBI–POSS/PAN dual-layer hollow fibre membranes. | PAN copolymer was provided by Prof. Hui-An Tsai from Chung Yuan Christian University, Taiwan | 0.5 wt % of POSS | Spinning (As-spun PBI–POSS/ 16 wt % PAN) | (PAN for inner substrate layer) (PBI and POSS for outer selective layer) | Salt (MgCl2 and NaCl) | FS: DI DS: 2 M MgCl2 for FO process DS: 1 M NaCl for PRO process | FO process Jw = 31.37 LMH R% of MgCl2 = 92.3 % R% of NaCl = 81.6 % | Outer dope flow rate = 6 m/min. | [151] |
| Hollow fibre PAN/ Ionic liquid | PAN, Mw = 324,000 Da. | - | Spinning (12 wt % PAN) + 80 wt % Ionic liquid | IP | Sucrose | FS: DI DS 1 M and 2 M sucrose | Jw = 6.7 LMH Js = 0 gMH | Flow rate of dope solution = 2.8 mL/min Flow rate of inner coagulant = 3 mL/min | [152] |
5.3. Commercial PAN-Based Membranes in FO Test
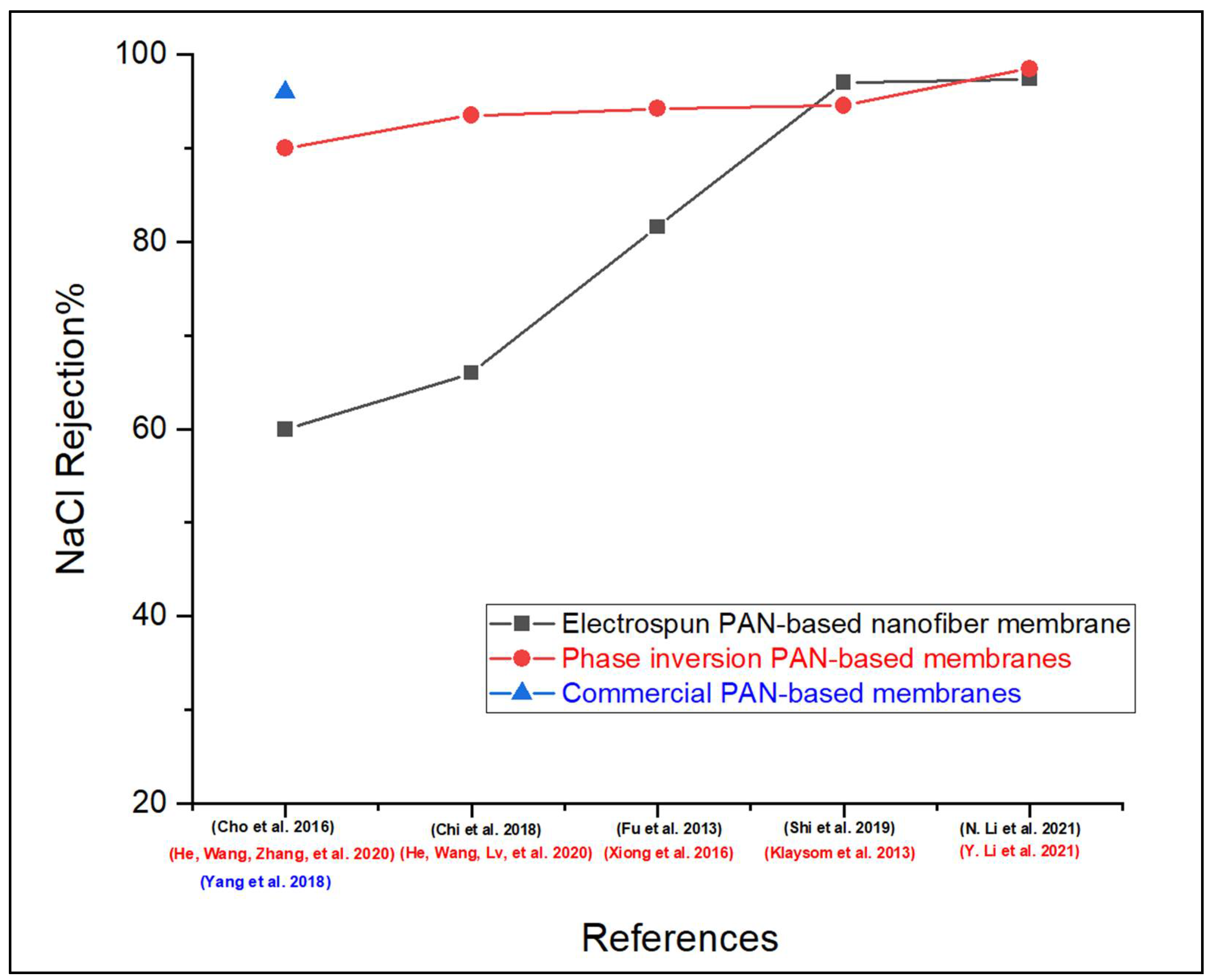
6. Performance Comparison of PAN-Based FO Membranes
7. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Riener, K.; Oswald, S.; Winkler, M.; Leichtfried, G.J. Influence of Storage Conditions and Reconditioning of AlSi10Mg Powder on the Quality of Parts Produced by Laser Powder Bed Fusion (LPBF). Addit. Manuf. 2021, 39, 101896. [Google Scholar] [CrossRef]
- Abounahia, N.; Ibrar, I.; Kazwini, T.; Altaee, A.; Samal, A.K.; Zaidi, S.J.; Hawari, A.H. Desalination by the Forward Osmosis: Advancement and Challenges. Sci. Total Environ. 2023, 886, 163901. [Google Scholar] [CrossRef]
- Han, G. Development and Fabrication of Thin Film Composite (TFC) Membranes for Engineered Osmosis Processes; National University of Singapore: Singapore, 2013. [Google Scholar]
- Altaee, A.; Braytee, A.; Millar, G.J.; Naji, O. Energy Efficiency of Hollow Fibre Membrane Module in the Forward Osmosis Seawater Desalination Process. J. Membr. Sci. 2019, 587, 117165. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X. Forward Osmosis Technology for Water Treatment: Recent Advances and Future Perspectives. J. Clean. Prod. 2020, 280, 124354. [Google Scholar] [CrossRef]
- Yadav, S.; Saleem, H.; Ibrar, I.; Naji, O.; Hawari, A.A.; Alanezi, A.A.; Zaidi, S.J.; Altaee, A.; Zhou, J. Recent Developments in Forward Osmosis Membranes Using Carbon-Based Nanomaterials. Desalination 2020, 482, 114375. [Google Scholar] [CrossRef]
- Kim, B.; Gwak, G.; Hong, S. Review on Methodology for Determining Forward Osmosis ( FO ) Membrane Characteristics: Water Permeability (A), Solute Permeability (B), and Structural Parameter (S). Desalination 2017, 422, 5–16. [Google Scholar] [CrossRef]
- Alihemati, Z.; Hashemifard, S.A.; Matsuura, T.; Ismail, A.F.; Hilal, N. Current Status and Challenges of Fabricating Thin Film Composite Forward Osmosis Membrane: A Comprehensive Roadmap. Desalination 2020, 491, 114557. [Google Scholar] [CrossRef]
- Worthley, C.H.; Constantopoulos, K.T.; Ginic-markovic, M.; Markovic, E.; Clarke, S. A Study into the Effect of POSS Nanoparticles on Cellulose Acetate Membranes. J. Membr. Sci. 2013, 431, 62–71. [Google Scholar] [CrossRef]
- Mertens, M.; Van Goethem, C.; Koeckelberghs, G.; Vankelecom, I.F.J. Crosslinked PVDF-Membranes Resistant Nanofiltration for Solvent Resistant Nanofiltration. J. Membr. Sci. 2018, 566, 223–230. [Google Scholar] [CrossRef]
- Abedi, M.; Sadeghi, M.; Pourafshari Chenar, M. Improving Antifouling Performance of PAN Hollow Fiber Membrane Using Surface Modification Method. J. Taiwan Inst. Chem. Eng. 2015, 55, 42–48. [Google Scholar] [CrossRef]
- Wu, D.; Han, Y.; Salim, W.; Kai, K.; Li, J.; Ho, W.S.W. Hydrophilic and Morphological Modification of Nanoporous Polyethersulfone Substrates for Composite Membranes in CO2 Separation. J. Membr. Sci. 2018, 565, 439–449. [Google Scholar] [CrossRef]
- Khan, A.; Sherazi, T.A.; Khan, Y.; Li, S.; Ali, S.; Naqvi, R. Fabrication and Characterization of Polysulfone/Modified Nanocarbon Black Composite Antifouling Ultra Filtration Membranes. J. Membr. Sci. 2018, 554, 71–82. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Raveshiyan, S.; Amini, Y.; Zadhoush, A. A Critical Review with Emphasis on the Rheological Behavior and Properties of Polymer Solutions and Their Role in Membrane Formation, Morphology, and Performance. Adv. Colloid Interface Sci. 2023, 319, 102986. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liew, S.R.; Bai, R. Simultaneous Alkaline Hydrolysis and Non-Solvent Induced Phase Separation Method for Polyacrylonitrile (PAN) Membrane with Highly Hydrophilic and Enhanced Anti-Fouling Performance. J. Membr. Sci. 2021, 635, 119499. [Google Scholar] [CrossRef]
- Hajighahremanzadeh, P.; Abbaszadeh, M.; Mousavi, S.A.; Soltanieh, M.; Bakhshi, H. Polyamide/Polyacrylonitrile Thin Film Composites as Forward Osmosis Membranes. J. Appl. Polym. Sci. 2016, 133, 44130. [Google Scholar] [CrossRef]
- Al-Furaiji, M.; Kadhom, M.; Kalash, K.; Waisi, B.; Albayati, N. Preparation of Thin-Film Composite Membranes Supported with Electrospun Nanofibers for Desalination by Forward Osmosis. Drink. Water Eng. Sci. 2020, 13, 51–57. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Qi, S.; Liu, X.; Li, Y.; Huo, F.; Tang, C.Y. Synthesis and Characterization of Silica Gel-Polyacrylonitrile Mixed Matrix Forward Osmosis Membranes Based on Layer-by-Layer Assembly. Sep. Purif. Technol. 2014, 124, 207–216. [Google Scholar] [CrossRef]
- Liu, X.; Qi, S.; Li, Y.; Yang, L.; Cao, B.; Tang, C.Y. Synthesis and Characterization of Novel Antibacterial Silver Nanocomposite Nanofiltration and Forward Osmosis Membranes Based on Layer-by-Layer Assembly. Water Res. 2013, 47, 3081–3092. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, Y.; Liu, Z.; Huang, H.; Fan, X.; Wang, Y.; Song, Y.; Song, C. Preparation and Characterization of High-Performance Electrospun Forward Osmosis Membrane by Introducing a Carbon Nanotube Interlayer. J. Membr. Sci. 2020, 616, 118563. [Google Scholar] [CrossRef]
