Abstract
The inadequate management of organic waste and excessive use of plastic containers cause damage to the environment; therefore, different studies have been carried out to obtain new biomaterials from agricultural subproducts. The objective of this work was to evaluate the feasibility of using the pectin extracted from the peel of Passiflora tripartita var. mollissima (PT), characterizing its type and viability for the production of edible biodegradable films. In addition, films of two thicknesses (23.45 ± 3.02 µm and 53.34 ± 2.28 µm) were prepared. The results indicated that PT is an excellent raw material for the extraction of pectin, with high yields (23.02 ± 0.02%), high galacturonic acid content (65.43 ± 2.241%), neutral sugars (ribose, xylose, glucose) and a high degree of esterification (76.93 ± 1.65%), classifying it as a high-methoxy pectin. Regarding the films, they were malleable and flexible, with a water vapor permeability from 2.57 × 10−10 ± 0.046 to 0.13 × 10−10 ± 0.029 g/s mPa according to thickness, being similar to other Passiflora varieties of edible films. The pectin extraction yield from PT makes this fruit a promising material for pectin production and its chemical composition a valuable additive for the food and pharmaceutical industries.
1. Introduction
Currently, environmental concerns regarding the production of non-biodegradable packaging have increased interest in its replacement with biomaterials. These materials are natural polymers, such as proteins and polysaccharides, which have been used in developing bio-based packaging films and edible coatings [1]. Among these biopolymers, pectin is widely used due to its high solubility in water, forming viscous solutions and, under appropriate conditions, its gelling capacity; this behavior varies depending on the number of carboxyl groups esterified with methanol [2].
Structurally, pectin is an acidic heteropolysaccharide, commonly named homogalacturonan, composed mainly of galacturonic acid (GalA), forming a linear backbone of (1 → 4)-linked α-d-GalA residues [3,4]. The carboxyl residues of the GalA unit can be esterified with methanol, which alters the electrical characteristics of the molecule. The degree and pattern of methoxylation of homogalacturonan, as well as its molecular weight, are essential parameters that determine the functional attributes of different pectins [5], giving rise to pectins’ classification into two groups: high and low methoxyl, dependent on the esterified carboxylic groups percentage.
High-methoxyl pectins are those that have more than 50% of esterified carboxylic groups [6], form gels in aqueous systems under pH conditions between 2.8 and 3.5 and have a soluble solids content between 60% and 70%. Low-methoxyl pectin is characterized by generating gel in the presence of polyvalent salts or in low-soluble solids systems, with a wide pH range [7].
The primary source of commercial pectin is citrus fruit peel and apple pomace, since they provide high yields (6–23%) [8]; however, the search to find other new sources is still ongoing. In this sense, the extraction of pectin from banana peel [9], prickly pear fruit and leaves [10,11,12], tejocote [13,14], cocoa [15], pineapple residues [16], guava [17] and different varieties of passion fruit peel [18,19,20,21], among others, has been mentioned, with all of them having low pectin yields (1.15–3.38%), with the exception of passion fruit with higher values (21.25–23.86%).
The genus Passiflora includes more than 500 species, and the most known are as follows: Passiflora edulis Sims, Passiflora ligularis Juss, Passiflora alta Curtis, Passiflora mollisima, and Passiflora edulis var. flavicarpa Degenerer [22]. From these species, P. mollissima (Kunth) L. H. Bailey, commonly known as “curuba de Castilla” or “banana passion fruit” [23], has been cited as a good source of vitamins A, B and C, with high antioxidant activity, as measured using FRAP, ABTS and phenolic compound content, as well as a good pectin source [23].
Regarding pectin extraction from passion fruit and its characterization, most reports are based mainly on Passiflora edulis f. flavicarpa Degener [19] and just a few on Passiflora mollissima [24], including, in most of the studies, the physicochemical properties of fruits at different stages of maturation, without characterizing the pectin. Furthermore, there is no information that allows one to establish whether the pectin extracted from Passiflora tripartita passion fruit var. mollissima is of the high- or low-methoxyl type. Likewise, there are no reports on applications, such as the preparation of biodegradable or edible films, based on this pectin. The objective of this work was to evaluate the feasibility of using the pectin extracted from the shell of “Passiflora tripartita var. mollissima to develop an edible-biodegradable film” for food applications, evaluating the pectin yield, characterizing its type and the feasibility of film production.
2. Materials and Methods
2.1. Materials
The fruits of Passiflora tripartita var. mollissima were obtained from the community of Guarda la Lagunita (Las Canoas), belonging to the municipality of San José del Rincón, State of Mexico. Citric pectin (P-1935, Sigma-Aldrich, México) was also used as a control sample. Glycerol (G5516, Sigma-Aldrich, Toluca, México), tween 20 (P1379, Sigma-Aldrich, Mex), hydrochloric acid (320331, Sigma-Aldrich, Toluca, México.), 96° ethanol and distilled water were also used.
2.2. Methods
2.2.1. Pectin Extraction
The extraction of pectin from the peel of “Passiflora tripartita var. mollissima” was carried out following the methodology reported by Chumbes (2010) [25], with some modifications. The extraction was carried out via acid hydrolysis, maintaining a 1:1 shell/water ratio, at 80 °C for 30 min, adjusting the pH to 3.5 with HCl. The solids were separated via centrifugation (2900× g; Metrix Lab Dynamica, Roterdam, The Netherlands), and the supernatant was precipitated with ethanol 96% in a 1:1 ratio while stirring gently. Subsequently, the precipitated solids were washed with ethanol 96% in a 1:1 ratio, dehydrated at 30 °C for 12 h and milled (KRUPS GX4100) until a fine powder was obtained. Finally, the pectin yield was calculated according to Equation (1).
2.2.2. Characterization of the Pectin of Passiflora tripartita var. mollissima
Methoxyl Group Content
The methoxyl group content was determined according to the methodology reported by Valdespino-León et al. (2020) [4]. Powder pectin (250 mg) was mixed in 50 mL of CO2-free water until completely dissolved, then titrated with 0.5 N NaOH using phenolphthalein until the formation of a faint pink color (initial titration), and then 10 mL of NaOH 0.5N was added and shaken vigorously and allowed to settle for 15 min. Subsequently, 10 mL of 0.5 N HCl was added until the pink coloration disappeared. Finally, a second titration with 0.5 N NaOH was carried out until the faint pink color was maintained (final titration).
The methoxyl group percentage was calculated (Equation (2)) considering that each mL of 0.5 N NaOH of the final titration is equivalent to 15.52 mg of methoxyl (OCH3).
Degree of Esterification
Two solutions, one of Passiflora tripartita var. mollissima (PPT) and the other of citric pectin (PC) in CO2-free water at a concentration of 0.1% (w/v), as reported by Valdespino-León et al. (2020) [4], were prepared. An aliquot of 10 mL was taken and titrated with 0.1 N NaOH using phenol phthalein as indicator (initial titration), and then 20 mL of 0.5 N NaOH was added to neutralize the solution. Finally, the final titration was carried out by placing 0.1 N NaOH until reaching a weak pink coloration. The calculation of the degree of esterification was carried out according to Equation (3).
where DE is the degree of esterification, A is the volume spent on titration A and B is the volume spent on titration B.
Acidity
Regarding the free acidity or acidity percentage, 20 mL of solution was prepared at a concentration of 0.5% (w/v) of powdered pectin (PPT or PC) in distilled water. The solution was heated to 70 °C in a boiling water bath. It was titrated with 0.1 N NaOH using 1% phenolphthalein as the indicator [26]. The results were calculated according to Equation 4 and expressed in terms of meq of free carboxyl using the meq of citric acid (mEAC) (A.O.A.C. 925.34) as a reference.
