Study of the Physical, Chemical, and Structural Properties of Low- and High-Methoxyl Pectin-Based Film Matrices Including Sunflower Waxes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film-Forming Solutions and Emulsions
2.3. Film Elaboration by Casting Method
2.4. Film Characterization
2.4.1. Film Thickness
2.4.2. Film Microstructure
2.4.3. Atomic Force Microscopy (AFM)
2.4.4. X-ray Diffraction
2.4.5. Raman Spectroscopy
2.4.6. Water Vapor Permeability (WVP) and Water Vapor Transmission Rate (WVTR)
2.4.7. Mechanical Properties
2.4.8. Statistical Analysis
3. Results and Discussion
3.1. Film Characterization
3.2. SEM Microscopy
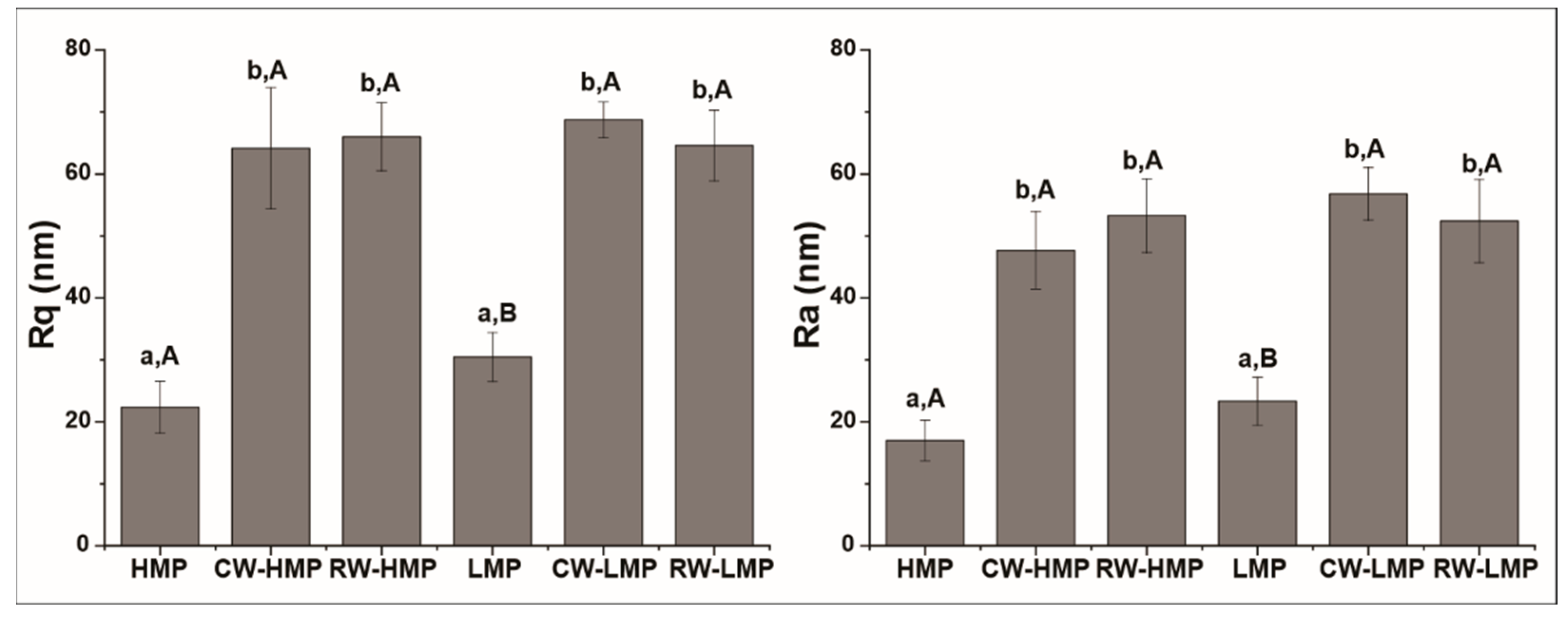
3.3. Atomic Force Microscopy (AFM)
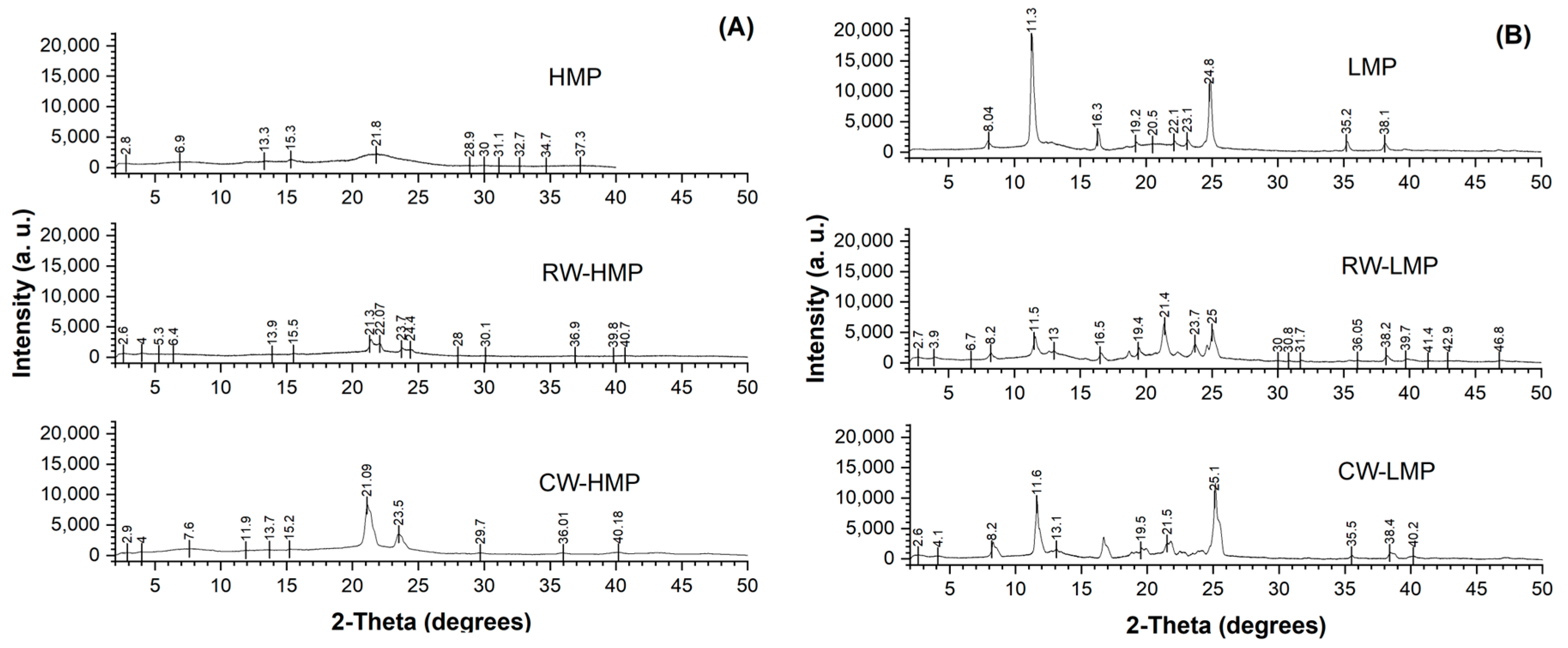
3.4. X-ray Diffraction
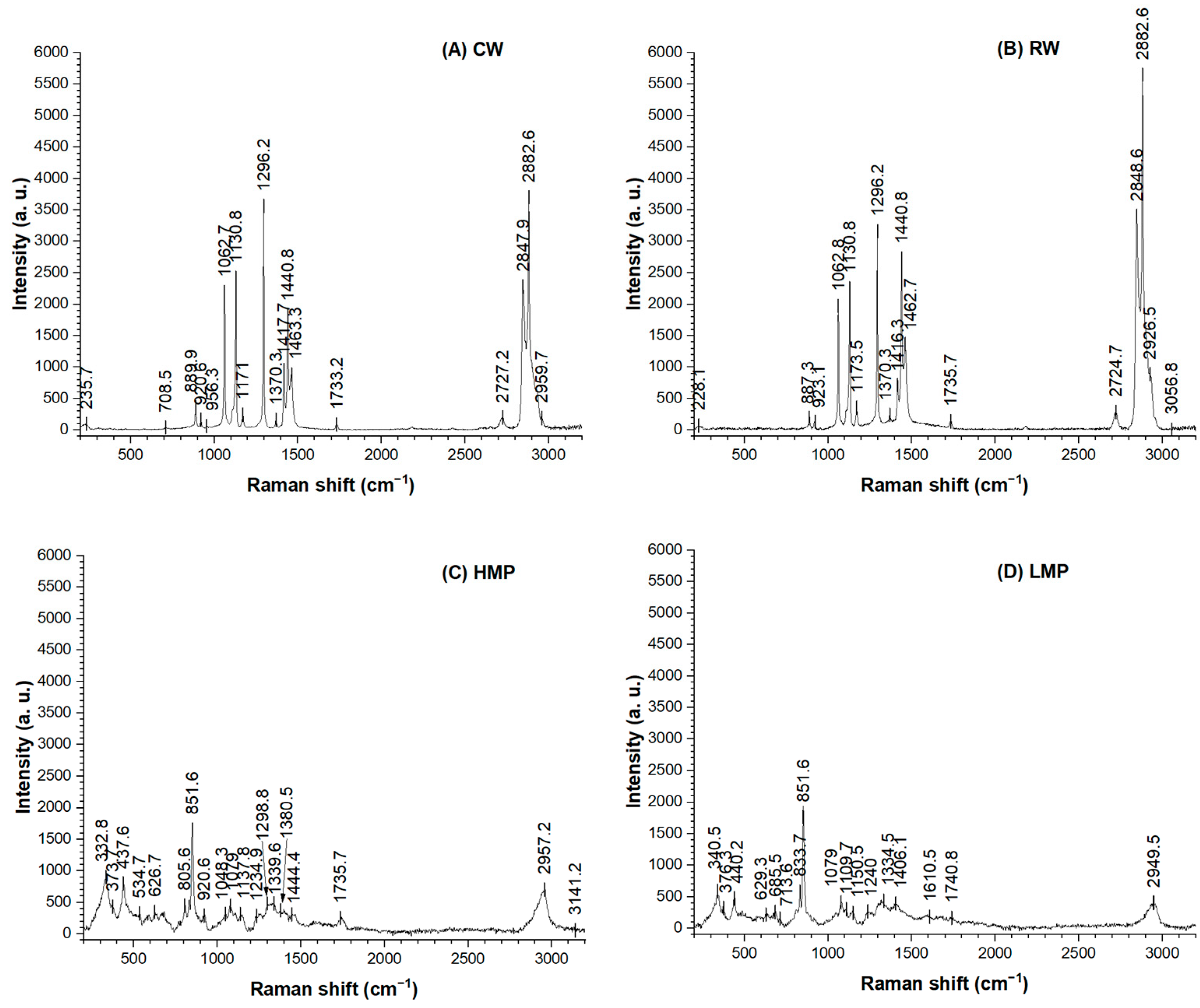
3.5. Raman Spectroscopy
3.6. Water Vapor Transmission Properties of Films
3.7. Mechanical Properties of Pectin Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mellinas, C.; Ramos, M.; Jiménez, A.; Garrigós, M.C. Recent trends in the use of pectin from agro-waste residues as a natural-based biopolymer for food packaging applications. Materials 2020, 13, 673. [Google Scholar] [CrossRef]
- Scopel, B.S.; Ribeiro, M.E.; Dettmer, A.; Baldasso, C. Cornstarch-gelatin films: Commercial gelatin versus chromed leather waste gelatin and evaluation of drying conditions. J. Polym. Environ. 2018, 26, 1998–2006. [Google Scholar] [CrossRef]
