Is It Possible to Prepare a “Super” Anion-Exchange Membrane by a Polypyrrole-Based Modification?
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment
2.1.1. Pristine Membrane
2.1.2. Membrane Modification
2.1.3. Membrane Characterization
Electrical Conductivity
Diffusion Permeability
Water Content
Ion-Exchange Capacity
2.1.4. Polypyrrole Properties
2.2. Theoretical Modelling
2.2.1. Model Description
Membrane Swelling
Calculation of Pristine Membrane Transport Characteristics
Accounting for the Presence of PPy
3. Results
3.1. Determination of the Input Parameters
3.2. Results of Simulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Helfferich, F. Ion Exchange, 1st ed.; Dover Publications, Inc.: New York, NY, USA, 1962; pp. 1–608. [Google Scholar]
- Strathmann, H. Applications of ion-exchange membrane separation processes. Membr. Sci. Technol. 2004, 9, 287–330. [Google Scholar] [CrossRef]
- Strathmann, H. Electrodialysis, a mature technology with a multitude of new applications. Desalination 2010, 264, 268–288. [Google Scholar] [CrossRef]
- Jiang, S.; Sun, H.; Wang, H.; Ladewig, B.P.; Yao, Z. A comprehensive review on the synthesis and applications of ion exchange membranes. Chemosphere 2021, 282, 130817–130836. [Google Scholar] [CrossRef]
- Ran, J.; Wu, L.; He, Y.; Yang, Z.; Wang, Y.; Jiang, C.; Ge, L.; Bakangura, E.; Xu, T. Ion exchange membranes: New developments and applications. J. Memb. Sci. 2017, 522, 267–291. [Google Scholar] [CrossRef]
- Zhao, W.-Y.; Zhou, M.; Yan, B.; Sun, X.; Liu, Y.; Wang, Y.; Xu, T.; Zhang, Y. Waste conversion and resource recovery from wastewater by ion exchange membranes: State-of-the-art and perspective. Ind. Eng. Chem. Res. 2018, 57, 6025–6039. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y. Circular economy-driven ammonium recovery from municipal wastewater: State of the art, challenges and solutions forward. Bioresour. Technol. 2021, 334, 125231–125242. [Google Scholar] [CrossRef]
- Sharma, M.; Das, P.P.; Chakraborty, A.; Purkait, M.K. Clean energy from salinity gradients using pressure retarded osmosis and reverse electrodialysis: A review. Sustain. Energy Technol. Assess. 2022, 49, 101687–101700. [Google Scholar] [CrossRef]
- Golubenko, D.V.; Van der Bruggen, B.; Yaroslavtsev, A.B. Ion exchange membranes based on radiation-induced grafted functionalized polystyrene for high-performance reverse electrodialysis. J. Power Sources 2021, 511, 230460–230471. [Google Scholar] [CrossRef]
