Preparation and Performance Study of the Anodic Catalyst Layer via Doctor Blade Coating for PEM Water Electrolysis
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Doctor Blade Coating
2.3. Spray Coating
2.4. Preparation of the CCM
2.5. Characterizations
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, G.; Hou, F.; Peng, S.; Wang, X.; Fang, B. Synthesis, physical properties and electrocatalytic performance of nickel phosphides for hydrogen evolution reaction of water electrolysis. Nanomaterials 2022, 12, 2935. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Fang, B.; Wang, Z.; Wang, C.; Liu, Z.; Liu, F.; Wang, W.; Alfantazi, A.; Wang, D.; Wilkinson, D. MoS2 nanosheets: A designed structure with high active site density for the hydrogen evolution reaction. ACS Catal. 2013, 3, 2101–2107. [Google Scholar] [CrossRef]
- Lu, L.; Zou, S.; Fang, B. The critical impacts of ligands on heterogeneous nanocatalysis: A review. ACS Catal. 2021, 11, 6020–6258. [Google Scholar] [CrossRef]
- Lu, L.; Zheng, H.; Li, Y.; Zhou, Y.; Fang, B. Ligand-free synthesis of noble metal nanocatalysts for electrocatalysis. Chem. Eng. J. 2022, 451, 138668. [Google Scholar] [CrossRef]
- Liao, G.; Li, C.; Liu, S.; Fang, B.; Yang, H. Z-scheme systems: From fundamental principles to characterization, synthesis, and photocatalytic fuel-conversion applications. Phys. Rep. 2022, 983, 1–41. [Google Scholar] [CrossRef]