- Bui, N.N.; McCutcheon, J.R. Nanoparticle-Embedded Nanofibers in Highly Permselective Thin-Film Nanocomposite Membranes for Forward Osmosis. J. Membr. Sci. 2016, 518, 338–346. [Google Scholar] [CrossRef]
- Lee, J.-Y.; She, Q.; Huo, F.; Tang, C.Y. Metal-Organic Framework-Based Porous Matrix Membranes for Improving Mass Transfer in Forward Osmosis Membranes. J. Membr. Sci. 2015, 492, 392–399. [Google Scholar] [CrossRef]
- Li, N.; Han, Y.; Yu, F.; Wang, W.; Liu, Q.; Qian, X.; Xu, Z.; Deng, H.; Shan, M. Decorating a Loose Defect-Free Hybrid Selective Layer on a Smooth Intermediary: An Effective Way for Unexpected Performances of Nanofiber-Based Forward Osmosis Membranes. ChemNanoMat 2021, 7, 184–199. [Google Scholar] [CrossRef]
- Cath, T.Y.; Childress, A.E.; Elimelech, M. Forward Osmosis: Principles, Applications, and Recent Developments. J. Membr. Sci. 2006, 281, 70–87. [Google Scholar] [CrossRef]
- Khamseh, A.G.; Ghorbanian, S.A. Experimental and Modeling Investigation of Thorium Biosorption by Orange Peel in a Continuous Fixed-Bed Column. J. Radioanal. Nucl. Chem. 2018, 317, 871–879. [Google Scholar] [CrossRef]
- Khani, M.H.; Khamseh, A.G. Statistical Analysis, Equilibrium and Dynamic Study on the Biosorption of Strontium Ions on Chlorella Vulgaris. J. Radioanal. Nucl. Chem. 2023, 332, 3325–3334. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.H.; Show, P.L. A Review on Conventional and Novel Materials towards Heavy Metal Adsorption in Wastewater Treatment Application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Al-Enezi, G.; Hamoda, M.F.; Fawzi, N. Ion Exchange Extraction of Heavy Metals from Wastewater Sludges. J. Environ. Sci. Health—Part A Toxic/Hazard. Subst. Environ. Eng. 2004, 39, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lv, P.; He, Y.; Li, S.; Chen, K.; Yin, S. Purification of Chlorine-Containing Wastewater Using Solvent Extraction. J. Clean. Prod. 2020, 273, 122863. [Google Scholar] [CrossRef]
- Zhao, S.; Zou, L.; Tang, C.Y.; Mulcahy, D. Recent Developments in Forward Osmosis: Opportunities and Challenges. J. Membr. Sci. 2012, 396, 1–21. [Google Scholar] [CrossRef]
- Phuntsho, S.; Hong, S.; Elimelech, M.; Shon, H.K. Osmotic Equilibrium in the Forward Osmosis Process: Modelling, Experiments and Implications for Process Performance. J. Membr. Sci. 2014, 453, 240–252. [Google Scholar] [CrossRef]
- Benavides, S.; Oloriz, A.S.; Phillip, W.A. Forward Osmosis Processes in the Limit of Osmotic Equilibrium. Ind. Eng. Chem. Res. 2015, 54, 480–490. [Google Scholar] [CrossRef]
- Shaffer, D.L.; Werber, J.R.; Jaramillo, H.; Lin, S.; Elimelech, M. Forward Osmosis: Where Are We Now? Desalination 2015, 356, 271–284. [Google Scholar] [CrossRef]
- Phuntsho, S.; Sahebi, S.; Majeed, T.; Lotfi, F.; Kim, J.E.; Shon, H.K. Assessing the Major Factors Affecting the Performances of Forward Osmosis and Its Implications on the Desalination Process. Chem. Eng. J. 2013, 231, 484–496. [Google Scholar] [CrossRef]
- Kim, W.J.; Campanella, O.; Heldman, D.R. A Stepwise Approach to Predict the Performance of Forward Osmosis Operation: Effect of Temperature and Flow Direction. Desalination 2022, 538, 115889. [Google Scholar] [CrossRef]
- Nguyen, T.P.N.; Jun, B.M.; Kwon, Y.N. The Chlorination Mechanism of Integrally Asymmetric Cellulose Triacetate (CTA)-Based and Thin Film Composite Polyamide-Based Forward Osmosis Membrane. J. Membr. Sci. 2017, 523, 111–121. [Google Scholar] [CrossRef]
- Lin, X.; He, Y.; Zhang, Y.; Yu, W.; Lian, T. Sulfonated Covalent Organic Frameworks (COFs) Incorporated Cellulose Triacetate/Cellulose Acetate (CTA/CA)-Based Mixed Matrix Membranes for Forward Osmosis. J. Membr. Sci. 2021, 638, 119725. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Yuan, B.; Wang, Z.; Li, X.; Ren, Y. Comparison of Biofouling Mechanisms between Cellulose Triacetate (CTA) and Thin-Film Composite (TFC) Polyamide Forward Osmosis Membranes in Osmotic Membrane Bioreactors. Bioresour. Technol. 2016, 202, 50–58. [Google Scholar] [CrossRef]
- Nguyen, T.P.N.; Yun, E.T.; Kim, I.C.; Kwon, Y.N. Preparation of Cellulose Triacetate/Cellulose Acetate (CTA/CA)-Based Membranes for Forward Osmosis. J. Membr. Sci. 2013, 433, 49–59. [Google Scholar] [CrossRef]
- Li, J.Y.; Ni, Z.Y.; Zhou, Z.Y.; Hu, Y.X.; Xu, X.H.; Cheng, L.H. Membrane Fouling of Forward Osmosis in Dewatering of Soluble Algal Products: Comparison of TFC and CTA Membranes. J. Membr. Sci. 2018, 552, 213–221. [Google Scholar] [CrossRef]
- Yu, Y.; Seo, S.; Kim, I.C.; Lee, S. Nanoporous Polyethersulfone (PES) Membrane with Enhanced Flux Applied in Forward Osmosis Process. J. Membr. Sci. 2011, 375, 63–68. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Z.; Zhang, J.; Song, P.; Liu, L. TIPS-Co-NIPS Method to Prepare PES Substrate with Enhanced Permeability for TFC-FO Membrane. J. Taiwan Inst. Chem. Eng. 2017, 80, 137–148. [Google Scholar] [CrossRef]
- Nasr, M.; Alfryyan, N.; Ali, S.S.; Abd El-Salam, H.M.; Shaban, M. Preparation, Characterization, and Performance of PES/GO Woven Mixed Matrix Nanocomposite Forward Osmosis Membrane for Water Desalination. RSC Adv. 2022, 12, 25654–25668. [Google Scholar] [CrossRef]
- Salehi, H.; Shakeri, A.; Lammertink, R.G.H. Thermo-Responsive Graft Copolymer PSf-g-PNIPM: Reducing the Structure Parameter via Morphology Control of Forward Osmosis Membrane Substrates. J. Membr. Sci. 2022, 661, 120794. [Google Scholar] [CrossRef]
- Ghalavand, R.; Mokhtary, M.; Shakeri, A.; Alizadeh, O. ZnO@PMMA Incorporated PSf Substrate for Improving Thin-Film Composite Membrane Performance in Forward Osmosis Process. Chem. Eng. Res. Des. 2022, 177, 594–603. [Google Scholar] [CrossRef]