High-Performance Liquid Chromatography (HPLC)
The evaluation of the pectic substances was performed using high-performance liquid chromatography (HPLC) according to the methodology reported by Valdespino-León et al. (2020) [4]. Briefly, 500 mg of the sample (PPT or PC) was enzymatically hydrolyzed by dissolving in 0.1 M citrate buffer (pH 4) in a 1:30 (w/v) ratio and adding 400 U (450 µL) of Aspergillus niger pectinase (P4716-100KU, Sigma-Aldrich, St. Louis, MO, USA). Subsequently, the samples were incubated in an orbital shaker (Barnstead International, MaxQ 4000, Dubuque, IA, USA) at 30 °C for 2.5 h and at 200 rpm; then, the hydrolyzed samples were stored and refrigerated at 4 °C for 24 h to allow for the precipitation of solids and recovering the supernatants. Consecutively, 2 mL aliquots of the hydrolyzed samples were taken, filtered through 0.2 μm syringe unit (Millex®, 13 mmØ CAT. SLGN013NL, Dublin, Ireland) and placed in glass vials (2 mL). Regarding the calibration standards, solutions of 10 mg of D (+) galacturonic acid monohydrate (47267 Sigma-Aldrich, St. Louis, MO, USA) and simple sugars (including mannose (92683), rhamnose (83650), glucuronic acid (G5269), glucose (G8270), galactose (PHR1206), xylose (PHR2102), arabinose (A3256) and fructose (93183)) (Sigma-Aldrich, St. Louis, MO, USA) were dissolved in 0.01 M H2SO4, to prepare a 0.01% solution, which was filtered in the same way as the pectin hydrolysates [27].
The samples were analyzed in an Agilent 1260 Infinity HPLC equipment (Agilent, Santa Clara, CA, USA) with an IR detector and an Agilent Hi-Plex H column (7.7 × 300 mm, 8 μm, PL1170-6830, Agilent, Santa Clara, CA, USA) following the methodology reported by Ball et al. (2011) [27] with some modifications. The mobile phase was 0.01 M H2SO4 with a flow of 0.4 mL/min; the sample injection volume was 20 μL; and the analysis temperature was 55 °C, both in the column and in the detector. All samples and standards were injected in triplicate, and the duration of each run was established as 25 min. All chromatograms were analyzed using OpenLab CDS v. 4.0 Agilent (Agilent, Santa Clara, CA, USA), reporting the presence of galacturonic acid and other sugars in the hydrolyzed samples, as well as their retention times and concentrations.
2.2.3. Film Preparation
The pectin solution (surface tension: 34.7 ± 1.09 N/m; density: 0.97 ± 0.15 g/mL; viscosity: 0.55 ± 0.04 Pa/s) was prepared following the methodology proposed by Gaona- Sanchez et al. (2016) [28] with some modifications. Pectin samples (2 g of pectin) were dissolved in 50 mL of distilled water by stirring at 850 rpm (Thermo Scientific SP131325 Vernon Hills, IL, USA) for 45 min at room temperature; then, glycerol (22% w/w) was added, maintaining the agitation for 30 min. Finally, Tween® 20 was added in a ratio of 1:10 (w/w) (Tween 20/pectin) at the same speed, temperature and time as described above.
Regarding the preparation of the films, the casting technique was used, in which the pectin solution (7 and 14 mL) was poured into circular Teflon molds (8.0 cm in diameter) and then dried at 30 °C for 12 h in an oven (AFOS, Hull, East Yorkshire, England) and stored at room temperature in a desiccator with Drierite™ anhydride desiccant (Drierite, Xenia, OH, USA) until further analysis.
2.2.4. Characterization of Pectin Films
Color
The readings of the reflection spectrum of the films were carried out according to the methodology reported by Gaona-Sánchez et al. (2015) [29] and Valdespino-León et al. (2020) [4] using a colorimeter (Konica Minolta CR-400, NJ, USES), which was previously calibrated with a reference plate (Y = 93.7, x = 0.3159, y = 0.3324). The result of the measurements was the average of the reading made at five different points. The coordinates of the CIELab color space were obtained, where L* represents lightness (values between 0 and 100), ±a* was the chromatic component from green (−) to red (+) and ±b* was the chromatic component from blue (−) to yellow (+) [30]. In addition, the transparency of the films (%T) was determined considering that L*=%T, assuming that a translucent film will generate the same luminosity values (L*) as the white calibration plate (L* 0 = 100) and that any difference will be the result of a more opaque material (L* < 100) [31]. For the measurements, a standard white plate was used as the background, and statistical analysis was performed with SigmaPlot 12.5 software using one-way ANOVA with p < 0.05. Reported values are the average of each independent triplicate.
Texture
The determination of tensile strength (TS) was performed following the methodology described by Ali et al. (2023) [32], with some modifications. A texture meter (Texture Analyzer CT3, Brookfield™, Chandler, AZ, USA) with a 4500 g load cell programmed with the TexturePro CV V1.6 software was used.
The films were cut into 70 × 33.9 mm rectangles and placed on specific double-grip pieces (TA-DGA accessory) for the tensile test using the following conditions: activation load of 450 g, speed of 0.3 mm/ s and return speed of 4.5 mm/s. The tensile strength (MPa) was calculated by dividing the maximum force (N) at the breaking point by the cross-sectional area (mm2) of the film block (Equation (5)), as described by Xie et al. (2023) [33], while the elongation-at-break values were obtained by recording the elongation at break divided by the initial length of the sample and multiplied by 100.
where TS is the tensile strength, L is the width (mm) and x is the thickness (mm) of the film. The statistical analysis was performed with SigmaPlot 12.5 software using the one-way ANOVA with p ≤ 0.05. The reported values are the average of each independent triplicate. At least five independent samples were employed to assure reproducibility.
Thickness
Films thickness was measured following the methodology reported by Arriaga (2019) [34] using a digital micrometer (Fowler 54860-001 Electronic IP54. Shanghai, China), taking the value that indicates the contact between the film and the probes. The measurements were made at a minimum of three points (central and extreme) and in at least three independent samples, reporting the average.
Water Vapor Permeability
This parameter was evaluated according to the methodology reported by Valdespino-León et al. (2020) [4], which is based on the ASTM E-96 method. A permeability cell and a cup with a lid were used, which were filled with distilled water; then, the film was placed in the mouth of the cup, which, in turn, was placed inside a container with Drierite™ anhydride desiccant (Drierite, Xenia, OH, USA) at 30 °C. The weight was measured for 4 h on an analytical balance with an accuracy of 0.0001 g (OHAUS® Pioneer ™, Parsippany, NJ, USA). The data were plotted, and the slope of the curve was calculated (weight vs time) (R2 > 0.99), obtaining the water vapor transmission rate (WVTR, gs−1 m−2) and dividing the value by the tested film area. WVP was calculated according to the combined laws of Fick and Henry for the diffusion of gases through films according to Equation 6, where x is the thickness of the film (m), and ΔP corresponds to the difference in vapor pressure within the system, whose value is 4246.9 Pa, corresponding to the saturation pressure of water at the saturation temperature at which the system is located (30 °C) (Table A4 water saturated: temperature table) [35].
Differential Scanning Calorimetry (DSC)
Differential scanning calorimetry (DSC) was carried out on DSC 2000 equipment equipment (TA Instruments, New Castle, DE, USA). Samples (5–6 mg) were placed in a standard aluminum container with a perforated lid and heated from 5 to 350 °C at a heating rate of 10 °C min−1 in a nitrogen atmosphere with a set flow rate of 20 mL min−1. An empty aluminum tray (<10 mg) was used as a reference probe. The experiments were performed in independent triplicates. In this analysis, the denaturation temperature (TD), the decomposition temperature (TDS), the melting temperature (Tm) and the glass transition temperature (Tg) are reported [36].
Statistical Analysis
All the analyses were made in independent triplicates, and the results were presented as mean values. Statistical differences were detected using one-way analysis of variance (ANOVA, Tukey’s test), and a value of p < 0.05 indicated statistical significance using the software SigmaPlot 12.5.
