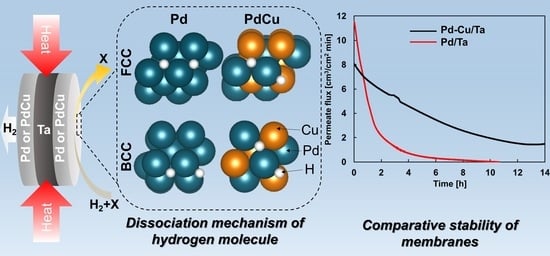
Experimental and Numerical Study of Pd/Ta and PdCu/Ta Composites for Thermocatalytic Hydrogen Permeation
Abstract
1. Introduction
- Providing evidence that fabrication of nanometer-thick Pd and PdCu on a dense support is achievable via plasma sputtering.
- Analysis of temporal stability of Pd/Ta and PdCu/Ta membranes.
2. Methodology
2.1. Materials
2.2. Membrane Preparation
2.3. Permeation Testing
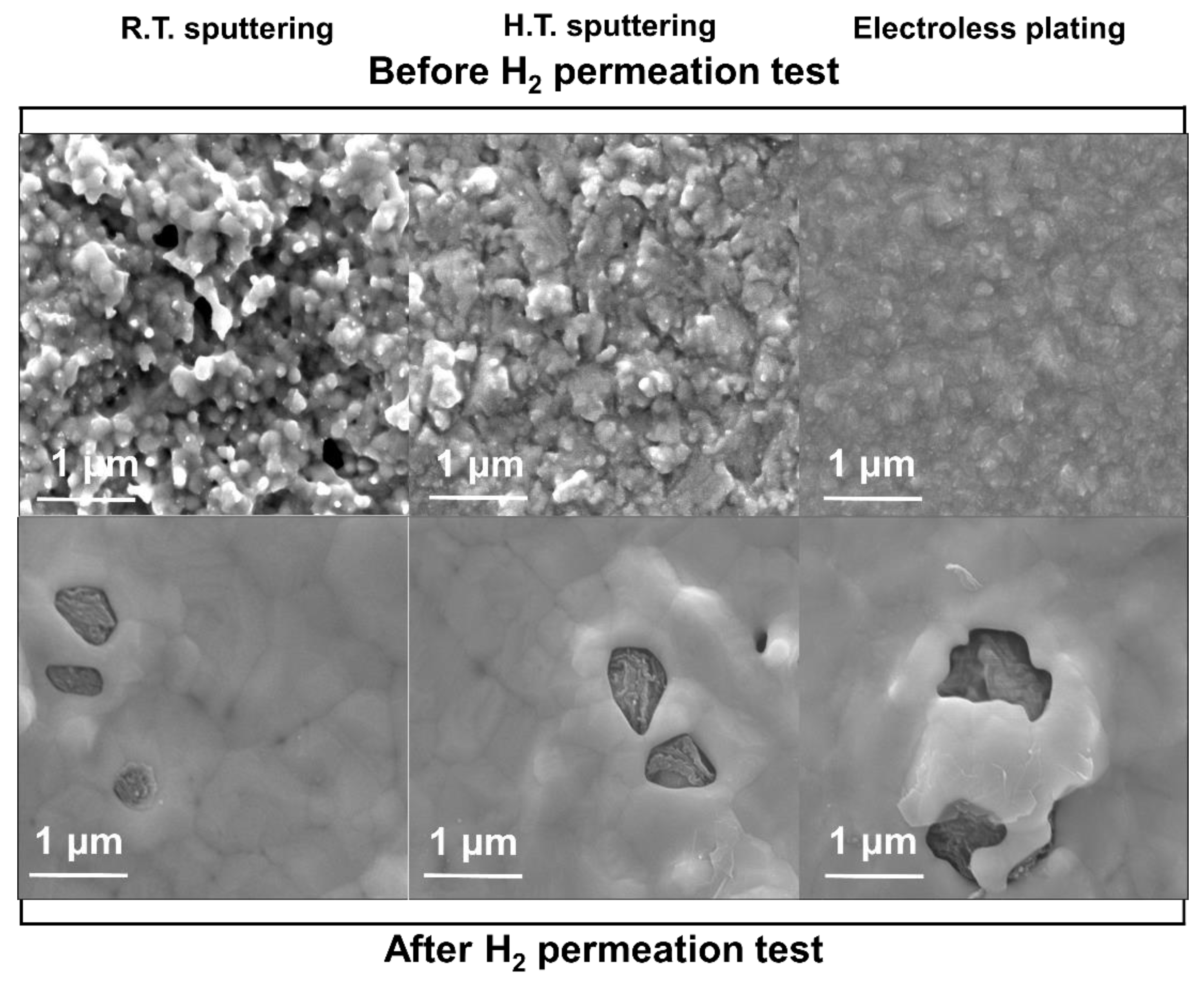
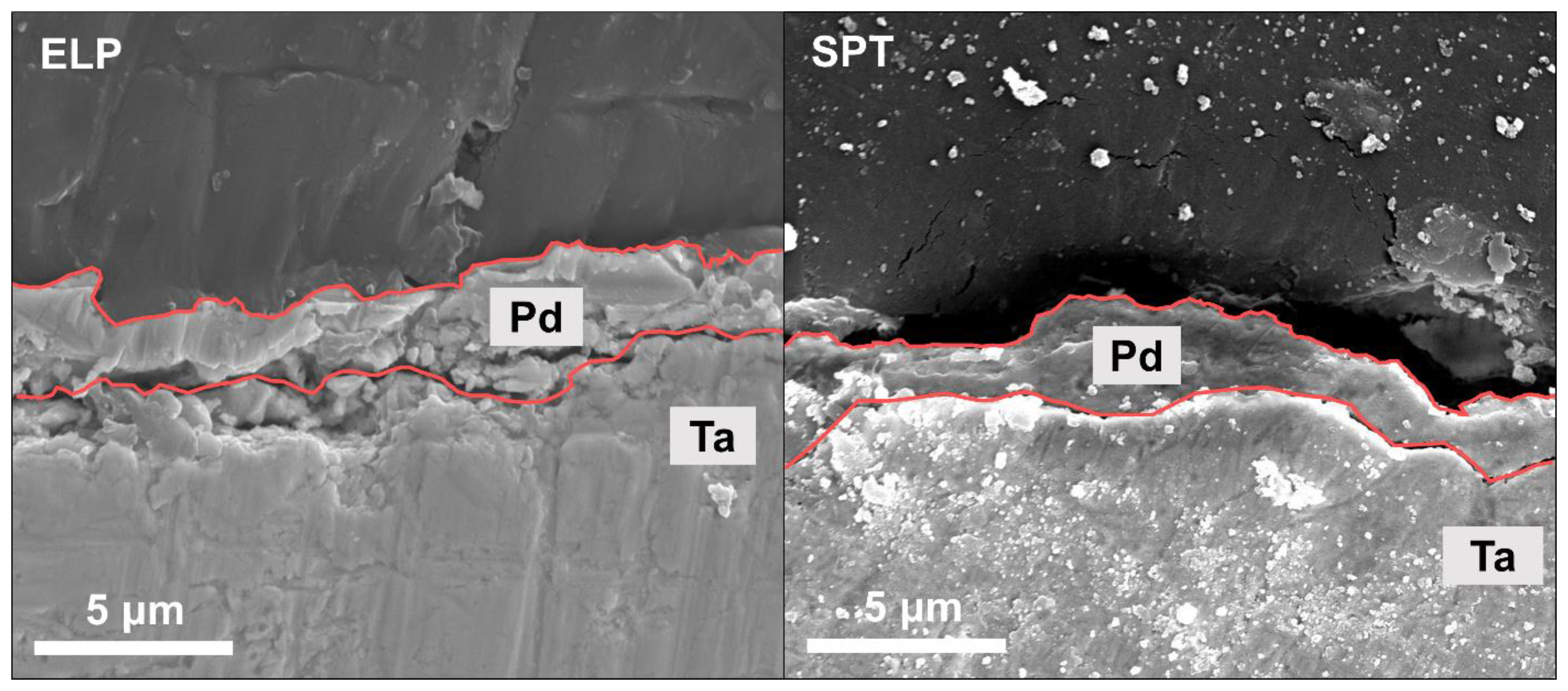
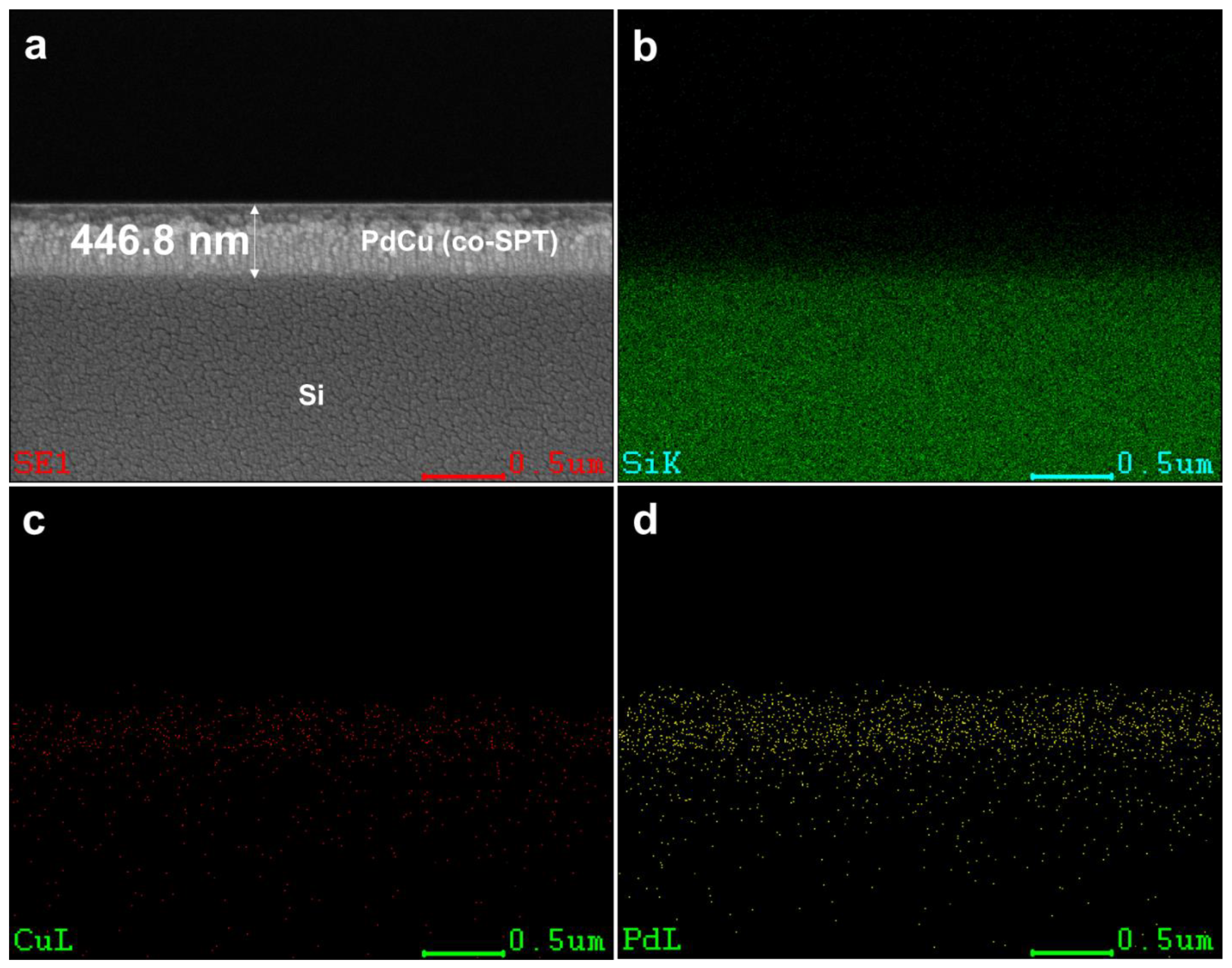
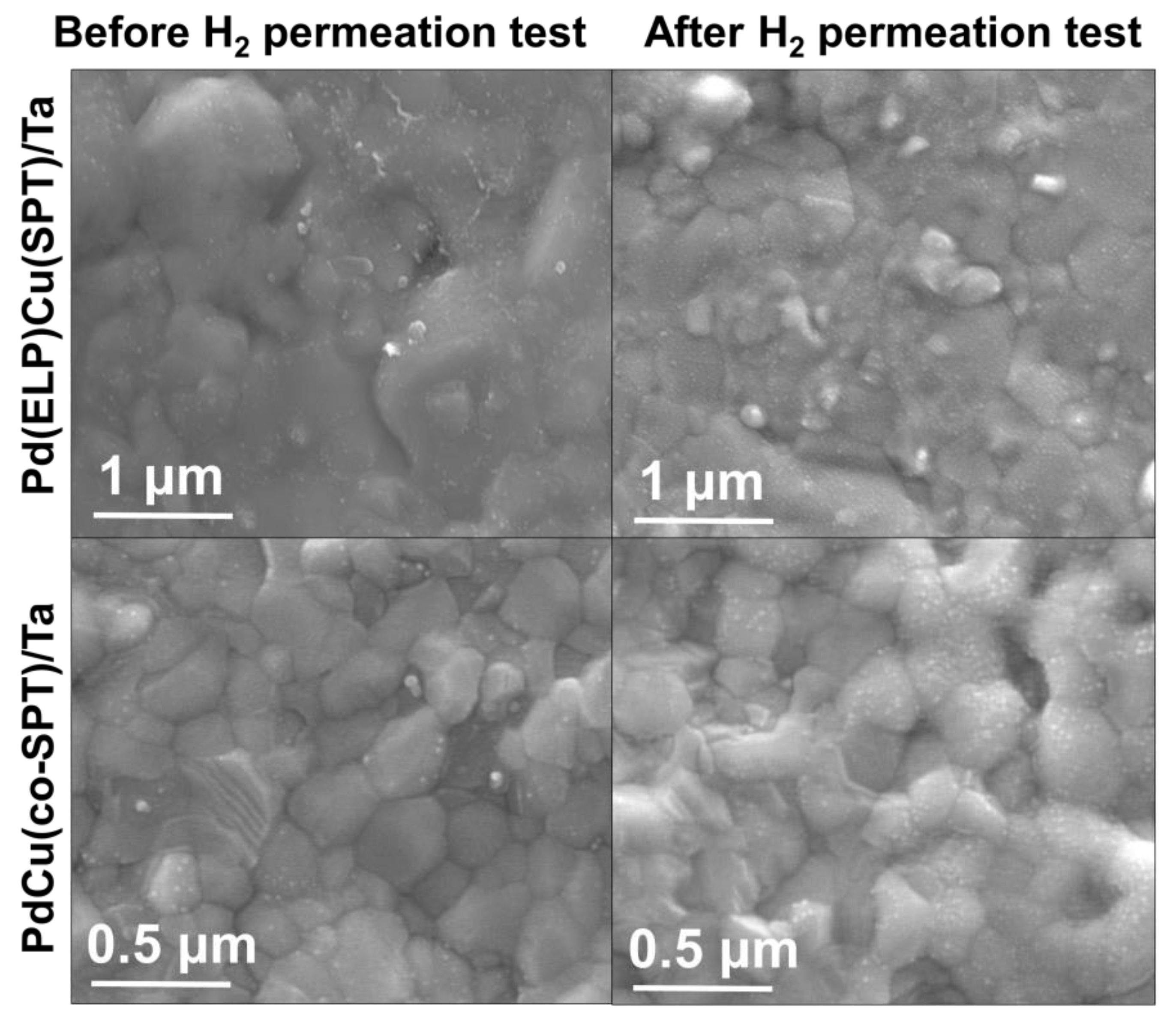
2.4. Material Characterizations
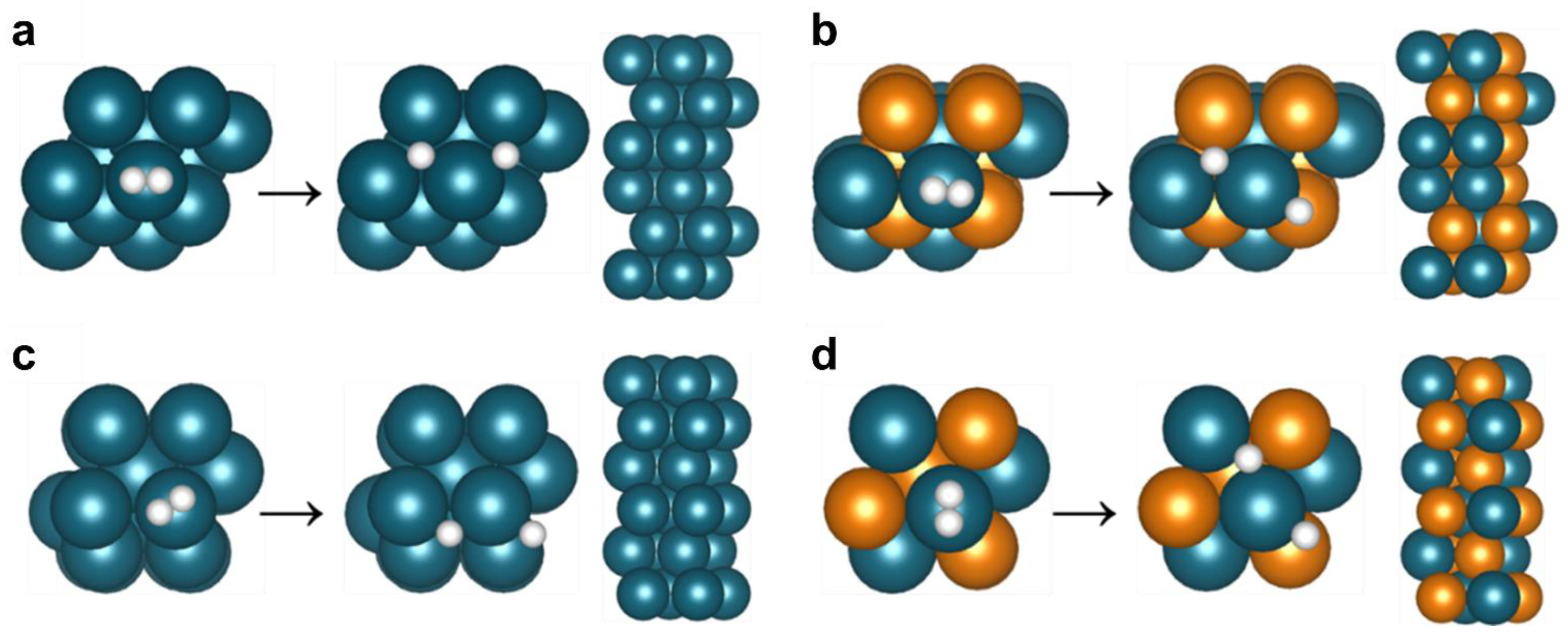
2.5. DFT Modeling
3. Results and Discussion
3.1. Pd/Ta Membrane
3.2. PdCu/Ta Membrane
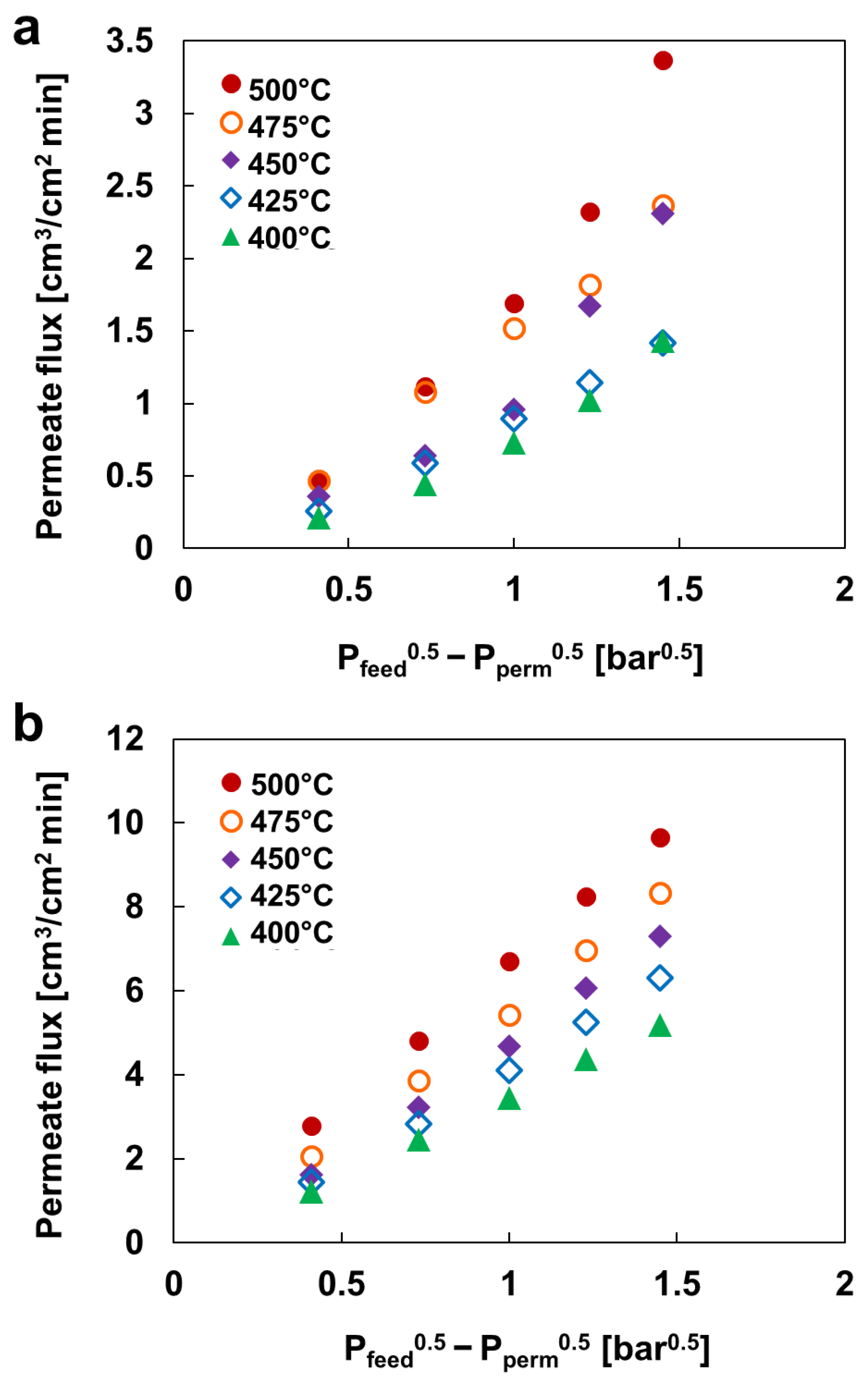
3.3. Arrhenius Plot
3.4. H2 Permeation Modeling
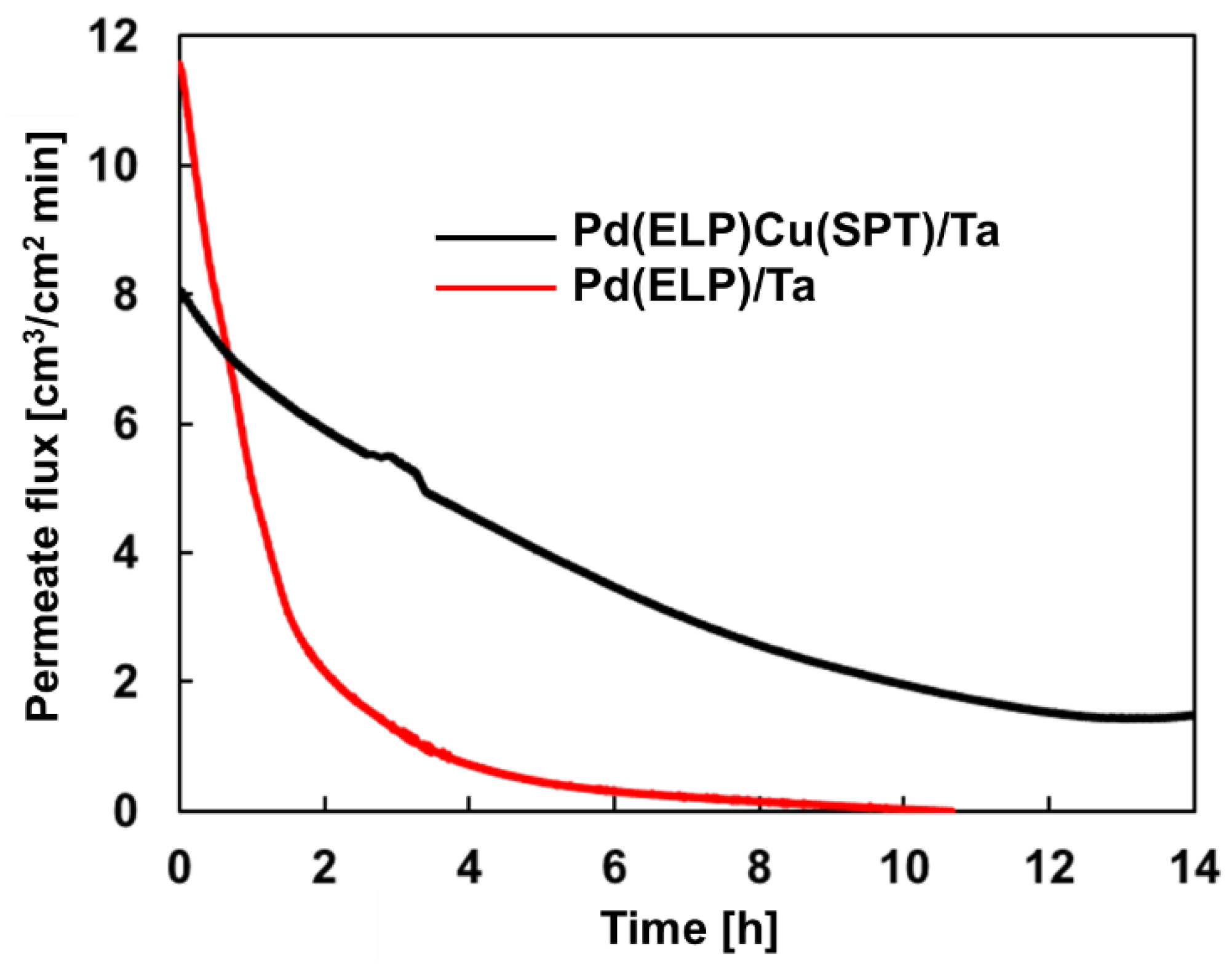
3.5. Comparative Temporal Stability Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shukla, P.R.; Skeg, J.; Buendia, E.C.; Masson-Delmotte, V.; Pörtner, H.O.; Roberts, D.C.; Zhai, P.; Slade, R.; Connors, S.; Van Diemen, S.; et al. Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; IPCC: Geneva, Switzerland, 2019; in press. [Google Scholar]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Badakhsh, A.; Cha, J.; Park, Y.; Lee, Y.-J.; Jeong, H.; Kim, Y.; Sohn, H.; Nam, S.W.; Yoon, C.W.; Park, C.W.; et al. Autothermal recirculating reactor (ARR) with Cu-BN composite as a stable reactor material for sustainable hydrogen release from ammonia. J. Power Sources 2021, 506, 230081. [Google Scholar] [CrossRef]
- alkuyeh, Y.K.; Saville, B.A.; MacLean, H.L. Techno-economic analysis and life cycle assessment of hydrogen production from natural gas using current and emerging technologies. Int. J. Hydrogen Energy 2017, 42, 18894–18909. [Google Scholar]