- Merle, G.; Wessling, M.; Nijmeijer, K. Anion exchange membranes for alkaline fuel cells: A review. J. Memb. Sci. 2011, 377, 1–35. [Google Scholar] [CrossRef]
- Yaroslavtsev, A.B.; Dobrovolsky, Y.A.; Shaglaeva, N.S.; Frolova, L.A.; Gerasimova, E.V.; Sanginov, E.A. Nanostructured materials for low-temperature fuel cells. Russ. Chem. Rev. 2012, 81, 191–220. [Google Scholar] [CrossRef]
- Thangarasu, S.; Oh, T.H. Progress in poly(phenylene oxide) based cation exchange membranes for fuel cells and redox flow batteries applications. Int. J. Hydrog. Energy 2021, 46, 38381–38415. [Google Scholar] [CrossRef]
- Ye, J.; Cheng, Y.; Sun, L.; Ding, M.; Wu, C.; Yuan, D.; Zhao, X.; Xiang, C.; Jia, C. A green SPEEK/lignin composite membrane with high ion selectivity for vanadium redox flow battery. J. Memb. Sci. 2019, 572, 110–118. [Google Scholar] [CrossRef]
- Dai, Q.; Liu, Z.; Huang, L.; Wang, C.; Zhao, Y.; Fu, Q.; Zheng, A.; Zhang, H.; Li, X. Thin-film composite membrane breaking the trade-off between conductivity and selectivity for a flow battery. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of ion exchange membranes: A review. J. Memb. Sci. 2018, 555, 429–454. [Google Scholar] [CrossRef]
- Xu, S.; Song, J.; Bi, Q.; Chen, Q.; Zhang, W.M.; Qian, Z.; Zhang, L.; Xu, S.; Tang, N.; He, T. Extraction of lithium from Chinese salt-lake brines by membranes: Design and practice. J. Memb. Sci. 2021, 635, 119441–119463. [Google Scholar] [CrossRef]
- Van der Bruggen, B. Chapter 7-Ion-exchange membrane systems-Electrodialysis and other electromembrane processes. In Fundamental Modelling of Membrane Systems; Elsevier: Amsterdam, The Netherlands, 2018; pp. 251–300. [Google Scholar] [CrossRef]
- Apel, P.Y.; Velizarov, S.; Volkov, A.V.; Eliseeva, V.; Nikonenko, V.V.; Parshina, A.V.; Pismenskaya, N.D.; Popov, K.I.; Yaroslavtsev, A.B. Fouling and membrane degradation in electromembrane and baromembrane processes. Membr. Membr. Technol. 2022, 4, 69–92. [Google Scholar] [CrossRef]
- Chen, Q.; Yao, Y.; Liao, J.; Li, J.; Xu, J.; Wang, T.; Tang, Y.; Xu, Y.; Ruan, H.; Shen, J. Subnanometer ion channel anion exchange membranes having a rigid benzimidazole structure for selective anion separation. ACS Nano 2022, 16, 4629–4641. [Google Scholar] [CrossRef]