3. Results and Discussion
3.1. Pectin Extraction Yield
The pectin extraction yield from Passiflora tripartita var. mollissima reached 23.2 ± 0.05% on a dry basis. This yield value is higher than that reported by Charchalac et al. (2008) [37] for passion fruit peel pectin (Passiflora edulis var. flavicarpa, 12.7–21.3%), Passiflora edulis f. flavicarpa passion fruit (7.52 ± 0.05%, [38] and Passiflora edulis f. flavicarpa Degener fruits (18.45%) [39]. It is also higher than the results of passion fruit peels reported by Kulkarni and Vijayanand (2010) [40], who cited values between 5.78% and 9.02%. Comparing with other pectin sources, the obtained yield can be considered very high, as most reports cited values below 10 percent, for example, for shells from curuba (9.7%), guava (1%) and badea (1.8%) [2], with the exception of apple pomace [41], which presented much higher values (7–23%). According to the above, Muñoz and Cuesta (2012) [18] mentioned that the yields can vary according to the fruits, maturity, extraction method, time and extraction temperature.
3.2. Pectin Chemical Characterization
In the literature, pectin is considered to be a high-methoxyl pectin when the percentage of galacturonic acid, the methoxyl degree and the degree of esterification are higher than 65%, 6.7% and 50%, respectively [4]. In this sense, Table 1 shows the chemical characterization Passiflora tripartita var. mollissima pectin (PPT), compared with citrus pectin (PC) as a control, with both of them being significantly different (p < 0.05) and classified as high-methoxyl pectins.

Table 1.
Chemical characterization of Passiflora tripartita var. mollissima and citric pectins.
According to the above, the percentage of galacturonic acid in PPT (65.4 ± 2.2) was lower than that of PC (72.4 ± 1.8), with similar values to those reported by Lin et al. (2020) [38] and Freitas de Oliveira et al. (2016) [42] on pectin from Passiflora edulis f. flavicarpa and Passiflora edulis Sims f. flavicarpa Degener (68.53 ± 1.40 and 66.27 ± 0.98), mentioning that the values depend on the extraction treatment, the degree of maturation, as well as the region where the fruits were obtained. In the same way, the degree of methoxylation (ME) of PPT was lower than that of PC. However, both were larger than 7%, being classified as high-methoxyl pectins, which agrees with the results reported by other authors for pectin obtained from Passiflora edulis f. flavicarpa. However, the PPT values are lower than those reported for other passion fruit peels, associated with a de-esterification with HCl during the extraction process, which affects its percentage and decreases methoxylation.
On the other hand, the results of the degree of esterification (DE) in both pectin samples were higher than 50% and similar to those reported by Freitas de Oliveira et al. in 2016 [42] (68.8% ± 0.57 to 77.4% ± 0.52), who mentioned that depending on the source and on the experimental conditions applied during the extraction process, the pectin will have different characteristics; in addition to the above, Mendoza-Vargas et al. (2017) [43] reported that during ripening, the tissues of the fruits present a variation in the soluble pectin content, and when the fruits are ripe, the pectin is fully esterified; adding to the above, Cerón-Salazar and Cardona-Alzate, (2011) [44] mentioned that in the immature state, the pectin is fully esterified, which gives the tissue greater rigidity. They also cited that at early stages of maturation, higher pectin yields are obtained with a high methoxyl percent, similar to that reported in this study [45].
Regarding the percentage of acidity, PPT presented a lower value than PC. The results are probably related to the galacturonic acid content of the pectin and possibly to a homogalacturonan skeleton, as reported by Valdespino-León et al. (2020) [4]. In this same sense, Cabarcas et al. (2012) [9] mentioned that pectins are neutral in their natural state; in solution, they have an acid character, which depends on the medium and the degree of esterification. However, the results are higher than those reported in 2016 by Campo-Vera et al. [46] (0.32 ± 0.13 to 0.43 ± 0.05). The differences are likely due to the parameters used in terms of the temperature and time of hydrolysis, affecting both the degree of esterification and the acidity, as reported by Durán et al. (2012) [47]. In addition to the above, Rodríguez-Mora et al. (2022) [15] reported a direct relationship between free acidity and extraction pH, which varies between 2.8 and 3.4 as a function of the degree of esterification. Therefore, the highest levels of acidity occur when the extraction medium shows extreme acidity conditions; in this sense, Cabarcas et al. (2012) [9] mentioned that the free acidity increases as the extraction pH is more acidic, causing a change in the chemical nature of the carboxyl groups, decreasing their state of form (salts or esters) and increasing their presence as acid groups.
High-Performance Liquid Chromatography (HPLC)
The enzymatic hydrolysis of both pectins (PC and PPT) generated significant fractions of glucuronic and galacturonic acids that correspond to the elution peaks at 13.62 ± 0.01 min and 14.03 ± 0.01 min, respectively, confirming what was reported by Valdespino-León et al. (2020) [4], who expressed that the presence of glucuronic acid together with galacturonic acid can constitute the pectic fraction of fruits and vegetables. In the case of the pectin obtained from the shell of Passiflora tripartita var. mollissima, it can be seen that the intensity of the glucuronic acid peak is higher than that of galacturonic acid (Figure 1), which implies that the purification process carried out is insufficient to eliminate this component, which is considered a contaminant in pectin extraction [48].
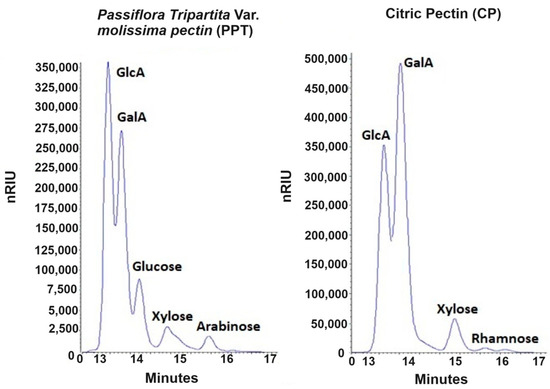
Figure 1.
HPLC chromatogram of hydrolyzed citric pectin (PC) and Passiflora tripartita var. mollissima pectin (PPT) samples. a PC; b PPT. Retention times: GlcA = 13.61 ± 0.02 min, GaIA = 14.03 ± 0.01 min; Glucose = 14.54 ± 0.03 min; Xylose = 15.36 ± 0.01 min; Rhamnose = 16.128 ± 0.05 min, arabinose = 16.63 ± 0.01 min.
When evaluating the concentration of the constituent sugars of the pectins, it was observed that citrus pectin (PC) is mainly composed of galacturonic acid (27.047 ± 0.149 mg/mL) and glucuronic acid (709.47± 0.88 mg/mL), with small proportions of other reducing sugars, such as xylose (2.688 ± 0.015 mg/mL), rhamnose (0.275 ± 0.003 mg/mL) and arabinose (0.119 ± 0.001 mg/mL), reflecting a predominantly linear homogalacturonan structure [49], with some substitutions of rhamnogalacturonan I [50], which, when hydrolyzed with pectinase, promotes the release of galacturonic acid units and other sugars.
Regarding the concentration of galacturonic acid present in PPT (13.933 ± 0.412 mg/mL), it was lower than that of PC; however, the concentration of glucuronic acid considerably exceeded this, indicating that pectin has a lower purity compared to PC [51], which means that a purifying step is needed.
3.3. Characterization of Pectin Films
3.3.1. Thickness
Regarding the thickness of the films, this parameter, as expected, was dependent on the solution volume employed. The films made with 7 mL of the solution presented thickness values of 23.45 ± 3.02 µ (PPT1), while those made with 14 mL of solution had 53.34 ± 2.28 µ (PPT2), the latter being almost twice that of the former, and with the small differences possibly due to the drying rate at which the film was formed. The thicker film also showed larger flexibility, less luminosity, more permeability to water vapor (Table 2) and it was easier to remove from the plate (Figure 2). These values for PPT2 are similar to those reported by Younis et al. (2019) [52] in chitosan-based films (54.37 μ), mentioning that as the thickness of the film increases, the diameter of the pores also increases. In addition to the above, Nascimento et al. (2012) [53] reported higher values (133 and 185 μ) in the thickness of starch films or mesocarp flour; this result is associated with a more significant amount of solution poured onto the plates and more solids. These results prove the feasibility of developing PPT films with similar physical properties to those reported for other pectin films prepared using the casting technique.

