Fabrication of Colored Polymeric Membrane Using Clay-Based Nano Pigments of Safranin O (SO) Dye
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Used
2.2. Synthesis of Organo Montmorillonite
2.3. Preparation of Stock Solution of SO Dye
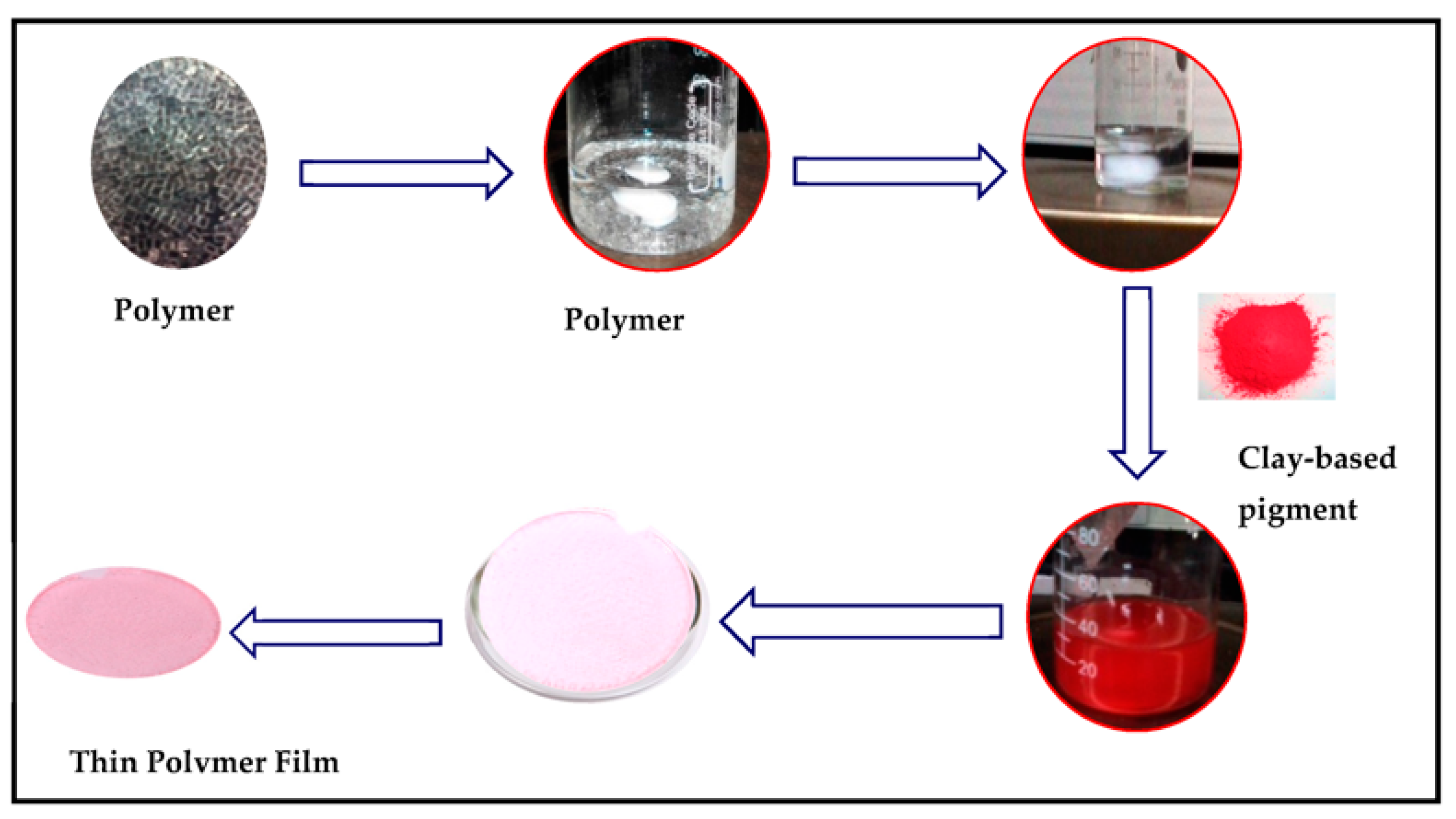
2.4. Synthesis of Clay-Based Nano Pigments
2.5. Characterization
3. Results and Discussion
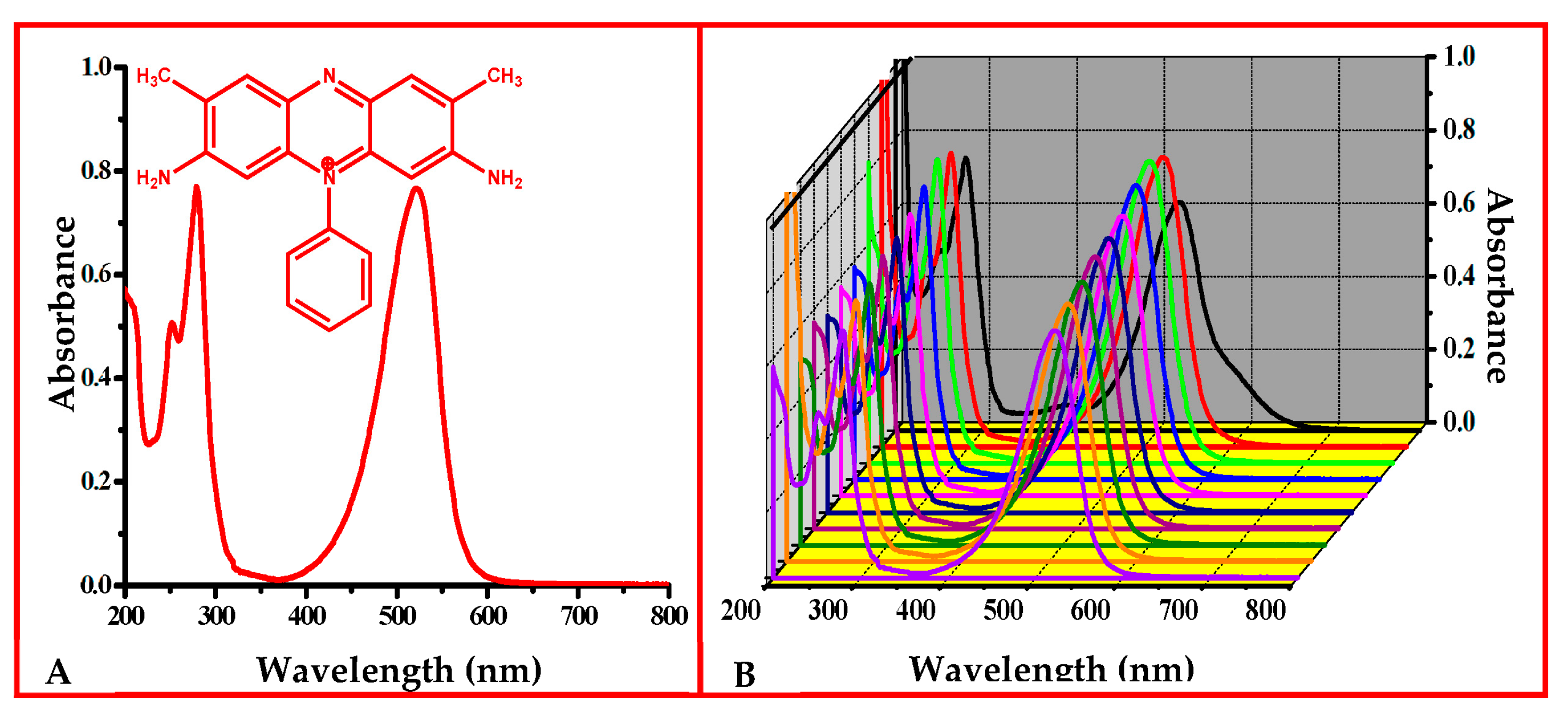
3.1. Effect of pH on SO Stability in Aqueous Media
3.2. Batch Extraction Studies
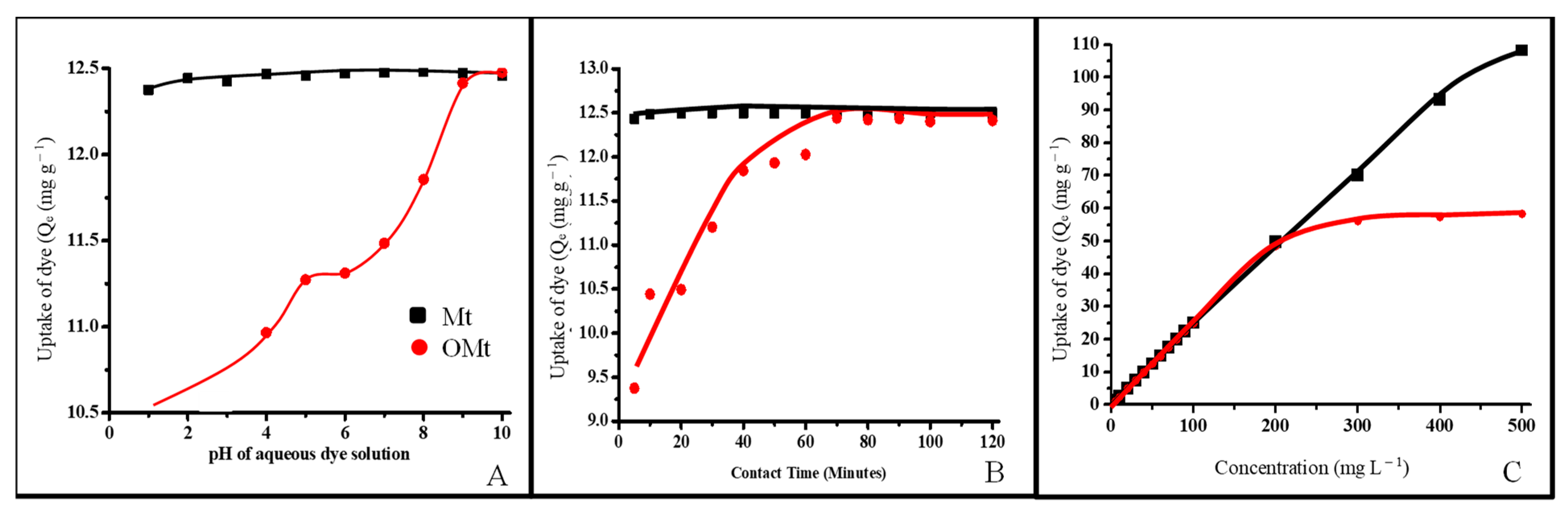
3.2.1. Adsorption of SO as a Function of pH
3.2.2. Adsorption of SO as a Function of Contact Time
3.2.3. Adsorption of SO as a Function of Initial Concentration
3.3. XRD Studies of Mt and OMt Based Nano Pigments
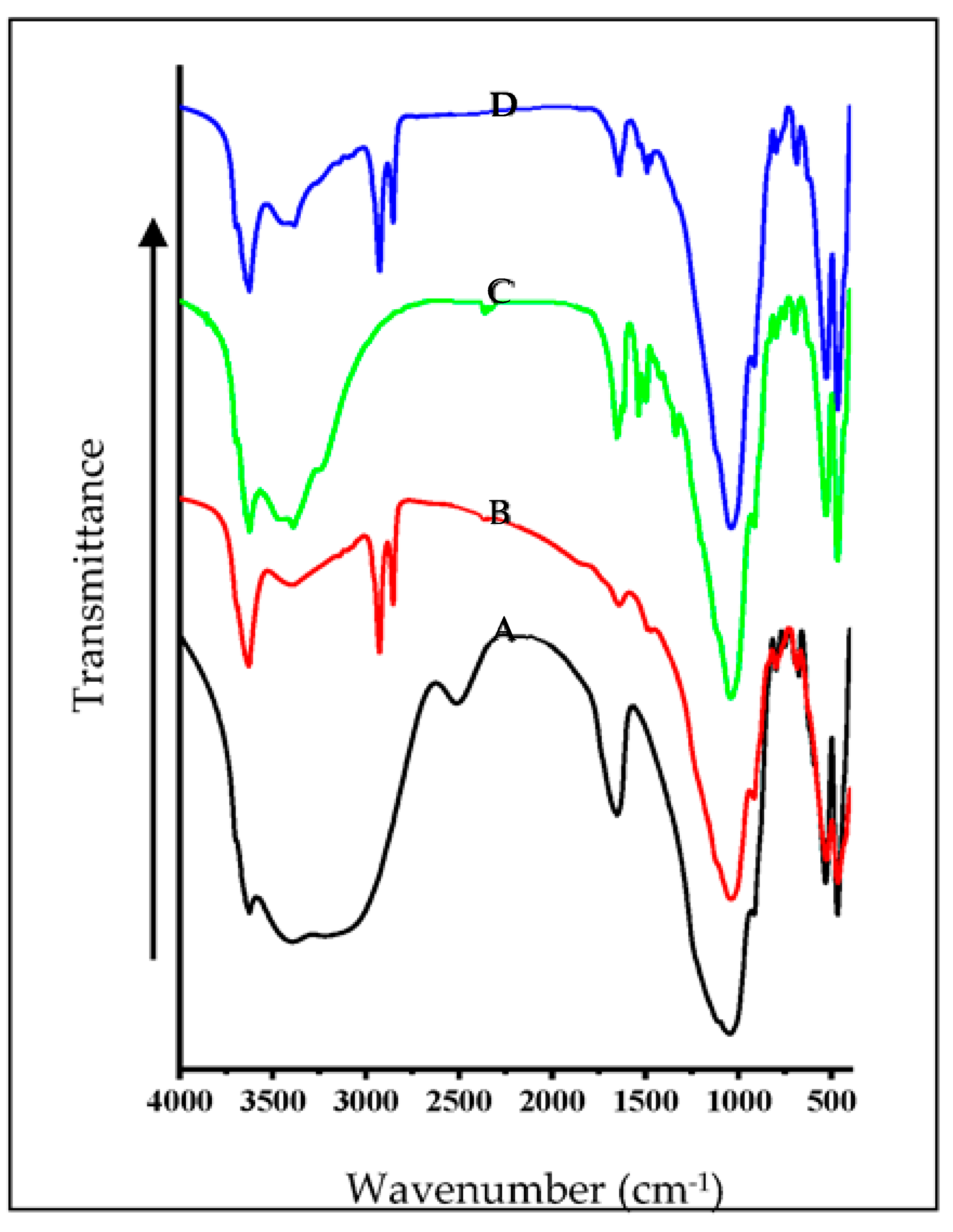
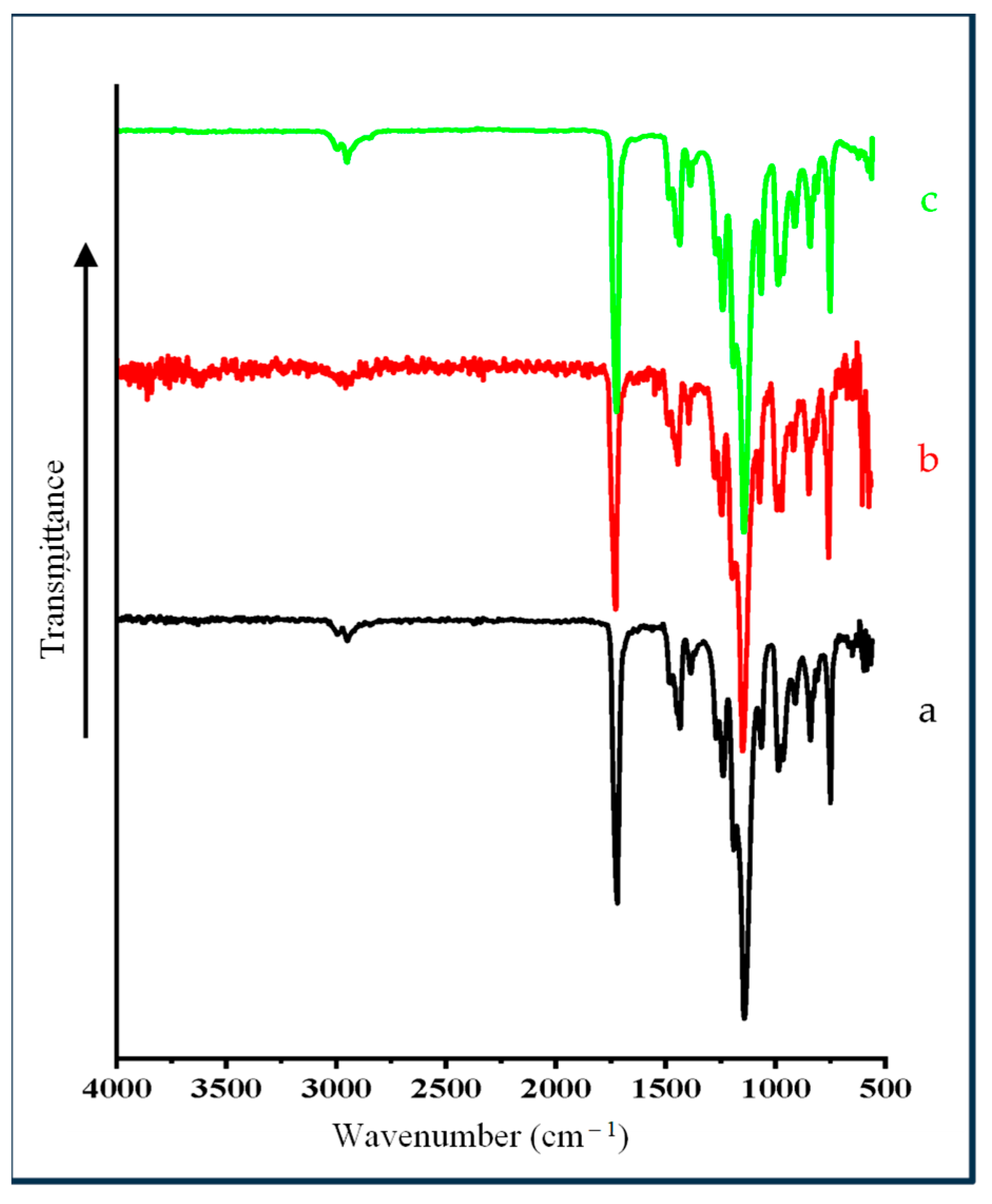