- Sanchez, A.; Ayala, O.; Hernandez-Sanchez, P.; Valdez-Vazquez, I.; de León-Rodríguez, A. An environment-economic analysis of hydrogen production using advanced biorefineries and its comparison with conventional technologies. Int. J. Hydrogen Energy 2020, 45, 27994–28006. [Google Scholar] [CrossRef]
- Badakhsh, A.; Kwak, Y.; Lee, Y.-J.; Jeong, H.; Kim, Y.; Sohn, H.; Nam, S.W.; Yoon, C.W.; Park, C.W.; Jo, Y.S. A compact catalytic foam reactor for decomposition of ammonia by the Joule-heating mechanism. Chem. Eng. J. 2021, 426, 130802. [Google Scholar] [CrossRef]
- Badakhsh, A.; Song, D.; Moon, S.; Jeong, H.; Sohn, H.; Nam, S.W.; Kim, P.S.; Seo, J.H.; Kim, Y.; Lee, J.; et al. COX-free LOHC dehydrogenation in a heatpipe reformer highly integrated with a hydrogen burner. Chem. Eng. J. 2022, 449, 137679. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Q.; Mei, D.; Wang, Y. A methanol fuel processing system with methanol steam reforming and CO selective methanation modules for PEMFC application. Int. J. Energy Res. 2021, 45, 6163–6173. [Google Scholar] [CrossRef]
- Makaruk, A.; Miltner, M.; Harasek, M. Membrane gas permeation in the upgrading of renewable hydrogen from biomass steam gasification gases. Appl. Therm. Eng. 2012, 43, 134–140. [Google Scholar] [CrossRef]
- Barreiro, M.M.; Maroño, M.; Sánchez, J.M. Hydrogen separation studies in a membrane reactor system: Influence of feed gas flow rate, temperature and concentration of the feed gases on hydrogen permeation. Appl. Therm. Eng. 2015, 74, 186–193. [Google Scholar] [CrossRef]
- Yurata, T.; Lei, H.; Tang, L.; Lu, M.; Patel, J.; Lim, S.; Piumsomboon, P.; Chalermsinsuwan, B.; Li, C. Feasibility and sustainability analyses of carbon dioxide—Hydrogen separation via de-sublimation process in comparison with other processes. Int. J. Hydrogen Energy 2019, 44, 23120–23134. [Google Scholar] [CrossRef]
