A Novel In Vitro Wound Healing Assay Using Free-Standing, Ultra-Thin PDMS Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Membrane Fabrication, Quality Management, and Surface Modifications
2.2. Cell Culture
2.3. Wound Model
2.4. Biaxial Mechanical Stimulation
2.5. Wound Closure Visualization
2.6. Quantification of Gap Closure
2.7. Statistical Analysis
3. Results and Discussion
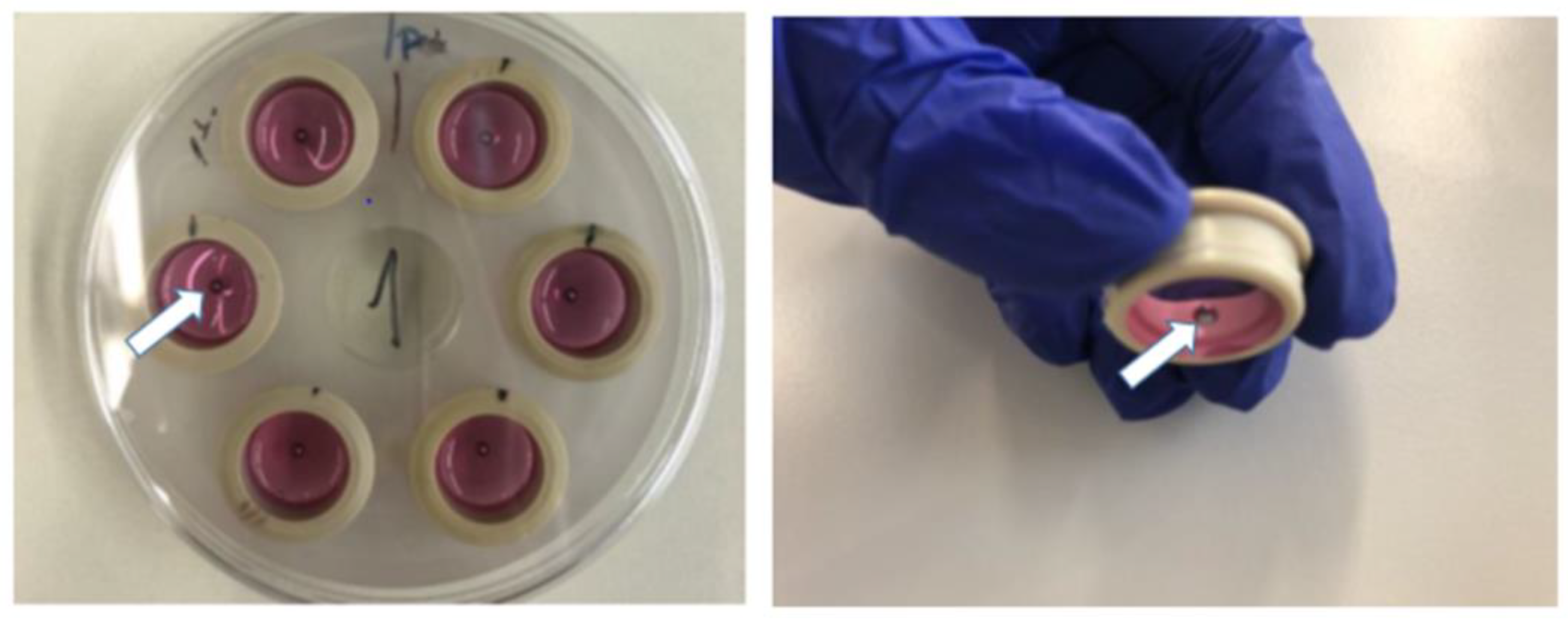
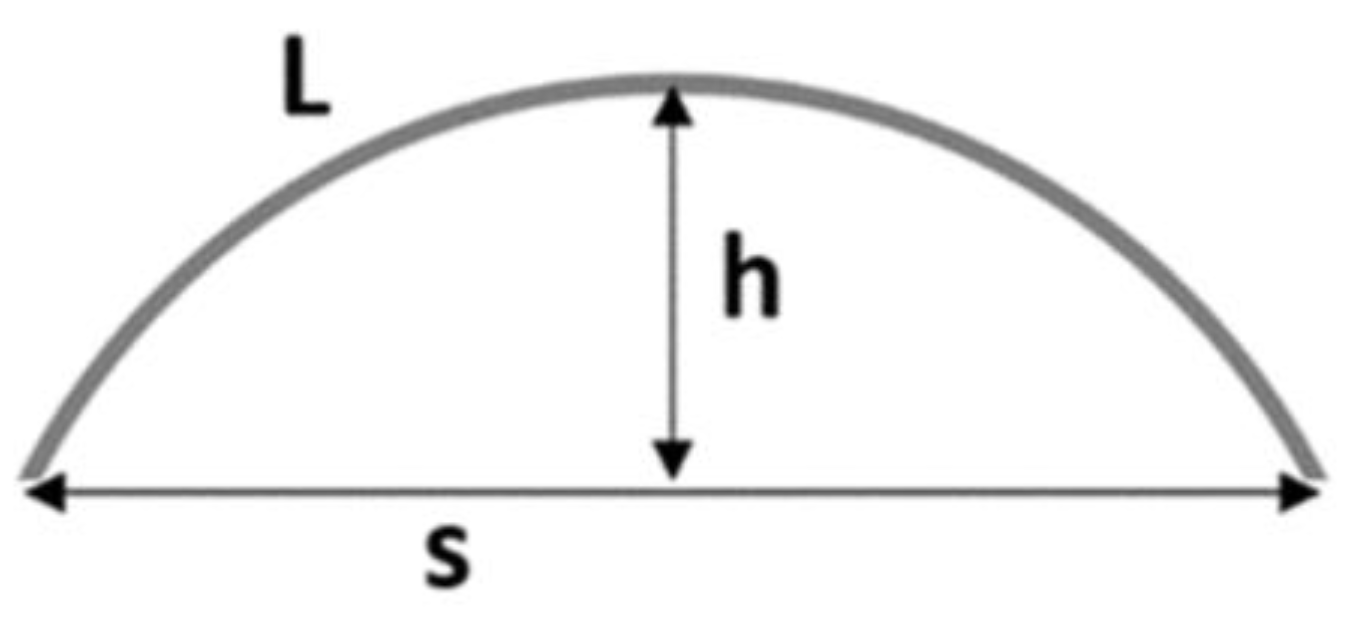
3.1. CellDrum Membrane Production
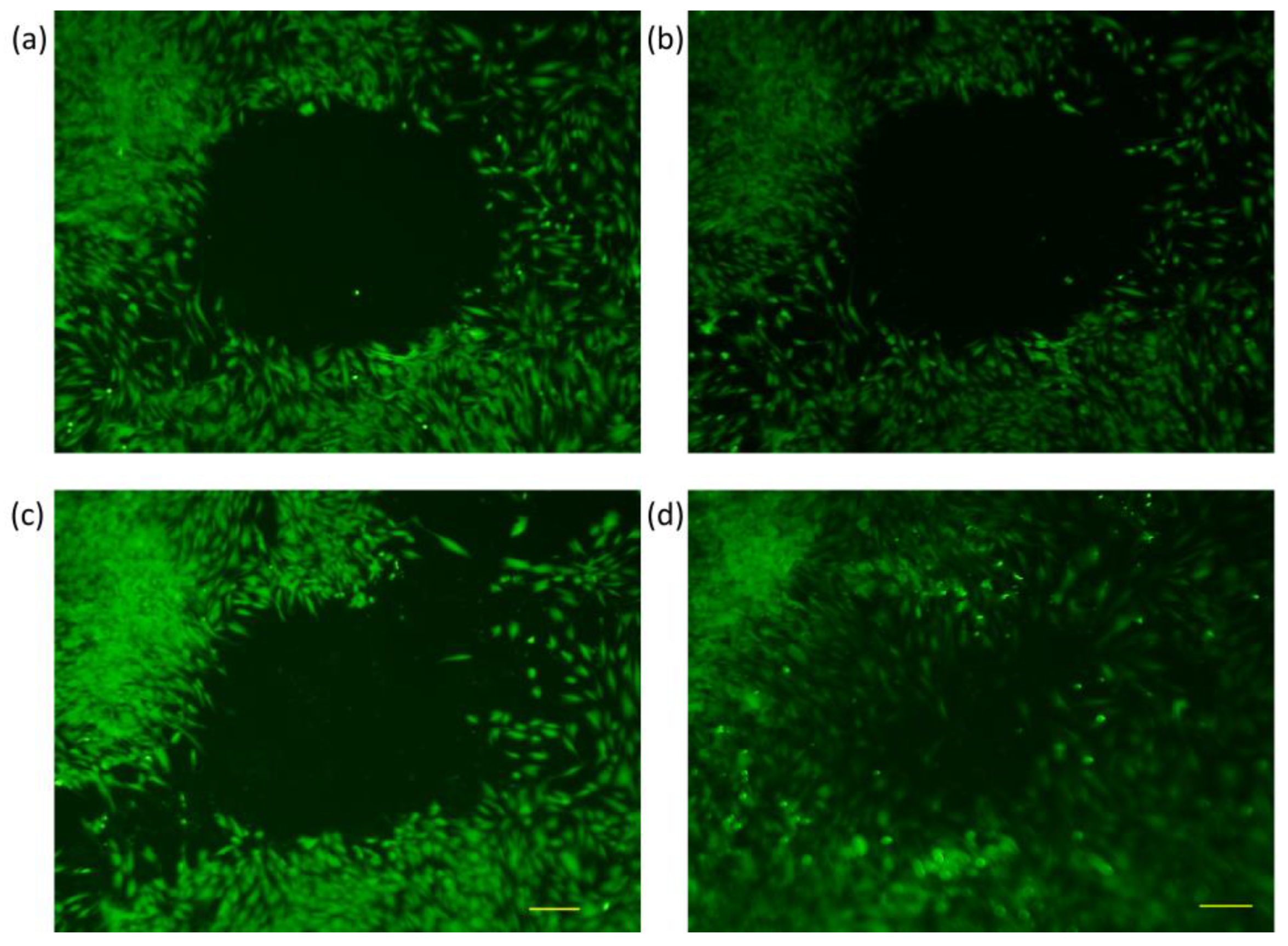
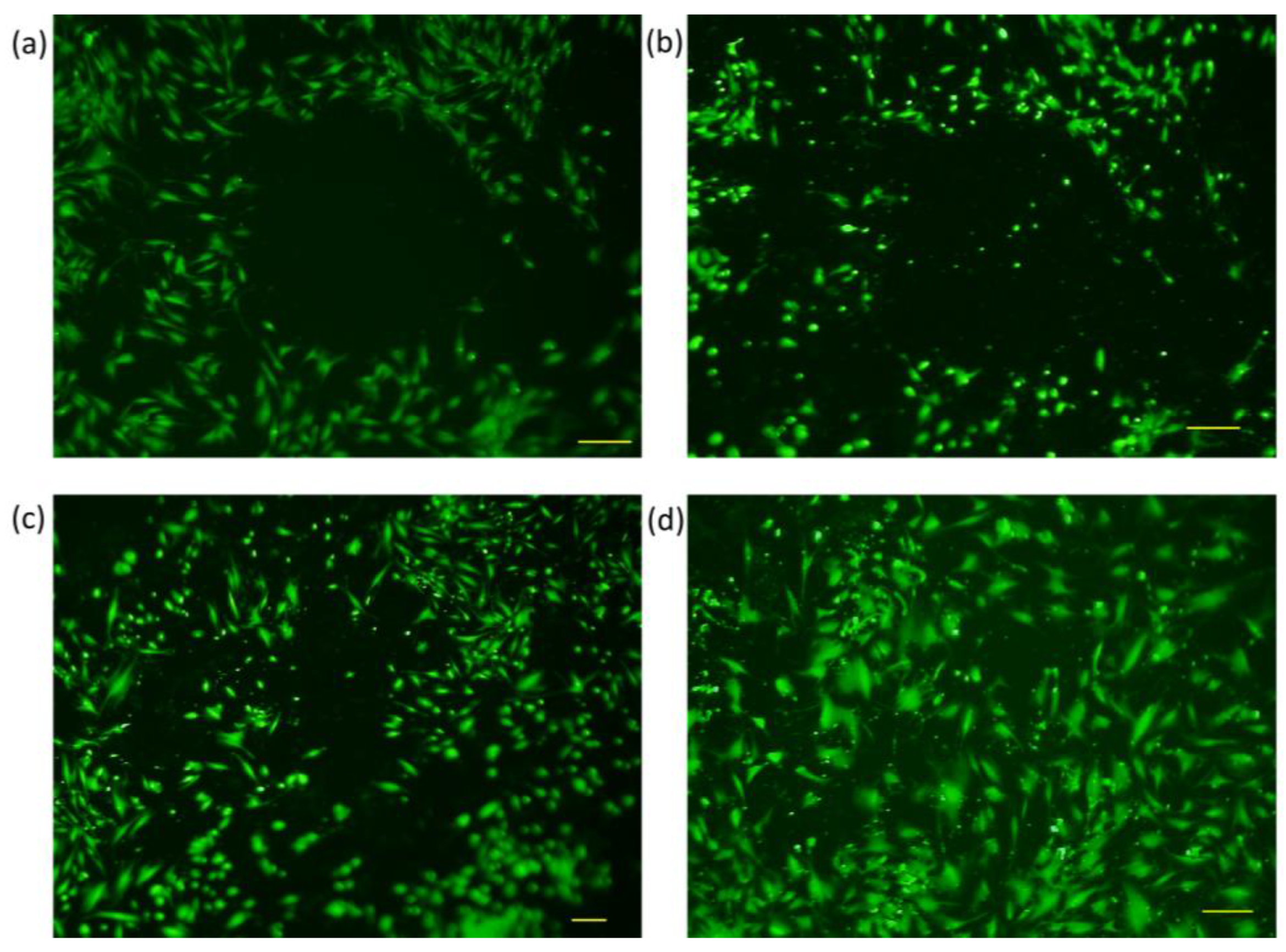
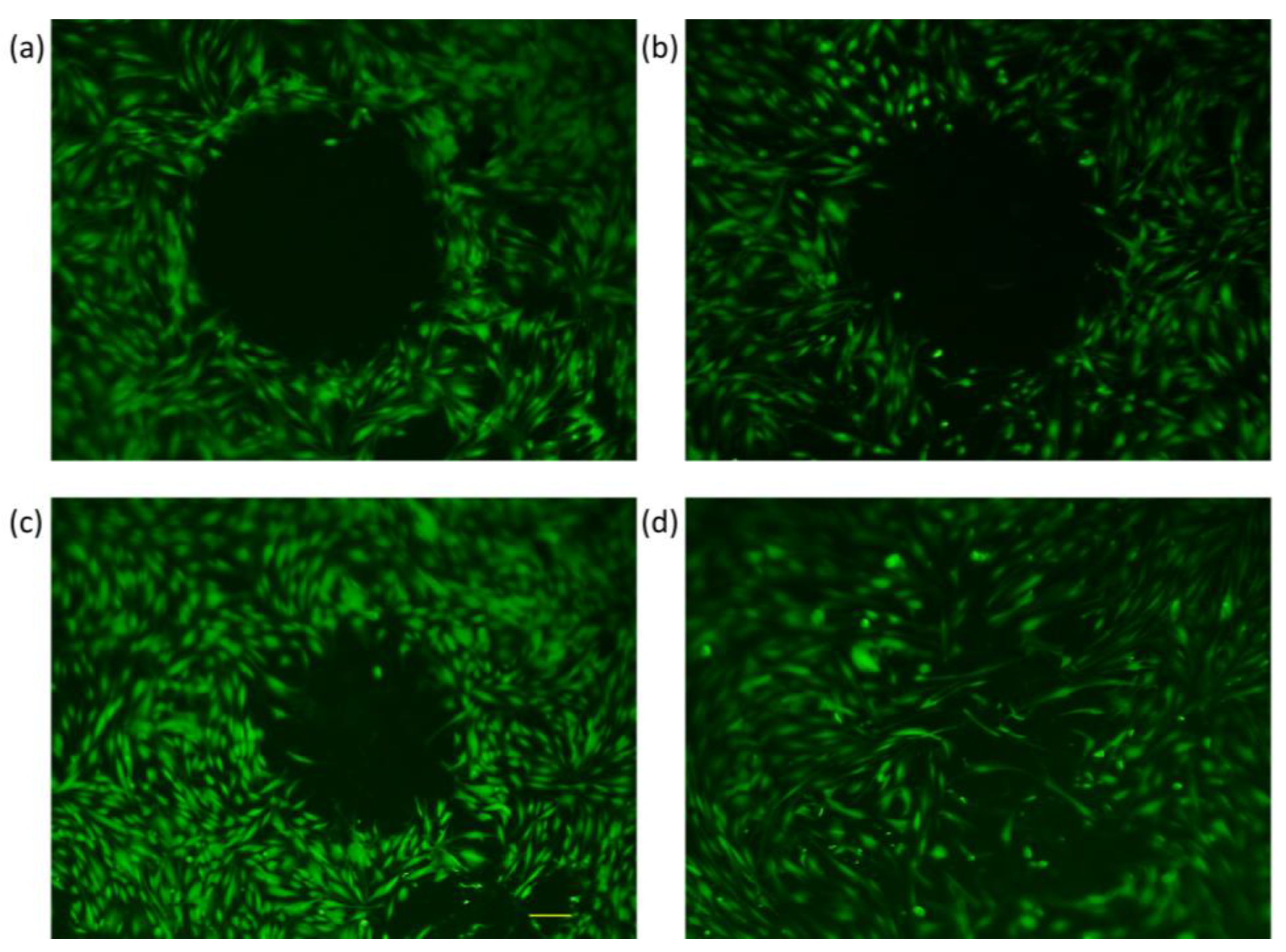
3.2. Trials with Different Methods of “Wound Generation” In-Vitro
3.3. Gap Perimeter Standardization
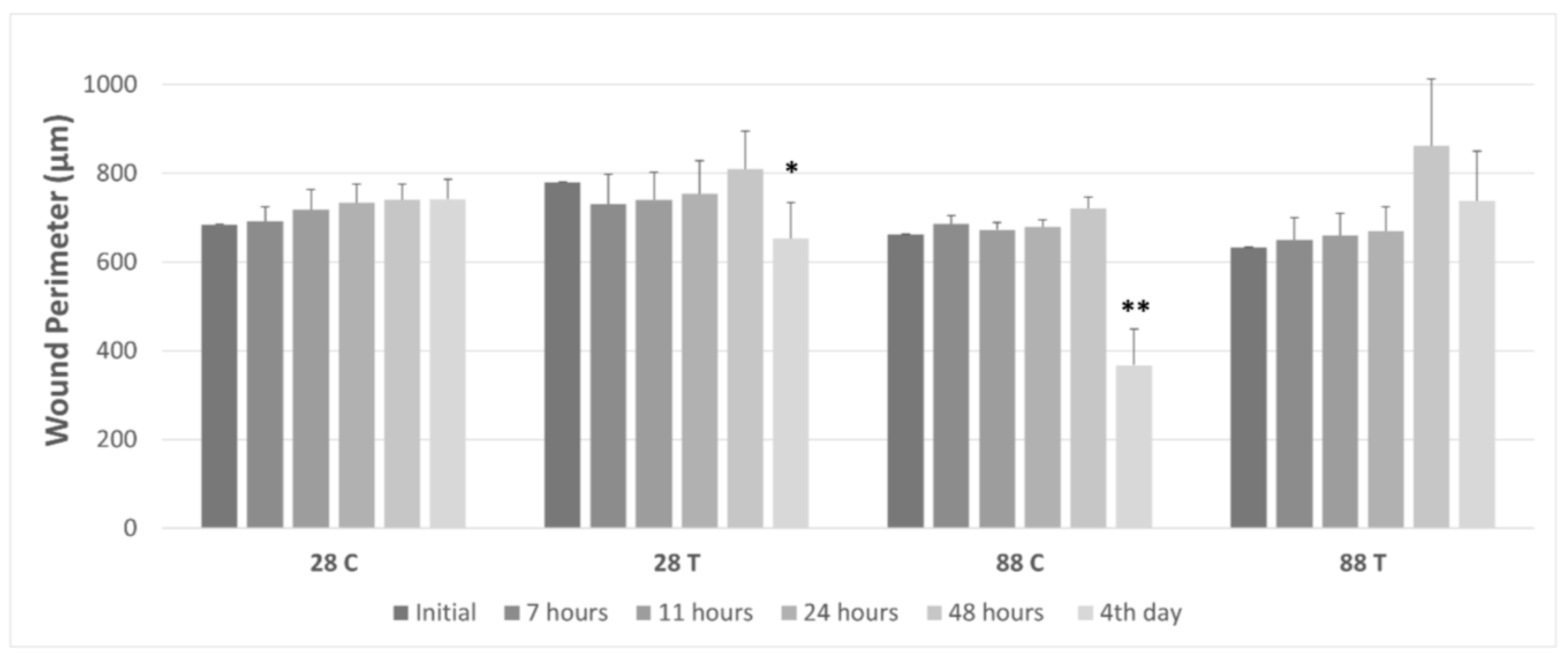
3.4. Gap Perimeter Change as Function of Wound Closure
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Characteristics, Applications and Properties of Polymers. In Polymer Engineering Science and Viscoelasticity; Springer: Newyork, NY, USA, 2008; pp. 55–97. [CrossRef]
- Victor, A.; Ribeiro, J.E.; Araújo, F.F. Study of PDMS characterization and its applications in biomedicine: A review. J. Mech. Eng. Biomech. 2019, 4, 1–9. [Google Scholar] [CrossRef]
- Gale, B.K.; Eddings, M.A.; Sundberg, S.O.; Hatch, A.; Kim, J.; Ho, T.; Karazi, S.M. Low-Cost MEMS Technologies. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Miranda, I.; Souza, A.; Sousa, P.; Ribeiro, J.; Castanheira, E.M.S.; Lima, R.; Minas, G. Properties and Applications of PDMS for Biomedical Engineering: A Review. J. Funct. Biomater. 2021, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, I.; Castro, I.; Carvalho, V.; Rodrigues, C.; Souza, A.; Lima, R.; Teixeira, S.; Ribeiro, J. Polydimethylsiloxane mechanical properties: A systematic review. AIMS Mater. Sci. 2021, 8, 952–973. [Google Scholar] [CrossRef]