3.4. FTIR Spectral Studies of Mt- and OMt-Based Nano Pigments
3.5. Thermogravimetric Analysis (TGA) of Mt- and OMt-Based Nano Pigments
3.6. Morphological Studies
4. Application of Clay-Based Nano Pigment for Colored Polymeric Membrane
4.1. XRD Pattern of PMMA Polymeric Membrane and Clay Based Nano Pigments Containing Polymeric Membrane
4.2. FTIR Spectra of PMMA Polymeric Membrane and Clay-Based Nano Pigments Containing Polymeric Membrane
4.3. High-Resolution Transmission Electron Microscopy (HR-TEM)
4.4. Mechanical Properties of PMMA Polymeric Membrane
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahmoodi, A.; Ebrahimi, M.; Khosravi, A.; Eivaz Mohammadloo, H. A hybrid dye-clay nano-pigment: Synthesis, characterization and application in organic coatings. Dyes Pigments 2017, 147, 234–240. [Google Scholar] [CrossRef]
- Tianyong, Z.; Xuening, F.; Jian, S.; Chunlong, Z. Properties of copper phthalocyanine microencapsulated in polystyrene by phase separation. Dyes Pigments 1999, 44, 1–7. [Google Scholar] [CrossRef]
- Herbst, W.; Hunger, K. Miscellaneous pigments. In Industrial Organic Pigments, Production, Properties, Applications, 2nd ed.; VCH: Weinheim, Germany, 1997; pp. 567–583. [Google Scholar]
- Farha, A.H.; Al Naim, A.F.; Mansour, S.A. Cost-Effective and Efficient Cool Nano pigments Based on Oleic-Acid-Surface-Modified ZnO Nanostructured. Materials 2023, 16, 2159. [Google Scholar] [CrossRef]
- Micó-Vicent, B.; Ramos, M.; Luzi, F.; Dominici, F.; Viqueira, V.; Torre, L.; Jiménez, A.; Puglia, D.; Garrigós, M.C. Effect of Chlorophyll Hybrid Nano pigments from Broccoli Waste on Thermomechanical and Colour Behaviour of Polyester-Based Bionanocomposites. Polymers 2020, 12, 2508. [Google Scholar] [CrossRef]