Table 2.
Properties of the films of Passiflora tripartita var. mollissima.
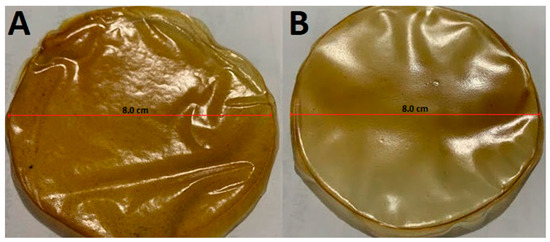
Figure 2.
Simple films based on pectin extracted from the peel of “Passiflora tripartita var. mollissima”. (A): PPT1: film with a thickness of 23.45 ± 3.02 µ; (B): PPT2 film with a thickness of 53.34 ± 2.28 µ.
3.3.2. Color and Texture
Regarding the color parameters (L*, a*, b*) of the films (Table 2), samples show a significant difference (p < 0.05) in luminosity (L*) (PPT1: 92.12; PP2: 85.24), red-green coordinates (a*) (PPT1: 11.72± 0.54; PP2: 9.27 ± 0.30) and the yellow-blue color (b*) (PPT1: 45.62 ± 3.17; PPT2: 65.61 ± 2.21).
The results agree with the visual appearance of the films since PPT1 is more transparent than PPT2, being almost colorless (transparent), which coincides with the high values obtained close to 100 reported for the parameter L*. At the same time, the significant difference found in b* is possibly associated with the increase in the solution concentration since the films became more yellowish. In contrast, for the values of a*, both films go from green to red. This finding indicated that, since the green color of the films became lighter and yellow became the dominant color, the color changes likely to come from the brownish character of the peel peptides of Passiflora tripartita var. mollissima denote a change in coloration, as reported by [34].
Brion-Espinoza et al. (2021) reported similar results [54] in edible films of pectin added with peptides from jackfruit leaves, obtaining values for L* from 91.31±0.01 to 94.01 ± 0.02 and b* from 5.27 ± 0.93 to 13.25 ± 0.059, attributed to the peptide compounds of jackfruit. In another work, Saurabh et al. (2015) [55] mentioned that the reduction in the values of L* and a* indicates an increase in the darkness and greenness of the films based on guar gum, while an increase in b* values means an increase in yellowing. According to the color values obtained, the films could be helpful for the protection of photosensitive compounds when applied as a coating, reducing the intensity of light that passes through them or even incorporating dyes that attract the consumer.
The mechanical properties of the films (deformation modulus, tensile strength (TS) and toughness) can be associated with their chemical structures [56]. The PPT1 and PPT2 films presented significant differences (p < 0.05), as shown in Table 2. The deformation modulus for PPT1 was higher (6.68 ± 0.13 Mpa) than for PPT2 (3.7 ± 0.17 Mpa), which could be related to the thickness of the film, since Márquez et al. (2008) [57] reported that films tend to be more brittle and deform more quickly when they are thinner; likewise, Trujillo (2014) [58] mentioned that the films made from more concentrated solutions of glycerol generate an increase in thickness, tension and deformation at the break. At the same time, Sood et al. (2022) [59] mentioned that the thickness of the film depends on the preparation method, the drying conditions, the composition of the film and the interaction between the components, in addition to being influenced by the structure of the films developed during drying, directly influencing the mechanical properties of said film.
Regarding tensile strength, PPT1 films presented lower values (4.07 ± 0.45 MPa) than PPT2 films (4.80 ± 0.33 MPa) and a higher toughness (3.7 ± 0.36). These results were attributed to the fact that the PPT2 pectin film contains more polar groups, which results in a more significant number of hydrogen bonds and a tighter internal structure. In this sense, Fu et al. (2022) [60] mentioned that the polymer chains were intertwined to form stronger intermolecular hydrogen bonding network structures, resulting in better mechanical properties of the films. Segura-Ceniceros et al. (2006) [61] presented similar results in films made with papain and pectin from passionflower edulis with a thickness of 40 microns, mentioning that the lower the TS of the films, the more fragile and difficult to manipulate. Similar information was reported by Valdespino-León et al. (2021) [4] for citrus pectin films (4.80 ± 0.33 Mpa) and by Sood et al. (2022) [59] for films composed of red grapefruit peel pectin, casein and egg albumin (1.34–9.65 MPa). These authors expressed that the tensile strength is related to the interaction between the polymers within the matrix and the constituents of the film, as well as the method of preparation. In the same way, López and Checa (2019) [62] related the mechanical properties to the effect of the plasticizer since it modifies the structure of the network formed by the biopolymer, achieving films with high elasticity but reducing the resistance of the materials.
3.3.3. Water Vapor Permeability
The application of biopolymer-based edible films seeks to reduce the exchange of moisture between food and the surrounding atmosphere or between two components of a food product [63]. Table 2 shows the water vapor permeability for PPT1 and PPT2 samples, where the values ranged from 0.128 × 10−10 ± 0.029 to 3.187 × 10−10 ± 0.080 g/s·m·Pa, respectively. In this sense, the lowest WVP values and the thinnest films are observed in the PPT1 sample (Table 2), representing a film that could protect most food products. However, the films did not follow a direct relationship with thickness, which was possibly a result of the drying process and film composition, mainly the higher quantity of exposed hydrophilic groups in the thicker samples. In this regard, Morillon et al. (2002) [64] mentioned that the increase in film permeability with thickness could be related to hydrophilic compounds, and Nguyen et al. (2014) [65] reported that an increase in the concentration of polar groups causes an increase in the availability of free hydroxyl groups in the film matrix to react with water, thus increasing the moisture sensitivity of the films. Therefore, the thickness of the film is a crucial parameter in the calculation of water vapor permeability values; in addition, the influence of the thickness varies with the composition of the film, as reported by Thakur et al. (2017) [66], but it remains the main affecting factor [67,68].
On the other hand, Salazar et al. 2015 [69] reported a water vapor permeability value of 0.758 × 10−10 g/m *s* Pa for a film of nopal mucilage, gelatin and beeswax, a lower value than that obtained for PPT2 films of pectin from Passiflora tripartita var. mollissima but higher than PPT1.
3.3.4. Differential Scanning Calorimetry (DSC)
Figure 3 shows the differential scanning calorimetric curves of pectin films of Passiflora tripartita var. mollissima at different thicknesses (A: 23.45 ± 3.02 µ; B: 53.34 ± 2.28 µ). Both thermograms had similar behavior, which was expected since the components are the same. Although the same peaks are observed in both thermograms, in B, there is a displacement, which is probably due to the thickness effect, indicating that the structure formed in the thinner film is less compact; therefore, it would require less energy to release the moisture, having lower transition temperatures. This could also be explained by the largest resistance to heat transfer of the thicker film, as expressed in heat conduction Fourier’s law. On the other hand, both samples presented three inflection points; the first was observed around 105.36 and 108.79 °C, respectively, the second point around 220.19–221.68 °C and, finally, the third was around 330.08 and 331.99 °C.
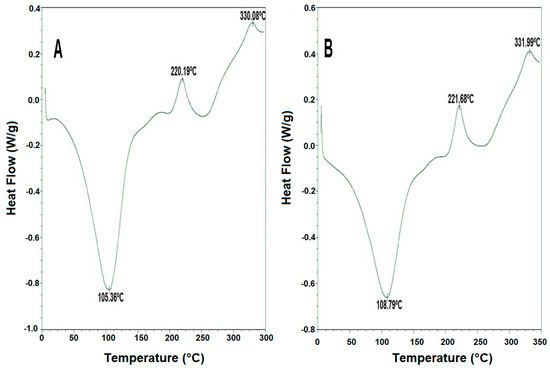
Figure 3.