- Liao, G.; Tao, X.; Fang, B. An innovative synthesis strategy for highly efficient and defects-switchable hydrogenated TiO2 photocatalysts. Matter 2022, 5, 377–379. [Google Scholar] [CrossRef]
- Liao, G.; Li, C.; Li, X.; Fang, B. Emerging polymeric carbon nitride Z-scheme systems for photocatalysis. Cell Rep. Phys. Sci. 2021, 2, 100355. [Google Scholar] [CrossRef]
- Liao, G.; Li, C.; Fang, B. Donor-acceptor organic semiconductor heterojunction nanoparticles for efficient photocatalytic H2 evolution. Matter 2022, 5, 1635–1637. [Google Scholar] [CrossRef]
- Liao, G.; Li, C.; Liu, S.; Fang, B.; Yang, H. Emerging frontiers of Z-scheme photocatalytic systems. Trends Chem. 2022, 4, 111–127. [Google Scholar] [CrossRef]
- Ursúa, A.; Gandía, L.M.; Sanchis, P. Hydrogen Production from Water Electrolysis: Current Status and Future Trends. Proc. IEEE 2012, 100, 410–426. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A Comprehensive Review on PEM Water Electrolysis. Int. J. Hydrog. Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Xiang, C.; Papadantonakis, K.M.; Lewis, N.S. Principles and Implementations of Electrolysis Systems for Water Splitting. Mater. Horiz. 2016, 3, 169–173. [Google Scholar] [CrossRef]
- Tong, W.; Forster, M.; Dionigi, F.; Dresp, S.; Sadeghi Erami, R.; Strasser, P.; Cowan, A.J.; Farràs, P. Electrolysis of Low-Grade and Saline Surface Water. Nat. Energy 2020, 5, 367–377. [Google Scholar] [CrossRef]