- Sales, F.C.; Ariati, R.M.; Noronha, V.T.; Ribeiro, J.E. Mechanical Characterization of PDMS with Different Mixing Ratios. Procedia Struct. Integr. 2022, 37, 383–388. [Google Scholar] [CrossRef]
- Vincent, L.; Engler, A.J. Effect of Substrate Modulus on Cell Function and Differentiation. In Comprehensive Biomaterials; Elsevier: New York, NY, USA, 2011; pp. 51–63. [Google Scholar] [CrossRef]
- Luttge, R. Basic technologies for microsystems. In Nano- and Microfabrication for Industrial and Biomedical Applications; William Andrew: Norwich, NY, USA, 2016; pp. 11–54. [Google Scholar] [CrossRef]
- Willerth, S. Synthetic biomaterials for engineering neural tissue from stem cells. In Engineering Neural Tissue from Stem Cells; Academic Press: Cambridge, MA, USA, 2017; pp. 127–158. [Google Scholar] [CrossRef]
- Rezai, P.; Wu, W.-I.; Selvaganapathy, P. Microfabrication of polymers for bioMEMS. In MEMS for Biomedical Applications; Woodhead Publishing: Sawston, UK, 2012; pp. 3–45. [Google Scholar] [CrossRef]
- Gohil, S.V.; Suhail, S.; Rose, J.; Vella, T.S.; Nair, L. Polymers and Composites for Orthopedic Applications. In Materials and Devices for Bone Disorders; Bose, S., Bandyopadhyay, A., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 349–403. [Google Scholar] [CrossRef]
- Nguyen, H.-T.; Massino, M.; Keita, C.; Salmon, J.-B. Microfluidic dialysis using photo-patterned hydrogel membranes in PDMS chips. Lab Chip 2020, 20, 2383–2393. [Google Scholar] [CrossRef]
- Uysal, K.; Creutz, T.; Firat, I.S.; Artmann, G.M.; Teusch, N.; Temiz Artmann, A. Bio-Functionalized Ultra-Thin, Large-Area and Waterproof Silicone Membranes for Biomechanical Cellular Loading and Compliance Experiments. Polymers 2022, 14, 2213. [Google Scholar] [CrossRef]
- Ulbricht, M. Advanced functional polymer membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef]