- Sun, K.-Q.; Li, F.-Y.; Li, J.-Y.; Li, J.-F.; Zhang, C.-W.; Chen, S.; Sun, X.; Cui, J.-F. Optimisation of compatibility for improving elongation at break of chitosan/starch films. RSC Adv. 2019, 9, 24451–24459. [Google Scholar] [CrossRef]
- Chaiwarit, T.; Ruksiriwanich, W.; Jantanasakulwong, K.; Jantrawut, P. Use of orange oil loaded pectin films as antibacterial material for food packaging. Polymers 2018, 10, 1144. [Google Scholar] [CrossRef]
- Nastasi, J.R.; Kontogiorgos, V.; Daygon, V.D.; Fitzgerald, M.A. Pectin-based films and coatings with plant extracts as natural preservatives: A systematic review. Trends Food Sci. Technol. 2022, 120, 193–211. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, J.; Zhang, S. The effect of degree of esterification of pectin on the interaction between pectin and wheat gluten protein. Food Hydrocoll. 2023, 136, 108272. [Google Scholar] [CrossRef]
- Cortés-Camargo, S.; Román-Guerrero, A.; Alpizar-Reyes, E.; Pérez-Alonso, C. New Sources of Pectin: Extraction, Processing, and Industrial Applications. In Utilization of Pectin in the Food and Drug Industries; IntechOpen: London, UK, 2023. [Google Scholar]
- Zhang, M.; Bai, B.; Cheng, H.; Ye, X.; Chang, J.; Chen, S.; Chen, J. A method for gel grade determination and application evaluation of two citrus pectins. Int. J. Biol. Macromol. 2023, 250, 126129. [Google Scholar] [CrossRef] [PubMed]