- Sirinupong, T.; Youravong, W.; Tirawat, D.; Lau, W.J.; Lai, G.S.; Ismail, A.F. Synthesis and Characterization of Thin Film Composite Membranes Made of PSF-TiO2/GO Nanocomposite Substrate for Forward Osmosis Applications. Arab. J. Chem. 2018, 11, 1144–1153. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, L.; Guan, C.Y.; Liu, C.X.; Lang, W.Z.; Wang, Y. Construction of SiO2@MWNTs Incorporated PVDF Substrate for Reducing Internal Concentration Polarization in Forward Osmosis. J. Membr. Sci. 2018, 564, 328–341. [Google Scholar] [CrossRef]
- Tian, M.; Qiu, C.; Liao, Y.; Chou, S.; Wang, R. Preparation of Polyamide Thin Film Composite Forward Osmosis Membranes Using Electrospun Polyvinylidene Fluoride (PVDF) Nanofibers as Substrates. Sep. Purif. Technol. 2013, 118, 727–736. [Google Scholar] [CrossRef]
- Park, M.J.; Gonzales, R.R.; Abdel-Wahab, A.; Phuntsho, S.; Shon, H.K. Hydrophilic Polyvinyl Alcohol Coating on Hydrophobic Electrospun Nanofiber Membrane for High Performance Thin Film Composite Forward Osmosis Membrane. Desalination 2018, 426, 50–59. [Google Scholar] [CrossRef]
- Huang, L.; Arena, J.T.; McCutcheon, J.R. Surface Modified PVDF Nanofiber Supported Thin Film Composite Membranes for Forward Osmosis. J. Membr. Sci. 2016, 499, 352–360. [Google Scholar] [CrossRef]
- Pan, S.F.; Ke, X.X.; Wang, T.Y.; Liu, Q.; Zhong, L.B.; Zheng, Y.M. Synthesis of Silver Nanoparticles Embedded Electrospun PAN Nanofiber Thin-Film Composite Forward Osmosis Membrane to Enhance Performance and Antimicrobial Activity. Ind. Eng. Chem. Res. 2019, 58, 984–993. [Google Scholar] [CrossRef]
- Klaysom, C.; Hermans, S.; Gahlaut, A.; Van Craenenbroeck, S.; Vankelecom, I.F.J. Polyamide/Polyacrylonitrile (PA/PAN) Thin Film Composite Osmosis Membranes: Film Optimization, Characterization and Performance Evaluation. J. Membr. Sci. 2013, 445, 25–33. [Google Scholar] [CrossRef]
- Kallem, P.; Banat, F.; Yejin, L.; Choi, H. High Performance Nanofiber-Supported Thin Film Composite Forward Osmosis Membranes Based on Continuous Thermal-Rolling Pretreated Electrospun PES/PAN Blend Substrates. Chemosphere 2020, 261, 127687. [Google Scholar] [CrossRef]
- Kallem, P.; Gaur, R.; Pandey, R.P.; Hasan, S.W.; Choi, H.; Banat, F. Thin Film Composite Forward Osmosis Membranes Based on Thermally Treated PAN Hydrophilized PVDF Electrospun Nanofiber Substrates for Improved Performance. J. Environ. Chem. Eng. 2021, 9, 106240. [Google Scholar] [CrossRef]
- Ndiaye, I.; Chaoui, I.; Eddouibi, J.; Vaudreuil, S.; Bounahmidi, T. Synthesis of Poly (Vinylidene Fluoride)/Polyacrylonitrile Electrospun Substrate-Based Thin-Film Composite Membranes for Desalination by Forward Osmosis Process. Chem. Eng. Process. Process Intensif. 2022, 181, 109132. [Google Scholar] [CrossRef]
- Matveev, D.N.; Plisko, T.V.; Volkov, V.V.; Vasilevskii, V.P.; Bazhenov, S.D.; Shustikov, A.A.; Chernikova, E.V.; Bildyukevich, A.V. Ultrafiltration Membranes Based on Various Acrylonitrile Copolymers. Membr. Membr. Technol. 2019, 1, 386–393. [Google Scholar] [CrossRef]
- Choi, W.; Jeon, S.; Kwon, S.J.; Park, H.; Park, Y.I.; Nam, S.E.; Lee, P.S.; Lee, J.S.; Choi, J.; Hong, S.; et al. Thin Film Composite Reverse Osmosis Membranes Prepared via Layered Interfacial Polymerization. J. Membr. Sci. 2017, 527, 121–128. [Google Scholar] [CrossRef]
- Nataraj, S.K.; Yang, K.S.; Aminabhavi, T.M. Polyacrylonitrile-Based Nanofibers—A State-of-the-Art Review. Prog. Polym. Sci. 2012, 37, 487–513. [Google Scholar] [CrossRef]
- Adegbola, T.A.; Agboola, O.; Fayomi, O.S.I. Review of Polyacrylonitrile Blends and Application in Manufacturing Technology: Recycling and Environmental Impact. Results Eng. 2020, 7, 100144. [Google Scholar] [CrossRef]
- Noor, A.B.M.N. Development of Polyacrylonitrile/Polyacrylonitrile-g- Poly(Vinyl Alcohol) Hollow Fiber Ultrafiltration Membranes with Enhanced Anti-Fouling Properties; Universiti Teknologi Malaysia: Johor, Malaysia, 2015; Volume 59. [Google Scholar]
- Liu, F.; Wang, L.; Li, D.; Liu, Q.; Deng, B. A Review: The Effect of the Microporous Support during Interfacial Polymerization on the Morphology and Performances of a Thin Film Composite Membrane for Liquid Purification. RSC Adv. 2019, 9, 35417–35428. [Google Scholar] [CrossRef] [PubMed]
- Scharnagl, N.; Buschatz, H. Polyacrylonitrile (PAN) Membranes for Ultra- and Microfiltration. Desalination 2001, 139, 191–198. [Google Scholar] [CrossRef]
- Palchikova, E.E.; Makarov, I.S.; Mironova, M.V.; Vinogradov, M.I.; Golova, L.K.; Kulichikhin, V.G. Phase Transformations in a PAN–N-Methylmorpholine-N-Oxide–Water System. Colloid J. 2022, 84, 730–740. [Google Scholar] [CrossRef]
- Makarov, I.S.; Vinogradov, M.I.; Golova, L.K.; Arkharova, N.A.; Shambilova, G.K.; Makhatova, V.E.; Naukenov, M.Z. Design and Fabrication of Membranes Based on PAN Copolymer Obtained from Solutions in N-Methylmorpholine-N-Oxide. Polymers 2022, 14, 2861. [Google Scholar] [CrossRef]
- Kulichikhin, V.; Golova, L.; Makarov, I.; Bondarenko, G.; Makarova, V.; Ilyin, S.; Skvortsov, I.; Berkovich, A. Solutions of Acrylonitrile Copolymers in N-Methylmorpholine-N-Oxide: Structure, Properties, Fiber Spinning. Eur. Polym. J. 2017, 92, 326–337. [Google Scholar] [CrossRef]
- Pérez-Álvarez, L.; Ruiz-Rubio, L.; Moreno, I.; Vilas-Vilela, J.L. Characterization and Optimization of the Alkaline Hydrolysis of Polyacrylonitrile Membranes. Polymers 2019, 11, 1843. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.; Neogi, S.; De, S. Treatment of Polyacrylonitrile Co-Polymer Membrane by Low Temperature Radio-Frequency Nitrogen Plasma. Polym. Adv. Technol. 2018, 29, 775–784. [Google Scholar] [CrossRef]
