Membrane Separation Processes and Post-Combustion Carbon Capture: State of the Art and Prospects
Abstract
:1. Introduction
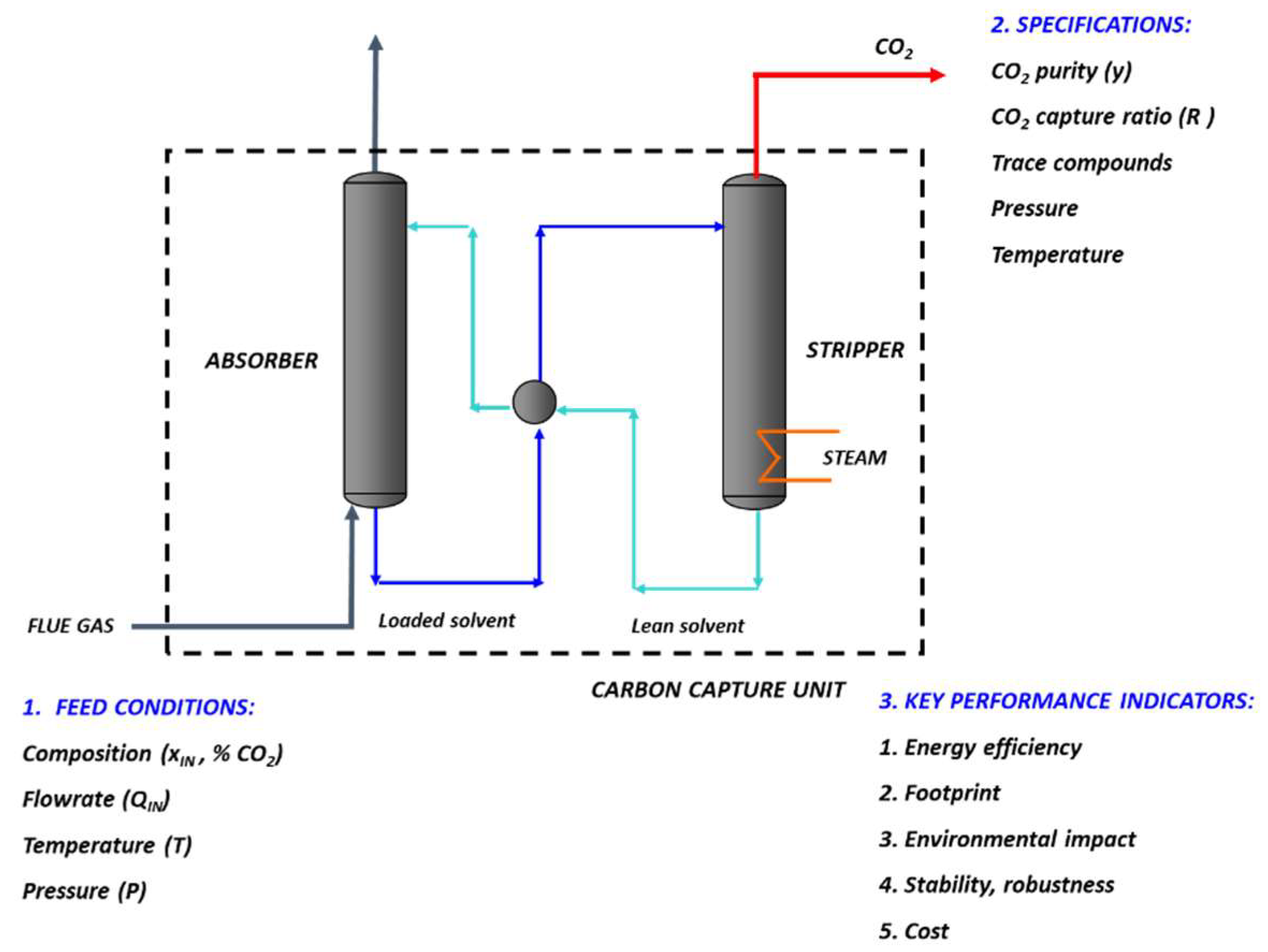
2. Carbon Capture Framework
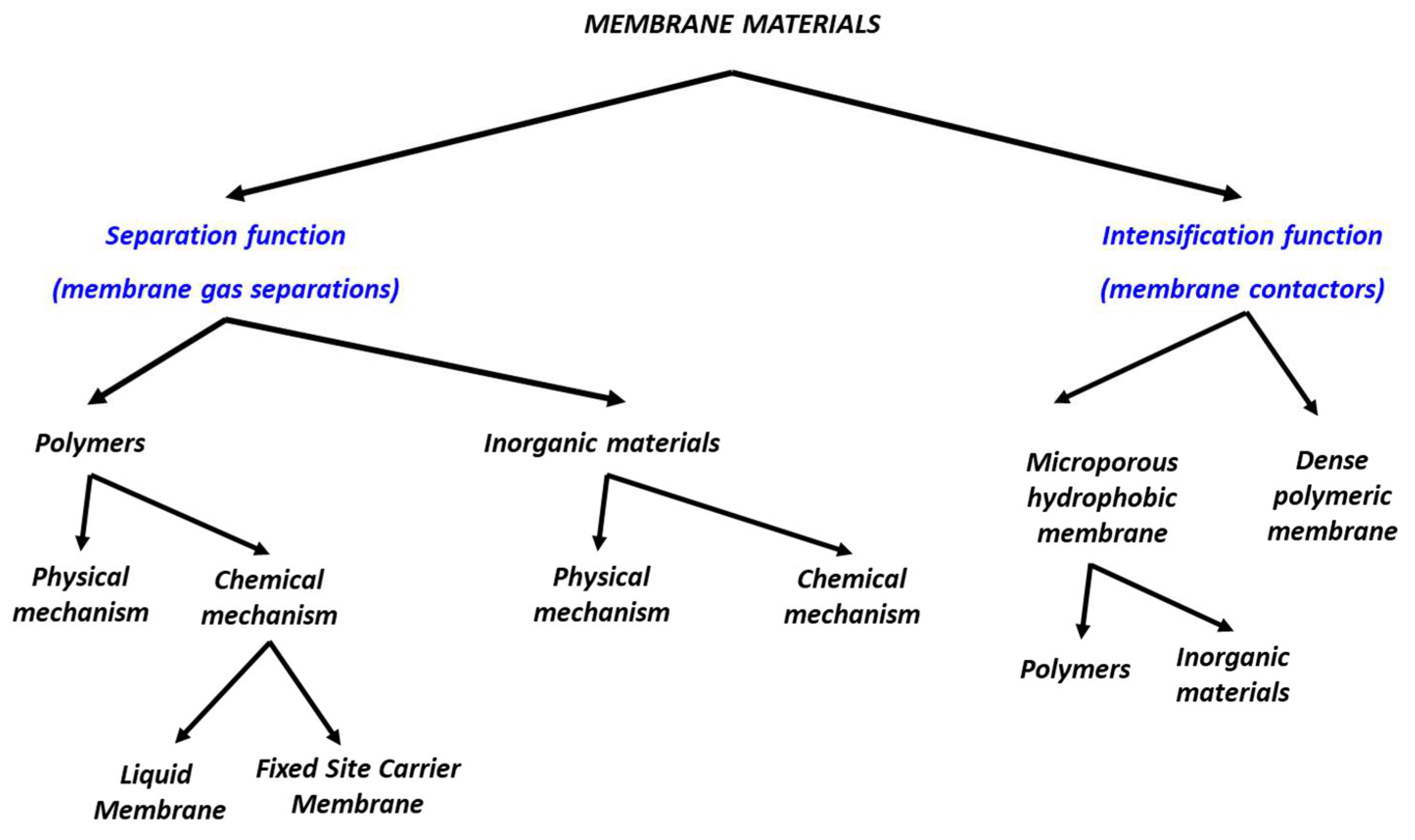
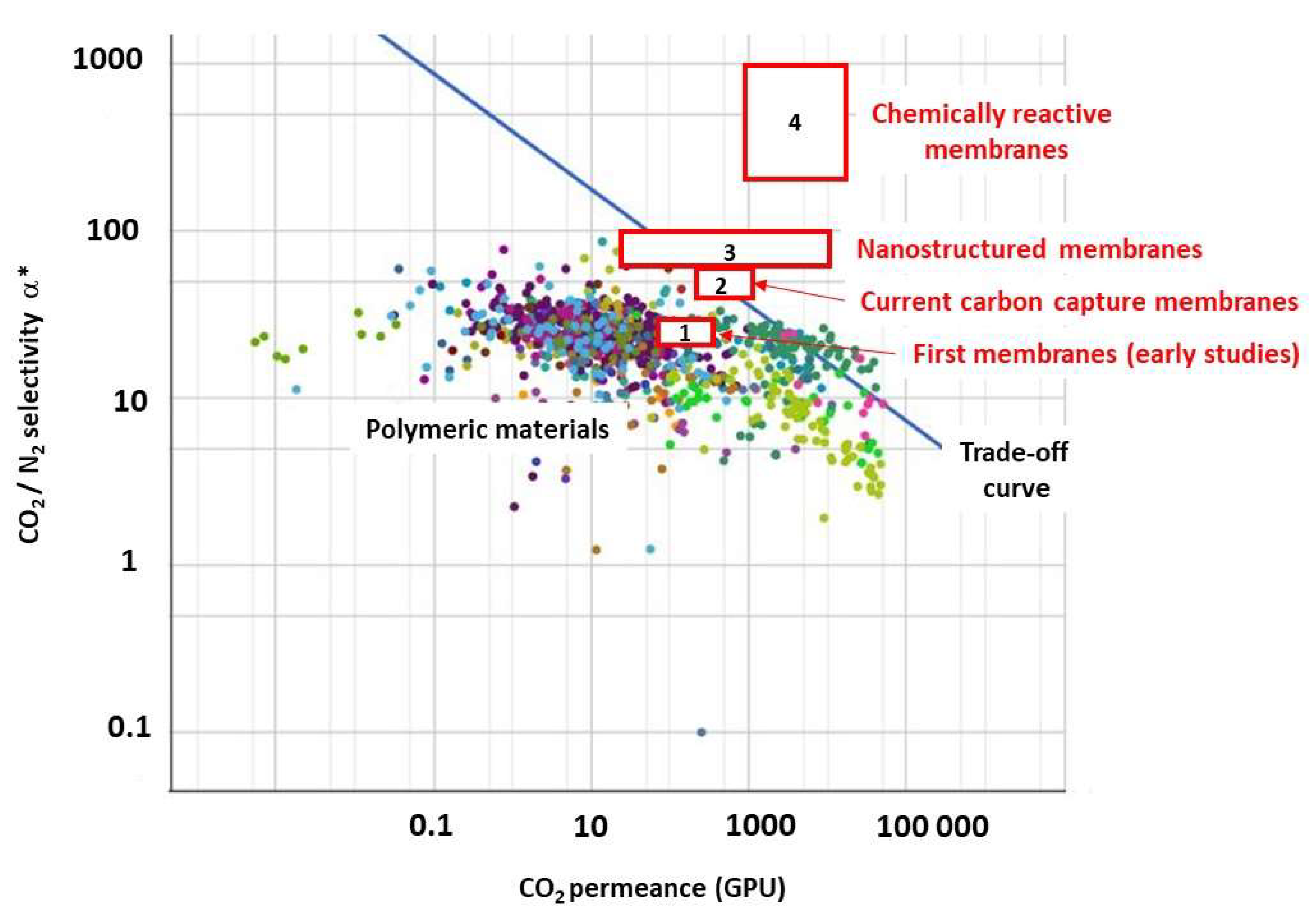
3. Membrane Materials
4. Process Engineering
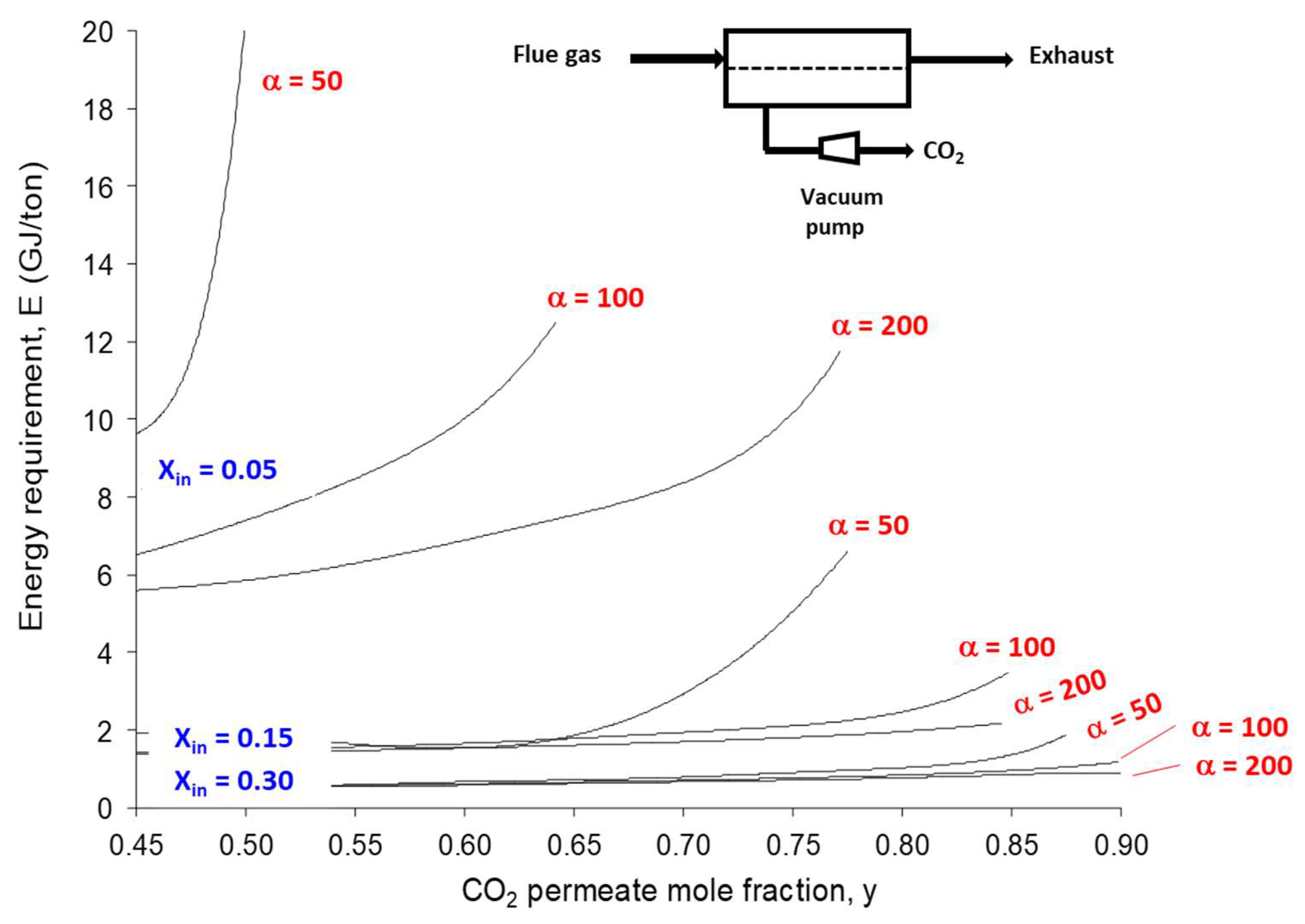