- Chalapud, M.C.; Baümler, E.R.; Carelli, A.A. Characterization of waxes and residual oil recovered from sunflower oil winterization waste. Eur. J. Lipid Sci. Technol. 2017, 119, 1500608. [Google Scholar] [CrossRef]
- Baümler, E.R.; Carelli, A.A.; Martini, S. Physical properties of aqueous solutions of pectin containing sunflower wax. J. Am. Oil Chem. Soc. 2013, 90, 791–802. [Google Scholar] [CrossRef]
- Baümler, E.R.; Carelli, A.A.; Martini, S. Preparation and physical properties of calcium pectinate films modified with sunflower wax. Eur. J. Lipid Sci. Technol. 2014, 116, 1534–1545. [Google Scholar] [CrossRef]
- Chalapud, M.C.; Baümler, E.R.; Carelli, A.A. Emulsions of sunflower wax in pectin aqueous solutions: Physical characterization and stability. Food Res. Int. 2018, 108, 216–225. [Google Scholar] [CrossRef]
- Akkaya, S.; Ozel, B.; Oztop, M.H.; Yanik, D.K.; Gogus, F. Physical characterization of high methoxyl pectin and sunflower oil wax emulsions: A low-field 1H NMR relaxometry study. J. Food Sci. 2020, 86, 120–128. [Google Scholar] [CrossRef]
- Chalapud, M.C.; Baümler, E.R.; Carelli, A.A. Edible films based on aqueous emulsions of low-methoxyl pectin with recovered and purified sunflower waxes. J. Sci. Food Agric. 2020, 100, 2675–2687. [Google Scholar] [CrossRef] [PubMed]
- Chalapud, M.C.; Baümler, E.R.; Carelli, A.A.; Salgado-Cruz, M.d.l.P.; Morales-Sánchez, E.; Rentería-Ortega, M.; Calderón-Domínguez, G. Pectin Films with Recovered Sunflower Waxes Produced by Electrospraying. Membranes 2022, 12, 560. [Google Scholar] [CrossRef] [PubMed]