- Ngamwonglumlert, L.; Devahastin, S.; Chiewchan, N. Natural colorants: Pigment stability and extraction yield enhancement via utilization of appropriate pretreatment and extraction methods. Crit. Rev. Food Sci. Nutr. 2017, 57, 3243–3259. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, L.; Li, J.; Oliveira, H.; Yang, N.; Jin, W.; Zhu, Z.; Li, S.; He, J. Microencapsulation of anthocyanins extracted from grape skin by emulsification/internal gelation followed by spray/freeze-drying techniques: Characterization, stability and bioaccessibility. LWT 2020, 123, 109097. [Google Scholar] [CrossRef]
- Serbanescu, O.S.; Pandele, A.M.; Miculescu, F.; Voicu, S.I. Synthesis and characterization of cellulose acetate membranes with self-indicating properties by changing the membrane surface color for separation of Gd(III). Coatings 2020, 10, 468. [Google Scholar] [CrossRef]
- Bello, O.S.; Olusegun, O.A.; Njoku, V.O. Fly Ash: An alternative to powdered activated carbon for the removal of eosin dye from aqueous solutions. Bull. Chem. Soc. Ethiop. 2013, 27, 191–204. [Google Scholar] [CrossRef]
- Edraki, M.; Zaarei, D. Evaluation of the anti-corrosion effect of clay-based nano pigments modified with organic azole compounds. Adv. Mat. New Coat. 2018, 6, 1641–1654. [Google Scholar]
- Mahmoodi, A.; Ebrahimi, M.; Khosravi, A. Preparation a Blue Color Epoxy/Clay Nanocomposite with a Better Color Performance, Iran. 2016. Available online: https://civilica.com/doc/578462/certificate/print/ (accessed on 15 March 2023).