- Ockwig, N.W.; Nenoff, T.M. Membranes for Hydrogen Separation. Chem. Rev. 2007, 107, 4078–4110. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Caravella, A.; Xu, H.Y. Recent progress in Pd-based composite membranes. J. Mater. Chem. A 2016, 4, 14069–14094. [Google Scholar] [CrossRef]
- Shu, J.; Grandjean, B.P.A.; Kaliaguine, S.; Giroir-Fendler, A.; Dalmon, J.-A. Hysteresis in hydrogen permeation through palladium membranes. J. Chem. Soc. Faraday Trans. 1996, 92, 2745–2751. [Google Scholar] [CrossRef]
- Yun, S.; Oyama, S.T. Correlations in palladium membranes for hydrogen separation: A review. J. Membr. Sci. 2011, 375, 28–45. [Google Scholar] [CrossRef]
- Lewis, F.A. The Palladium/Hydrogen System; Academic Press: Cambridge, MA, USA, 1967. [Google Scholar]
- Jo, Y.S.; Lee, C.H.; Kong, S.Y.; Lee, K.-Y.; Yoon, C.W.; Nam, S.W.; Han, J. Characterization of a Pd/Ta composite membrane and its application to a large scale high-purity hydrogen separation from mixed gas. Sep. Purif. Technol. 2018, 200, 221–229. [Google Scholar] [CrossRef]
- Ryi, S.-K.; Ahn, H.-S.; Park, J.-S.; Kim, D.-W. Pd–Cu alloy membrane deposited on CeO2 modified porous nickel support for hydrogen separation. Int. J. Hydrogen Energy 2014, 39, 4698–4703. [Google Scholar] [CrossRef]
- Hwang, K.-R.; Oh, D.-K.; Lee, S.-W.; Park, J.-S.; Song, M.-H.; Rhee, W.-H. Porous stainless steel support for hydrogen separation Pd membrane; fabrication by metal injection molding and simple surface modification. Int. J. Hydrogen Energy 2017, 42, 14583–14592. [Google Scholar] [CrossRef]
- SKong, S.Y.; Kim, D.H.; Henkensmeier, D.; Kim, H.-J.; Ham, H.C.; Han, J.; Yoon, S.P.; Yoon, C.W.; Choi, S.H. Ultrathin layered Pd/PBI–HFA composite membranes for hydrogen separation. Sep. Purif. Technol. 2017, 179, 486–493. [Google Scholar]