- Mendes, J.; Norcino, L.; Manrich, A.; Pinheiro, A.; Oliveira, J.; Mattoso, L. Characterization of pectin films integrated with cocoa butter by continuous casting: Physical, thermal and barrier properties. J. Polym. Environ. 2020, 28, 2905–2917. [Google Scholar] [CrossRef]
- Kumar, S.; Reddy, A.R.L.; Basumatary, I.B.; Nayak, A.; Dutta, D.; Konwar, J.; Purkayastha, M.D.; Mukherjee, A. Recent progress in pectin extraction and their applications in developing films and coatings for sustainable food packaging: A review. Int. J. Biol. Macromol. 2023, 239, 124281. [Google Scholar] [CrossRef]
- Panahirad, S.; Naghshiband-Hassani, R.; Mahna, N. Pectin-based edible coating preserves antioxidative capacity of plum fruit during shelf life. Food Sci. Technol. Int. 2020, 26, 583–592. [Google Scholar] [CrossRef]
- Shulga, O.; Hrybkov, S.; Shulga, S. Ecological packaging materials for bakery and confectionery products based on a new pectin modification. Ukr. Food J. 2022, 11, 390–402. [Google Scholar] [CrossRef]
- Shahrampour, D.; Khomeiri, M.; Razavi, S.M.A.; Kashiri, M. Development and characterization of alginate/pectin edible films containing Lactobacillus plantarum KMC 45. LWT 2020, 118, 108758. [Google Scholar] [CrossRef]
- Koster Keunen. Sunflower Wax. Available online: https://www.kosterkeunen.com/product/sunflower-wax/ (accessed on 25 May 2021).
