Design of an Antibiotic-Releasing Polymer: Physicochemical Characterization and Drug Release Patterns
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sulfonation of PEEK and Membrane Casting
2.3. Degree of Sulfonation (DS)
2.3.1. Confirmation of Polymer Sulfonation
2.3.2. Determination of the Degree of Sulfonation
2.4. Membrane Characterization
2.5. Solubility and Water Uptake Studies
2.6. Physicochemical Studies
2.6.1. Distribution, Diffusion, and Permeability Coefficients
2.6.2. Drug Release Kinetics
2.7. In Vitro Biocompatibility Studies
2.8. Statistical Analysis
3. Results and Discussion
3.1. Degree of Sulfonation of SPEEK
3.1.1. Reaction Optimization and Confirmation of Sulfonation
3.1.2. EDS Analysis to Confirm the Sulfonation of the Polymer
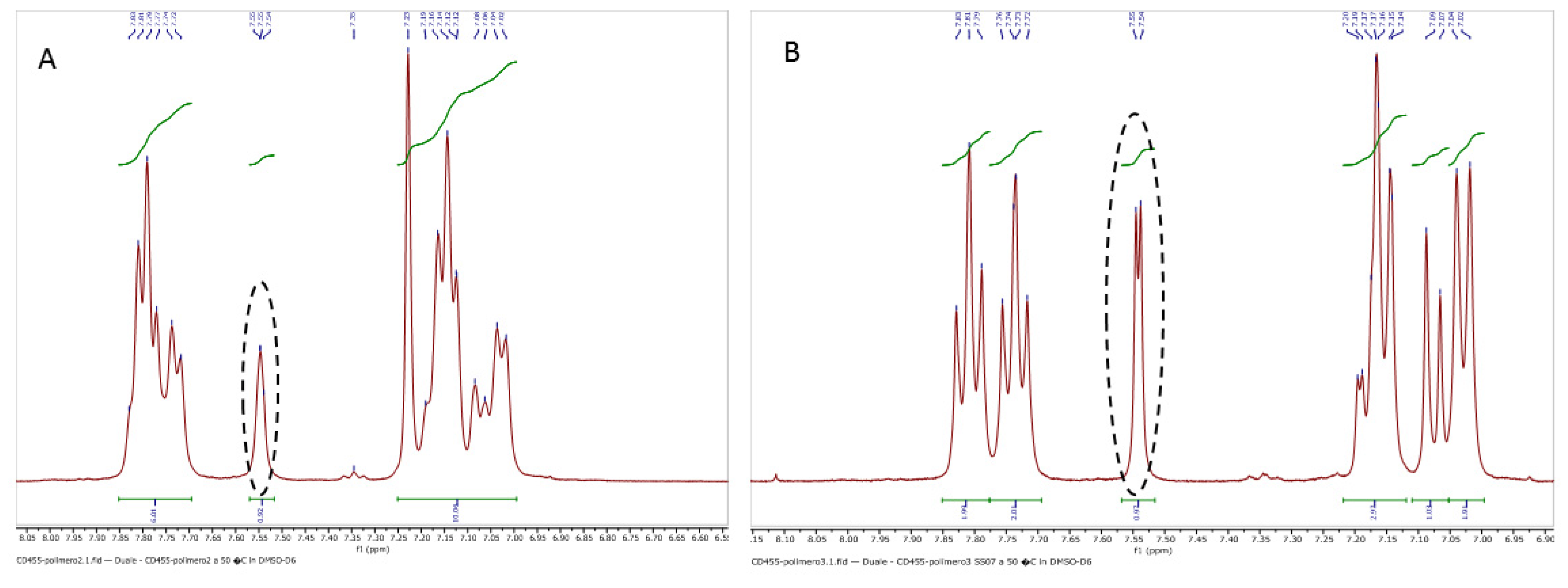
3.1.3. Determination of the Degree of Sulfonation by NMR Spectroscopy
3.2. Casting of the SPEEK Membrane and Its Characterization
3.2.1. Mechanical Characterization
3.2.2. FT-IR Spectra of the SPEEK Membrane
3.2.3. Water Uptake Studies
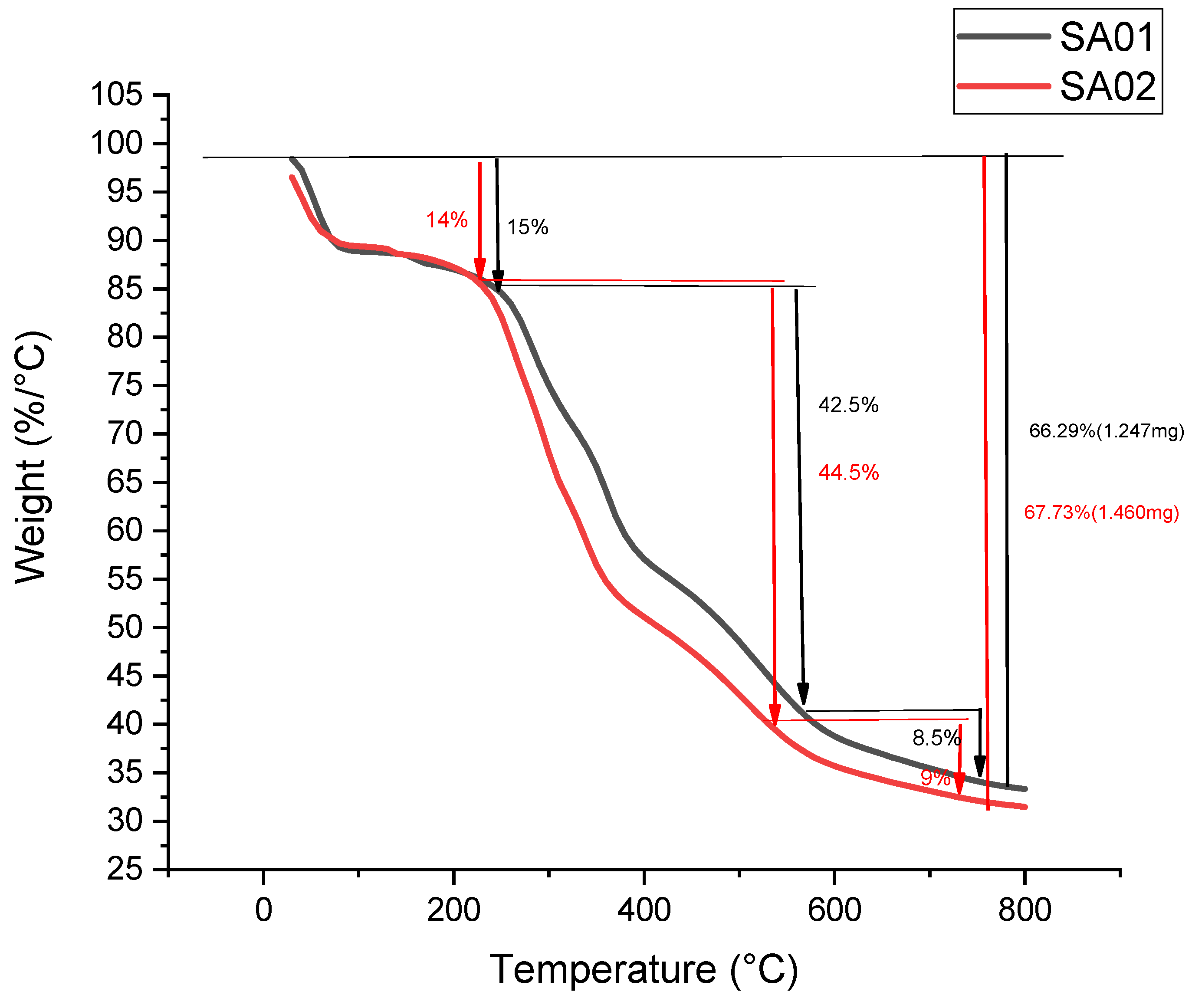
3.2.4. Thermogravimetric Analysis of the Membrane
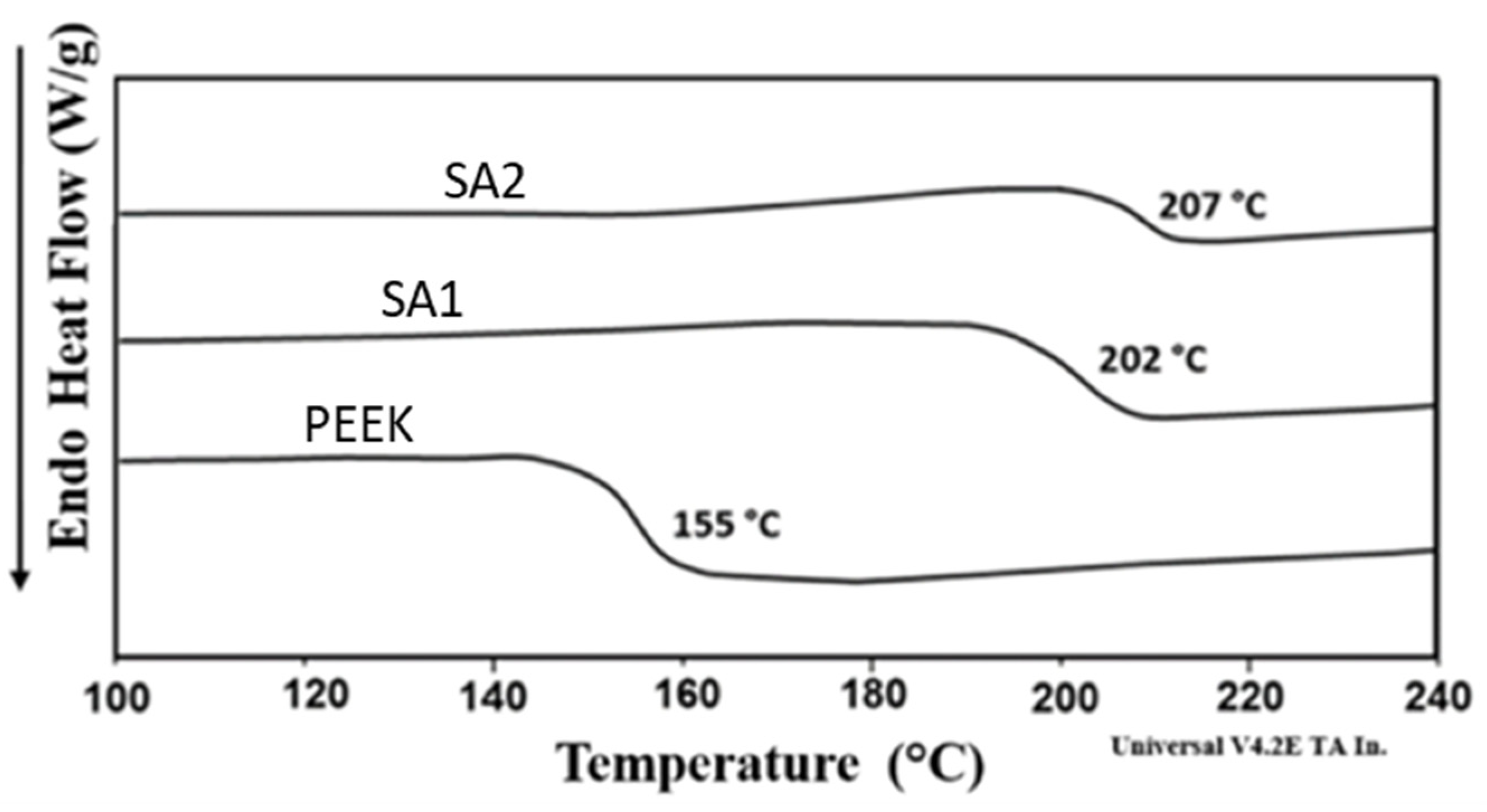
3.2.5. DSC Analysis of Membrane
3.2.6. SEM Images of the Drug-Loaded SPEEK Membranes
3.3. Physicochemical Studies of the Drug-Loaded SPEEK Membrane
3.3.1. Distribution, Diffusion, and Permeability Coefficients
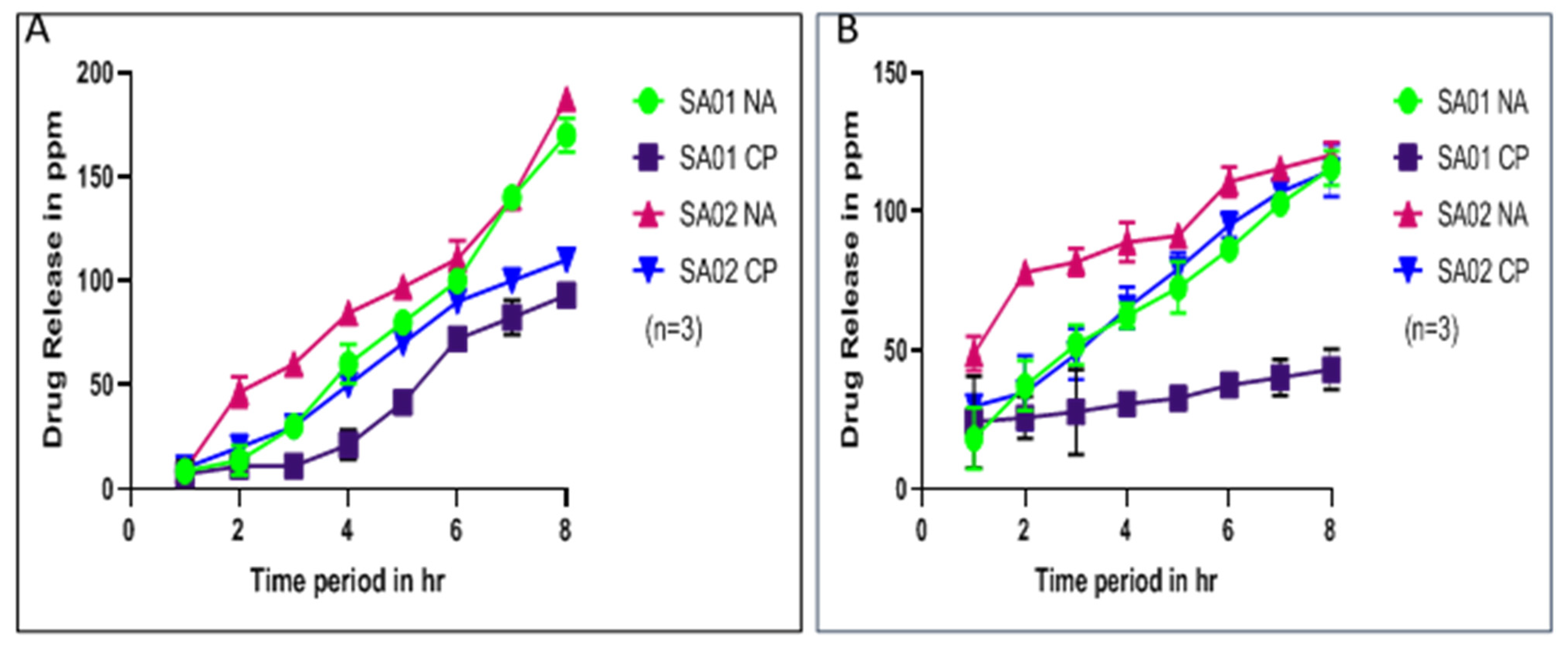
3.3.2. Drug Release Kinetics
3.4. Mathematical Models of Drug Release
3.5. In Vitro Cytotoxicity Studies of the SPEEK Membrane