- Hung, Y.; Tawfik, H.; Mahajan, D. Durability and Characterization Studies of Polymer Electrolyte Membrane Fuel Cell’s Coated Aluminum Bipolar Plates and Membrane Electrode Assembly. J. Power Sources 2009, 186, 123–127. [Google Scholar] [CrossRef]
- Feng, Q.; Yuan, X.-Z.; Liu, G.; Wei, B.; Zhang, Z.; Li, H.; Wang, H. A Review of Proton Exchange Membrane Water Electrolysis on Degradation Mechanisms and Mitigation Strategies. J. Power Sources 2017, 366, 33–55. [Google Scholar] [CrossRef]
- Liu, G.; Xu, J.; Jiang, J.; Peng, B.; Wang, X. Nanosphere-Structured Composites Consisting of Cs-Substituted Phosphotungstates and Antimony Doped Tin Oxides as Catalyst Supports for Proton Exchange Membrane Liquid Water Electrolysis. Int. J. Hydrog. Energy 2014, 39, 1914–1923. [Google Scholar] [CrossRef]
- Liu, G.; Xu, J.; Wang, Y.; Jiang, J.; Wang, X. A Novel Catalyst Coated Membrane Embedded with Cs-Substituted Phosphotungstates for Proton Exchange Membrane Water Electrolysis. Int. J. Hydrog. Energy 2014, 39, 14531–14539. [Google Scholar] [CrossRef]
- Liu, G.; Xu, J.; Wang, Y.; Wang, X. An Oxygen Evolution Catalyst on an Antimony Doped Tin Oxide Nanowire Structured Support for Proton Exchange Membrane Liquid Water Electrolysis. J. Mater. Chem. A 2015, 3, 20791–20800. [Google Scholar] [CrossRef]