- ASTM Standard E96/E96M-05; Standard Test Methods for Water Vapor Transmission of Materials. ASTM International: West Conshohocken, PA, USA, 2005.
- ASTM Standard D882-09; Standard Test Methods for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2009.
- Valdespino-León, M.; Calderón-Domínguez, G.; De La Paz Salgado-Cruz, M.; Rentería-Ortega, M.; Farrera-Rebollo, R.R.; Morales-Sánchez, E.; Gaona-Sánchez, V.A.; Terrazas-Valencia, F. Biodegradable electrosprayed pectin films: An alternative to valorize coffee mucilage. Waste Biomass Valorization 2021, 12, 2477–2494. [Google Scholar] [CrossRef]
- Di Rienzo, J. InfoStat Versión 2009; Grupo InfoStat, FCA, Universidad Nacional de Córdoba: Córdoba, Argentina, 2009; Available online: https://www.infostat.com.ar (accessed on 27 August 2023).
- Çavdaroğlu, E.; Büyüktaş, D.; Farris, S.; Yemenicioğlu, A.J.F.H. Novel edible films of pectins extracted from low-grade fruits and stalk wastes of sun-dried figs: Effects of pectin composition and molecular properties on film characteristics. Food Hydrocoll. 2023, 135, 108136. [Google Scholar] [CrossRef]
- Assifaoui, A.; Loupiac, C.; Chambin, O.; Cayot, P. Structure of calcium and zinc pectinate films investigated by FTIR spectroscopy. Carbohydr. Res. 2010, 345, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Nesic, A.; Meseldzija, S.; Onjia, A.; Cabrera-Barjas, G. Impact of Crosslinking on the Characteristics of Pectin Monolith Cryogels. Polymers 2022, 14, 5252. [Google Scholar] [CrossRef]
- Ngouémazong, D.E.; Tengweh, F.F.; Fraeye, I.; Duvetter, T.; Cardinaels, R.; Van Loey, A.; Moldenaers, P.; Hendrickx, M. Effect of de-methylesterification on network development and nature of Ca2+-pectin gels: Towards understanding structure–function relations of pectin. Food Hydrocoll. 2012, 26, 89–98. [Google Scholar] [CrossRef]
- Han, W.; Meng, Y.; Hu, C.; Dong, G.; Qu, Y.; Deng, H.; Guo, Y. Mathematical model of Ca2+ concentration, pH, pectin concentration and soluble solids (sucrose) on the gelation of low methoxyl pectin. Food Hydrocoll. 2017, 66, 37–48. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Kim, S.-M.; Rhim, J.-W. Pectin/pullulan blend films for food packaging: Effect of blending ratio. Food Chem. 2021, 347, 129022. [Google Scholar] [CrossRef]
- Gaona Sánchez, V.A.; Calderón Domínguez, G.; Morales Sánchez, E.; Chanona Pérez, J.J.; Arzate Vázquez, I.; Terrés Rojas, E. Pectin-based films produced by electrospraying. J. Appl. Polym. Sci. 2016, 133, 1–10. [Google Scholar] [CrossRef]
- Sartori, T.; Feltre, G.; do Amaral Sobral, P.J.; da Cunha, R.L.; Menegalli, F.C. Properties of films produced from blends of pectin and gluten. Food Packag. Shelf Life 2018, 18, 221–229. [Google Scholar] [CrossRef]
- Bharti, D.; Kim, D.; Cerqueira, M.A.; Mohanty, B.; Habibullah, S.; Banerjee, I.; Pal, K. Effect of biodegradable hydrophilic and hydrophobic emulsifiers on the oleogels containing sunflower wax and sunflower oil. Gels 2021, 7, 133. [Google Scholar] [CrossRef] [PubMed]