- Makvandi, P.; Iftekhar, S.; Pizzetti, F.; Zarepour, A.; Zare, E.N.; Ashrafizadeh, M.; Agarwal, T.; Padil, V.V.T.; Mohammadinejad, R.; Sillanpaa, M.; et al. Functionalization of polymers and nanomaterials for water treatment, food packaging, textile and biomedical applications: A review. Environ. Chem. Lett. 2020, 19, 583–611. [Google Scholar] [CrossRef]
- Trzewik, J.; Linder, P.; Zerlin, K.F. How Strong is the Beating of Cardiac Myocytes?—The Cell Drum Solution. In Bioengineering in Cell and Tissue Research; Springer: Berlin/Heidelberg, Germany, 2008; pp. 351–369. [Google Scholar] [CrossRef]
- Artmann, A.T.; Demirci, E.K.; Fırat, I.S.; Oflaz, H.; Artmann, G.M. Recombinant Activated Protein C (rhAPC) Affects Lipopolysaccharide-Induced Mechanical Compliance Changes and Beat Frequency of mESC-Derived Cardiomyocyte Monolayers. Shock 2022, 57, 544–552. [Google Scholar] [CrossRef]
- Creutz, T. Cyclic Mechanical Stimulation Effects on Cell Cytoskeleton in 2D and 3D Cell Culture Models via New CellDrum PulSElect Using Optimal Parameters. Master’s Thesis, Aachen University of Applied Sciences, Aachen, Germany, 2018. [Google Scholar]
- Ohashi, K.; Fujiwara, S.; Mizuno, K. Roles of the cytoskeleton, cell adhesion and rho signalling in mechanosensing and mechanotransduction. J. Biochem. 2017, 161, 245–254. [Google Scholar] [CrossRef]
- Carver, W.; Nagpal, M.L.; Nachtigal, M.; Borg, T.K.; Terracio, L. Collagen expression in mechanically stimulated cardiac fibroblasts. Circ. Res. 1991, 69, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Hendriks F, F. Mehanical behaviour of human epidermal and dermal layers in vivo. J Biology. 2005, 0472. [Google Scholar] [CrossRef]