DSC thermograms of the film of “Passiflora tripartita var. mollissima”. (A): film with a thickness of 23.45 ± 3.02 µ; (B): film with a thickness of 53.34 ± 2.28 µ.
According to the above, exothermic peaks with a temperature between 220.19 and 221.68 °C according to Nisar et al. (2018) [70] are related to the thermal degradation of polymers and pectin. These values are similar to those reported by Linares (2015) [71] and Muñoz (2016) [72] in commercial pectin and hawthorn pectin. In another work, Nisar et al. (2018) [70] mentioned that at 230 °C, there is an exothermic transition peak in citrus pectin, responsible for its degradation, a value similar to that reported by Muñoz (2016) [72], with degradation temperatures ranging between 220 and 240 °C. In this regard, Pasini-Cabello et al. (2015) [73] reported that the endothermic pre-peaks, before the degradation temperatures, are related to a conformational change that could be the transformation of the more stable 4C1 chair conformation of the galacturonan ring through a 1,4B boat conformation to the reverse 1C4 chair conformation.
In another study, Rezvanian et al. (2017) [74] reported that citrus pectin films with sodium alginate exhibited two stages of thermal degradation. The first stage was up to 125 °C, attributed to the loss of different types of water, including free water (released at 40 to 60 °C), water interacting with hydroxyl groups (lost up to 120 °C) and water bound to carboxyl groups (up to 160 °C), and the second stage between 185 and 370 °C.
In this sense, Del Angel (2019) [75] indicated that from 290 °C to 423 °C, the decomposition of polymeric residues occurs, and a high Tg is possibly attributed to the plasticizing effect of these sugars and good chemical stability of the films [76], as well as intramolecular and intermolecular interactions and steric effects [77]; therefore, the high value of Tg in the films of Passiflora tripartita var. mollissima could be governed by its rigid structure or by the presence of sugars [78], while low Tg values imply excellent flexibility of the films, even at refrigeration temperatures [77].
4. Conclusions
It was confirmed that it is possible to obtain pectin from the shell of Passiflora tripartita var. mollissima using the acid hydrolysis technique, with a good extraction yield, resulting in high-methoxyl pectin, with glucuronic acid, galacturonic acid, glucose, xylose and arabinose, characteristic pectin components. In addition to the above, it was possible to make simple films using the plate casting technique; the films are malleable and flexible, with a greenish-yellowish tendency, which highlights the feasibility of this technique for the production of films of different thicknesses and allows for the uniformity of the films. In addition, the color of the films could favor its use for the protection of photosensitive compounds. On the other hand, the films presented good mechanical properties, being resistant films. The thickness and characteristics of the film affected the thermal stability and the diffusion rate of water vapor, presenting a low permeability to water vapor, suggesting a good homogenization of the polymeric matrix and, therefore, a high barrier to water vapor. Likewise, the films were continuous, with certain imperfections, and bright designs were visualized that could indicate the presence of macromolecular aggregates. Finally, pectin was successfully extracted from Passiflora tripartita var. mollissima, which is a little-consumed and -studied fruit and contributes to a reduction in environmental impacts, diversifying the materials for the extraction of feasible compounds in the elaboration of films, with possible applications in the areas of medicine, pharmaceuticals and food.
Author Contributions
Conceptualization: M.R.-O. and G.C.-D.; methodology: E.B.L.-E., M.R.-O., M.V.-L., V.A.G.-S. and G.C.-D.; software: M.V.-L., M.R.-O. and M.C.C.; validation: M.R.-O. and G.C.-D.; formal analysis: A.B.G.-H., M.C.C., M.d.L.C.-A. and F.S.S.-V.; investigation: A.B.G.-H., M.R.-O., V.A.G.-S. and G.C.-D.; resources: M.R.-O., V.A.G.-S. and G.C.-D.; Original draft preparation: V.A.G.-S. and M.R.-O.; writing—reviewing and editing: M.R.-O., M.V.-L., F.S.S.-V. and G.C.-D.; visualization: G.C.-D.; supervision: G.C.-D.; project administration: M.R.-O. and G.C.-D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded through the proyects; 20221471; 20221510; 20230985; and 20230993, from the Instituto Politécnico Nacional (IPN, Mexico) a proyect CAT2022-0011 from catedras COMECYT and FICDTEM-2023-97 from COMECYT-Estado de México.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Minerva Rentería-Ortega thanks Tecnológico de Estudios Superiores de San Felipe del Progreso and Instituto Politécnico Nacional for economical support. Fátima Sarahí Serrano Villa thanks Instituto Politécnico Nacional (BEIFI-IPN) and CONACYT, and Alitzel Belem García Hernández thanks COMECyT (CAT2022-0011) for the scholarships provided.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gahruie, H.H.; Eskandari, M.H.; Van der Meeren, P.; Hosseini, S.M.H. Study on hydrophobic modification of basil seed gum-based (BSG) films by octenyl succinate anhydride (OSA). Carbohydr. Polym. 2019, 219, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, B.L.; Anzola, C. Estudio del efecto fisiológico del consumo de arepas enriquecidas con pectina extraída de la cáscara de curuba (Passiflora tripartita var. mollissima). Rev. Colomb. Química 2018, 47, 5–11. [Google Scholar] [CrossRef]
- Santos, E.E.; Amaro, R.C.; Cid Bustamante, C.C.; Andrade Guerra, M.H.; Soares, L.C.; Froes, R.E.S. Extraction of pectin from agroindustrial residue with an ecofriendly solvent: Use of FTIR and chemometrics to differentiate pectins according to degree of methyl esterification. Food Hydrocoll. 2020, 107, 105921. [Google Scholar] [CrossRef]
- Valdespino-León, M.; Calderón-Domínguez, G.; De La Paz Salgado-Cruz, M.; Rentería-Ortega, M.; Farrera-Rebollo, R.R.; Morales-Sánchez, E.; Terrazas-Valencia, F. Biodegradable electrosprayed pectin films: An alternative to valorize coffee mucilage. Waste Biomass Valorization 2021, 12, 2477–2494. [Google Scholar] [CrossRef]
- Funami, T.; Nakauma, M.; Ishihara, S.; Tanaka, R.; Inoue, T.; Phillips, G.O. Structural modifications of sugar beet pectin and the relationship of structure to functionality. Food Hydrocoll. 2011, 25, 221–229. [Google Scholar] [CrossRef]
- Matta, E.; Bertola, N. Development and characterization of high methoxyl pectin film by using isomalt as plasticizer. J. Food Process. Preserv. 2020, 44, e14568. [Google Scholar] [CrossRef]
- Benavides, Y.E.L.; Moreano, H.L.M.; Chasoy, S.A.P. Obtención de pectina a partir de la cáscara de maracuyá, fuente para la elaboración de plástico biodegradable. Boletín Inf. CEI 2022, 9, 107–110. Available online: https://revistas.umariana.edu.co/index.php/BoletinInformativoCEI/article/view/3018 (accessed on 12 February 2023).
- Ibarra-Egas, J.P.; Ordoñez-Villegas, J.A.; Ortiz-Cabrera, I.A. Obtención de pectina a partir del albedo de maracuyá y limón tahití a través de hidrólisis química. Boletín Inf. CEI 2022, 9, 189–198. Available online: https://revistas.umariana.edu.co/index.php/BoletinInformativoCEI/article/view/3180 (accessed on 15 February 2023).
- Cabarcas, E.; Guerra, A.; Henao, C. Extracción y Caracterización de Pectina a Partir de Cáscaras de Plátano Para Desarrollar un Diseño General del Proceso de Producción. Ph.D. Thesis, Universidad de Cartagena, Cartagena, Colombia, 2012. Available online: https://repositorio.unicartagena.edu.co/handle/11227/109 (accessed on 15 February 2023).