- Park, Y.-C.; Tokiwa, H.; Kakinuma, K.; Watanabe, M.; Uchida, M. Effects of Carbon Supports on Pt Distribution, Ionomer Coverage and Cathode Performance for Polymer Electrolyte Fuel Cells. J. Power Sources 2016, 315, 179–191. [Google Scholar] [CrossRef]
- Chlistunoff, J.; Pivovar, B. Effects of Ionomer Morphology on Oxygen Reduction on Pt. J. Electrochem. Soc. 2015, 162, F890–F900. [Google Scholar] [CrossRef]
- Healy, J.; Hayden, C.; Xie, T.; Olson, K.; Waldo, R.; Brundage, M.; Gasteiger, H.; Abbott, J. Aspects of the Chemical Degradation of PFSA Ionomers Used in PEM Fuel Cells. Fuel Cells 2005, 5, 302–308. [Google Scholar] [CrossRef]
- Zawodzinski, T.A.; Neeman, M.; Sillerud, L.O.; Gottesfeld, S. Determination of Water Diffusion Coefficients in Perfluorosulfonate Ionomeric Membranes. J. Phys. Chem. 1991, 95, 6040–6044. [Google Scholar] [CrossRef]
- Chen, M.; Wang, M.; Yang, Z.; Ding, X.; Li, Q.; Wang, X. A Novel Catalyst Layer Structure Based Surface-Patterned Nafion® Membrane for High-Performance Direct Methanol Fuel Cell. Electrochim. Acta 2018, 263, 201–208. [Google Scholar] [CrossRef]
- Liu, G.; Peng, S.; Hou, F.; Fang, B.; Wang, X. Au-TiO2/Ti Hybrid Coating as a Liquid and Gas Diffusion Layer with Improved Performance and Stability in Proton Exchange Membrane Water Electrolyzer. Molecules 2022, 27, 6644. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, S.; Wang, D.; Zhang, H.; Shan, D.; Peng, S.; Shen, G.; Wang, L.; Wang, X. Modifying Ti-Based Gas Diffusion Layer Passivation for Polymer Electrolyte Membrane Water Electrolysis via Electrochemical Nitridation. ACS Appl. Mater. Interfaces 2022, 14, 15728–15735. [Google Scholar] [CrossRef]