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug Delivery Systems and Materials for Wound Healing Applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human Skin Wounds: A Major and Snowballing Threat to Public Health and the Economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Posnett, J.; Gottrup, F.; Lundgren, H.; Saal, G. The Resource Impact of Wounds on Health-Care Providers in Europe. J. Wound Care 2009, 18, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.A.; Rhodes, C.T.; Shah, N.H.; Malick, A.W. Controlled-Release Drug Delivery Systems for Prolonged Gastric Residence: An Overview. Drug Dev. Ind. Pharm. 2008, 22, 531–539. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound Healing Dressings and Drug Delivery Systems: A Review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Kim, H.S.; Sun, X.; Lee, J.H.; Kim, H.W.; Fu, X.; Leong, K.W. Advanced Drug Delivery Systems and Artificial Skin Grafts for Skin Wound Healing. Adv. Drug Deliv. Rev. 2019, 146, 209–239. [Google Scholar] [CrossRef]
- Mirani, B.; Pagan, E.; Currie, B.; Siddiqui, M.A.; Hosseinzadeh, R.; Mostafalu, P.; Zhang, Y.S.; Ghahary, A.; Akbari, M. An Advanced Multifunctional Hydrogel-Based Dressing for Wound Monitoring and Drug Delivery. Adv. Healthc. Mater. 2017, 6, 1700718. [Google Scholar] [CrossRef]
- Yee, R.S.L.; Zhang, K.; Ladewig, B.P. The Effects of Sulfonated Poly (Ether Ether Ketone) Ion Exchange Preparation Conditions on Membrane Properties. Membranes 2013, 3, 182–195. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef]
- Shanmuga Sundar, S.; Sangeetha, D. Investigation on Sulphonated PEEK Beads for Drug Delivery, Bioactivity and Tissue Engineering Applications. J. Mater. Sci. 2012, 47, 2736–2742. [Google Scholar] [CrossRef]