- Chaparro, S.P.; Márquez, R.A.; Sánchez, J.P.; Vargas, M.L.; Gil, J.H. Extracción de pectina del fruto del higo (Opuntia ficus indica) y su aplicación en un dulce de piña. Rev. UDCA Actual. Divulg. Científica 2015, 18, 435–443. [Google Scholar]
- Bello-Lara, J.E.; Balois-Morales, R.; Juárez-López, P.; Alia-Tejacal, I.; Peña-Valdivia, C.B.; Jiménez-Zurita, J.O.; Jiménez-Ruíz, E.I. Recubrimientos a base de almidón y pectina de plátano ‘Pera’(Musa ABB), y quitosano aplicados a frutos de mango ‘Ataulfo’en postcosecha. Rev. Chapingo. Ser. Hortic. 2016, 22, 209–218. [Google Scholar] [CrossRef]
- Díaz Bustamante, G. Evaluación del Rendimiento en la Extracción de Pectina de Tuna (Opuntia Ficus Indica). Bachelor’s Thesis, Universidad Nacional de Cajamarga, Cajamarca, Peru, 2019. Available online: https://190.116.36.86/handle/20.500.14074/3703 (accessed on 25 April 2023).
- Lozano-Grande, M.A.; Valle-Guadarrama, S.; Aguirre-Mandujano, E.; Lobato-Calleros, C.S.; Huelitl-Palacios, F. Películas basadas en emulsiones de pectina de frutos de tejocote (Crataegus spp.) y cera de candelilla: Caracterización y aplicación en Pleurotus ostreatus. Agrociencia 2016, 50, 849–866. [Google Scholar]
- Martínez-Mendoza, A.A.; Mora, O.F.; Sánchez-Pale, J.R.; Rodríguez-Núñez, J.R.; Castañeda-Vildózola, A. Evaluación de recubrimiento comestible a base de pectina de tejocote en postcosecha de tihuixocote (Ximenia americana L.; olacaceae). Acta Agrícola y Pecu. 2020, 6, 0061004. [Google Scholar] [CrossRef]
- Rodríguez-Mora, D.A.; Ramírez, A.F.; Altamar, A.D. Extraction of Pectin from the Acid Hydrolysis of Cocoa (Theobroma Cacao L.) and its Application to Obtain Biofilms. Mutis 2022, 13. Available online: https://hdl.handle.net/20.500.12010/28717 (accessed on 27 March 2023).
- Guevara, B.; Garavito, E.C.A.; Cerquera, J.P. Extracción y caracterización de pectina a partir de residuos de cáscaras de piña (ananas comosus) por el método de hidrólisis ácida. ECBTI Work. Pap. 2020, 1. [Google Scholar]
- Kamal, M.M.; Akhtaruzzaman, M.; Sharmin, T.; Rahman, M.; Mondal, S.C. Optimization of extraction parameters for pectin from guava pomace using response surface methodology. J. Agric. Res. 2023, 11, 100530. [Google Scholar] [CrossRef]
- Muñoz, R.; Cuesta, M. Extracción de pectina a partir de la corteza de maracuyá (Passiflora edulis var. flavicarpa degener). Rev. Politécnica 2012, 31. Available online: https://revistapolitecnica.epn.edu.ec/ojs2/index.php/revista_politecnica2/article/view/195 (accessed on 17 June 2023).
- Sindoni, M.; Hidalgo, P.R.; Castellano, G.; Núñez, K.; Burgos, M.E.; Méndez, R.R. Efecto de dos fases de maduración sobre la cantidad de pectina obtenida en dos variedades de parchita (Passiflora edulis f. flavicarpa degener) de diferente procedencia. Rev. Iberoam. De. Tecnol. Postcosecha 2013, 14, 93–100. Available online: https://www.redalyc.org/pdf/813/81329290001.pdf (accessed on 26 May 2023).
- Lliuyacc Laurente, R. Efecto de la Temperatura, Tiempo y ph en el Rendimiento de Extracción de Pectina en Cáscara de Tumbo Serrano (Passiflora tripartita L.). Bachelor’s Thesis, Universidad de Acobamba–Huancavelica, Acobamba, Peru, 2018. Available online: https://repositorio.unh.edu.pe/items/850b1840-ee05-4789-a11e-d663612d7a0d (accessed on 23 March 2023).
- Rea-Jara, L.C.R.; Moreno, A.M.; Veloz, M.A.L. Determinación del poder gelificante de pectina extraída de cáscara de maracuyá en medio ácido y su aplicación en postres. Ecuadorian J. Steam 2020, 1, 33–43. Available online: https://cimogsys.espoch.edu.ec/direccion-publicaciones/public/docs/books/2021-01-21-164459-ARTICULOS%20CIENTIFICOS%20231120.pdf#page=236 (accessed on 16 April 2023).
- Pereira, Z.C.; dos Anjos Cruz, J.M.; Corrêa, R.F.; Sanches, E.A.; Campelo, P.H.; de Araújo Bezerra, J. Passion fruit (Passiflora spp.) pulp: A review on bioactive properties, health benefits and technological potential. J. Food Res. Int. 2023, 166, 112626. [Google Scholar] [CrossRef]
- Narain, N.; Shanmugam, S.; de Souza Araújo, A.A. Antioxidant, antimicrobial, analgesic, anti-inflammatory and antipyretic effects of bioactive compounds from Passiflora species. Med. Plants Farm. Pharm. 2019, 243–274. [Google Scholar] [CrossRef]
- Vera-Cieza, R.; Rufasto-Pérez, E. Caracterización fisicoquímica y reológica en frutos de “poro poro” Passiflora mollissima (Kunth) LH Bailey. (Passifloraceae) en la provincia de Chota: Physicochemical and rheological characterization in “poro poro” fruits Passiflora mollissima (Kunth) LH Bailey. (Passifloraceae) in the province of Chota. Rev. Cienc. Nor@Ndina 2019, 2, 66–71. [Google Scholar] [CrossRef]
- Chumbes Montes, M.M. Hidrólisis ácida de la Cáscara de Maracuyá (Passiflora edulis) Para la Obtención de Pectina Como Agente gelificante. Tesis de Ingeniería, Universidad Nacional José Faustino Sánchez Carrión, Huacho, Peru, 2010. Available online: https://hdl.handle.net/20.500.14067/4411 (accessed on 17 August 2023).
- Owens, H.S.; McCready, R.K.; Sheperd, A.D.; Schultz, T.H.; Pippen, E.L.; Swenson, H.A.; Miers, J.C.; Erlandsen, R.F.; Maclay, W.D. Methods Used at Western Regional Research Laboratory or Extraction and Analysis of Pectic materials. Western. Anal. Chem. 1952, 24, 54–59. [Google Scholar]
- Ball, S.; Bullock, S.; Lloyd, L.; Mapp, K. Analysis of carbohydrates, alcohols and organic acids by ion-exchange chromatography. In Agilent Hi Plex Columns Applications Compendium; Agilent Technologies Inc.: Pirmasens, Germany, 2011; pp. 1–98. [Google Scholar]
- Gaona-Sánchez, V.A.; Calderón-Domínguez, G.; Morales-Sánchez, E.; Chanona-Pérez, J.J.; Arzate-Vázquez, I.; Terrés-Rojas, E. Pectin-based films produced by electrospraying. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Gaona-Sánchez, V.A.; Calderon-Dominguez, G.; Morales-Sanchez, E.; Chanona-Perez, J.J.; Velazquez-De La Cruz, G.; Mendez-Mendez, J.V.; Farrera-Rebollo, R.R. Preparation and characterisation of zein films obtained by electrospraying. Food Hydrocoll. 2015, 49, 1–10. [Google Scholar] [CrossRef]
- Calderon-Dominguez, G.; Vera-Dominguez, M.; Farrera-Rebollo, R.; Arana-Errasquin, R.; Mora-Escobedo, R. Rheological changes of dough and bread quality prepared from a sweet dough: Effect of temperature and mixing time. Int. J. Food Prop. 2004, 7, 165–174. [Google Scholar] [CrossRef]
- Escamilla-García, M.; Calderón-Domínguez, G.; ChanonaPérez, J.J.; Farrera-Rebollo, R.R.; Andraca-Adame, J.A.; ArzateVázquez, I.; Méndez-Méndez, J.V.; Moreno-Ruiz, L.A. Physical and structural characterisation of zein and chitosan edible flms using nanotechnology tools. Int. J. Biol. Macromol. 2013, 61, 196–203. [Google Scholar] [CrossRef]
- Ali, S.M.A.; Niaz, T.; Munir, A.; Shahid, R.; Shabbir, S.; Noor, T.; Imran, M. Potential of pectin-chitosan based composite films embedded with quercetin-loaded nanofillers to control meat associated spoilage bacteria. Food Biosci. 2023, 53, 102547. [Google Scholar]
- Xie, J.; Zhang, Y.; Klomklao, S.; Simpson, B.K. Pectin from plantain peels: Green recovery for transformation into reinforced packaging films. Waste Manag. 2023, 161, 225–233. [Google Scholar] [CrossRef]
- Arriaga Perea, J.A. Evaluación de las Propiedades Físicas de Películas de Gliadinas con el uso de Formaldehído Como Agente Entrecruzante. Tesis de ingeniería, Universidad de La Salle, Bogotá, Colombia, 2019. Available online: https://ciencia.lasalle.edu.co/ing_alimentos/260 (accessed on 13 May 2023).