- Mauger, S.A.; Wang, M.; Cetinbas, F.C.; Dzara, M.J.; Park, J.; Myers, D.J.; Ahluwalia, R.K.; Pylypenko, S.; Hu, L.; Litster, S.; et al. Development of High-Performance Roll-to-Roll-Coated Gas-Diffusion-Electrode-Based Fuel Cells. J. Power Sources 2021, 506, 230039. [Google Scholar] [CrossRef]
- Kwan, J.T.H.; Bonakdarpour, A.; Afonso, G.; Wilkinson, D.P. Bridging Fundamental Electrochemistry with Applied Fuel Cell Testing: A Novel and Economical Rotating Disk Electrode Tip for Electrochemical Assessment of Catalyst-Coated Membranes. Electrochim. Acta 2017, 258, 208–219. [Google Scholar] [CrossRef]
- Klingele, M.; Britton, B.; Breitwieser, M.; Vierrath, S.; Zengerle, R.; Holdcroft, S.; Thiele, S. A Completely Spray-Coated Membrane Electrode Assembly. Electrochem. Commun. 2016, 70, 65–68. [Google Scholar] [CrossRef]
- Park, I.S.; Li, W.; Manthiram, A. Fabrication of Catalyst-Coated Membrane-Electrode Assemblies by Doctor Blade Method and Their Performance in Fuel Cells. J. Power Sources 2010, 195, 7078–7082. [Google Scholar] [CrossRef]
- Stähler, M.; Stähler, A.; Scheepers, F.; Carmo, M.; Stolten, D. A Completely Slot Die Coated Membrane Electrode Assembly. Int. J. Hydrog. Energy 2019, 44, 7053–7058. [Google Scholar] [CrossRef]