- Xi, J.; Wu, Z.; Teng, X.; Zhao, Y.; Chen, L.; Qiu, X. Self-assembled polyelectrolyte multilayer modified Nafion membrane with suppressed vanadium ion crossover for vanadium redox flow batteries. J. Mater. Chem. 2008, 18, 1232–1238. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, H.; Li, X.; Mai, Z.; Wei, W. Silica modified nanofiltration membranes with improved selectivity for redox flow battery application. Energy Environ. Sci. 2012, 5, 6299–6303. [Google Scholar] [CrossRef]
- Khoiruddin, K.; Ariono, D.; Subagjo, S.; Wenten, I.G. Structure and transport properties of polyvinyl chloride-based heterogeneous cation-exchange membrane modified by additive blending and sulfonation. J. Electroanal. Chem. 2020, 873, 114304–114310. [Google Scholar] [CrossRef]
- Porozhnyy, M.V.; Shkirskaya, S.A.; Butylskii, D.Y.; Dotsenko, V.V.; Safronova, E.Y.; Yaroslavtsev, A.B.; Deabate, S.; Huguet, P.; Nikonenko, V.V. Physicochemical and electrochemical characterization of Nafion-type membranes with embedded silica nanoparticles: Effect of functionalization. Electrochim. Acta 2021, 370, 137689–137702. [Google Scholar] [CrossRef]
- Kozmai, A.; Pismenskaya, N.; Nikonenko, V. Mathematical description of the increase in selectivity of an anion-exchange membrane due to its modification with a perfluorosulfonated ionomer. Int. J. Mol. Sci. 2022, 23, 2238–2253. [Google Scholar] [CrossRef] [PubMed]
- Stenina, I.; Golubenko, D.; Nikonenko, V.; Yaroslavtsev, A. Selectivity of transport processes in ion-exchange membranes: Relationship with the structure and methods for its improvement. Int. J. Mol. Sci. 2020, 21, 5517–5550. [Google Scholar] [CrossRef]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356, eaab0530–eaab0542. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Yaroslavtsev, A.B.; Karavanova, Y.A.; Safronova, E.Y. Ionic conductivity of hybrid membranes. Pet. Chem. 2011, 51, 473–479. [Google Scholar] [CrossRef]
- Reig, M.; Farrokhzad, H.; Van der Bruggen, B.; Gibert, O.; Cortina, J.L. Synthesis of a monovalent selective cation exchange membrane to concentrate reverse osmosis brines by electrodialysis. Desalination 2015, 375, 1–9. [Google Scholar] [CrossRef]
- Golubenko, D.V.; Karavanova, Y.A.; Melnikov, S.S.; Achoh, A.R.; Pourcelly, G.; Yaroslavtsev, A.B. An approach to increase the permselectivity and mono-valent ion selectivity of cation-exchange membranes by introduction of amorphous zirconium phosphate nanoparticles. J. Memb. Sci. 2018, 563, 777–784. [Google Scholar] [CrossRef]
- Epsztein, R.; DuChanois, R.M.; Ritt, C.L.; Noy, A.; Elimelech, M. Towards single-species selectivity of membranes with subnanometre pores. Nat. Nanotechnol. 2020, 15, 426–436. [Google Scholar] [CrossRef]