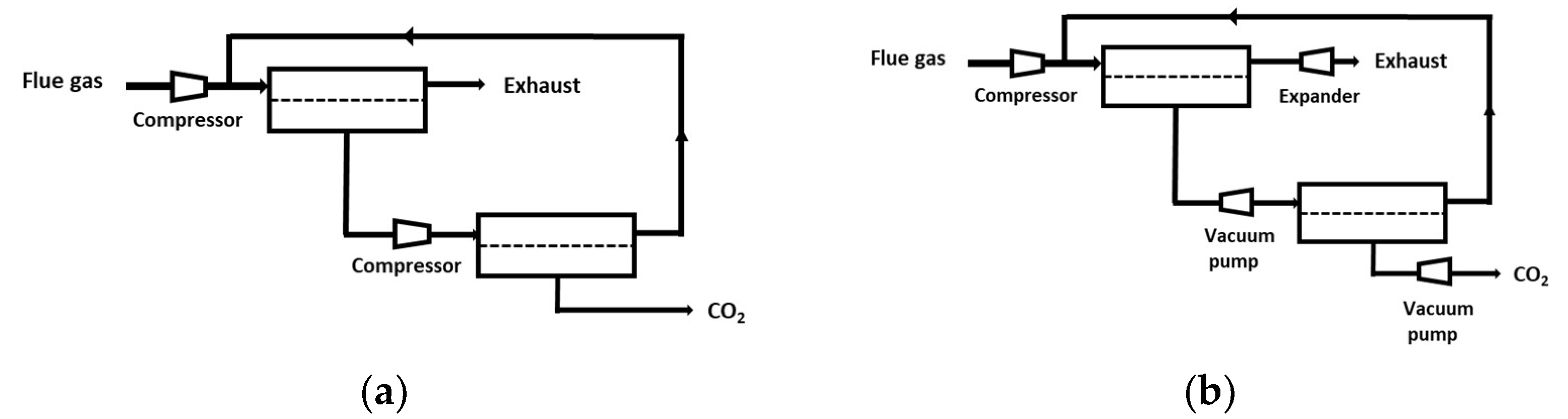
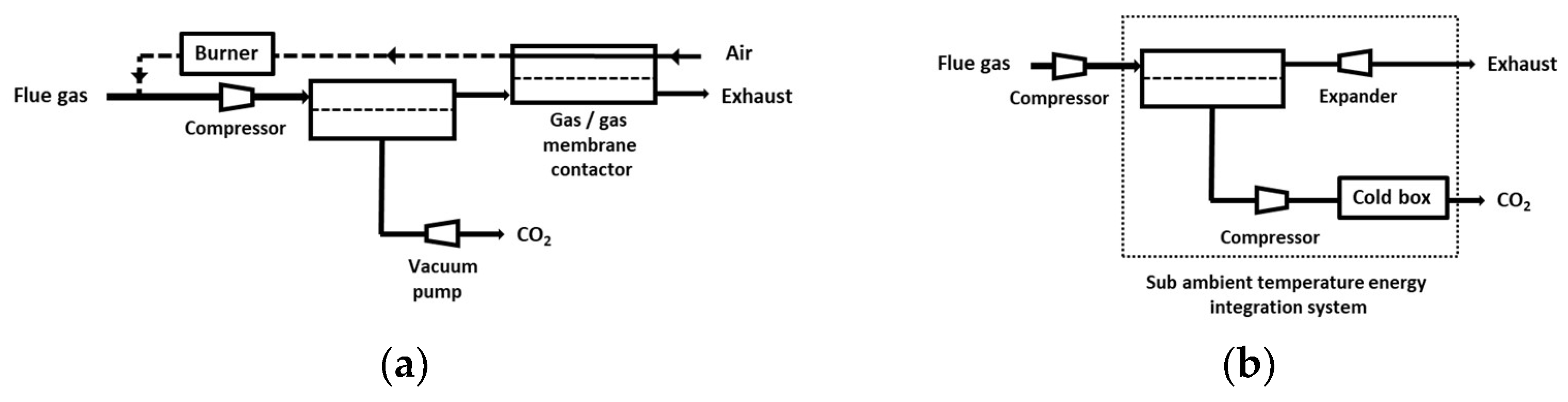
4.1. Single-Stage Performances and Limits
- -
- Based on the currently available membrane materials (e.g., Polaris in Table 2 [19]), a two-stage (or more) process is needed. Alternatively, a hybrid process combing a membrane concentration step and a cryogenic polishing unit can