- Cruces, F.; García, M.G.; Ochoa, N.A. Reduction of Water Vapor Permeability in Food Multilayer Biopackaging by Epitaxial Crystallization of Beeswax. Food Bioprocess Technol. 2021, 14, 1244–1255. [Google Scholar] [CrossRef]
- Szymańska-Chargot, M.; Chylińska, M.; Pieczywek, P.M.; Rösch, P.; Schmitt, M.; Popp, J.; Zdunek, A. Raman imaging of changes in the polysaccharides distribution in the cell wall during apple fruit development and senescence. Planta 2016, 243, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Chylińska, M.; Szymańska-Chargot, M.; Zdunek, A. FT-IR and FT-Raman characterization of non-cellulosic polysaccharides fractions isolated from plant cell wall. Carbohydr. Polym. 2016, 154, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Engelsen, S.B.; Nørgaard, L. Comparative vibrational spectroscopy for determination of quality parameters in amidated pectins as evaluated by chemometrics. Carbohydr. Polym. 1996, 30, 9–24. [Google Scholar] [CrossRef]
- Zhuang, Y.; Sterr, J.; Kulozik, U.; Gebhardt, R. Application of confocal Raman microscopy to investigate casein micro-particles in blend casein/pectin films. Int. J. Biol. Macromol. 2015, 74, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Synytsya, A.; Čopíková, J.; Matějka, P.; Machovič, V. Fourier transform Raman and infrared spectroscopy of pectins. Carbohydr. Polym. 2003, 54, 97–106. [Google Scholar] [CrossRef]
- Edwards, H.; Falk, M. Fourier-transform Raman spectroscopic study of unsaturated and saturated waxes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1997, 53, 2685–2694. [Google Scholar] [CrossRef]
- Nogales-Bueno, J.; Baca-Bocanegra, B.; Rooney, A.; Hernández-Hierro, J.M.; Byrne, H.J.; Heredia, F.J. Study of phenolic extractability in grape seeds by means of ATR-FTIR and Raman spectroscopy. Food Chem. 2017, 232, 602–609. [Google Scholar] [CrossRef]
- de Oliveira Filho, J.G.; Bezerra, C.C.d.O.N.; Albiero, B.R.; Oldoni, F.C.A.; Miranda, M.; Egea, M.B.; de Azeredo, H.M.C.; Ferreira, M.D. New approach in the development of edible films: The use of carnauba wax micro-or nanoemulsions in arrowroot starch-based films. Food Packag. Shelf Life 2020, 26, 100589. [Google Scholar] [CrossRef]
- Muscat, D.; Adhikari, R.; McKnight, S.; Guo, Q.; Adhikari, B. The physicochemical characteristics and hydrophobicity of high amylose starch–glycerol films in the presence of three natural waxes. J. Food Eng. 2013, 119, 205–219. [Google Scholar] [CrossRef]
- Mehraj, S.; Sistla, Y.S.; Garg, M.; Santra, B.; Grewal, H.S.; Kanjilal, A. Improvement of Moisture Barrier and Tensile Properties of Pectin Films by Incorporating Terminalia catappa Linn. Leaf Wax and Xylitol. J. Polym. Environ. 2023, 31, 3522–3537. [Google Scholar] [CrossRef]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Pascall, M.A. Evaluation of structural and functional properties of citrus pectin film enriched with green tea extract. Polym. Eng. Sci. 2023, 63, 2522–2533. [Google Scholar] [CrossRef]