- Qin, Y.; Yang, H.; Xu, Z.; Li, F. Surface Modification of Polyacrylonitrile Membrane by Chemical Reaction and Physical Coating: Comparison between Static and Pore-Flowing Procedures. ACS Omega 2018, 3, 4231–4241. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.G.; Wan, L.S.; Xu, Z.K. Surface Engineerings of Polyacrylonitrile-Based Asymmetric Membranes towards Biomedical Applications: An Overview. J. Membr. Sci. 2007, 304, 8–23. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Reis, R.; Chen, Z.; Milne, N.; Winther-Jensen, B.; Kong, L.; Dumée, L.F. Plasma Modification and Synthesis of Membrane Materials—A Mechanistic Review. Membranes 2018, 8, 56. [Google Scholar] [CrossRef]
- Pal, D.; Neogi, S.; De, S. Improved Antifouling Characteristics of Acrylonitrile Co-Polymer Membrane by Low Temperature Pulsed Ammonia Plasma in the Treatment of Oil–Water Emulsion. Vacuum 2016, 131, 293–304. [Google Scholar] [CrossRef]
- Zhou, H.; Su, Y.; Chen, X.; Luo, J.; Tan, S.; Wan, Y. Plasma Modification of Substrate with Poly(Methylhydrosiloxane) for Enhancing the Interfacial Stability of PDMS/PAN Composite Membrane. J. Membr. Sci. 2016, 520, 779–789. [Google Scholar] [CrossRef]
- Hochart, F.; Levalois-Mitjaville, J.; De Jaeger, R.; Gengembre, L.; Grimblot, J. Plasma Surface Treatment of Poly(Acrylonitrile) Films by Fluorocarbon Compounds. Appl. Surf. Sci. 1999, 142, 574–578. [Google Scholar] [CrossRef]
- Yalcinkaya, F.; Siekierka, A.; Bryjak, M. Surface Modification of Electrospun Nanofibrous Membranes for Oily Wastewater Separation. RSC Adv. 2017, 7, 56704–56712. [Google Scholar] [CrossRef]
- Al-Jumaili, A.; Alancherry, S.; Grant, D.; Kumar, A.; Bazaka, K.; Jacob, M.V. Plasma Treatment of Polymeric Membranes; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128131527. [Google Scholar]
- Kang, Y.H.; Ahn, K.; Jeong, S.Y.; Bae, J.S.; Jin, J.S.; Kim, H.G.; Hong, S.W.; Cho, C.R. Effect of Plasma Treatment on Surface Chemical-Bonding States and Electrical Properties of Polyacrylonitrile Nanofibers. Thin Solid Films 2011, 519, 7090–7094. [Google Scholar] [CrossRef]
- Tran, T.D.; Mori, S.; Suzuki, M. Plasma Modification of Polyacrylonitrile Ultrafiltration Membrane. Thin Solid Films 2007, 515, 4148–4152. [Google Scholar] [CrossRef]
- Ulbricht, M.; Belfort, G. Surface Modification of Ultrafiltration Membranes by Low Temperature Plasma II. Graft Polymerization onto Polyacrylonitrile and Polysulfone. J. Membr. Sci. 1996, 111, 193–215. [Google Scholar] [CrossRef]
- Roy, D.; Semsarilar, M.; Guthrie, J.T.; Perrier, S. Cellulose Modification by Polymer Grafting: A Review. Chem. Soc. Rev. 2009, 38, 2046–2064. [Google Scholar] [CrossRef] [PubMed]
- Desmet, T.; Morent, R.; De Geyter, N.; Leys, C.; Schacht, E.; Dubruel, P. Nonthermal Plasma Technology as a Versatile Strategy for Polymeric Biomaterials Surface Modification: A Review. Biomacromolecules 2009, 10, 2351–2378. [Google Scholar] [CrossRef]
- Nouzaki, K.; Araib, M.N.J.; Idemotob, Y.; Kourab, N.; Yanagishita, H.; Negishi, H.; Kitamoto, D.; Ikegami, T.; Haraya, K. Preparation of Polyacrylonitrile Ultrafiltration Membranes for Wastewater Treatment. Desalination 2002, 144, 53–59. [Google Scholar] [CrossRef]
- Surface Modification by Graft Polymerization. In Surface Engineering of Polymer Membranes. Advanced Topics in Science and Technology in China; Springer: Berlin/Heidelberg, Germany, 2009; pp. 80–149. [CrossRef]
- Ulbricht, M.; Matuschewski, H.; Oechel, A.; Hicke, H.G. Photo-Induced Graft Polymerization Surface Modifications for the Preparation of Hydrophilic and Low-Protein-Adsorbing Ultrafiltration Membranes. J. Membr. Sci. 1996, 115, 31–47. [Google Scholar] [CrossRef]
- Lai, C.L.; Chao, W.C.; Hung, W.S.; An, Q.; De Guzman, M.; Hu, C.C.; Lee, K.R. Physicochemical Effects of Hydrolyzed Asymmetric Polyacrylonitrile Membrane Microstructure on Dehydrating Butanol. J. Membr. Sci. 2015, 490, 275–281. [Google Scholar] [CrossRef]
- Yang, S.; Zhen, H.; Su, B. Polyimide Thin Film Composite (TFC) Membranes: Via Interfacial Polymerization on Hydrolyzed Polyacrylonitrile Support for Solvent Resistant Nanofiltration. RSC Adv. 2017, 7, 42800–42810. [Google Scholar] [CrossRef]
- Zhang, G.; Meng, H.; Ji, S. Hydrolysis Differences of Polyacrylonitrile Support Membrane and Its Influences on Polyacrylonitrile-Based Membrane Performance. Desalination 2009, 242, 313–324. [Google Scholar] [CrossRef]
- Cheraghali, R.; Maghsoud, Z. Enhanced Modification Technique for Polyacrylonitrile UF Membranes by Direct Hydrolysis in the Immersion Bath. J. Appl. Polym. Sci. 2020, 137, 48583. [Google Scholar] [CrossRef]
- Krentsel, L.B.; Kudryavtsev, Y.V.; Rebrov, A.I.; Litmanovich, A.D.; Platé, N.A. Acidic Hydrolysis of Polyacrylonitrile: Effect of Neighboring Groups. Macromolecules 2001, 34, 5607–5610. [Google Scholar] [CrossRef]
- Yang, W.; Chen, J.; Yan, J.; Liu, S.; Yan, Y.; Zhang, Q. Advance of Click Chemistry in Anion Exchange Membranes for Energy Application. J. Polym. Sci. 2022, 60, 627–649. [Google Scholar] [CrossRef]
- Rein, C.; Nissen, S.; Grzelakowski, M.; Meldal, M. Click-Chemistry of Polymersomes on Nanoporous Polymeric Surfaces. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 2032–2039. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, J.; Wang, T.; Dong, W.; Li, W.; Xing, W. A Novel Catalytic Composite Membrane with Anti-Swelling for Enhancing Esterification of Acetic Acid with Ethanol. Chem. Eng. J. Adv. 2021, 6, 100088. [Google Scholar] [CrossRef]
- Wang, T.; Shi, J.; Liang, Y.; Han, J.; Tong, Y.; Li, W. Novel SPVA/g-C3N4-Sa/PAN Pervaporation Membranes with Porous Catalytic Layers for Esterification Enhancement. Ind. Eng. Chem. Res. 2021, 60, 6089–6100. [Google Scholar] [CrossRef]
- Pérez-Manríquez, L.; Aburabi’E, J.; Neelakanda, P.; Peinemann, K.V. Cross-Linked PAN-Based Thin-Film Composite Membranes for Non-Aqueous Nanofiltration. React. Funct. Polym. 2015, 86, 243–247. [Google Scholar] [CrossRef]