- Ounissi, T.; Dammak, L.; Fauvarque, J.-F.; Hmida, E.S.B.H. Ecofriendly lithium-sodium separation by diffusion processes using lithium composite membrane. Sep. Purif. Technol. 2021, 275, 119134–119144. [Google Scholar] [CrossRef]
- Falina, I.; Loza, N.; Loza, S.; Titskaya, E.; Romanyuk, N. Permselectivity of cation exchange membranes modified by polyaniline. Membranes 2021, 11, 227. [Google Scholar] [CrossRef]
- Luo, T.; Roghmans, F.; Wessling, M. Ion mobility and partition determine the counter-ion selectivity of ion exchange membranes. J. Memb. Sci. 2020, 597, 117645–117656. [Google Scholar] [CrossRef]
- Sarapulova, V.; Pismenskaya, N.; Titorova, V.; Sharafan, M.; Wang, Y.; Xu, T.; Zhang, Y.; Nikonenko, V. Transport characteristics of CJMAEDTM homogeneous anion exchange membranes in sodium chloride and sodium sulfate solutions. Int. J. Mol. Sci. 2021, 22, 1415. [Google Scholar] [CrossRef] [PubMed]
- Kingsbury, R.S.; Coronell, O. Modeling and validation of concentration dependence of ion exchange membrane permselectivity: Significance of convection and Manning’s counter-ion condensation theory. J. Memb. Sci. 2021, 620, 118411–118425. [Google Scholar] [CrossRef]
- Salmeron-Sanchez, I.; Asenjo-Pascual, J.; Avilés-Moreno, J.R.; Pérez-Flores, J.C.; Mauleón, P.; Ocón, P. Chemical physics insight of PPy-based modified ion exchange membranes: A fundamental approach. J. Memb. Sci. 2022, 643, 120020–120033. [Google Scholar] [CrossRef]
- Salmeron-Sanchez, I.; Asenjo-Pascual, J.; Avilés-Moreno, J.R.; Ocón, P. Microstructural description of ion exchange membranes: The effect of PPy-based modification. J. Memb. Sci. 2022, 659, 120771–120783. [Google Scholar] [CrossRef]
- Akberova, E.M.; Vasil’eva, V.I.; Zabolotsky, V.I.; Novak, L. A study of ralex membrane morphology by SEM. Membranes 2019, 9, 169. [Google Scholar] [CrossRef]
- Sarapulova, V.; Shkorkina, I.; Mareev, S.; Pismenskaya, N.; Kononenko, N.; Larchet, C.; Dammak, L.; Nikonenko, V. Transport characteristics of fujifilm ion-exchange membranes as compared to homogeneous membranes AMX and CMX and to heterogeneous membranes MK-40 and MA-41. Membranes 2019, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Sata, T. Studies on anion exchange membranes having permselectivity for specific anions in electrodialysis—Effect of hydrophilicity of anion exchange membranes on permselectivity of anions. J. Memb. Sci. 2000, 167, 1–31. [Google Scholar] [CrossRef]