- Ashour, S.; Kattan, N. Simultaneous Determination of Miconazole Nitrate and Metronidazole in Different Pharmaceutical Dosage Forms by Gas Chromatography and Flame Ionization Detector (GC-FID). Int. J. Biomed. Sci. 2010, 6, 13. [Google Scholar] [PubMed] [PubMed Central]
- Arul Joseph Helen Therese, J.B.; Selvakumar, K.; Gayathri, R.; Ramesh Prabhu, M.; Sivakumar, P. In Situ Polymerization of Poly Aniline—SPEEK-PMA-Based Proton Exchange Membrane for DMFC Application. J. Thermoplast. Compos. Mater. 2019, 34, 221–237. [Google Scholar] [CrossRef]
- Aravind, K.; Sangeetha, D. Characterization and in vitro studies of sulfonated polyether ether ketone/polyether sulfone/nano hydroxyapatite composite. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 220–227. [Google Scholar] [CrossRef]
- Rambabu, G.; Bhat, S.D. Sulfonated Fullerene in SPEEK Matrix and Its Impact on the Membrane Electrolyte Properties in Direct Methanol Fuel Cells. Electrochim. Acta 2015, 176, 657–669. [Google Scholar] [CrossRef]
- Charcosset, C.; Bernard, S.; Fiaty, K.; Bechelany, M.; Cornu, D. Membrane techniques for the preparation of nanomaterials: Nanotubes, nanowires and nanoparticles: A review. Dyn. Biochem. Process Biotechnol. Mol. Biology. Glob. Sci. Books 2007, 1, 15–23. [Google Scholar]
- Liu, X.; He, S.; Shi, Z.; Zhang, L.; Lin, J. Effect of Residual Casting Solvent Content on the Structure and Properties of Sulfonated Poly (Ether Ether Ketone) Membranes. J. Membr. Sci. 2015, 492, 48–57. [Google Scholar] [CrossRef]