- Masson-Meyers, D.S.; Andrade, T.A.M.; Caetano, G.F.; Guimaraes, F.R.; Leite, M.N.; Leite, S.N.; Frade, M.A.C. Experimental models and methods for cutaneous wound healing assessment. Int. J. Exp. Pathol. 2020, 101, 21–37. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Siriwattanasatorn, M.; Itharat, A.; Thongdeeying, P.; Ooraikul, B. In Vitro Wound Healing Activities of Three Most Commonly Used Thai Medicinal Plants and Their Three Markers. Evid.-Based Complement. Altern. Med. 2020, 2020, 6795383. [Google Scholar] [CrossRef] [PubMed]
- Stamm, A.; Reimers, K.; Strauß, S.; Vogt, P.; Scheper, T.; Pepelanova, I. In vitro wound healing assays—State of the art. BioNanoMaterials 2016, 17, 79–87. [Google Scholar] [CrossRef]
- Riahi, R.; Yang, Y.; Zhang, D.D.; Wong, P.K. Advances in Wound-Healing Assays for Probing Collective Cell Migration. J. Lab. Autom. 2012, 17, 59–65. [Google Scholar] [CrossRef]
- Main, K.A.; Mikelis, C.M.; Doçi, C.L. In Vitro Wound Healing Assays to Investigate Epidermal Migration. In Epidermal Cells; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2019; Volume 2109, pp. 147–154. [Google Scholar] [CrossRef]
- Vang Mouritzen, M.; Jenssen, H. Optimized Scratch Assay for In Vitro Testing of Cell Migration with an Automated Optical Camera. J. Vis. Exp. 2018, 138, e57691. [Google Scholar] [CrossRef]
- Rodriguez, L.G.; Wu, X.; Guan, J.-L. Wound-Healing Assay. In Cell Migration; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2005; Volume 294, pp. 23–30. [Google Scholar] [CrossRef]
- Lin, J.-Y.; Lo, K.-Y.; Sun, Y.-S. A microfluidics-based wound-healing assay for studying the effects of shear stresses, wound widths, and chemicals on the wound-healing process. Sci. Rep. 2019, 9, 20016. [Google Scholar] [CrossRef]
- Molinie, N.; Gautreau, A. Directional Collective Migration in Wound Healing Assays. In Cell Migration; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2018; Volume 1749, pp. 11–19. [Google Scholar] [CrossRef]