be proposed (e.g., Air Liquide low-temperature Cryocap process [37]).
- -
- Vacuum pumping is usually favored, associated to a moderate feed compression, in order to reach the energy requirement. From an industrial point of view, vacuum operation is most often unwanted, but for carbon capture, this option is almost unavoidable. A moderate vacuum, typically around 10 to 100 mBar, can be operated for large-scale installations based on liquid ring or primary dry pumps, which generate a large footprint area. Lower vacuum levels can be achieved at lab scale, but vacuum pumps energy efficiency often drops for low-pressure levels, and leaks make low pressure very difficult to attain.
- -
- Because of the very high sensitivity of inlet CO2 content on purity and energy, a strategy of partial exhaust CO2 recycling in order to increase inlet CO2 content can be of interest (e.g., MTR process [19]).
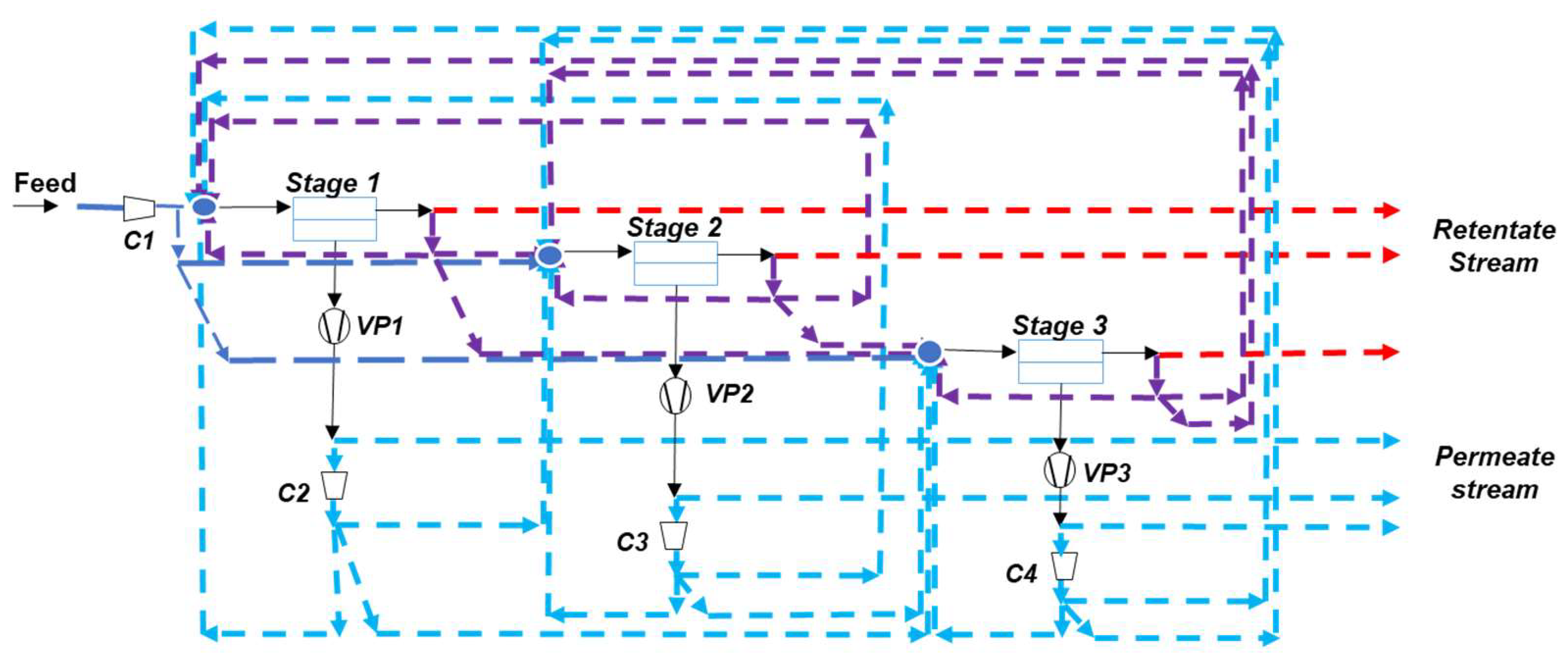
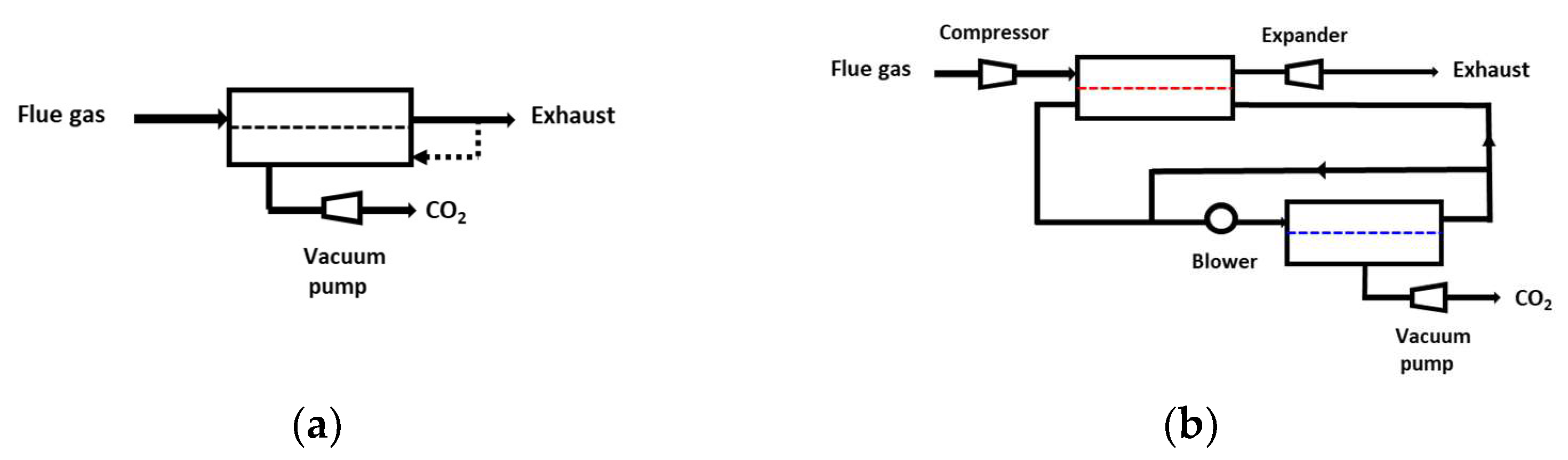
4.2. Multistage Processes
- -
- Two-stage designs, including one or two recycling loops, are usually favored for sake of simplicity (Figure 5). It is interesting to note that the most frequent design of simulation/optimization studies for a coal power plant flue gas, shown on Figure 5b, is similar to the structure of natural gas or biogas upgrading membrane processes [11]. This solution is typical of a purity/recovery constraint when membranes are used [13].