- Kong, L.; Palacios, E.; Guan, X.; Shen, M.; Liu, X. Mechanisms for enhanced transport selectivity of like-charged ions in hydrophobic-polymer-modified ion-exchange membranes. J. Membr. Sci. 2022, 658, 120645. [Google Scholar] [CrossRef]
- Zabolotsky, V.I.; Nikonenko, V.V. Effect of structural membrane inhomogeneity on transport properties. J. Membr. Sci. 1993, 79, 181–198. [Google Scholar] [CrossRef]
- Slavinskaya, G.V.; Kurenkova, O.V. On the multifunctional character of strong basic anion-exchange resin. Sorp. Chromatograph. Proc. 2019, 19, 101–110. [Google Scholar] [CrossRef]
- Lteif, R.; Dammak, L.; Larchet, C.; Auclair, B. Conductivité électrique membranaire: Étude de l’effet de la concentration, de la nature de l’electrolyte et de la structure membranaire. Eur. Polym. J. 1999, 35, 1187–1195. [Google Scholar] [CrossRef]
- Berezina, N.P.; Kononenko, N.A.; Dyomina, O.A.; Gnusin, N.P. Characterization of ion-exchange membrane materials: Properties vs. structure. Adv. Colloid Interfac. 2008, 139, 3–28. [Google Scholar] [CrossRef] [PubMed]
- Trchova, M.; Stejskal, J. Resonance raman spectroscopy of conducting polypyrrole nanotubes: Disordered surface versus ordered body. J. Phys. Chem. A 2018, 122, 9298–9306. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Wang, Y.; Liu, P. Polypyrrole nanoparticles with high dispersion stability via chemical oxidative polymerization in presence of an anionic-non-ionic bifunctional polymeric surfactant. Powder Technol. 2012, 217, 134–139. [Google Scholar] [CrossRef]
- Stejskal, J.; Trchová, M.; Bober, P.; Morávková, Z.; Kopecký, D.; Vrňata, M.; Prokeš, J.; Varga, M.; Watzlová, E. Polypyrrole salts and bases: Superior conductivity of nanotubes and their stability towards the loss of conductivity by deprotonation. RSC Adv. 2016, 6, 88382–88391. [Google Scholar] [CrossRef]
- Ge, H.; Wallace, G.G. Ion exchange properties of polypyrrole. React. Polym. 1992, 18, 133–140. [Google Scholar] [CrossRef]
- Lopez Cascales, J.J.; Otero, T.F. Molecular dynamic simulation of the hydration and diffusion of chloride ions from bulk water to polypyrrole matrix. J. Chem. Phys. 2004, 120, 1951–1957. [Google Scholar] [CrossRef]
- Chalmers, E.; Lee, H.; Zhu, C.; Liu, X. Increasing the conductivity and adhesion of polypyrrole hydrogels with electropolymerized polydopamine. Chem. Mater. 2020, 32, 234–244. [Google Scholar] [CrossRef]