- Cengel, Y.A.; Boles, M.A.; Kanoğlu, M. Thermodynamics: An Engineering Approach; McGraw-hill: New York, NY, USA, 2011; Volume 5, p. 445. [Google Scholar]
- García-Hernández, A.B.; Morales-Sánchez, E.; Berdeja-Martínez, B.M.; Escamilla-García, M.; Salgado-Cruz, M.P.; Rentería-Ortega, M.; Calderón-Domínguez, G. PVA-based electrospun biomembranes with hydrolyzed collagen and ethanolic extract of Hypericum perforatum for potential use as wound dressing: Fabrication and characterization. Polymers 2022, 14, 1981. [Google Scholar] [CrossRef]
- Charchalac, L.R. Efecto del Agente de Extracción y Tiempo de Hidrólisis Ácida en el Rendimiento de Pectina de Cáscaras de Maracuyá (Passiflora Edulis var. Flavicarpa). Ph.D. Thesis, Escuela Agricola Panamericana, Zamorano, Honduras, 2008. Available online: https://bdigital.zamorano.edu/handle/11036/5401 (accessed on 20 February 2023).
- Lin, Y.; An, F.; He, H.; Geng, F.; Song, H.; Huang, Q. Structural and rheological characterization of pectin from passion fruit (Passiflora edulis f. flavicarpa) peel extracted by high-speed shearing. Food Hydrocoll. 2020, 114, 106555. [Google Scholar] [CrossRef]
- D’Addosio, R.; Paéz, G.; Marı’n, M.; Ma’rmol, Z.; Ferrer, J. Obtainment and characterization of pectin since of the peel of passion fruit (Passiflora edulis F. Flavicarpa Degener). Rev. Fac. Agron. J—LUZ 2005, 22, 240–249. Available online: https://ve.scielo.org/scielo.php?script=sci_arttext&pid=S0378-78182005000300004&lng=es&nrm=iso> (accessed on 19 June 2023).
- Kulkarni, S.G.; Vijayanand, P. Effect of extraction conditions on the quality characteristics of pectin from passion fruit peel (Passiflora edulis f. flavicarpa L.). LWT-Food Sci. Technol. 2010, 43, 1026–1031. [Google Scholar] [CrossRef]
- Calvete-Torre, I.; Muñoz-Almagro, N.; Pacheco, M.T.; Antón, M.J.; Dapena, E.; Ruiz, L.; Moreno, F.J. Apple pomaces derived from mono-varietal Asturian ciders production are potential source of pectins with appealing functional properties. Carbohydr. Polym. 2021, 264, 117980. [Google Scholar] [CrossRef] [PubMed]
- Freitas de Oliveira, C.F.; Giordani, D.; Lutckemier, R.; Gurak, P.D.; Cladera-Olivera, F.; Marczak, L.D.F. Extraction of pectin from passion fruit peel assisted by ultrasound. LWT-Food Sci. Technol. 2016, 71, 110–115. [Google Scholar] [CrossRef]
- Mendoza-Vargas, L.; Jiménez-Forero, J.; Ramírez-Niño, M. Evaluation Of Pectin Extracted Enzymatically From Cocoa (Theobroma Cacao L.) Pod Husks. Rev. UDCA Actual. Divulg. Científica 2017, 20, 131–138. Available online: http://www.scielo.org.co/pdf/rudca/v20n1/v20n1a15.pdf (accessed on 29 April 2023).
- Cerón-Salazar, I.; Cardona-Alzate, C. Evaluación del proceso integral para la obtención de aceite esencial y pectina a partir de cáscara de naranja. Ing. Y Cienc. 2011, 7, 65–86. Available online: http://www.scielo.org.co/pdf/ince/v7n13/v7n13a04.pdf (accessed on 13 May 2023).
- Fredes Monsalves, C.; Loyola López, N.; Muñoz Cruz, J.C. Extracción de pectinas de Vitis labrusca CV. Concord para producir jaleas. Idesia 2009, 27, 9–14. [Google Scholar] [CrossRef]
- Campo-Vera, Y.E.; Villada-Castillo, D.C.; Meneses-Ortega, J.D. Effect of The Pre-Tratamiento With Ultrasound In The Extraction Of Pectin Contained In The Albedo Of The Maracuyá (Passiflora edulis). Biotecnol. En. El Sect. Agropecu. Y Agroindustrial 2016, 14, 103–109. [Google Scholar] [CrossRef]
- Duran, V.; Honores, M.; Cáceres, P.; Obtención de Pectina en Polvo a Partir de la Cáscara de Maracuyá (Passiflora edulis). FIMCP 2012. Available online: https://www.dspace.espol.edu.ec/handle/123456789/20660 (accessed on 19 June 2023).
- Yapo, B.M. Pectic substances: From simple pectic polysaccharides to complex pectins—A new hypothetical model. Carbohydr. Polym. 2011, 86, 373–385. [Google Scholar] [CrossRef]
- Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef]
- Seixas, F.L.; Fukuda, D.L.; Turbiani, F.R.B.; Garcia, P.S.; Petkowicz, C.L.d.O.; Jagadevan, S.; Gimenes, M.L. Extraction of pectin from passion fruit peel (Passiflora edulis f. flavicarpa) by microwave-induced heating. Food Hydrocoll. 2014, 38, 186–192. [Google Scholar] [CrossRef]
- Dranca, F.; Oroian, M. Extraction, purification and characterization of pectin from alternative sources with potential technological applications. Food Res. Int. 2018, 113, 327–350. [Google Scholar] [CrossRef] [PubMed]
- Younis, H.G.R.; Abdellatif, H.R.S.; Ye, F.; Zhao, G. Tuning the physicochemical properties of apple pectin films by incorporating chitosan/pectin fiber. Int. J. Biol. Macromol. 2020, 159, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, T.A.; Calado, V.; Carvalho, C.W.P. Development and characterization of flexible film based on starch and passion fruit mesocarp flour with nanoparticles. Food Res. Int. 2012, 49, 588–595. [Google Scholar] [CrossRef]
- Brion-Espinoza, I.A.; Iniguez-Moreno, M.; Ragazzo-Sánchez, J.A.; Barros-Castillo, J.C.; Calderón-Chiu, C.; Calderón-Santoyo, M. Edible pectin film added with peptides from jackfruit leaves obtained by high-hydrostatic pressure and pepsin hydrolysis. Food Chem. X 2021, 12, 100170. [Google Scholar] [CrossRef]
- Saurabh, C.K.; Gupta, S.; Bahadur, J.; Mazumder, S.; Variyar, P.S.; Sharma, A. Mechanical and barrier properties of guar gum based nano-composite films. Carbohydr. Polym. 2015, 124, 77–84. [Google Scholar] [CrossRef]
- Otoni, C.G.; de Moura, M.R.; Aouada, F.A.; Camilloto, G.P.; Cruz, R.S.; Lorevice, M.V.; Soares, N.F.F.; Mattoso, L.H.C. Antimicrobial and physical-mechanical properties of pectin/papaya puree/cinnamaldehyde nanoemulsion edible composite films. Food Hydrocoll. 2014, 41, 188–194. [Google Scholar] [CrossRef]
- Márquez, R.; Escobar, D.; Sala, A.; Silvera, C.; Repiso, L. Elaboración, caracterización y comparación de películas comestibles en base a aislado de proteínas de suero lácteo (WPI). INNOTEC (3 ene-dic) 2008, 57–62. [Google Scholar] [CrossRef]
- Trujillo Rivera, C.T. Obtención de películas biodegradables a partir de almidón de yuca (Manihot esculente Crantz) doblemente modificado para uso en empaque de alimentos. Bachelor’s Thesis, Universidad Nacional Amazónica de Madre de Dios, Puerto Maldonado, Peru, 2014. Available online: https://hdl.handle.net/20.500.14070/65 (accessed on 26 April 2023).