- Ma, X.; Yang, C.; Chen, H.; Lv, Q.; Sun, K.; Li, W. Hydrogen permeation and chemical stability of Ni–BaCe0.7In0.2Ta0.1O3−δ cermet membrane. Sep. Purif. Technol. 2020, 236, 116276. [Google Scholar] [CrossRef]
- Saini, N.; Awasthi, K. Insights into the progress of polymeric nano-composite membranes for hydrogen separation and purification in the direction of sustainable energy resources. Sep. Purif. Technol. 2021, 282, 120029. [Google Scholar] [CrossRef]
- Dolan, M. Non-Pd BCC alloy membranes for industrial hydrogen separation. J. Membr. Sci. 2010, 362, 12–28. [Google Scholar] [CrossRef]
- Lee, C.H.; Jo, Y.S.; Park, Y.; Jeong, H.; Kim, Y.; Sohn, H.; Yoon, C.W.; Nam, S.W.; Ham, H.C.; Han, J. Unconventional hydrogen permeation behavior of Pd/BCC composite membranes and significance of surface reaction kinetics. J. Membr. Sci. 2019, 595, 117506. [Google Scholar] [CrossRef]
- Pujari, M.; Agarwal, A.; Uppaluri, R.; Verma, A. Role of electroless nickel diffusion barrier on the combinatorial plating characteristics of dense Pd/Ni/PSS composite membranes. Appl. Surf. Sci. 2014, 305, 658–664. [Google Scholar] [CrossRef]
- Cooney, D.A.; Way, J.D.; Wolden, C.A. A comparison of the performance and stability of Pd/BCC metal composite membranes for hydrogen purification. Int. J. Hydrogen Energy 2014, 39, 19009–19017. [Google Scholar] [CrossRef]
- Kozhakhmetov, S.; Sidorov, N.; Piven, V.; Sipatov, I.; Gabis, I.; Arinov, B. Alloys based on Group 5 metals for hydrogen purification membranes. J. Alloy Compd. 2015, 645, S36–S40. [Google Scholar] [CrossRef]
- Adams, B.D.; Chen, A. The role of palladium in a hydrogen economy. Mater. Today 2011, 14, 282–289. [Google Scholar] [CrossRef]
- Zhang, K.; Way, J.D. Palladium-copper membranes for hydrogen separation. Sep. Purif. Technol. 2017, 186, 39–44. [Google Scholar] [CrossRef]