- -
- Vacuum pumping is most often applied, and it is useful for energy requirement decrease constraints, but this translates into low driving forces, that is, a very large membrane surface area.
- -
- Very high membrane permeance levels are needed (mostly due to the previous item).
- -
- The impact of water is most often neglected (a dry inlet mixture is assumed), but it can affect the set of performances [45]. More specifically, chemically reactive membranes require a humid gas in order to enable the chemical reaction to take place on both sides of the membrane [46]. The resulting process simulation problem is tricky (variable permeability, correct computation of water fluxes).
- -
- The same membrane type (i.e., same selectivity and permeance) is used for the different stages except in very few studies.
- -
- Membrane pre-treatment operation, such as dust or SOx/NOx removal, are not considered in the analysis.
- -
5. Open Questions, Further Research, and Prospects
5.1. Materials
5.2. Processes
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Metz, B. Intergovernmental Panel on Climate Change. In IPCC Special Report on Carbon Dioxide Capture and Storage; Cambridge University Press for the Intergovernmental Panel on Climate Change: Cambridge, UK, 2005. [Google Scholar]
- Steeneveldt, R.; Berger, B.; Torp, T.A. CO2 capture and storage: Closing the knowing-doing gap. Chem. Eng. Res. Des. 2006, 84, 739–763. [Google Scholar] [CrossRef]
- Wilcox, J. Carbon Capture; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Figueroa, J.D.; Fout, T.; Plasynski, S.; McIlvried, H.; Srivastava, R.D. Advances in CO2 capture technology—The, U.S. Department of Energy’s Carbon Sequestration Program. Int. J. Greenh. Gas Control 2008, 2, 9–20. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. A Research Agenda for Transforming Separation Science; The National Academies Press: Washington, DC, USA, 2019. [Google Scholar] [CrossRef]
- de Visser, E.; Hendriks, C.; Barrio, M.; Mølnvik, M.J.; de Koeijer, G.; Liljemark, S.; Le Gallo, Y. Dynamis CO2 quality recommendations. Int. J. Greenh. Gas Control 2008, 2, 478–484. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications; J. Wiley: Chichester, NY, USA, 2004. [Google Scholar]
- Prasad, R.; Shaner, R.L.; Doshi, K.J. Comparison of Membranes with Other Gas Separation Technologies. In Polymeric Gas Separation Membranes; Paul, D.R., Yampol’skii, Y.P., Eds.; CRC Press Inc.: Boca Raton, FL, USA, 1994; pp. 531–614. [Google Scholar]
- Favre, E.; Svendsen, H. Membrane contactors for intensified post-combustion carbon dioxide capture by gas–liquid absorption processes. J. Membr. Sci. 2012, 407–408, 1–7. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, Y.G.; Kim, M.K.; Lee, S.H.; Park, J.H. Study on CO2 absorption performance of lab-scale ceramic hollow fiber membrane contactor by gas/liquid flow direction and module design. Sep. Purif. Technol. 2019, 220, 189–196. [Google Scholar] [CrossRef]
- Baker, R.W.; Low, B.T. Gas Separation Membrane Materials: A Perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356, 1137. [Google Scholar] [CrossRef]
- Favre, E. Polymeric Membranes for Gas Separation. In Comprehensive Membrane Science and Technology; Drioli, E., Giorno, L., Eds.; Elsevier: New York, NY, USA, 2017; Volume II, pp. 155–212. ISBN 978-0-08-093250-7. [Google Scholar]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Herzog, H.; Golomb, D.; Zemba, S. Feasibility, modeling and economics of sequestering power plant CO2 emissions in the deep ocean. Environ. Prog. 1991, 10, 64–74. [Google Scholar] [CrossRef]
- Van der Sluis, J.P.; Hendriks, C.A.; Blok, K. Feasibility of polymer membranes for carbon dioxide recovery from flue gases. Energy Convers. Manag. 1992, 33, 429–436. [Google Scholar] [CrossRef]