- Neoh, K.G.; Young, T.T.; Kang, E.T.; Tan, K.L. Structural and mechanical degradation of polypyrrole films due to aqueous media and heat treatment and the subsequent redoping characteristics. J. Appl. Polym. Sci. 1997, 64, 519–526. [Google Scholar] [CrossRef]
- Xie, H.; Yan, M.; Jiang, Z. Transition of polypyrrole from electroactive to electroinactive state investigated by use of in situ FTIR spectroscopy. Electrochim. Acta 1997, 42, 2361–2367. [Google Scholar] [CrossRef]
- Kozmai, A.E.; Nikonenko, V.V.; Zyryanova, S.; Pismenskaya, N.D.; Dammak, L. A simple model for the response of an anion-exchange membrane to variation in concentration and pH of bathing solution. J. Memb. Sci. 2018, 567, 127–138. [Google Scholar] [CrossRef]
- Kozmai, A.E.; Nikonenko, V.V.; Zyryanova, S.; Pismenskaya, N.D.; Dammak, L.; Baklouti, L. Modelling of anion-exchange membrane transport properties with taking into account the change in exchange capacity and swelling when varying bathing solution concentration and pH. J. Memb. Sci. 2019, 590, 117291–117304. [Google Scholar] [CrossRef]
- Gregor, H.P. Gibbs-Donnan equilibria in ion exchange resin systems. J. Am. Chem. Soc. 1951, 73, 642–650. [Google Scholar] [CrossRef]
- Kreuer, K.-D.; Paddison, S.J.; Spohr, E.; Schuster, M. Transport in proton conductors for fuel-cell applications: Simulations, elementary reactions, and phenomenology. Chem. Rev. 2004, 104, 4637–4678. [Google Scholar] [CrossRef]
- Mackie, J.S.; Meares, P. The diffusion of electrolytes in a cation-exchange resin membrane I. Theoretical. Proc. R. Soc. Lond. A 1955, 232, 498–509. [Google Scholar] [CrossRef]
- Gierke, T.D.; Munn, G.E.; Wilson, F.C. The morphology in nafion perfluorinated membrane products, as determined by wide- and small-angle x-ray studies. J. Polym. Sci. Polym. Phys. Ed. 1981, 19, 1687–1704. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Moore, R.B. State of understanding of Nafion. Chem. Rev. 2004, 104, 4535–4586. [Google Scholar] [CrossRef]
- Porozhnyy, M.; Huguet, P.; Cretin, M.; Safronova, E.; Nikonenko, V. Mathematical modeling of transport properties of proton-exchange membranes containing immobilized nanoparticles. Int. J. Hydrog. Energy 2016, 41, 15605–15614. [Google Scholar] [CrossRef]
- Simons, R. Strong electric field effects on proton transfer between membrane-bound amines and water. Nature 1979, 280, 824–826. [Google Scholar] [CrossRef]
- Tanaka, Y. Ion Exchange Membranes. Fundamentals and Applications, 2nd ed.; Chapter 7—Water dissociation; Elsevier: Amsterdam, The Netherlands, 2015; pp. 123–159. [Google Scholar] [CrossRef]