- Fernandez, E.; Medrano, J.A.; Melendez, J.; Parco, M.; Viviente, J.L.; Annaland, M.V.S.; Gallucci, F.; Tanaka, D.P. Preparation and characterization of metallic supported thin Pd–Ag membranes for hydrogen separation. Chem. Eng. J. 2016, 305, 182–190. [Google Scholar] [CrossRef]
- Kamakoti, P.; Morreale, B.D.; Ciocco, M.V.; Howard, B.H.; Killmeyer, R.P.; Cugini, A.V.; Sholl, D.S. Prediction of Hydrogen Flux Through Sulfur-Tolerant Binary Alloy Membranes. Science 2005, 307, 569–573. [Google Scholar] [CrossRef]
- Morreale, B.; Ciocco, M.; Howard, B.; Killmeyer, R.; Cugini, A.; Enick, R. Effect of hydrogen-sulfide on the hydrogen permeance of palladium?copper alloys at elevated temperatures. J. Membr. Sci. 2004, 241, 219–224. [Google Scholar] [CrossRef]
- United States Geological Survey. Mineral Commodity Summaries: Nitrogen (Fixed)—Ammonia, January 2021. Available online: https://pubs.usgs.gov/periodicals/mcs2021/mcs2021-nitrogen.pdf (accessed on 2 August 2022).
- Park, Y.; Kwak, Y.; Yu, S.; Badakhsh, A.; Lee, Y.-J.; Jeong, H.; Kim, Y.; Sohn, H.; Nam, S.W.; Yoon, C.W.; et al. Degradation mechanism of a Pd/Ta composite membrane: Catalytic surface fouling with inter-diffusion. J. Alloy Compd. 2020, 854, 157196. [Google Scholar] [CrossRef]
- Ohtsu, N.; Ishikawa, K.; Kobori, Y. Hydrogen permeability degradation of Pd-coated Nb⿿TiNi alloy caused by its interfacial diffusion. Appl. Surf. Sci. 2016, 360, 566–571. [Google Scholar] [CrossRef]
- Papaderakis, A.; Mintsouli, I.; Georgieva, J.; Sotiropoulos, S. Electrocatalysts Prepared by Galvanic Replacement. Catalysts 2017, 7, 80. [Google Scholar] [CrossRef]
- Kresse, G. VASP the Guide. Available online: http://cms.mpi.univie.ac.at/vasp/ (accessed on 10 October 2021).
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Blöchl, P.E.; Jepsen, O.; Andersen, O.K. Improved tetrahedron method for Brillouin-zone integrations. Phys. Rev. B 1994, 49, 16223–16233. [Google Scholar] [CrossRef]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef]