- Mauger, S.A.; Neyerlin, K.C.; Yang-Neyerlin, A.C.; More, K.L.; Ulsh, M. Gravure Coating for Roll-to-Roll Manufacturing of Proton-Exchange-Membrane Fuel Cell Catalyst Layers. J. Electrochem. Soc. 2018, 165, F1012–F1018. [Google Scholar] [CrossRef]
- Debe, M.K. Electrocatalyst Approaches and Challenges for Automotive Fuel Cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wei, C.; Huang, Z.F.; Liu, C.; Zeng, L.; Wang, X.; Xu, Z.J. A Review on Fundamentals for Designing Oxygen Evolution Electrocatalysts. Chem. Soc. Rev. 2020, 49, 2196–2214. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Aili, D.; Li, Q.; Pan, C.; Christensen, E.; Jensen, J.O.; Zhang, W.; Liu, G.; Wang, X.; Bjerrum, N.J. Antimony Doped Tin Oxide Modified Carbon Nanotubes as Catalyst Supports for Methanol Oxidation and Oxygen Reduction Reactions. J. Mater. Chem. A 2013, 1, 9737–9745. [Google Scholar] [CrossRef]
- Yu, H.M.; Ziegler, C.; Oszcipok, M.; Zobel, M.; Hebling, C. Hydrophilicity and Hydrophobicity Study of Catalyst Layers in Proton Exchange Membrane Fuel Cells. Electrochim. Acta 2006, 51, 1199–1207. [Google Scholar] [CrossRef]
- Park, J.; Kang, Z.; Bender, G.; Ulsh, M.; Mauger, S.A. Roll-to-Roll Production of Catalyst Coated Membranes for Low-Temperature Electrolyzers. J. Power Sources 2020, 479, 228819. [Google Scholar] [CrossRef]