- Sood, A.; Saini, C.S. Red pomelo peel pectin based edible composite films: Effect of pectin incorporation on mechanical, structural, morphological and thermal properties of composite films. Food Hydrocoll. 2022, 123, 107135. [Google Scholar] [CrossRef]
- Fu, X.; Chang, X.; Ding, Z.; Xu, H.; Kong, H.; Chen, F.; Ding, S. Fabrication and Characterization of Eco-Friendly Polyelectrolyte Bilayer Films Based on Chitosan and Different Types of Edible Citrus Pectin. Foods 2022, 11, 3536. [Google Scholar] [CrossRef]
- Segura-Ceniceros, E.P.; Ilyinб, A.D.; Montalvo-Arredondo, J.I.; Zaragoza-Contreras, A.; Flores-Gallardo, S.G.; Vargas-Dominguez, C.I. Evaluation of the effect of pectin-papain interactions on the enzyme stability and mechanical properties of maracuya’s pectin films for the treatment of skin wounds. Вестник Мoскoвскoгo Университета Серия 2. Химия 2006, 47, 66–72. [Google Scholar]
- López, D.F.; Osorio, O.; Checa, O.E. Propiedades mecánicas de un material de pectina para revestimiento de fibras naturales utilizadas en aplicaciones agrícolas. Inf. Tecn. 2019, 30, 189–198. [Google Scholar] [CrossRef]
- Xu, Y.; Chu, Y.; Feng, X.; Gao, C.; Wu, D.; Cheng, W.; Meng, L.; Zhang, Y.; Tang, X. Effects of zein stabilized clove essential oil Pickering emulsion on the structure and properties of chitosan-based edible films. Int. J. Biol. Macromol. 2020, 156, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Morillon, V.; Debeaufort, F.; Blond, G.; Capelle, M.; Voilley, A. Factors affecting the moisture permeability of lipid-based edible films: A review. Crit. Rev. Food Sci. Nutr. 2002, 42, 67–89. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.A.; Gregersen, Ø.W.; Männle, F.; Brachet, P. Effects of hydrophobic polyhedral oligomeric silsesquioxane coating on water vapour barrier and water resistance properties of paperboard. J. Sol-Gel Sci. Technol. 2014, 69, 237–249. [Google Scholar] [CrossRef]
- Thakur, R.; Saberi, B.; Pristijono, P.; Stathopoulos, C.E.; Golding, J.B.; Scarlett, C.J.; Bowyer, M.; Vuong, Q.V. Use of response surface methodology (RSM) to optimize pea starch–chitosan novel edible film formulation. J. Food Sci. Technol. 2017, 54, 2270–2278. [Google Scholar] [CrossRef]
- Fadini, A.L.; Rocha, F.S.; Alvim, I.D.; Sadahira, M.S.; Queiroz, M.B.; Alves, R.M.V.; Silva, L.B. Mechanical properties and water vapour permeability of hydrolysed collagen–cocoa butter edible films plasticised with sucrose. Food Hydrocoll. 2013, 30, 625–631. [Google Scholar] [CrossRef]
- Mali, S.; Grossmann, M.V.E.; García, M.A.; Martino, M.N.; Zaritzky, N.E. Barrier, mechanical and optical properties of plasticized yam starch films. Carbohydr. Polym. 2004, 56, 129–135. [Google Scholar] [CrossRef]
- Salazar, V.M.S.; Márquez, M.A.T.; Vargas, A.L. Propiedades físicas, mecánicas y de barrera de películas comestibles a base de mucílago de Nopal como alternativa para la aplicación en frutos. Rev. Iberoam. De. Tecnol. Postcosecha 2015, 16, 193–198. Available online: https://www.redalyc.org/articulo.oa?id=81343176007 (accessed on 9 May 2023).
- Nisar, T.; Wang, Z.-C.; Yang, X.; Tian, Y.; Iqbal, M.; Guo, Y. Characterization of citrus pectin films integrated with clove bud essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Int. J. Biol. Macromol. 2018, 106, 670–680. [Google Scholar] [CrossRef]
- Linares García, J.A. Estudio de las Propiedades Físicas y Texturales de Geles de Pectinas de alto y Bajo Metoxilo Obtenidas de Crataegus pubescens (Tejocote). Ph.D. Thesis, Centro de Investigación y Estudios Avanzados del Instituto Politécnico Nacional, Ciudad de México, Mexico, 2015. Available online: https://repositorio.cinvestav.mx/bitstream/handle/cinvestav/1377/SSIT0013102.pdf?sequence=1 (accessed on 18 July 2023).
- Muñoz Labrador, A. Caracterización de pectinas industriales de cítricos y su aplicación como recubrimientos de fresas. Master’s Thesis, Universidad Autónoma de Madrid, Madrid, Spain, 2016. Available online: https://digital.csic.es/bitstream/10261/176559/1/LabradorTFMpectinasfresas.pdf (accessed on 13 June 2023).
- Pasini Cabello, S.D.; Takara, E.A.; Marchese, J.; Ochoa, N.A. Influence of plasticizers in pectin films: Microstructural changes. Mater. Chem. Phys. 2015, 162, 491–497. [Google Scholar] [CrossRef]
- Rezvanian, M.; Ahmad, N.; Mohd Amin, M.C.I.; Ng, S.-F. Optimization, characterization, and in vitro assessment of alginate-pectin ionic cross-linked hydrogel film for wound dressing applications. Int. J. Biol. Macromol. 2017, 97, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Del Angel Purata, F.M. Películas Para Recubrimiento De Alimentos Base Pectina, Alginato Y Quitosano. Master’s Thesis, Tecnológico Nacional de México, Ciudad Madero, Mexico, 2019. Available online: https://200.188.131.162:8080/jspui/handle/123456789/395 (accessed on 25 August 2023).
- Ghanbarzadeh, B.; Oromiehie, A.R.; Musavi, M.; D-Jomeh, Z.E.; Rad, E.R.; Milani, J. Effect of plasticizing sugars on rheological and thermal properties of zein resins and mechanical properties of zein films. Food Res. Int. 2006, 39, 882–890. [Google Scholar] [CrossRef]
- Azeredo, H.M.; Mattoso, L.H.C.; Wood, D.; Williams, T.G.; Avena-Bustillos, R.J.; McHugh, T.H. Nanocomposite Edible Films from Mango Puree Reinforced with Cellulose Nanofibers. J. Food Sci. 2009, 74, N31–N35. [Google Scholar] [CrossRef] [PubMed]
- Falcaorodrigues, M.; Moldaomartins, M.; Beiraodacosta, M. Dsc As A Tool To Assess Physiological Evolution Of Apples Preserved By Edibles Coatings. Food Chem. 2007, 102, 475–480. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).