- Keese, C.R.; Wegener, J.; Walker, S.R.; Giaever, I. Electrical wound-healing assay for cells in vitro. Proc. Natl. Acad. Sci. USA 2004, 101, 1554–1559. [Google Scholar] [CrossRef]
- Ashby, W.J.; Zijlstra, A. Established and novel methods of interrogating two-dimensional cell migration. Integr. Biol. 2012, 4, 1338–1350. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-Y.; Sun, Y.-S.; Cheng, K.-C.; Lo, K.-Y. A Wound-Healing Assay Based on Ultraviolet Light Ablation. SLAS Technol. Transl. Life Sci. Innov. 2017, 22, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Sui, G.; Wang, J.; Lee, C.-C.; Lu, W.; Lee, S.P.; Leyton, J.V.; Wu, A.A.M.; Tseng, H.-R. Solution-Phase Surface Modification in Intact Poly(dimethylsiloxane) Microfluidic Channels. Anal. Chem. 2006, 78, 5543–5551. [Google Scholar] [CrossRef] [PubMed]
- Witucki, G.L. A silane primer: Chemistry and applications of alkoxy silanes. J. Coat. Technol. 1993, 65, 57–60. [Google Scholar]
- Campos, D.M.; Gritsch, K.; Salles, V.; Attik, G.N.; Grosgogeat, B. Surface Entrapment of Fibronectin on Electrospun PLGA Scaffolds for Periodontal Tissue Engineering. BioRes. Open Access 2014, 3, 117–126. [Google Scholar] [CrossRef]
- Syedain, Z.H.; Meier, L.A.; Bjork, J.W.; Lee, A.; Tranquillo, R.T. Implantable arterial grafts from human fibroblasts and fibrin using a multi-graft pulsed flow-stretch bioreactor with noninvasive strength monitoring. Biomaterials 2011, 32, 714–722. [Google Scholar] [CrossRef]
- Fırat, I.S. Effects of Aging and Equibiaxial Mechanical Stimulation on Dermal Fibroblasts: A Study of Quantification of ROMO1 Gene Expression via RT-qPCR, Quantification of Actin Cytoskeleton Reassembly Using Cell Morphology Index, and Measurement of Cellular Tension Forces with Tissue Tension Analyzer. Master’s Thesis, Aachen University of Applied Sciences, Aachen, Germany, 2020. [Google Scholar]