- Pearson, W.B. A Handbook of Lattice Spacings and Structures of Metals and Alloys; Pergamon: Oxford, UK, 1959. [Google Scholar]
- Hansen, M.; Anderko, K.; Salzberg, H.W. Constitution of Binary Alloys. J. Electrochem. Soc. 1958, 105, 260C. [Google Scholar] [CrossRef]
- Frank, F.C.; Kasper, J.S. Complex alloy structures regarded as sphere packings. II. Analysis and classification of representative structures. Acta Crystallogr. 1959, 12, 483–499. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lin, C.-N.; Chi, Y.-H.; Lin, Y.-L. Permeation characteristics of hydrogen through palladium membranes in binary and ternary gas mixtures. Int. J. Energy Res. 2017, 41, 1579–1595. [Google Scholar] [CrossRef]
- Livshits, A. The hydrogen transport through the metal alloy membranes with a spatial variation of the alloy composition: Potential diffusion and enhanced permeation. Int. J. Hydrogen Energy 2017, 42, 13111–13119. [Google Scholar] [CrossRef]
- Choi, S.H.; Hwang, C.S.; Lee, H.-W.; Kim, J. Fabrication of Gd2O3-Doped CeO2 Thin Films for Single-Chamber-Type Solid Oxide Fuel Cells and Their Characterization. J. Electrochem. Soc. 2009, 156, B185–B381. [Google Scholar] [CrossRef]
- Macfie, G.; Cooper, A.; Cardosi, M.F. Room temperature formation, electro-reduction and dissolution of surface oxide layers on sputtered palladium films. Electrochim. Acta 2011, 56, 8394–8402. [Google Scholar] [CrossRef]
- Checchetto, R.; Bazzanella, N.; Patton, B.; Miotello, A. Palladium membranes prepared by r.f. magnetron sputtering for hydrogen purification. Surf. Coat. Technol. 2004, 177-178, 73–79. [Google Scholar] [CrossRef]
- Al-Mufachi, N.; Rees, N.; Steinberger-Wilkens, R. Hydrogen selective membranes: A review of palladium-based dense metal membranes. Renew. Sustain. Energy Rev. 2015, 47, 540–551. [Google Scholar] [CrossRef]