- Palacio, D.A.; Rivas, B.L.; Vega, C.M. Effect of Solvent Behavior of Nalidixic Acid by Ultraviolet Spectroscopy. J. Chil. Chem. Soc. 2020, 65, 4885–4887. [Google Scholar]
- Baladhandapania, A.; Suresh, S.; Sindhu, S.; Ramani, P. Physico-Chemical Studies of Amoxycillin Loaded Sulfonated Polymer. Mater. Today Proc. 2018, 5, 16146–16151. [Google Scholar] [CrossRef]
- Adiranan Smarandache, R.L.P. UV-VIS and FTIR Spectroscopic Investigations of Gamma-Ray Irradiated Antibiotics. Rom. Rep. Phys. 2017, 70, 602. [Google Scholar]
- Huang, R.Y.M.; Shao, P.; Burns, C.M.; Feng, X. Sulfonation of Poly (Ether Ether Ketone) (PEEK): Kinetic Study and Characterization. J. Appl. Polym. Sci. 2001, 82, 2651–2660. [Google Scholar] [CrossRef]
- Long, Y.; Zhao, X.; Liu, S.; Chen, M.; Liu, B.; Ge, J.; Jia, Y.-G.; Ren, L. Collagen–Hydroxypropyl Methylcellulose Membranes for Corneal Regeneration. ACS Omega 2018, 3, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Li, Q.; Wang, L.; Zhang, X.; Fang, L.; Luo, Z.; Xue, B.; Ma, L. Mechanical Properties and in Vivo Study of Modified-Hydroxyapatite/Polyetheretherketone Biocomposites. Mater. Sci. Eng. C 2017, 73, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Reyna-Valencia, A.; Kaliaguine, S.; Bousmina, M. Tensile Mechanical Properties of Sulfonated Poly (Ether Ether Ketone) (SPEEK) and BPO4/SPEEK Membranes. J. Appl. Polym. Sci. 2005, 98, 2380–2393. [Google Scholar] [CrossRef]
- Indong, E.S.E.; Sekak, K.A. Electrospun Strontium Cross-Linked Sulfonated Poly (Ether Ether Ketone) Speek Nanofiber for Proton Exchange Membrane Fuel Cell. J. Teknol. 2020, 82, 65–70. [Google Scholar] [CrossRef]