- De Ieso, M.L.; Pei, J.V. An accurate and cost-effective alternative method for measuring cell migration with the circular wound closure assay. Biosci. Rep. 2018, 38, BSR20180698. [Google Scholar] [CrossRef]
- Zorin, V.; Zorina, A.; Smetanina, N.; Kopnin, P.; Ozerov, I.V.; Leonov, S.; Isaev, A.; Klokov, D.; Osipov, A.N. Diffuse colonies of human skin fibroblasts in relation to cellular senescence and proliferation. Aging 2017, 9, 1404–1413. [Google Scholar] [CrossRef]
- Swarnabala, S.; Gattu, M.; Perry, B.; Cho, Y.; Lockey, R.F.; Kolliputi, N. ROMO1 links oxidative stress to mitochondrial integrity. J. Cell Commun. Signal. 2014, 9, 73–75. [Google Scholar] [CrossRef]
- Li, B.; Wang, J.H.-C. Application of Sensing Techniques to Cellular Force Measurement. Sensors 2010, 10, 9948–9962. [Google Scholar] [CrossRef]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.-C.; Thampatty, B.P.; Lin, J.-S.; Im, H.-J. Mechanoregulation of gene expression in fibroblasts. Gene 2007, 391, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.-H.; Zhang, G.-A.; Wang, C.; Ning, F.-G. Quantification of type I and III collagen content in normal human skin in different age groups. Zhonghua Shao Shang Za Zhi = Zhonghua Shaoshang Zazhi = Chin. J. Burn. 2008, 24, 51–53. [Google Scholar]
- Ouyang, X.; Xie, Y.; Wang, G. Mechanical stimulation promotes the proliferation and the cartilage phenotype of mesenchymal stem cells and chondrocytes co-cultured in vitro. Biomed. Pharmacother. 2019, 117, 109146. [Google Scholar] [CrossRef]
- Susanne, J.; Lauren, B.; Kathrin, S.; Marcel, H.; Johannes, K.; Sonja, S. Mechanical Stimulation Increases the Proliferation and Differentiation Potential of Human Adipose-Derived Stromal Cells. Int. J. Stem Cell Res. Ther. 2018, 5, 056. [Google Scholar] [CrossRef]