- Zhao, J.; Yangong, Y. Study on Structure and Properties of Polyacrylonitrile Fiber Modified by Hydrazine Hydrate. Adv. Mater. Res. 2012, 548, 24–28. [Google Scholar] [CrossRef]
- Yushkin, A.A.; Efimov, M.N.; Vasilev, A.A.; Karpacheva, G.P.; Volkov, A.V. PAN Filtration Membranes with Extended Solvent Stability. J. Phys. Conf. Ser. 2018, 1099, 012031. [Google Scholar] [CrossRef]
- Li, F.; Dong, Y.; Kang, W.; Cheng, B.; Cui, G. Enhanced Removal of Azo Dye Using Modified PAN Nanofibrous Membrane Fe Complexes with Adsorption/Visible-Driven Photocatalysis Bifunctional Roles. Appl. Surf. Sci. 2017, 404, 206–215. [Google Scholar] [CrossRef]
- Satyanarayana, S.V.; Bhattacharya, P.K. Pervaporation of Hydrazine Hydrate: Separation Characteristics of Membranes with Hydrophilic to Hydrophobic Behaviour. J. Membr. Sci. 2004, 238, 103–115. [Google Scholar] [CrossRef]
- Chaudhary, B.K.; Farrell, J. Preparation and Characterization of Homopolymer Polyacrylonitrile-Based Fibrous Sorbents for Arsenic Removal. Environ. Eng. Sci. 2014, 31, 593–601. [Google Scholar] [CrossRef]
- Pinem, J.A.; Wardani, A.K.; Aryanti, P.T.P.; Khoiruddin, K.; Wenten, I.G. Hydrophilic Modification of Polymeric Membrane Using Graft Polymerization Method: A Mini Review. IOP Conf. Ser. Mater. Sci. Eng. 2019, 547, 012054. [Google Scholar] [CrossRef]
- Zaidi, S.J.; Mauritz, K.A.; Hassan, M.K. Membrane Surface Modification and Functionalization. In Functional Polymers; Springer: Cham, Switzerland, 2018; pp. 1–26. [Google Scholar] [CrossRef]
- Alkhouzaam, A.; Qiblawey, H.; Khraisheh, M. Polydopamine Functionalized Graphene Oxide as Membrane Nanofiller: Spectral and Structural Studies. Membranes 2021, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Qiblawey, H.; Judd, S.; Benamor, A.; Nasser, M.S.; Mohammadian, A. Fabrication of High Flux Nanofiltration Membrane via Hydrogen Bonding Based Co-Deposition of Polydopamine with Poly(Vinyl Alcohol). J. Membr. Sci. 2018, 552, 222–233. [Google Scholar] [CrossRef]
- Abounahia, N.; Qiblawey, H.; Zaidi, S.J. Progress for Co-Incorporation of Polydopamine and Nanoparticles for Improving Membranes Performance. Membranes 2022, 12, 675. [Google Scholar] [CrossRef]
- Alkhouzaam, A.; Qiblawey, H. Functional GO-Based Membranes for Water Treatment and Desalination: Fabrication Methods, Performance and Advantages. A Review. Chemosphere 2021, 274, 129853. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Zhang, X.; Tian, L.; Li, Z.; Ding, C.; Yi, M.; Han, C.; Yu, X.; Wang, Y. Constructing Substrate of Low Structural Parameter by Salt Induction for High-Performance TFC-FO Membranes. J. Membr. Sci. 2020, 600, 117866. [Google Scholar] [CrossRef]
- Shao, M.; Li, Y.; Meng, L.; Guo, J.; Gao, Y.; Liu, Y.; Huang, M. Simultaneous Removal of Antimony, Chromium and Aniline by Forward Osmosis Membrane: Preparation, Performance and Mechanism. Desalination 2021, 520, 115363. [Google Scholar] [CrossRef]
- Yao, Z.; Peng, L.E.; Guo, H.; Qing, W.; Mei, Y.; Tang, C.Y. Seawater Pretreatment with an NF-like Forward Osmotic Membrane: Membrane Preparation, Characterization and Performance Comparison with RO-like Membranes. Desalination 2019, 470, 114115. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Wang, Y.; Tang, C.Y.; Huo, F. Mesoporous Silica Gel-Based Mixed Matrix Membranes for Improving Mass Transfer in Forward Osmosis: Effect of Pore Size of Filler. Sci. Rep. 2015, 5, 16808. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, J.; Liu, S.; Hao, X.; Li, L.; Zou, G.; Hou, H.; Ji, X. Channel Regulation of TFC Membrane with Hydrophobic Carbon Dots in Forward Osmosis. Chin. Chem. Lett. 2021, 32, 2882–2886. [Google Scholar] [CrossRef]
- Tsai, M.; Chung, L.; Lin, G.; Chang, M.; Lee, C.; Tai, N. Layered Carbon Nanotube/Polyacrylonitrile Thin-Film Composite Membrane for Forward Osmosis Application. Sep. Purif. Technol. 2020, 241, 116683. [Google Scholar] [CrossRef]
- Kwon, H.E.; Kwon, S.J.; Park, S.J.; Shin, M.G.; Park, S.H.; Park, M.S.; Park, H.; Lee, J.H. High Performance Polyacrylonitrile-Supported Forward Osmosis Membranes Prepared via Aromatic Solvent-Based Interfacial Polymerization. Sep. Purif. Technol. 2019, 212, 449–457. [Google Scholar] [CrossRef]
- Li, Y.; Huang, M.; Chen, D.; Chen, G. Fabrication of Carbon Nanotube Membrane for Enhanced Performance in Forward Osmosis Process. In Proceedings of the International Conference on Energy, Power and Environmental Engineering ICEPEE, Shanghai, China, 23–24 April 2017; pp. 458–462. [Google Scholar] [CrossRef]
- Shen, L.; Xiong, S.; Wang, Y. Graphene Oxide Incorporated Thin-Film Composite Membranes for Forward Osmosis Applications. Chem. Eng. Sci. 2016, 143, 194–205. [Google Scholar] [CrossRef]
- He, M.; Wang, L.; Zhang, Z.; Zhang, Y.; Zhu, J.; Wang, X.; Lv, Y.; Miao, R. Stable Forward Osmosis Nanocomposite Membrane Doped with Sulfonated Graphene Oxide@Metal-Organic Frameworks for Heavy Metal Removal. ACS Appl. Mater. Interfaces 2020, 12, 57102–57116. [Google Scholar] [CrossRef]
- He, M.; Wang, L.; Lv, Y.; Wang, X.; Zhu, J.; Zhang, Y.; Liu, T. Novel Polydopamine/Metal Organic Framework Thin Film Nanocomposite Forward Osmosis Membrane for Salt Rejection and Heavy Metal Removal. Chem. Eng. J. 2020, 389, 124452. [Google Scholar] [CrossRef]
- Li, Z.; Han, Q.; Sun, F.Y.; Li, S.; Liu, J.; Liu, X.; Lu, J.J.; Li, W. Unraveling Effects of Multivalent Salts on Internal Fouling by Proteins in NF-like Forward Osmosis. J. Membr. Sci. 2023, 668, 121236. [Google Scholar] [CrossRef]
- Wang, S.; Cai, J.; Ding, W.; Xu, Z.; Wang, Z. Bio-Inspired Aquaporinz Containing Double-Skinned Forward Osmosis Membrane Synthesized through Layer-by-Layer Assembly. Membranes 2015, 5, 369–384. [Google Scholar] [CrossRef]