- Fischer, H.R.; Batenburg, L.F. Coloring Pigment. US6648959B1, 18 November 2003. [Google Scholar]
- Marchante, V.; Benavente, V.; Marcilla, A.; Martínez-Verdú, F.M.; Beltrán, M.I. Ethylene vinyl acetate/nanoclay-based pigment composites: Morphology, rheology, and mechanical, thermal, and colorimetric properties. J. Appl. Polym. Sci. 2013, 130, 2987–2994. [Google Scholar] [CrossRef]
- Raha, S.; Ivanov, I.; Quazi, N.H.; Bhattacharya, S.N. Photo-stability of rhodamine-B/montmorillonite nano pigments in polypropylene matrix. Appl. Clay Sci. 2009, 42, 661–666. [Google Scholar]
- Smitha, V.S.; Manjumol, K.A.; Ghosh, S.; Brahmakumar, M.; Pavithran, C.; Perumal, P. Rhodamine 6G intercalated montmorillonite nano pigments–polyethylene composites: Facile synthesis and ultraviolet stability study. J. Am. Ceram. Soc. 2011, 94, 1731–1736. [Google Scholar] [CrossRef]
- Trigueiro, P.; Pereira, F.A.; Guillermin, D.; Rigaud, B.; Balme, S.; Janot, J.M.; dos Santos, I.M.G.; Fonseca, M.G.; Walter, P.; Jaber, M. When anthraquinone dyes meet pillared montmorillonite: Stability or fading upon exposure to light? Dyes Pigments 2018, 159, 384–394. [Google Scholar] [CrossRef]
- Szadkowski, B.; Kuśmierek, M.; Kozanecki, M.; Nowakowska, J.; Rogowski, J.; Maniukiewicz, W.; Marzec, A. Ecological hybrid pigments with improved thermal, light, and chemical stability based on purpurin dye and different minerals for applications in polymer materials. Dyes Pigments 2023, 217, 111430. [Google Scholar] [CrossRef]
- Cavalcanti, G.R.; Rodrigues, F.; Zhuang, G.; Balme, S.; Janot, J.M.; Fonseca, M.G.; Jaber, M. Inorganic-organic hybrid pigments based on carminic acid and clay minerals. Dyes Pigments 2023, 190, 109306. [Google Scholar] [CrossRef]
- Hussain, A.F.; Halboos, M.H. Adsorption of safranin dye from their aqueous solutions by using CA and Nano FeO/CA. J. Phys. Conf. Ser. 2020, 1660, 012080. [Google Scholar] [CrossRef]
- Sayhood, A.A.; Mohammed, H.J. Synthesis of new azo reagent for determination of Pd(II), Ag(I) and applied to enhance the properties of silver nano particles. Int. J. Chem. Sci. 2015, 13, 1123–1136. [Google Scholar]
- Ghosh, I.; Kar, S.; Chatterjee, T.; Bar, T.; Das, S.K. Adsorptive removal of Safranin-O dye from aqueous medium using coconut coir and its acid-treated forms: Adsorption study, scale-up design, MPR and GA-ANN modeling. Sustain. Chem. Pharm. 2021, 19, 100374. [Google Scholar] [CrossRef]
- Azimvand, J.; Didehban, K.; Mirshokraie, S. Safranin-O removal from aqueous solutions using lignin nanoparticle-g-polyacrylic acid adsorbent: Synthesis, properties, and application. Adsorpt. Sci. Technol. 2021, 36, 1422–1440. [Google Scholar] [CrossRef]
- Shah, K.; Parmar, A. Removal of Safranin O dye from synthetic wastewater by Activated Carbon prepared from Tamarind seeds. Int. J. Appl. Eng. Res. 2018, 13, 10105–10107. [Google Scholar]