- Wang, W.; Luo, C.J.; Huang, J.; Edirisinghe, M. PEEK Surface Modification by Fast Ambient-Temperature Sulfonation for Bone Implant Applications. J. R. Soc. Interface 2019, 16, 20180955. [Google Scholar] [CrossRef] [PubMed]
- Behnecke, M.; Petersen, S.; Behnecke, M.; Petersen, S. Establishment of PEEK-Associated Drug Delivery Systems—Limits and Perspectives. J. Mater. Sci. Chem. Eng. 2020, 8, 32–41. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic Modeling on Drug Release from Controlled Drug Delivery Systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar]
- Bruschi, M.L. Strategies to Modify the Drug Release from Pharmaceutical Systems. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Sawston, UK, 2015; pp. 1–199. [Google Scholar] [CrossRef]
- Patel, H.A.; Somani, R.S.; Bajaj, H.C.; Jasra, R.V. Nanoclays for polymer nanocomposites, paints, inks, greases and cosmetics formulations, drug delivery vehicle and waste water treatment. Bull. Mater. Sci. 2006, 29, 133–145. [Google Scholar] [CrossRef]
- Harting, R.; Barth, M.; Bührke, T.; Pfefferle, R.S.; Petersen, S. Functionalization of polyethetherketone for application in dentistry and orthopedics. BioNanoMaterials 2017, 18, 1–2. [Google Scholar] [CrossRef]
- Mohandas, A.; Nimal, T.R.; Das, V.; Shankarappa, S.A.; Biswas, R.; Jayakumar, R. Drug Loaded Bi-Layered Sponge for Wound Management in Hyperfibrinolytic Conditions. J. Mater. Chem. B 2015, 3, 5795–5805. [Google Scholar] [CrossRef]