- Qi, S.; Qiu, C.Q.; Zhao, Y.; Tang, C.Y. Double-Skinned Forward Osmosis Membranes Based on Layer-by-Layer Assembly-FO Performance and Fouling Behavior. J. Membr. Sci. 2012, 405–406, 20–29. [Google Scholar] [CrossRef]
- Lin, C.F.; Chung, L.H.; Lin, G.Y.; Chang, M.C.; Lee, C.Y.; Tai, N.H. Enhancing the Efficiency of a Forward Osmosis Membrane with a Polydopamine/Graphene Oxide Layer Prepared Via the Modified Molecular Layer-by-Layer Method. ACS Omega 2020, 5, 18738–18745. [Google Scholar] [CrossRef]
- Duong, P.H.H.; Zuo, J.; Chung, T. Highly Crosslinked Layer-by-Layer Polyelectrolyte FO Membranes: Understanding Effects of Salt Concentration and Deposition Time on FO Performance. J. Membr. Sci. 2013, 427, 411–421. [Google Scholar] [CrossRef]
- Qi, S.; Li, W.; Zhao, Y.; Ma, N.; Wei, J.; Chin, T.W.; Tang, C.Y. Influence of the Properties of Layer-by-Layer Active Layers on Forward Osmosis Performance. J. Membr. Sci. 2012, 423–424, 536–542. [Google Scholar] [CrossRef]
- Kwon, S.B.; Lee, J.S.; Kwon, S.J.; Yun, S.T.; Lee, S.; Lee, J.H. Molecular Layer-by-Layer Assembled Forward Osmosis Membranes. J. Membr. Sci. 2015, 488, 111–120. [Google Scholar] [CrossRef]
- Saren, Q.; Qiu, C.Q.; Tang, C.Y. Synthesis and Characterization of Novel Forward Osmosis Membranes Based on Layer-by-Layer Assembly. Environ. Sci. Eng. 2011, 45, 5201–5208. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Qi, S.; Tang, C.Y. Synthesis of High Flux Forward Osmosis Membranes by Chemically Crosslinked Layer-by-Layer Polyelectrolytes. J. Membr. Sci. 2011, 381, 74–80. [Google Scholar] [CrossRef]
- Liu, X.; Liu, G.; Li, W.; Wang, Q.; Deng, B. Effects of the Substrate on Interfacial Polymerization: Tuning the Hydrophobicity via Polyelectrolyte Deposition. Membranes 2020, 10, 259. [Google Scholar] [CrossRef]
- Li, Y.; Deng, W.; Li, H.; Su, F.; Huang, X.; Mo, F.; Zhang, R.; Ren, X. Toward Enhancing the Chlorine Resistance of Forward Osmosis Membranes: An Effective Strategy via Grafting Cyclohexylamine. Water Supply 2021, 21, 3449–3458. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Z.; Qiu, G.; Li, J.; Xie, J.; Lee, J.Y. Surface Reaction Route To Increase the Loading of Antimicrobial Ag Nanoparticles in Forward Osmosis Membranes. ACS Sustain. Chem. Eng. 2015, 3, 2959–2966. [Google Scholar] [CrossRef]
- Duong, P.H.H.; Chung, T.S. Application of Thin Film Composite Membranes with Forward Osmosis Technology for the Separation of Emulsified Oil-Water. J. Membr. Sci. 2014, 452, 117–126. [Google Scholar] [CrossRef]
- Xiong, S.; Zuo, J.; Ma, Y.G.; Liu, L.; Wu, H.; Wang, Y. Novel Thin Film Composite Forward Osmosis Membrane of Enhanced Water Flux and Anti-Fouling Property with N-[3-(Trimethoxysilyl) Propyl] Ethylenediamine Incorporated. J. Membr. Sci. 2016, 520, 400–414. [Google Scholar] [CrossRef]
- Duong, P.H.H.; Chung, T.S.; Wei, S.; Irish, L. Highly Permeable Double-Skinned Forward Osmosis Membranes for Anti-Fouling in the Emulsified Oil-Water Separation Process. Environ. Sci. Technol. 2014, 48, 4537–4545. [Google Scholar] [CrossRef]
- Qi, S.; Li, Y.; Zhao, Y.; Li, W.; Tang, C.Y. Highly Efficient Forward Osmosis Based on Porous Membranes-Applications and Implications. Environ. Sci. Technol. 2015, 49, 4690–4695. [Google Scholar] [CrossRef]
- Kwon, D.; Kwon, S.J.; Kim, J.; Lee, J.H. Feasibility of the Highly-Permselective Forward Osmosis Membrane Process for the Post-Treatment of the Anaerobic Fluidized Bed Bioreactor Effluent. Desalination 2020, 485, 114451. [Google Scholar] [CrossRef]
- Peng, L.E.; Yao, Z.; Chen, J.; Guo, H.; Tang, C.Y. Highly Selective Separation and Resource Recovery Using Forward Osmosis Membrane Assembled by Polyphenol Network. J. Membr. Sci. 2020, 611, 118305. [Google Scholar] [CrossRef]
- Ahmad, T.; Rehman, L.M.; Al-Nuaimi, R.; de Levay, J.P.B.B.; Thankamony, R.; Mubashir, M.; Lai, Z. Thermodynamics and Kinetic Analysis of Membrane: Challenges and Perspectives. Chemosphere 2023, 337, 139430. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Zhang, X.; Ding, C.; Xiong, S.; Yu, X.; Wang, Y. Improved Performance of Thin-Film Composite Membrane Supported by Aligned Nanofibers Substrate with Slit-Shape Pores for Forward Osmosis. J. Membr. Sci. 2020, 612, 118447. [Google Scholar] [CrossRef]
- Cho, M.; Lee, S.H.; Lee, D.; Dennis, P.; Kim, I.; Diallo, M.S. Osmotically Driven Membrane Processes: Exploring the Potential of Branched Polyethyleneimine as Draw Solute Using Porous FO Membranes with NF Separation Layers. J. Membr. Sci. 2016, 511, 278–288. [Google Scholar] [CrossRef]
- Bui, N.N.; McCutcheon, J.R. Nanofiber Supported Thin-Film Composite Membrane for Pressure- Retarded Osmosis. Environ. Sci. Technol. 2014, 48, 4129–4136. [Google Scholar] [CrossRef]
- Ren, J.; McCutcheon, J.R. Polyacrylonitrile Supported Thin Film Composite Hollow Fiber Membranes for Forward Osmosis. Desalination 2015, 372, 67–74. [Google Scholar] [CrossRef]
- Koyuncu, I.; Guclu, S.; Eyvaz, M.; Aslan, T.; Yuksekdag, A.; Yuksel, E. Manufacturing of a Nanofiber Forward Osmosis Membrane with Tubular Shape. U.S. Patent 10,583,406, 10 March 2020. [Google Scholar]
- Chi, X.Y.; Zhang, M.X.; Xu, Z.L.; Xia, B.G. New Insights into the Interaction between Surface-Charged Membranes and Positively-Charged Draw Solutes in the Forward Osmosis Process. J. Water Process Eng. 2020, 37, 101439. [Google Scholar] [CrossRef]
- Wang, T.; Qiblawey, H.; Sivaniah, E.; Mohammadian, A. Novel Methodology for Facile Fabrication of Nano Filtration Membranes Based on Nucleophilic Nature of Polydopamine. J. Membr. Sci. 2016, 511, 65–75. [Google Scholar] [CrossRef]
- Pan, S.F.; Dong, Y.; Zheng, Y.M.; Zhong, L.B.; Yuan, Z.H. Self-Sustained Hydrophilic Nanofiber Thin Film Composite Forward Osmosis Membranes: Preparation, Characterization and Application for Simulated Antibiotic Wastewater Treatment. J. Membr. Sci. 2017, 523, 205–215. [Google Scholar] [CrossRef]