- Didehban, K.H.; Mirshokraie, S.A.; Azimvand, J. Safranin-O dye removal from aqueous solution using super-absorbent lignin nanoparticle/polyacrylic acid hydrogel. Bulg. Chem. Commun. 2018, 50, 180–187. [Google Scholar] [CrossRef]
- Pakdel, P.M.; Peighambardoust, S.J.; Arsalani, N.; Aghdasinia, H. Safranin-O cationic dye removal from wastewater using carboxymethyl cellulose-grafted-poly(acrylic acid-co-itaconic acid) nanocomposite hydrogel. Environ. Res. 2022, 212, 113201. [Google Scholar] [CrossRef] [PubMed]
- Sieren, B.; Baker, J.; Wang, X.; Rozzoni, S.J.; Carlson, K.; McBain, A.; Kerstan, D.; Allen, L.; Li, L.Z. Sorptive Removal of color dye safranin o by fibrous clay minerals and zeolites. Adv. Mat. Sci. Eng. 2020, 2020, 8845366. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, X.; Wang, X.; Carlson, K.; Li, Z. Removal of Toluidine blue and Safranin O from single and binary solutions using Zeolite. Crystals 2021, 11, 1181. [Google Scholar] [CrossRef]
- Chanra, J.; Budianto, E.; Soegijono, B. Surface modification of montmorillonite by the use of organic cations via conventional ion exchange method. IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 012057. [Google Scholar] [CrossRef]
- Guegan, T. Organoclay applications and limits in the environment. Comptes Rendus Chim. 2018, 22, 132–141. [Google Scholar] [CrossRef]
- Kumari, J.; Singh, A. Green synthesis of nanostructured silver particles and their catalytic application in dye degradation. J. Gen. Eng. Biotechnol. 2016, 14, 311–317. [Google Scholar] [CrossRef]
- Avila, M.C.; Lick, L.D.; Comelli, N.A.; Ruiz, M.L. Adsorption of an anionic dye from aqueous solution on a treated clay. Groundw. Sustain. Dev. 2021, 15, 100688. [Google Scholar] [CrossRef]
- Romdhane, D.F.; Satlaoui, Y.; Nasraoui, R.; Charef, A.; Azouzi, R. Adsorption, modeling, thermodynamic, and kinetic studies of methyl red removal from textile-polluted water using natural and purified organic matter rich clays as low-cost adsorbent. J. Chem. 2020, 2020, 4376173. [Google Scholar] [CrossRef]
- Sivalingam, R.; Sivasamy, A.; Muthukaruppan, A.; Chandrasekar, F. Synthesis and characterization of poly(n-vinyl-2-pyrrolidone)-organo-modified montmorillonite (OMMT) clay hybrid nanocomposites. J. Comp. Mater. 2011, 45, 1483–1489. [Google Scholar]
- Kumari, N.; Mohan, C.; Negi, A. An investigative study on the structural, thermal and mechanical properties of clay-based PVC polymer composite films. Polymers 2023, 15, 1922. [Google Scholar] [CrossRef]
- Elhossein, A.; Moawed, A.; Abulkibash, B. Selective separation of Light green and Safranin O from aqueous solution using Salvadora persica (Miswak) powder as a new biosorbent. J. Saudi Chem. Soc. 2016, 20, S178–S185. [Google Scholar] [CrossRef]