- Thattaruparambil Raveendran, N.; Mohandas, A.; Ramachandran Menon, R.; Somasekharan Menon, A.; Biswas, R.; Jayakumar, R. Ciprofloxacin-and Fluconazole-Containing Fibrin-Nanoparticle-Incorporated Chitosan Bandages for the Treatment of Polymicrobial Wound Infections. ACS Appl. Bio. Mater. 2019, 2, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Siepmann, F. Mathematical Modeling of Drug Delivery. Int. J. Pharm. 2008, 364, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhao, S.; Wang, Z.; Tian, X.; Shi, M.; Wang, J.; Wang, S. Improved Performance of Polyamide Thin-Film Composite Nanofiltration Membrane by Using Polyetersulfone/Polyaniline Membrane as the Substrate. J. Membr. Sci. 2015, 493, 263–274. [Google Scholar] [CrossRef]
- Ambika, A.P.; Nair, S.N. Wound Healing Activity of Plants from the Convolvulaceae Family. Adv. Wound Care 2019, 8, 28–37. [Google Scholar] [CrossRef]
- Ma, R.; Tang, T. Current Strategies to Improve the Bioactivity of PEEK. Int. J. Mol. Sci. 2014, 15, 5426–5445. [Google Scholar] [CrossRef]




| Temperature (°C) | Time of Reaction (in Hours) | Yield (%) | Dissolution Time (in Hours) |
|---|---|---|---|
| 40 | 1.5 | 73.33 ± 4.6 | 12 |
| 40 | 8 | 65.7 ± 3.5 | 4 |
| 50 | 1.5 | 59.2 ± 2.1 | 9 |
| 50 | 8 | 53.7 ± 1.4 | 3 |
| Sample Name | C (%) | S (%) | H (%) | O (%) | Sample Weight (mg) |
|---|---|---|---|---|---|
| PEEK | 78.69 | 0 | 1.53 | 19.79 | 7.96 |
| SA-01 | 61.75 | 3.88 | 4.13 | 30.24 | 6.85 |
| SA-02 | 45.64 | 14.50 | 9.02 | 33.75 | 8.08 |
| Samples | Young’s Modulus (N/sq.mm) | Elongation Factor (%) | Stress Yield (N/sq.mm) | Stain Yield (mm) |
|---|---|---|---|---|
| SA-01 | 88.1 ± 0.87 | 77 | 20.73 ± 0.09 | 62.88 |
| SA-02 | 32.9 ± 1.21 | 17.44 | 4.9 ± 0.1 | 5.33 |