- Khandavalli, S.; Park, J.H.; Kariuki, N.N.; Zaccarine, S.F.; Pylypenko, S.; Myers, D.J.; Ulsh, M.; Mauger, S.A. Investigation of the Microstructure and Rheology of Iridium Oxide Catalyst Inks for Low-Temperature Polymer Electrolyte Membrane Water Electrolyzers. ACS Appl. Mater. Interfaces 2019, 11, 45068–45079. [Google Scholar] [CrossRef]
- Kabir, S.; Myers, D.J.; Kariuki, N.; Park, J.; Wang, G.; Baker, A.; Macauley, N.; Mukundan, R.; More, K.L.; Neyerlin, K.C. Elucidating the Dynamic Nature of Fuel Cell Electrodes as a Function of Conditioning: An Ex Situ Material Characterization and in Situ Electrochemical Diagnostic Study. ACS Appl. Mater. Interfaces 2019, 11, 45016–45030. [Google Scholar] [CrossRef]
- Li, C.; LIU, S.; Zeng, Y.; Liu, Y.; Wu, G.; Cullen, D.A.; Xie, J. Effects of Ink Formulation on the Structure and Performance of PGM-Free Catalyst Layer in PEMFCs. ECS Trans. 2021, 104, 327–333. [Google Scholar] [CrossRef]
- Sone, Y. Proton Conductivity of Nafion 117 as Measured by a Four-Electrode AC Impedance Method. J. Electrochem. Soc. 1996, 143, 1254. [Google Scholar] [CrossRef]
- Wang, M.; Park, J.H.; Kabir, S.; Neyerlin, K.C.; Kariuki, N.N.; Lv, H.; Stamenkovic, V.R.; Myers, D.J.; Ulsh, M.; Mauger, S.A. Impact of Catalyst Ink Dispersing Methodology on Fuel Cell Performance Using In-Situ X-Ray Scattering. ACS Appl. Energy Mater. 2019, 2, 6417–6427. [Google Scholar] [CrossRef]
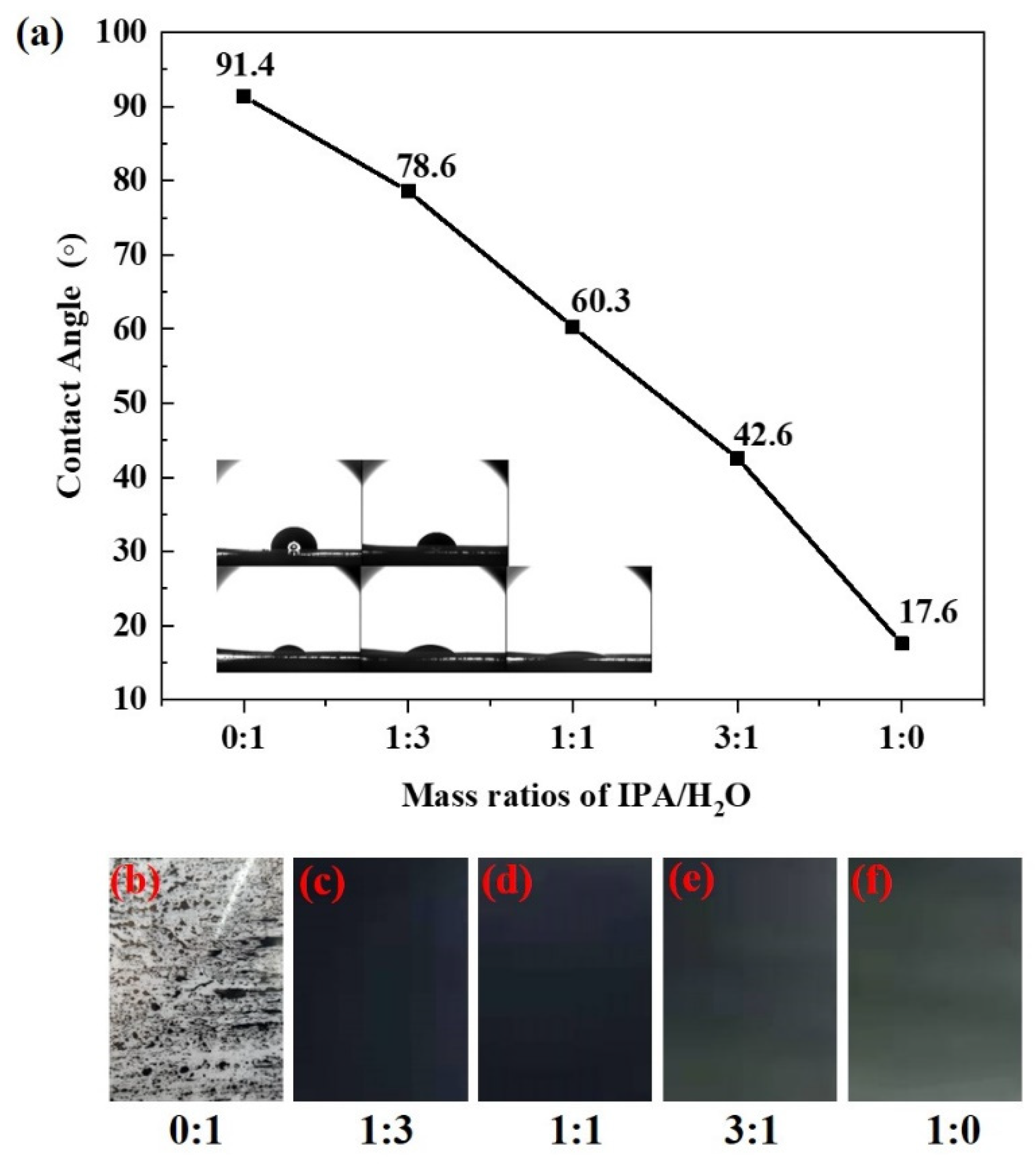
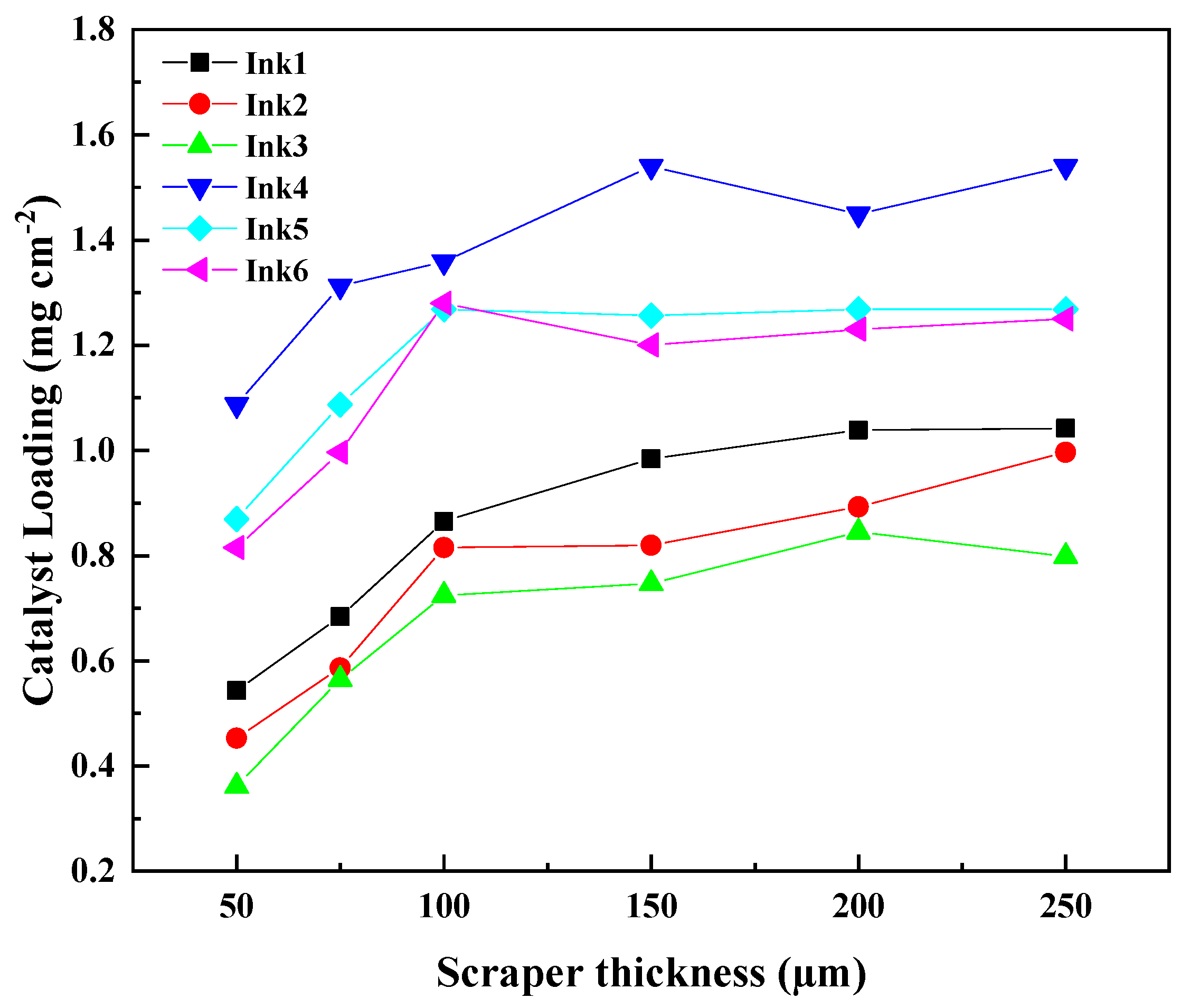

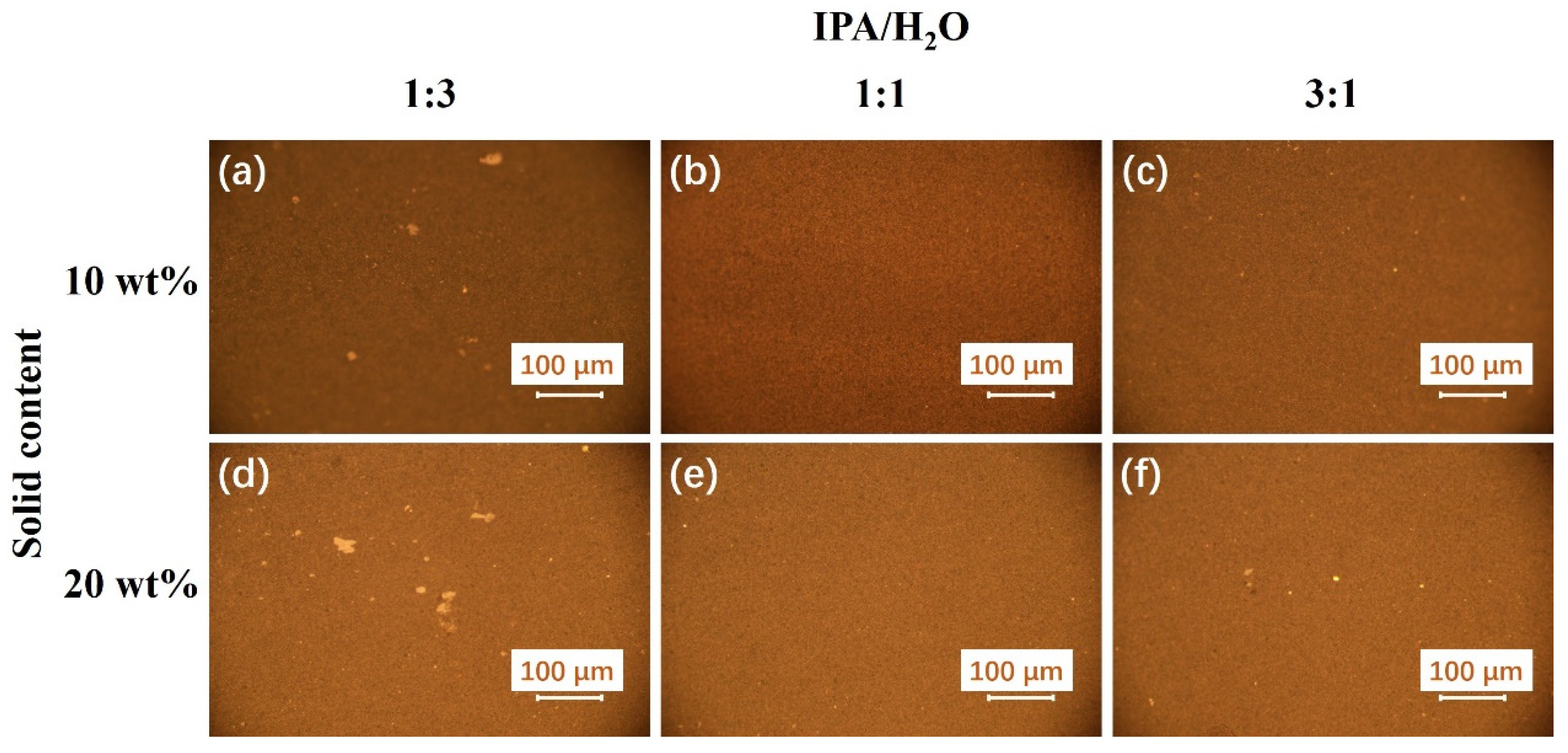