- Wahlsten, A.; Rütsche, D.; Nanni, M.; Giampietro, C.; Biedermann, T.; Reichmann, E.; Mazza, E. Mechanical stimulation induces rapid fibroblast proliferation and accelerates the early maturation of human skin substitutes. Biomaterials 2021, 273, 120779. [Google Scholar] [CrossRef]
- Zhou, S.; Salisbury, J.; Preedy, V.R.; Emery, P.W. Increased Collagen Synthesis Rate during Wound Healing in Muscle. PLoS ONE 2013, 8, e58324. [Google Scholar] [CrossRef]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased Collagen Production in Chronologically Aged Skin: Roles of Age-Dependent Alteration in Fibroblast Function and Defective Mechanical Stimulation. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef]
- Schulze, C.; Wetzel, F.; Kueper, T.; Malsen, A.; Muhr, G.; Jaspers, S.; Blatt, T.; Wittern, K.-P.; Wenck, H.; Käs, J.A. Stiffening of Human Skin Fibroblasts with Age. Biophys. J. 2010, 99, 2434–2442. [Google Scholar] [CrossRef]
- DiLoreto, R.; Murphy, C.T. The cell biology of aging. Mol. Biol. Cell 2015, 26, 4524–4531. [Google Scholar] [CrossRef]
- Gerstein, A.D.; Phillips, T.J.; Rogers, G.S.; Gilchrest, B.A. Wound Healing and Aging. Dermatol. Clin. 1993, 11, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Kaisers, W.; Boukamp, P.; Stark, H.-J.; Schwender, H.; Tigges, J.; Krutmann, J.; Schaal, H. Age, gender and UV-exposition related effects on gene expression in in vivo aged short term cultivated human dermal fibroblasts. PLoS ONE 2017, 12, e0175657. [Google Scholar] [CrossRef] [PubMed]
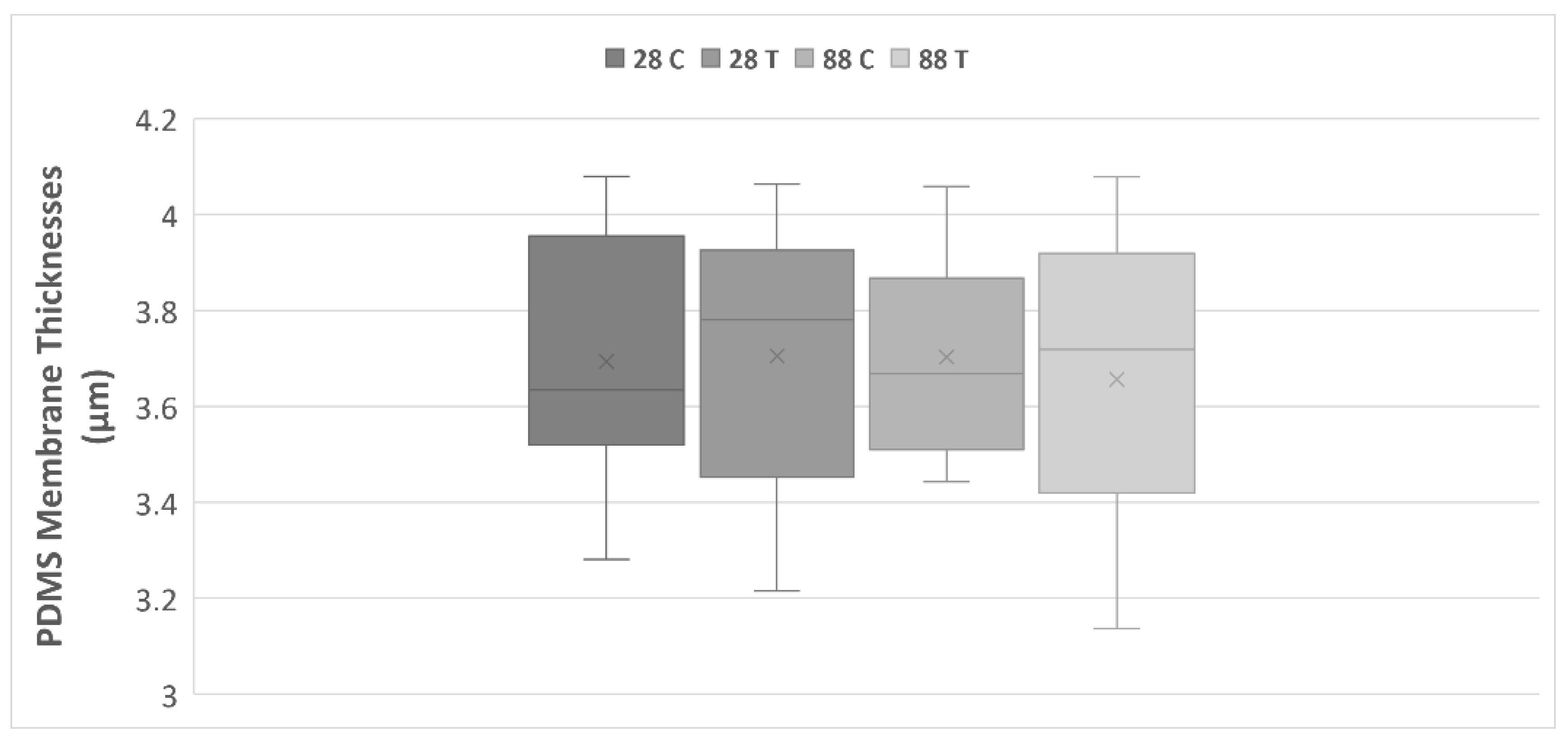

| Membrane Thickness in µm | |||||
|---|---|---|---|---|---|
| Experimental Groups | Average | SEM | Total Average | Total SEM | |
| n = 10 | 28 T | 3.70 | 0.09 | 3.69 | 0.04 |
| 28 C | 3.69 | 0.08 | |||
| 88 T | 3.66 | 0.10 | |||
| 88 C | 3.70 | 0.07 | |||
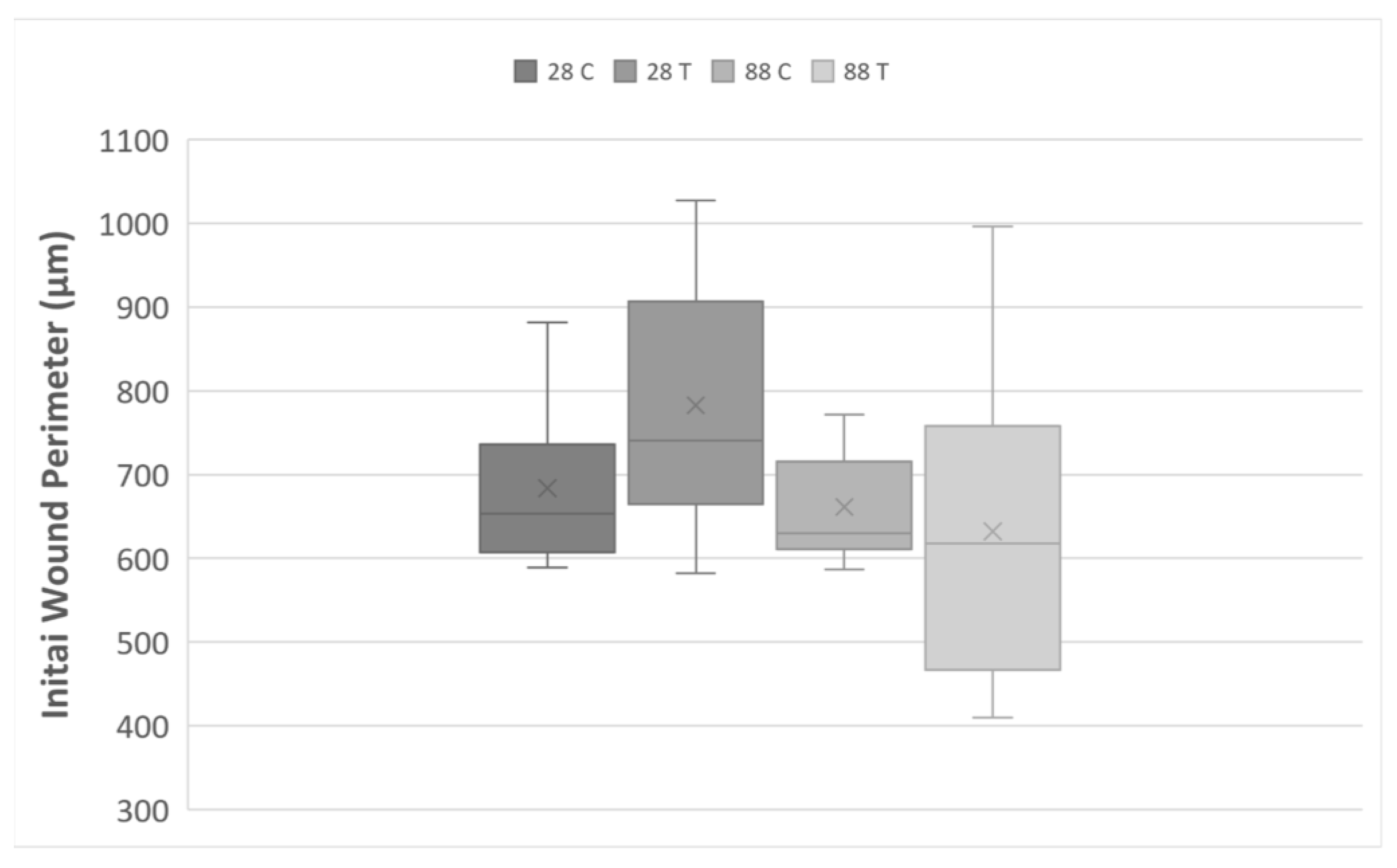
| Average Wound Perimeter in µm | |||||||