- Shi, J.; Kang, H.; Li, N.; Teng, K.; Sun, W.; Xu, Z.; Qian, X.; Liu, Q. Chitosan Sub-Layer Binding and Bridging for Nanofiber-Based Composite Forward Osmosis Membrane. Appl. Surf. Sci. 2019, 478, 38–48. [Google Scholar] [CrossRef]
- Chi, X.Y.; Xia, B.G.; Xu, Z.L.; Zhang, M.X. Impact of Cross-Linked Chitosan Sublayer Structure on the Performance of TFC FO PAN Nanofiber Membranes. ACS Omega 2018, 3, 13009–13019. [Google Scholar] [CrossRef]
- Shokrollahzadeh, S.; Tajik, S. Fabrication of Thin Film Composite Forward Osmosis Membrane Using Electrospun Polysulfone/Polyacrylonitrile Blend Nanofibers as Porous Substrate. Desalination 2018, 425, 68–76. [Google Scholar] [CrossRef]
- Bui, N.N.; McCutcheon, J.R. Hydrophilic Nanofibers as New Supports for Thin Film Composite Membranes for Engineered Osmosis. Environ. Sci. Technol. 2013, 47, 1761–1769. [Google Scholar] [CrossRef]
- Cheng, B.; Wang, Y.; Wu, X.; Fang, M.; Min, X.; Huang, Z.; Liu, Y.; Mi, R. Preparation and Characterization of Novel Thin Film Composite Forward Osmosis Membrane with Halloysite Nanotube Interlayer. Polymer 2022, 254, 125096. [Google Scholar] [CrossRef]
- Luo, F.; Wang, J.; Yao, Z.; Zhang, L.; Chen, H. Polydopamine Nanoparticles Modified Nanofiber Supported Thin Film Composite Membrane with Enhanced Adhesion Strength for Forward Osmosis. J. Membr. Sci. 2021, 618, 118673. [Google Scholar] [CrossRef]
- Wu, W.; Yu, L.; Li, L.; Li, Z.; Kang, J.; Pu, S.; Chen, D.; Ma, R.; An, K.; Liu, G.; et al. Electrospun Nanofiber Based Forward Osmosis Membrane Using Graphene Oxide as Substrate Modifier for Enhanced Water Flux and Rejection Performance. Desalination 2021, 518, 115283. [Google Scholar] [CrossRef]
- Chen, S.C.; Fu, X.Z.; Chung, T.S. Fouling Behaviors of Polybenzimidazole (PBI)-Polyhedral Oligomeric Silsesquioxane (POSS)/Polyacrylonitrile (PAN) Hollow Fiber Membranes for Engineering Osmosis Processes. Desalination 2014, 335, 17–26. [Google Scholar] [CrossRef]
- Fu, F.J.; Zhang, S.; Sun, S.P.; Wang, K.Y.; Chung, T.S. POSS-Containing Delamination-Free Dual-Layer Hollow Fiber Membranes for Forward Osmosis and Osmotic Power Generation. J. Membr. Sci. 2013, 443, 144–155. [Google Scholar] [CrossRef]
- Kim, D.; Moreno, N.; Nunes, S.P. Fabrication of Polyacrylonitrile Hollow Fiber Membranes from Ionic Liquid Solutions. Polym. Chem. 2016, 7, 113–124. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, J.; Song, P.; Wang, Z. Layer-by-Layer Assembly for Preparation of High-Performance Forward Osmosis Membrane. IOP Conf. Ser. Mater. Sci. Eng. 2018, 301, 012032. [Google Scholar] [CrossRef]
- Chen, Y.; Song, X.; Zhang, N.; Zhang, X.; Su, G.; Huang, M.; Jiang, H. Polyethyleneimine-Mediated Polyamide Composite Membrane with High Perm-Selectivity for Forward Osmosis. Macromol. Mater. Eng. 2021, 306, 818. [Google Scholar] [CrossRef]
- Farman, A.A.; Irfan, M.; Amin, N.U.; Jahan, Z.; Song, X.; Jiang, H.; Gul, S. Evaluation of Sodium Acetate and Glucose as Minor Additives with Calcium Chloride as Optimum Mixed Draw Solutes for Fruit Juice Concentration via Forward Osmosis. Korean J. Chem. Eng. 2022, 39, 3102–3108. [Google Scholar] [CrossRef]
- Xu, S.; Li, F.; Su, B.; Hu, M.Z.; Gao, X.; Gao, C. Novel Graphene Quantum Dots (GQDs)-Incorporated Thin Film Composite (TFC) Membranes for Forward Osmosis (FO) Desalination. Desalination 2018, 451, 219–230. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Lalia, B.S.; Hashaikeh, R. A Review on Electrospinning for Membrane Fabrication: Challenges and Applications. Desalination 2015, 356, 15–30. [Google Scholar] [CrossRef]
- Bui, N.N.; Lind, M.L.; Hoek, E.M.V.; McCutcheon, J.R. Electrospun Nanofiber Supported Thin Film Composite Membranes for Engineered Osmosis. J. Membr. Sci. 2011, 385–386, 10–19. [Google Scholar] [CrossRef]
- Huang, L.; McCutcheon, J.R. Impact of Support Layer Pore Size on Performance of Thin Film Composite Membranes for Forward Osmosis. J. Membr. Sci. 2015, 483, 25–33. [Google Scholar] [CrossRef]
- Obaid, M.; Abdelkareem, M.A.; Kook, S.; Kim, H.Y.; Hilal, N.; Ghaffour, N.; Kim, I.S. Breakthroughs in the Fabrication of Electrospun-Nanofiber-Supported Thin Film Composite/Nanocomposite Membranes for the Forward Osmosis Process: A Review. Crit. Rev. Environ. Sci. Technol 2020, 50, 1727–1795. [Google Scholar] [CrossRef]
- Díez, B.; Rosal, R. A Critical Review of Membrane Modification Techniques for Fouling and Biofouling Control in Pressure-Driven Membrane Processes. Nanotechnol. Environ. Eng. 2020, 5, 15. [Google Scholar] [CrossRef]
- Dong, X.; Lu, D.; Harris, T.A.L.; Escobar, I.C. Polymers and Solvents Used in Membrane Fabrication: A Review Focusing on Sustainable Membrane Development. Membranes 2021, 11, 309. [Google Scholar] [CrossRef] [PubMed]
- Lalia, B.S.; Kochkodan, V.; Hashaikeh, R.; Hilal, N. A Review on Membrane Fabrication: Structure, Properties and Performance Relationship. Desalination 2013, 326, 77–95. [Google Scholar] [CrossRef]
- Dommati, H.; Ray, S.S.; Wang, J.C.; Chen, S.S. A Comprehensive Review of Recent Developments in 3D Printing Technique for Ceramic Membrane Fabrication for Water Purification. RSC Adv. 2019, 9, 16869–16883. [Google Scholar] [CrossRef]
- Shiohara, A.; Prieto-Simon, B.; Voelcker, N.H. Porous Polymeric Membranes: Fabrication Techniques and Biomedical Applications. J. Mater. Chem. B 2021, 9, 2129–2154. [Google Scholar] [CrossRef]
- Saqib, J.; Aljundi, I.H. Membrane Fouling and Modification Using Surface Treatment and Layer-by-Layer Assembly of Polyelectrolytes: State-of-the-Art Review. J. Water Process Eng. 2016, 11, 68–87. [Google Scholar] [CrossRef]
- Suzaimi, N.D.; Goh, P.S.; Ismail, A.F.; Mamah, S.C.; Malek, N.A.N.N.; Lim, J.W.; Wong, K.C.; Hilal, N. Strategies in Forward Osmosis Membrane Substrate Fabrication and Modification: A Review. Membranes 2020, 10, 332. [Google Scholar] [CrossRef]