- Ramachandran, A.; Tucho, W.; Mejdell, A.; Stange, M.; Venvik, H.; Walmsley, J.; Holmestad, R.; Bredesen, R.; Borg, A. Surface characterization of Pd/Ag23wt% membranes after different thermal treatments. Appl. Surf. Sci. 2010, 256, 6121–6132. [Google Scholar] [CrossRef]
- Nobari, N.; Behboudnia, M.; Maleki, R. Palladium-free electroless deposition of pure copper film on glass substrate using hydrazine as reducing agent. Appl. Surf. Sci. 2016, 385, 9–17. [Google Scholar] [CrossRef]
- Hsu, H.-H.; Lin, K.-H.; Lin, S.-J.; Yeh, J.-W. Electroless Copper Deposition for Ultralarge-Scale Integration. J. Electrochem. Soc. 2001, 148, C47–C53. [Google Scholar] [CrossRef]
- Howard, B.; Killmeyer, R.; Rothenberger, K.; Cugini, A.; Morreale, B.; Enick, R.; Bustamante, F. Hydrogen permeance of palladium—Copper alloy membranes over a wide range of temperatures and pressures. J. Membr. Sci. 2004, 241, 207–218. [Google Scholar] [CrossRef]
- Liguori, S.; Kian, K.; Buggy, N.; Anzelmo, B.H.; Wilcox, J. Opportunities and challenges of low-carbon hydrogen via metallic membranes. Prog. Energy Combust. Sci. 2020, 80, 100851. [Google Scholar] [CrossRef]
- Godbole, B.; Badera, N.; Shrivastava, S.B.; Jain, D.; Ganesan, V. Investigation of Fe-Doped and Undoped Nio Nanocrystalline Films. Surf. Rev. Lett. 2007, 14, 1113–1119. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Z.-X. Alloying effect on the C–C coupling reactions in acetylene hydrogenation by palladium-coinage metal alloys, a DFT study and microkinetic modeling. Appl. Surf. Sci. 2022, 575, 1154–1160. [Google Scholar] [CrossRef]
- Wei, L.; Yu, J.; Hu, X.; Wang, R.; Huang, Y. Effects of Sn residue on the high temperature stability of the H2-permeable palladium membranes prepared by electroless plating on Al2O3 substrate after SnCl2–PdCl2 process: A case study. Chin. J. Chem. Eng. 2016, 24, 151513. [Google Scholar] [CrossRef]