| Samples | DS (%) | Distribution Coefficient | Thickness (mm) | Permeability Coefficient (cm/s) | ||
|---|---|---|---|---|---|---|
| Nalidixic Acid Sodium Salt | Ciprofloxacin | Nalidixic Acid Sodium Salt | Ciprofloxacin | |||
| SA-01 | 61.6 | 0.2469 ± 0.07 | 0.3066 ± 0.05 | 0.088 | 6.5752 ± 1 × 10−6 | 5.4430 ± 3.1 × 10−6 |
| SA-02 | 98.9 | 0.2968 ± 0.06 | 0.2302 ± 0.07 | 0.11 | 5.9829 ± 0.8 × 10−6 | 7.5697 ± 1.7 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padinjarathil, H.; Mudradi, S.; Balasubramanian, R.; Drago, C.; Dattilo, S.; Kothurkar, N.K.; Ramani, P. Design of an Antibiotic-Releasing Polymer: Physicochemical Characterization and Drug Release Patterns. Membranes 2023, 13, 102. https://doi.org/10.3390/membranes13010102
Padinjarathil H, Mudradi S, Balasubramanian R, Drago C, Dattilo S, Kothurkar NK, Ramani P. Design of an Antibiotic-Releasing Polymer: Physicochemical Characterization and Drug Release Patterns. Membranes. 2023; 13(1):102. https://doi.org/10.3390/membranes13010102
Chicago/Turabian StylePadinjarathil, Himabindu, Srikrishna Mudradi, Rajalakshmi Balasubramanian, Carmelo Drago, Sandro Dattilo, Nikhil K. Kothurkar, and Prasanna Ramani. 2023. "Design of an Antibiotic-Releasing Polymer: Physicochemical Characterization and Drug Release Patterns" Membranes 13, no. 1: 102. https://doi.org/10.3390/membranes13010102
APA StylePadinjarathil, H., Mudradi, S., Balasubramanian, R., Drago, C., Dattilo, S., Kothurkar, N. K., & Ramani, P. (2023). Design of an Antibiotic-Releasing Polymer: Physicochemical Characterization and Drug Release Patterns. Membranes, 13(1), 102. https://doi.org/10.3390/membranes13010102