- Franco-Urquiza, E.A. Clay-based polymer nanocomposites: Essential work of fracture. Polymers 2021, 13, 2399. [Google Scholar] [CrossRef]
- Yadav, S.; Yusoh, K. Modification of pristine nanoclay and its application in wood-plastic composite. e-Polymers 2016, 16, 447–461. [Google Scholar] [CrossRef]
- Kumar, M.; Arun, S.; Upadhyaya, P. Properties of PMMA/clay nanocomposites prepared using various compatibilizers. Int. J. Mech. Mater. Eng. 2015, 10, 7. [Google Scholar] [CrossRef]
- Naderi-Samani, H.; Razavi, R.S.; Loghman-Estarki, M.R.; Ramazani, M. The effects of organoclay on the morphology and mechanical properties of PAI/clay nanocomposites coatings prepared by the ultrasonication assisted process. Ultrason. Sonochem. 2017, 38, 306–316. [Google Scholar] [CrossRef]
- Pandey, S.; Jana, K.K.; Aswal, V.K.; Rana, D.; Maiti, P. Effect of nanoparticle on the mechanical and gas barrier properties of thermoplastic polyurethane. Appl. Clay Sci. 2017, 146, 468–474. [Google Scholar] [CrossRef]
- Adak, B.; Butola, B.S.; Joshi, M. Effect of organoclay-type and clay-polyurethane interaction chemistry for tuning the morphology, gas barrier and mechanical properties of clay/polyurethane nanocomposites. Appl. Clay Sci. 2018, 161, 343–353. [Google Scholar] [CrossRef]
- Kumar, M.; Chakraborty, S.; Upadhyaya, P.; Pugazhenthi, G. Morphological, mechanical, and thermal features of PMMA nanocomposites containing two-dimensional Co–Al layered double hydroxide. J. Appl. Polym. Sci. 2017, 135, 45774. [Google Scholar] [CrossRef]





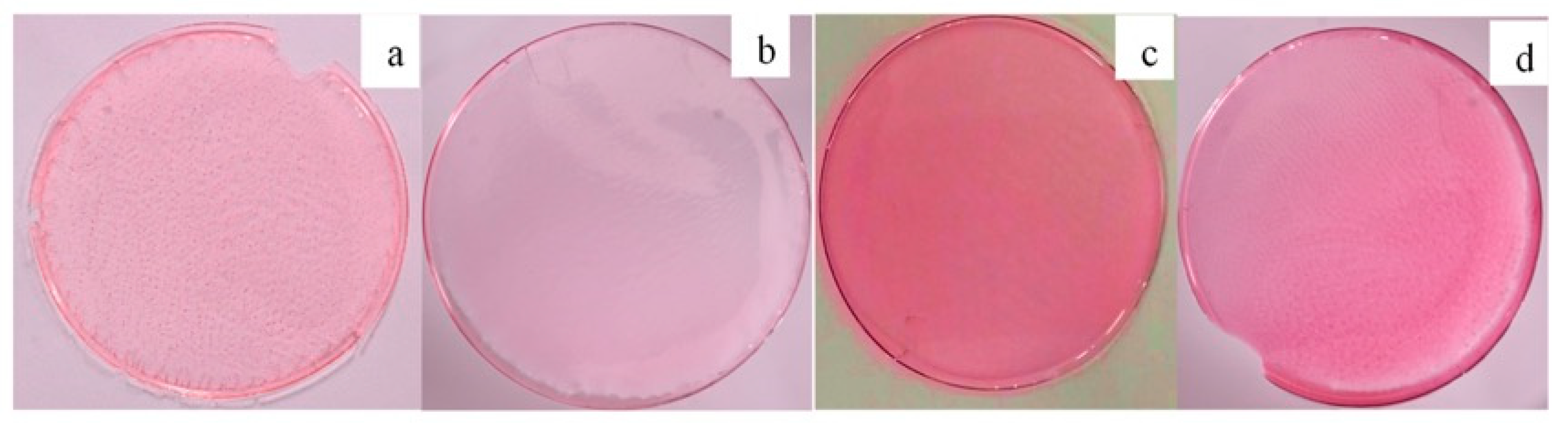


| S. No. | Sample Code | Stress (Pascal) | Strain (mm/mm) | Tensile Strength (MPa) | Young’s Modulus (MPa) |
|---|---|---|---|---|---|
| 01 | PMMA + SO | 10.72 | 0.038 | 3.06 | 346.28 |
| 02 | PMMA + MtSO | 18.45 | 0.040 | 5.27 | 523.12 |
| 03 | PMMA + OMtSO | 27.61 | 0.045 | 7.89 | 748.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohan, C.; Kumari, P.; Kumari, N.; Negi, A. Fabrication of Colored Polymeric Membrane Using Clay-Based Nano Pigments of Safranin O (SO) Dye. Membranes 2023, 13, 619. https://doi.org/10.3390/membranes13070619
Mohan C, Kumari P, Kumari N, Negi A. Fabrication of Colored Polymeric Membrane Using Clay-Based Nano Pigments of Safranin O (SO) Dye. Membranes. 2023; 13(7):619. https://doi.org/10.3390/membranes13070619
Chicago/Turabian StyleMohan, Chandra, Priyanka Kumari, Neeraj Kumari, and Arvind Negi. 2023. "Fabrication of Colored Polymeric Membrane Using Clay-Based Nano Pigments of Safranin O (SO) Dye" Membranes 13, no. 7: 619. https://doi.org/10.3390/membranes13070619
APA StyleMohan, C., Kumari, P., Kumari, N., & Negi, A. (2023). Fabrication of Colored Polymeric Membrane Using Clay-Based Nano Pigments of Safranin O (SO) Dye. Membranes, 13(7), 619. https://doi.org/10.3390/membranes13070619