|---|---|---|---|---|---|---|---|
| Experimental Groups | Initial | 7 h | 11 h | 24 h | 48 h | 4 days | |
| n = 10 | 28 C | 683.7 ± 32.9 | 692.0 ± 33.3 | 717.7 ± 45.8 | 733.8 ± 41.6 | 740.1 ± 35.6 | 741.7 ± 44.7 |
| 28 T | 779.3 ± 45.2 | 730.6 ± 67.0 | 739.7 ± 63.0 | 753.4 ± 74.6 | 809.1 ± 85.3 | 652.8 ± 80.2 * | |
| 88 C | 661.2 ± 19.4 | 685.1 ± 18.7 | 672.3 ± 16.8 | 678.6 ± 16.6 | 720.2 ± 26.6 | 367.4 ± 81.6 ** | |
| 88 T | 632.1 ± 54.4 | 649.6 ± 49.8 | 659.4 ± 49.9 | 669.2 ± 55.9 | 861.4 ± 151.2 | 737.2 ± 112.6 | |
| Gap Closure Rate (%) | ||||
|---|---|---|---|---|
| Experimental Groups | 24 h | 48 h | 4 days | |
| n = 10 | 28 C | −10 | −27 | −10 |
| 28 T | 6 | 1 | 33 * | |
| 88 C | −3 | −17 | 55 ** | |
| 88 T | −14 | −111 | −49 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uysal, K.; Firat, I.S.; Creutz, T.; Aydin, I.C.; Artmann, G.M.; Teusch, N.; Temiz Artmann, A. A Novel In Vitro Wound Healing Assay Using Free-Standing, Ultra-Thin PDMS Membranes. Membranes 2023, 13, 22. https://doi.org/10.3390/membranes13010022
Uysal K, Firat IS, Creutz T, Aydin IC, Artmann GM, Teusch N, Temiz Artmann A. A Novel In Vitro Wound Healing Assay Using Free-Standing, Ultra-Thin PDMS Membranes. Membranes. 2023; 13(1):22. https://doi.org/10.3390/membranes13010022
Chicago/Turabian StyleUysal, Karya, Ipek Seda Firat, Till Creutz, Inci Cansu Aydin, Gerhard M. Artmann, Nicole Teusch, and Aysegül Temiz Artmann. 2023. "A Novel In Vitro Wound Healing Assay Using Free-Standing, Ultra-Thin PDMS Membranes" Membranes 13, no. 1: 22. https://doi.org/10.3390/membranes13010022
APA StyleUysal, K., Firat, I. S., Creutz, T., Aydin, I. C., Artmann, G. M., Teusch, N., & Temiz Artmann, A. (2023). A Novel In Vitro Wound Healing Assay Using Free-Standing, Ultra-Thin PDMS Membranes. Membranes, 13(1), 22. https://doi.org/10.3390/membranes13010022