| Film | Thickness (µm) | Film | Thickness (µm) |
|---|---|---|---|
| HMP | A 22 ± 3 a | LMP | A 21 ± 4 a |
| CW-HMP | A 35 ± 2 b | CW-LMP | A 32 ± 4 b |
| RW-HMP | A 33 ± 4 b | RW-LMP | A 31 ± 8 b |
| Film | DC (%) | Film | DC (%) |
|---|---|---|---|
| HMP | A 4.4 ± 1.2 a | LMP | B 50.4 ± 4.6 c |
| CW-HMP | A 3.6 ± 0.9 a | CW-LMP | B 26.9 ± 3.0 b |
| RW-HMP | A 3.5 ± 1.7 a | RW-LMP | B 14.3 ± 0.4 a |
| Film | WVTR (kg·m−2·s−1) | WVP × 10−7 (kg·Pa−1·m−1·s−1) | Film | WVTR (kg·m−2·s−1) | WVP × 10−7 (kg·Pa−1·m−1·s−1) |
|---|---|---|---|---|---|
| HMP | A 25.40 ± 1.17 a | A 13.10 ± 0.55 a | LMP | A 24.60 ± 1.64 c | A 12.20 ± 0.16 a |
| CW-HMP | B 23.90 ± 0.97 a | B 19.20 ± 0.27 b | CW-LMP | A 20.70 ± 0.19 b | A 15.60 ± 0.27 b |
| RW-HMP | B 22.80 ± 2.31 a | B 17.80 ± 0.13 b | RW-LMP | A 18.40 ± 0.83 a | A 13.30 ± 0.55 ab |
| Film | %E | TS (MPa) | ε (MPa) |
|---|---|---|---|
| HMP | 6.32 ± 0.47 a, A | 7.80 ± 0.58 a, B | 142.97 ± 2.44 a, B |
| LMP | 5.36 ± 0.20 a, A | 6.06 ± 0.40 a, A | 116.51 ± 8.27 a, A |
| CW-HMP | 6.59 ± 1.37 a, A | 8.55 ± 0.39 a, B | 280.68 ± 11.29 b, B |
| CW-LMP | 8.91 ± 0.13 b, A | 6.17 ± 1.02 a, A | 122.68 ± 3.94 a, A |
| RW-HMP | 4.79 ± 0.71 a, A | 8.20 ± 1.23 a, B | 289.61 ± 8.26 b, B |
| RW-LMP | 6.97 ± 1.02 a, B | 6.18 ± 0.02 a, A | 121.19 ± 4.74 a, A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalapud, M.C.; Salgado-Cruz, M.d.l.P.; Baümler, E.R.; Carelli, A.A.; Morales-Sánchez, E.; Calderón-Domínguez, G.; García-Hernández, A.B. Study of the Physical, Chemical, and Structural Properties of Low- and High-Methoxyl Pectin-Based Film Matrices Including Sunflower Waxes. Membranes 2023, 13, 846. https://doi.org/10.3390/membranes13100846
Chalapud MC, Salgado-Cruz MdlP, Baümler ER, Carelli AA, Morales-Sánchez E, Calderón-Domínguez G, García-Hernández AB. Study of the Physical, Chemical, and Structural Properties of Low- and High-Methoxyl Pectin-Based Film Matrices Including Sunflower Waxes. Membranes. 2023; 13(10):846. https://doi.org/10.3390/membranes13100846
Chicago/Turabian StyleChalapud, Mayra C., Ma. de la Paz Salgado-Cruz, Erica R. Baümler, Amalia A. Carelli, Eduardo Morales-Sánchez, Georgina Calderón-Domínguez, and Alitzel B. García-Hernández. 2023. "Study of the Physical, Chemical, and Structural Properties of Low- and High-Methoxyl Pectin-Based Film Matrices Including Sunflower Waxes" Membranes 13, no. 10: 846. https://doi.org/10.3390/membranes13100846
APA StyleChalapud, M. C., Salgado-Cruz, M. d. l. P., Baümler, E. R., Carelli, A. A., Morales-Sánchez, E., Calderón-Domínguez, G., & García-Hernández, A. B. (2023). Study of the Physical, Chemical, and Structural Properties of Low- and High-Methoxyl Pectin-Based Film Matrices Including Sunflower Waxes. Membranes, 13(10), 846. https://doi.org/10.3390/membranes13100846