- Lu, X.; Elimelech, M. Fabrication of Desalination Membranes by Interfacial Polymerization: History, Current Efforts, and Future Directions. Chem. Soc. Rev. 2021, 50, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Seah, M.Q.; Lau, W.J.; Goh, P.S.; Tseng, H.H.; Wahab, R.A.; Ismail, A.F. Progress of Interfacial Polymerization Techniques for Polyamide Thin Film (Nano)Composite Membrane Fabrication: A Comprehensive Review. Polymers 2020, 12, 2817. [Google Scholar] [CrossRef] [PubMed]





| PAN Surface Modification Techniques | Pros | Cons | References |
|---|---|---|---|
| Plasma treatment | Stable water flux. Anti-fouling. Smooth surface roughness. | Laboratory cost-effective. Complex modification. | [71,99] |
| Graft polymerization | Anti-swelling. Unaltered chemical and physical properties. Chemical resistance. | Complexity Time-consuming. | [83] |
| Alkaline hydrolysis | Stable water flux. Fast cross-linking reaction. Sustain high temperatures. Increase salt rejection. Cost effective. | Physical change in the backing layer. Reduce pore size. | [86] |
| Acidic hydrolysis | Slow reaction. Cost effective. | Weak bond formation. | [88] |
| Click Chemistry | Rapid synthesis and high yield. High chemical resistance. | Complex chemistry. Alkyne homocoupling. | [90,100] |
| Static And Pore flowing modification using Ethanolamine. | Multilayer surface formation. Uniform layer. Increase surface hydrophilicity. Enhanced mechanical properties. | Lower selectivity. | [68] |
| Esterification. | Increase membrane stability. Anti-swelling properties. | Requires multiple stages. More chemical usage. | [91,92] |
| Hydrazine Cross-linking. | Increase membrane stability. Improve resistance to chemical attacks. Increase membrane mechanical strength. | Complex reaction. | [93,97] |
| Type of PAN Membrane | MWCO KDa | Fillers-Optimal Loading wt % | Fabrication Method | Modification Techniques | Solute Type/Applications | DS and FS | Achieved Parameters under FO Test | References |
|---|---|---|---|---|---|---|---|---|
| Commercial PAN membrane | Mean pore size of 0.1 μm | - | Commercial membrane Supplied by Beijing Ande Membrane Technology, China. | Hydrolysis LBL IP | Salt (NaCl) | FS: DI DS: 1 M NaCl | Optimal conditions for LBL-8. Jw PRO/FO: 14.4/7.8 LMH Js PRO/FO = 10/5.4 gMH R% = 96% by RO test. | [153] |
| Commercial PAN membrane | NA | - | NA | Hydrolysis electrostatic interaction using PEI. IP. | Salt (MgCl2) | FS: DI DS: 2 M MgCl2 | Optimal conditions for TFC-PEI-1.5 membrane at FO mode. Jw = 16.1 LMH Js = 1.25 gMH | [154] |
| Commercial PAN UF- membrane | (PAN-50,000 Da) | 0.05 wt % GQDs | Commercial membrane Supplied by Suntar Membrane Technology (Xiamen, China). | Hydrolysis IP (GQDs into PEI aqueous phase). | Salt (MgCl2) Humic acid BSA | FS: DI DS: 0.5 M MgCl2 | At FO mode. Jw = 12.9 LMH Js = 1.41 gMH | [156] |
| Commercial PAN UF- membrane | NA | - | NA | Hydrolysis PEI interlayer coating. IP | CaCl2 Glucose Sodium acetate (CH3COONa) | FS: DI DS: 5% CH3COONa with CaCl2 | At PRO mode Jw = 23.9 LMH Js = 6.64 gMH | [155] |
| Technique. | Pros | Cons | References | |
|---|---|---|---|---|
| Fabrication technique | Phase inversion | Uniform thickness distribution. Good flatness. High flux. | Limited to specific polymers. Depend on many parameters. No- uniformity in pore size distribution. Uncontrolled pore size and pore diameter. Low mechanical strength. Time-consuming technique. High surface roughness. | [161,162,163,164] |
| Electrospinning | Large surface area-to-volume ratio. High porosity. Formation of interconnected pores. Easily combined with different materials. High mechanical strength. High flux. | Depend on many parameters. Jet instability. High-voltage power supply. High surface roughness. Require post-treatment. | [163,164,165] | |
| Modification technique | Layer By Layer assembly | Finely tuneable. Control membrane thickness, roughness, and surface charge. | Time-consuming. Require an appropriate crosslinker. Not appropriate for large-scale production. | [163,166,167] |
| Interfacial polymerization | Simple technique. High anti-fouling properties. High retention. Low surface roughness. Easily combined with different materials. High surface charge. Low width of pore size distribution. | Low Flux. At the industrial manufacturing scale, it is not economically viable and environmentally friendly because of the high chemical demand. | [168,169] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abounahia, N.; Shahab, A.A.; Khan, M.M.; Qiblawey, H.; Zaidi, S.J. A Comprehensive Review of Performance of Polyacrylonitrile-Based Membranes for Forward Osmosis Water Separation and Purification Process. Membranes 2023, 13, 872. https://doi.org/10.3390/membranes13110872
Abounahia N, Shahab AA, Khan MM, Qiblawey H, Zaidi SJ. A Comprehensive Review of Performance of Polyacrylonitrile-Based Membranes for Forward Osmosis Water Separation and Purification Process. Membranes. 2023; 13(11):872. https://doi.org/10.3390/membranes13110872
Chicago/Turabian StyleAbounahia, Nada, Arqam Azad Shahab, Maryam Mohammad Khan, Hazim Qiblawey, and Syed Javaid Zaidi. 2023. "A Comprehensive Review of Performance of Polyacrylonitrile-Based Membranes for Forward Osmosis Water Separation and Purification Process" Membranes 13, no. 11: 872. https://doi.org/10.3390/membranes13110872
APA StyleAbounahia, N., Shahab, A. A., Khan, M. M., Qiblawey, H., & Zaidi, S. J. (2023). A Comprehensive Review of Performance of Polyacrylonitrile-Based Membranes for Forward Osmosis Water Separation and Purification Process. Membranes, 13(11), 872. https://doi.org/10.3390/membranes13110872