| System | Alloy Composition [at.% Pd] | Lattice Constants [Å] | Experimental Lattice Constants [Å] | Experimental Alloy Composition [at.% Pd] |
|---|---|---|---|---|
| FCC pure Pd | 100 | 3.94 | 3.89 | 100 |
| FCC PdCu | 50 | 3.81 | 3.77 | 52 |
| BCC pure Pd | 100 | 3.23 | N/A | N/A |
| BCC PdCu | 50 | 2.99 | 2.97 | 47 |
| BCC pure Ta | 0 | 3.31 | 3.31 | 0 |
| System | H2 Adsorption Site | H2 Adsorption Energy [eV] | 2H Adsorption Site | 2H Adsorption Energy [eV] | Activation Energy [eV] |
|---|---|---|---|---|---|
| FCC Pd (111) | top | −0.23 | fcc–fcc | −5.65 | 0.02 |
| FCC PdCu (111) | top | −0.22 | fcc1–hcp1 | −5.35 | 0.10 |
| BCC Pd (110) | top | −0.41 | hollow–hollow | −5.83 | 0.06 |
| BCC PdCu (110) | top | −0.27 | hollow1–hollow1 | −5.43 | 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, S.; Badakhsh, A.; Oh, J.G.; Ham, H.C.; Sohn, H.; Yoon, S.P.; Choi, S.H. Experimental and Numerical Study of Pd/Ta and PdCu/Ta Composites for Thermocatalytic Hydrogen Permeation. Membranes 2023, 13, 23. https://doi.org/10.3390/membranes13010023
Ryu S, Badakhsh A, Oh JG, Ham HC, Sohn H, Yoon SP, Choi SH. Experimental and Numerical Study of Pd/Ta and PdCu/Ta Composites for Thermocatalytic Hydrogen Permeation. Membranes. 2023; 13(1):23. https://doi.org/10.3390/membranes13010023
Chicago/Turabian StyleRyu, Seungbo, Arash Badakhsh, Je Gyu Oh, Hyung Chul Ham, Hyuntae Sohn, Sung Pil Yoon, and Sun Hee Choi. 2023. "Experimental and Numerical Study of Pd/Ta and PdCu/Ta Composites for Thermocatalytic Hydrogen Permeation" Membranes 13, no. 1: 23. https://doi.org/10.3390/membranes13010023
APA StyleRyu, S., Badakhsh, A., Oh, J. G., Ham, H. C., Sohn, H., Yoon, S. P., & Choi, S. H. (2023). Experimental and Numerical Study of Pd/Ta and PdCu/Ta Composites for Thermocatalytic Hydrogen Permeation. Membranes, 13(1), 23. https://doi.org/10.3390/membranes13010023