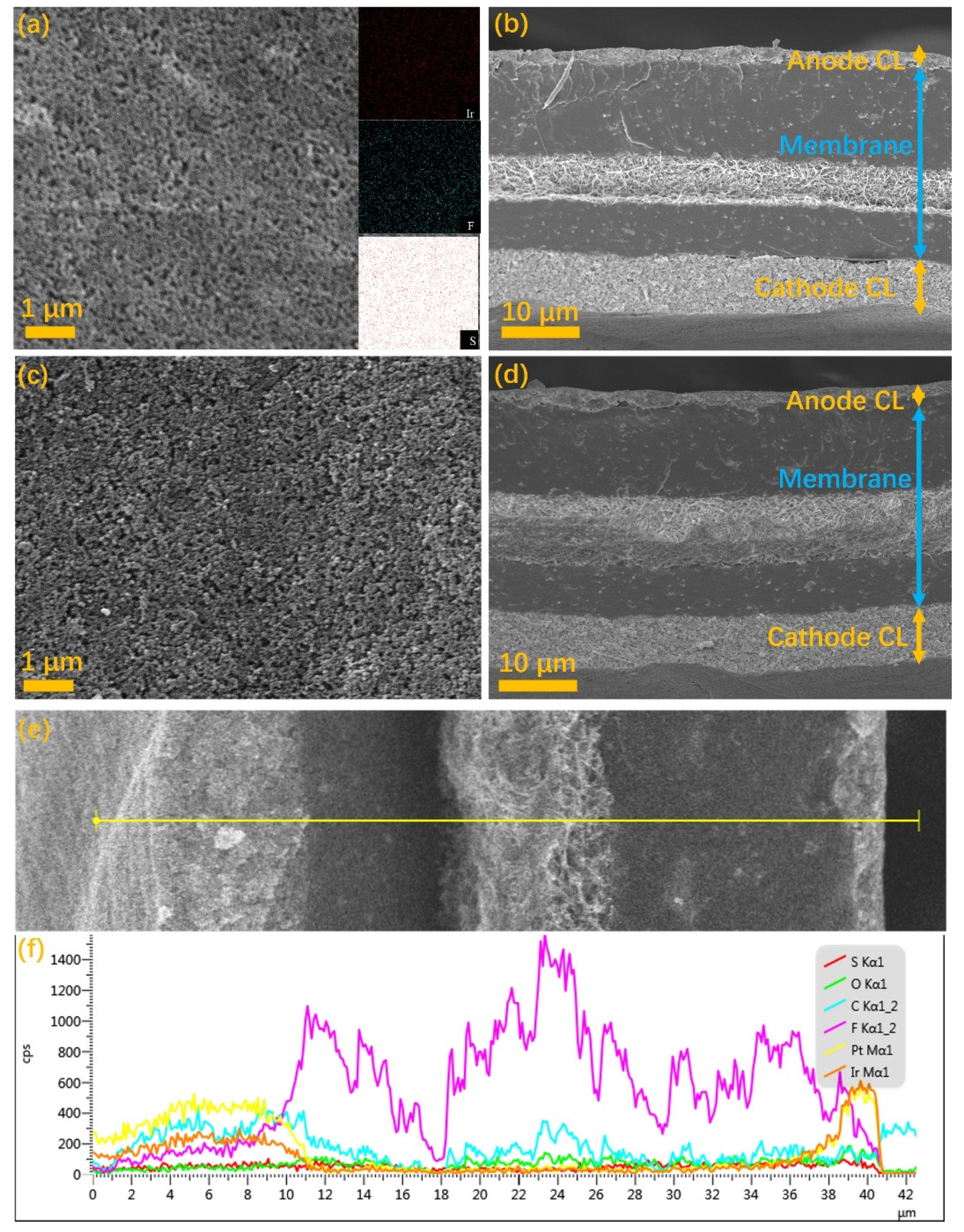
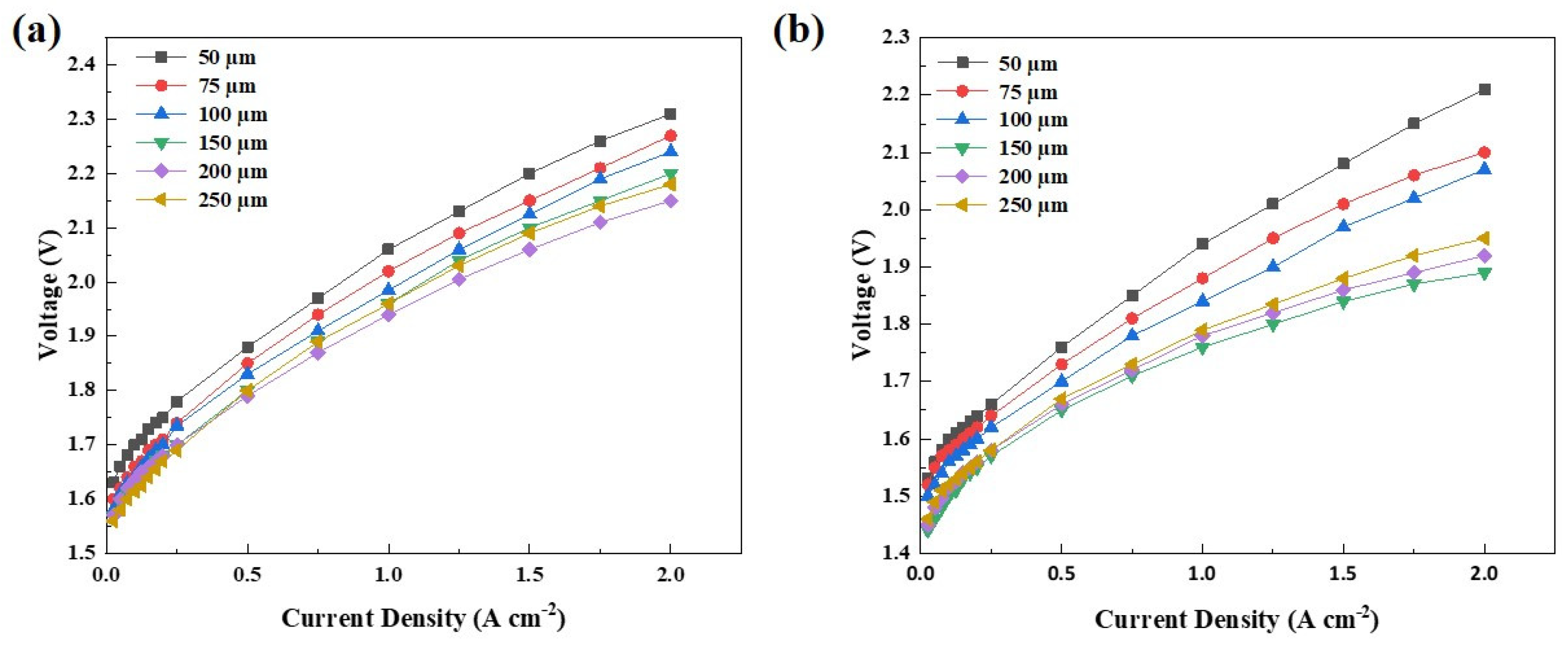
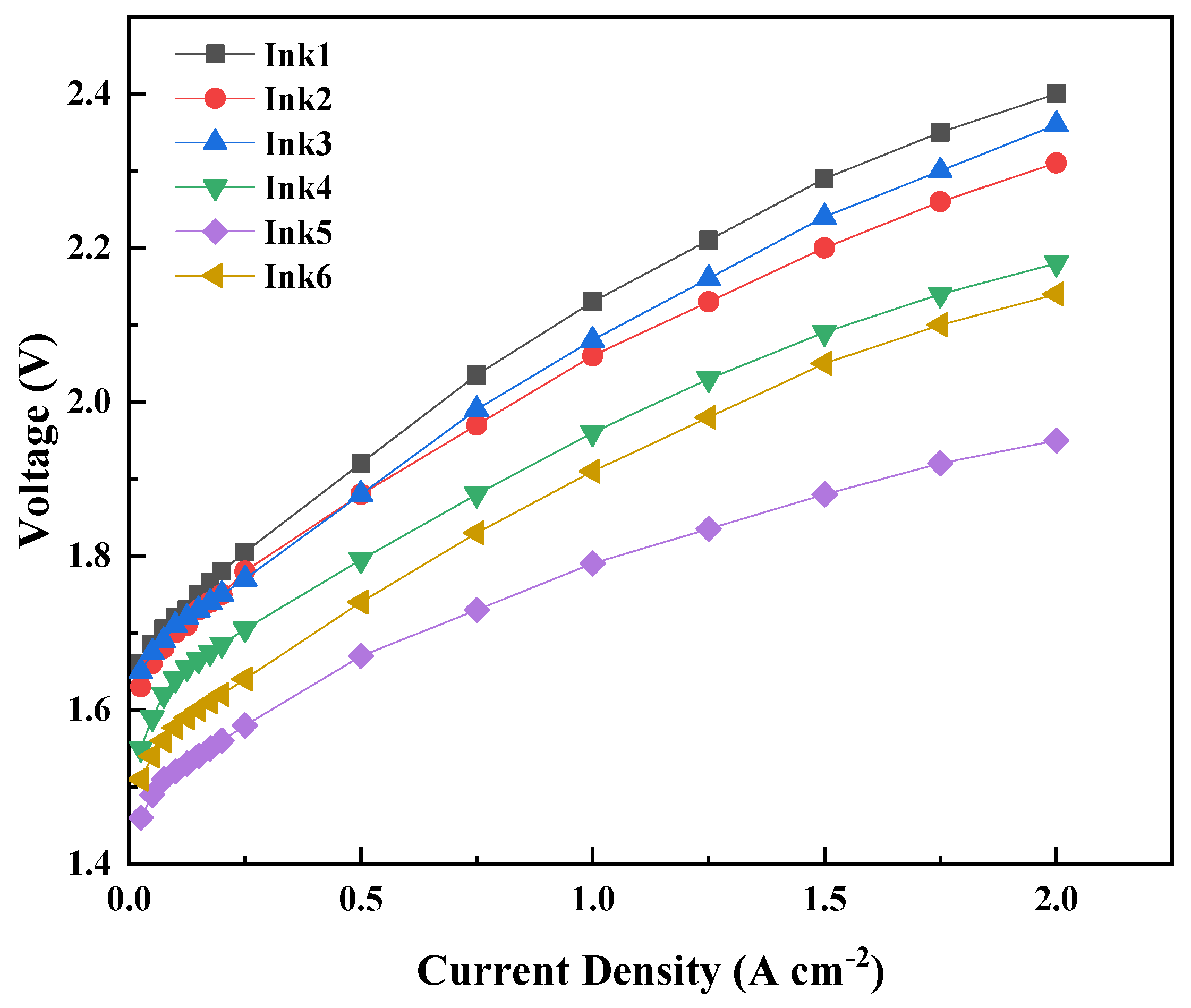
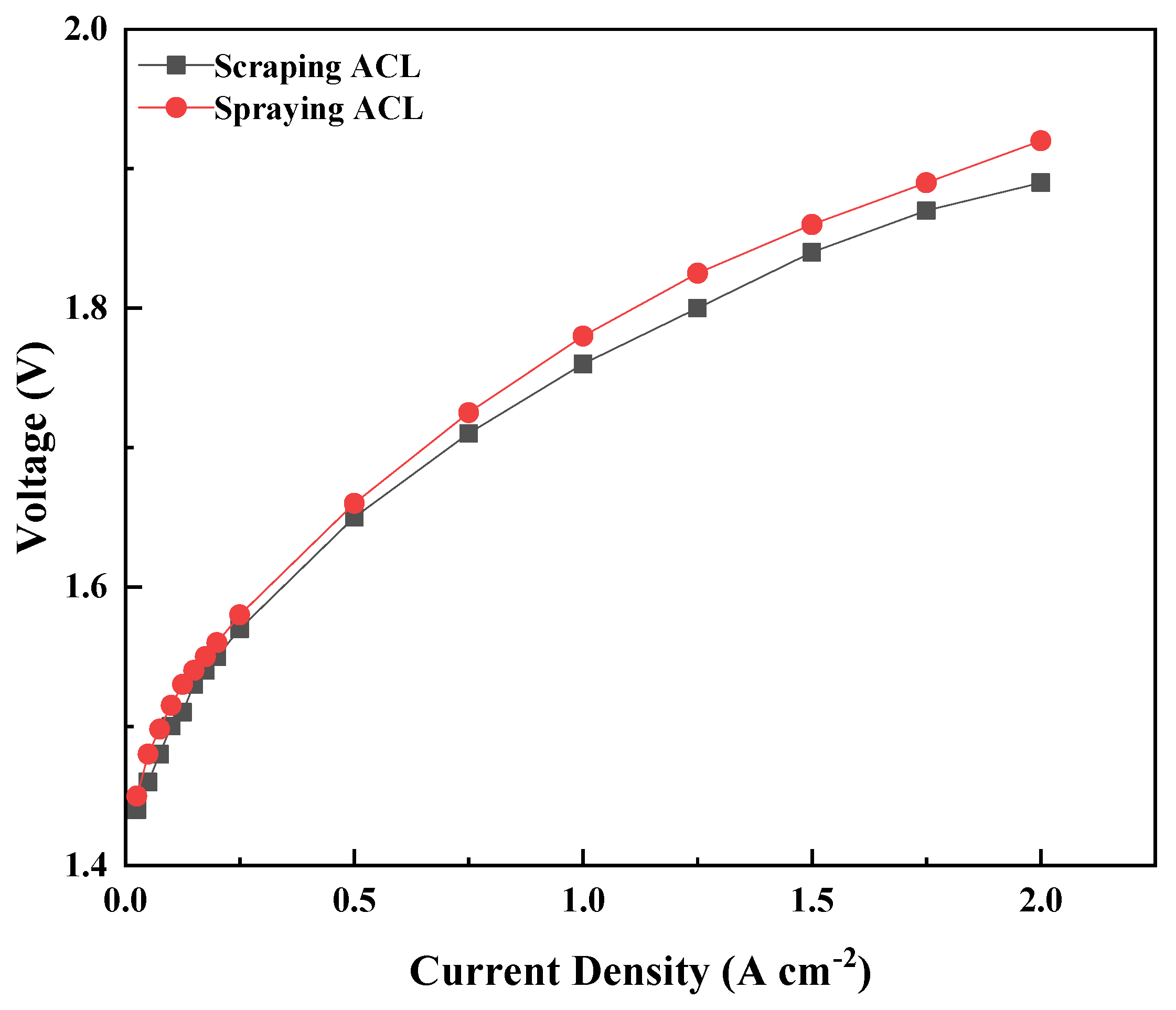
| Ink No. | Solid Content (IrO2 wt%) | IPA/H2O | I/C |
|---|---|---|---|
| Ink 1 | 10 | 1:3 | 6:1 |
| Ink 2 | 10 | 1:1 | 6:1 |
| Ink 3 | 10 | 3:1 | 6:1 |
| Ink 4 | 20 | 1:3 | 6:1 |
| Ink 5 | 20 | 1:1 | 6:1 |
| Ink 6 | 20 | 3:1 | 6:1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Peng, S.; Hou, F.; Wang, X.; Fang, B. Preparation and Performance Study of the Anodic Catalyst Layer via Doctor Blade Coating for PEM Water Electrolysis. Membranes 2023, 13, 24. https://doi.org/10.3390/membranes13010024
Liu G, Peng S, Hou F, Wang X, Fang B. Preparation and Performance Study of the Anodic Catalyst Layer via Doctor Blade Coating for PEM Water Electrolysis. Membranes. 2023; 13(1):24. https://doi.org/10.3390/membranes13010024
Chicago/Turabian StyleLiu, Gaoyang, Shanlong Peng, Faguo Hou, Xindong Wang, and Baizeng Fang. 2023. "Preparation and Performance Study of the Anodic Catalyst Layer via Doctor Blade Coating for PEM Water Electrolysis" Membranes 13, no. 1: 24. https://doi.org/10.3390/membranes13010024
APA StyleLiu, G., Peng, S., Hou, F., Wang, X., & Fang, B. (2023). Preparation and Performance Study of the Anodic Catalyst Layer via Doctor Blade Coating for PEM Water Electrolysis. Membranes, 13(1), 24. https://doi.org/10.3390/membranes13010024