- Nikonenko, V.V.; Pismenskaya, N.D.; Belova, E.I.; Sistat, P.; Huguet, P.; Pourcelly, G.; Larchet, C. Intensive current transfer in membrane systems: Modelling, mechanisms and application in electrodialysis. Adv. Colloid Interface Sci. 2010, 160, 101–123. [Google Scholar] [CrossRef] [PubMed]
- Vasil’eva, V.I.; Pismenskaya, N.D.; Akberova, E.M.; Nebavskaya, K.A. Effect of thermochemical treatment on the surface morphology and hydrophobicity of heterogeneous ion-exchange membranes. Russ. J. Phys. Chem. 2014, 88, 1293–1299. [Google Scholar] [CrossRef]
- Vasil’eva, V.I.; Kranina, N.A.; Malykhin, M.D.; Akberova, E.M.; Zhiltsova, A.V. The surface inhomogeneity of ion-exchange membranes by SEM and AFM data. J. Surf. Investig. X-Ray Synchrotron Neutron Tech. 2013, 7, 144–153. [Google Scholar] [CrossRef]
- Kononenko, N.; Nikonenko, V.; Grande, D.; Larchet, C.; Dammak, L.; Fomenko, M.; Volfkovich, Y. Porous structure of ion exchange membranes investigated by various techniques. Adv. Colloid Interface Sci. 2017, 246, 196–216. [Google Scholar] [CrossRef]
- Robinson, R.A.; Stokes, R.H. Electrolyte Solutions, 2nd ed.; Butterworths Scientific Publications: London, UK, 1959; pp. 1–644. [Google Scholar]
- Korolev, V.P. Hydration numbers of Na+ and Cl− ions in an aqueous urea solution. J. Struct. Chem. 2011, 52, 737–742. [Google Scholar] [CrossRef]
- Larchet, C.; Dammak, L.; Auclair, B.; Parchikov, S.; Nikonenko, V. A simplified procedure for ion-exchange membrane characterization. New J. Chem. 2004, 28, 1260. [Google Scholar] [CrossRef]
- Drozdov, A.D.; de Claville Christiansen, J. Modeling the effects of pH and ionic strength on swelling of polyelectrolyte gels. J. Chem. Phys. 2015, 142, 114904–114923. [Google Scholar] [CrossRef]
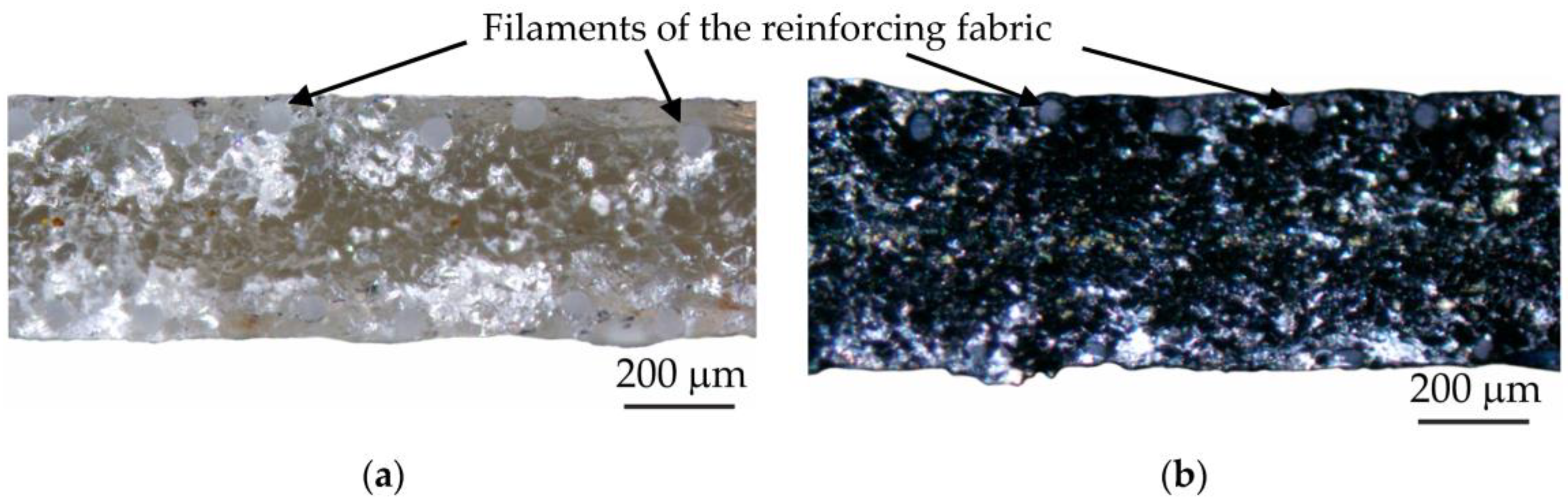
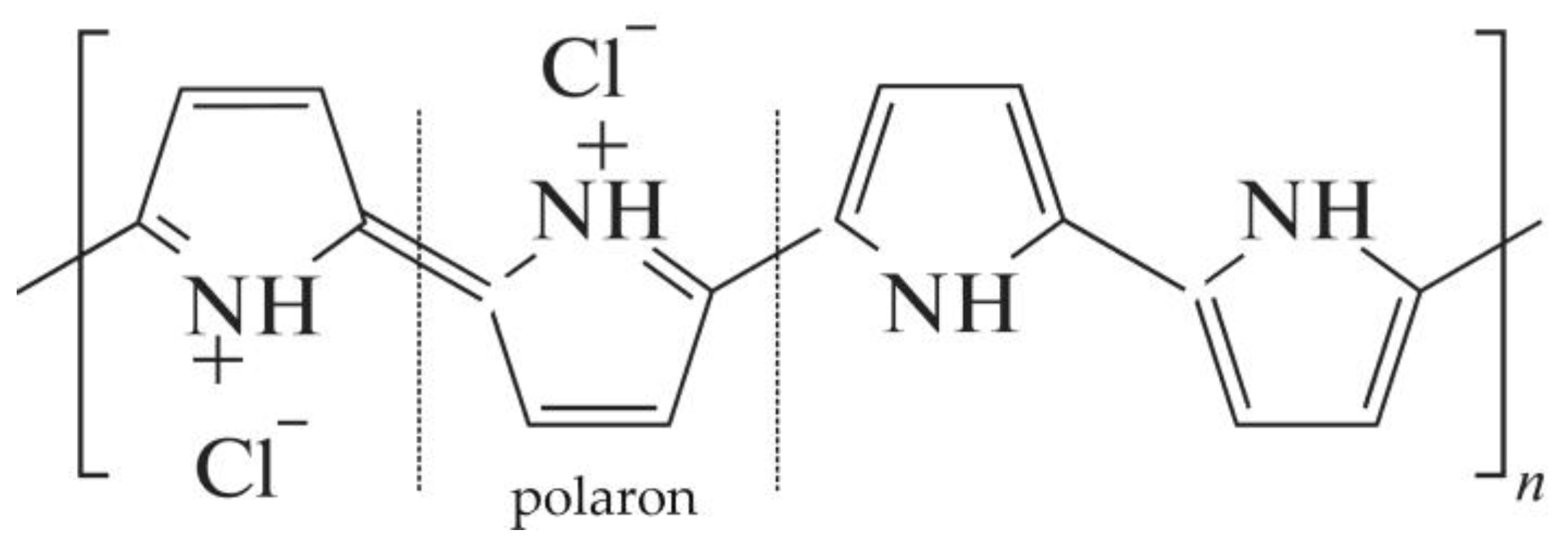
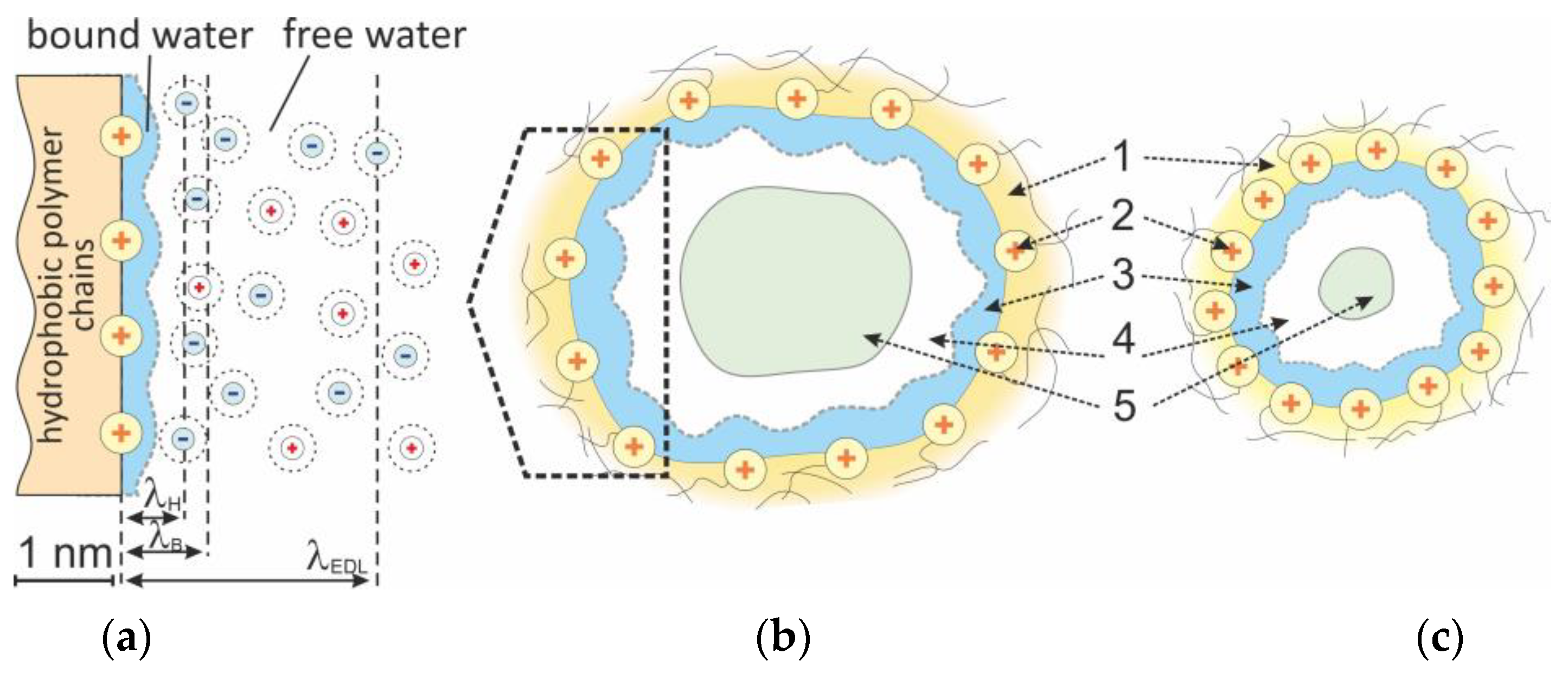


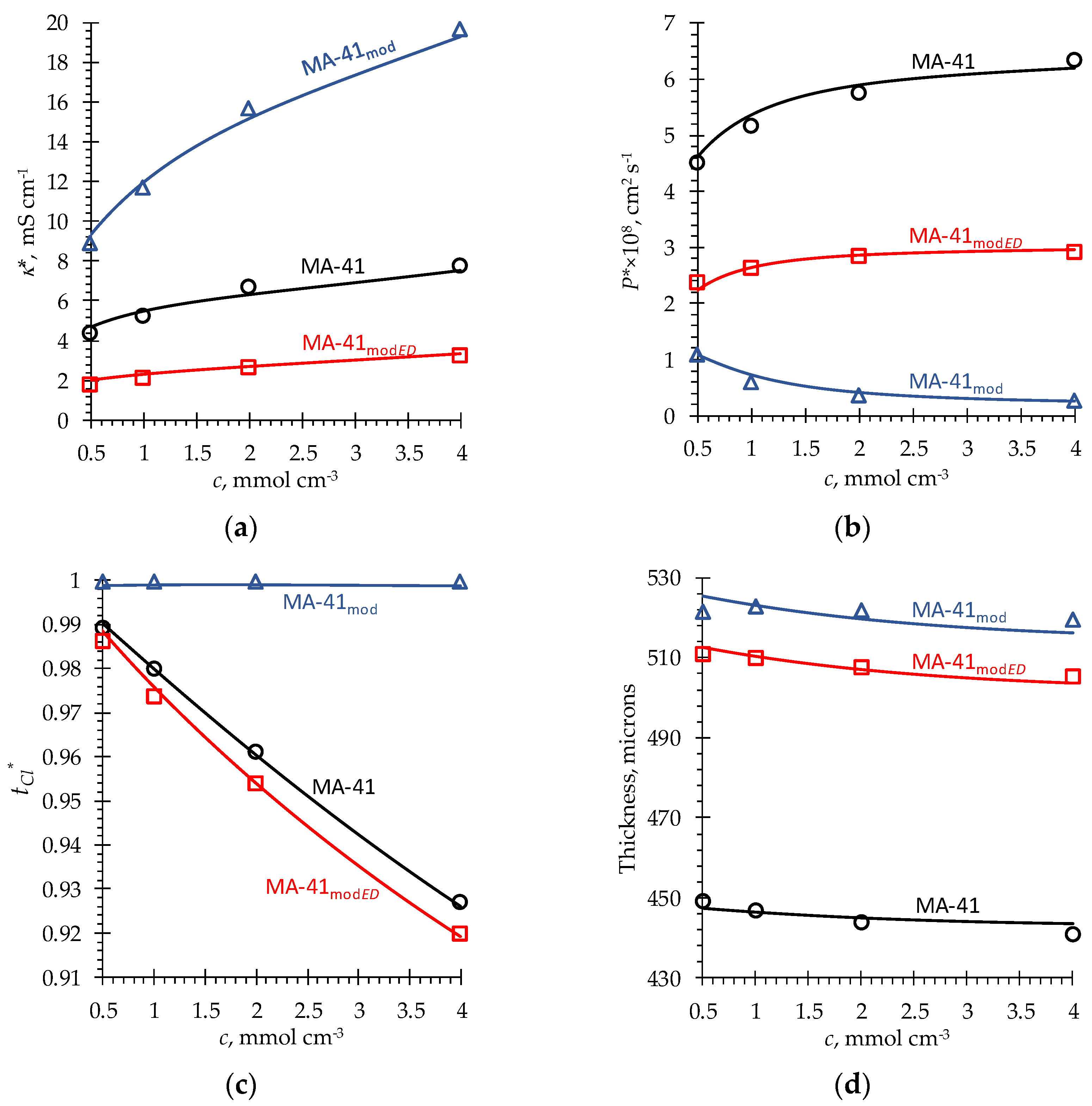
| Parameter | MA-41 | MA-41mod | MA-41modED | PPy | PPyED | Description |
|---|---|---|---|---|---|---|
| Thermodynamic parameters | ||||||
| Q, mmol/cm3 swollen membrane | 1.47 | 1.47 | 1.47 | 2.0 | 0.05 | Membrane exchange capacity |
| KD | 0.035 | 0.09 | 0.05 | 0.01 | 0.10 | Donnan equilibrium constant |
| Structural parameters | ||||||
| fmacro | 0.10 | 0.10 | 0.10 | 0.83 | 0.40 | Volume fraction of macropores |
| 0.15 | 0.42 | 0.23 | −0.28 | 0.08 | Parameter of the membrane structure | |
| , cm3/mmol | 0.48 | 0.48 | 0.48 | 0.03 | 0.03 | Volume of dry polyelectrolyte gel containing 1 mmol of fixed functional groups with counterions |
| hR | 1.7 | Hydration number of membrane fixed groups | ||||
| hCl | 0.5 | Hydration number of Cl− in the membrane pore solution | ||||
| hNa | 1.5 | Hydration number of Na+ in the membrane pore solution | ||||
| Kinetic parameters | ||||||
| , cm2/s | 3.4 × 10−7 | 5.8 × 10−7 | 2.0 × 10−7 | 5.4 × 10−7 | 1.1 × 10−7 | The values of Cl− and Na+ diffusion coefficients in the gel phase at c = 1 M NaCl, Equation (24) |
| , cm2/s | 6.0 × 10−8 | 3.5 × 10−9 | 3.2 × 10−8 | 6.4 × 10−10 | 4.6 × 10−8 | |
| Mechanical parameter | ||||||
| E, bar | 3800 | 2000 | 2000 | - | - | Young’s modulus |
| Parameter | MA-41 | MA-41mod | MA-41modED | PPy | PPyED | Description |
|---|---|---|---|---|---|---|
| f2meso | 0.1 | 0.12 | 0.14 | 0.12 | 0.14 | The volume fraction of the electroneutral solution localized in the membrane mesopores, Equation (20) |
| f2 | 0.2 | 0.24 | 0.24 | 0.95 | 0.55 | The volume fraction of the intergel spaces, Equation (19) |
| qwb | 2.2 | 3.0 | 2.4 | - | - | The amount of bound water in the membrane |
| qw | 6.0 | 9.7 | 9.0 | - | - | The amount of free water in the membrane |
| ε | 0.088 | 0.120 | 0.118 | - | - | Relative elongation, Equation (10) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozmai, A.; Porozhnyy, M.; Ruleva, V.; Gorobchenko, A.; Pismenskaya, N.; Nikonenko, V. Is It Possible to Prepare a “Super” Anion-Exchange Membrane by a Polypyrrole-Based Modification? Membranes 2023, 13, 103. https://doi.org/10.3390/membranes13010103
Kozmai A, Porozhnyy M, Ruleva V, Gorobchenko A, Pismenskaya N, Nikonenko V. Is It Possible to Prepare a “Super” Anion-Exchange Membrane by a Polypyrrole-Based Modification? Membranes. 2023; 13(1):103. https://doi.org/10.3390/membranes13010103
Chicago/Turabian StyleKozmai, Anton, Mikhail Porozhnyy, Valentina Ruleva, Andrey Gorobchenko, Natalia Pismenskaya, and Victor Nikonenko. 2023. "Is It Possible to Prepare a “Super” Anion-Exchange Membrane by a Polypyrrole-Based Modification?" Membranes 13, no. 1: 103. https://doi.org/10.3390/membranes13010103
APA StyleKozmai, A., Porozhnyy, M., Ruleva, V., Gorobchenko, A., Pismenskaya, N., & Nikonenko, V. (2023). Is It Possible to Prepare a “Super” Anion-Exchange Membrane by a Polypyrrole-Based Modification? Membranes, 13(1), 103. https://doi.org/10.3390/membranes13010103