- Koros, W.J.; Zhang, C. Materials for next-generation molecularly selective synthetic membranes. Nat. Mater. 2017, 16, 289. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, X.; Wu, H.; Tian, Z.; Xin, Q.; He, G.; Peng, D.; Chen, S.; Yin, Y.; Jiang, Z.; et al. Advances in high permeability polymer-based membrane materials for CO2 separations. Energy Environ. Sci. 2016, 9, 1863–1890. [Google Scholar] [CrossRef]
- Merkel, M.T.; Lin, H.; Wei, X.; Baker, R. Power plant post-combustion carbon dioxide capture: An opportunity for membranes. J. Membr. Sci. 2010, 359, 126–139. [Google Scholar] [CrossRef]
- Brinkmann, T.; Lillepärg, J.; Notzke, H.; Pohlmann, J.; Shishatskiy, S.; Wind, J.; Wolff, T. Development of CO2 Selective Poly(Ethylene Oxide)-Based Membranes: From Laboratory to Pilot Plant Scale. Engineering 2017, 3, 485–493. [Google Scholar] [CrossRef]
- Xomeritakis, G.; Tsai, C.Y.; Chen, Z.; Jiang, Y.; Khin, R.; Johnson, P.E.; Tsai, C.; Shah, P.B.; Khalil, S.; Singh, S.; et al. Anodic alumina supported dual-layer microporous silica membranes. J. Membr. Sci. 2007, 287, 157–161. [Google Scholar] [CrossRef]
- Guo, H.; Zhu, G.; Li, H.; Zou, X.; Yin, X.; Yang, W.; Qiu, S.; Xu, R. Hierarchical growth of large-scale ordered zeolite silicalite-1 membranes with high permeability and selectivity for recycling CO2. Angew. Chem. Int. Ed. 2006, 45, 7053–7056. [Google Scholar] [CrossRef]
- Li, S.; Fan, C.Q. High-flux SAPO-34 membrane for CO2/N2 separation. Ind. Eng. Chem. Res. 2010, 49, 4399–4404. [Google Scholar] [CrossRef]
- Liu, B.; Tang, C.; Li, X.; Wang, B.; Zhou, R. High-performance SAPO-34 membranes for CO2 separations from simulated flue gas. Microporous Mesoporous Mater. 2020, 292, 109712. [Google Scholar] [CrossRef]
- Lo, B.T.; Chung, T.S. Carbon molecular sieve membranes derived from pseudo-interpenetrating polymer networks for gas separation and carbon capture. Carbon 2011, 49, 2014–2112. [Google Scholar]
- Micari, M.; Dakhchoune, M.; Agrawal, K.V. Techno-economic assessment of postcombustion carbon capture using high-performance nanoporous single-layer graphene membranes. J. Membr. Sci. 2021, 624, 119103. [Google Scholar] [CrossRef]
- Deng, L.; Hägg, M.-B. Swelling behavior and gas permeation performance of PVAm/PVA blend FSC membrane. J. Membr. Sci. 2010, 363, 295–301. [Google Scholar] [CrossRef]
- Sandru, M.; Sandru, E.M.; Ingram, W.F.; Deng, J.; Stenstad, P.M.; Deng, L.; Spontak, R.J. An Integrated Materials Approach to Ultrapermeable and Ultraselective CO2 Polymer Membranes. Science 2022, 376, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.K.; Salim, W.; Han, Y.; Wu, D.; Ho, W.S.W. Fabrication and scale-up of multi-leaf spiral-wound membrane modules for CO2 capture from flue gas. J. Membr. Sci. 2020, 595, 117504. [Google Scholar] [CrossRef]
- Han YSalim, W.; Chen, K.K.; Wu, D.; Ho, W.S.W. Field trial of spiral-wound facilitated transport membrane module for CO2 capture from flue gas. J. Membr. Sci. 2019, 575, 242–251. [Google Scholar]
- Fu, Y.; Jiang, Y.B.; Dunphy, D.; Xiong, H.; Coker, E.; Chou, S.S.; Zhang, H.; Vanegas, J.M.; Croissant, J.G.; Cecchi, J.L.; et al. Ultra-thin enzymatic liquid membrane for CO2 separation and capture. Nat. Commun. 2018, 9, 990. [Google Scholar] [CrossRef]
- Rui, Z.B.; Anderson, M.; Li, Y.D.; Lin, Y.S. Ionic conducting ceramic and carbonate dual phase membranes for carbon dioxide separation. J. Membr. Sci. 2012, 417, 174–182. [Google Scholar] [CrossRef]
- Cerón, M.R.; Lai, L.S.; Amiri, A.; Monte, M.; Katta, S.; Kelly, J.C.; Worsley, M.A.; Merrill, M.D.; Kim, S.; Campbell, P.G. Surpassing the conventional limitations of CO2 separation membranes with hydroxide/ceramic dual-phase membranes. J. Membr. Sci. 2018, 567, 191–198. [Google Scholar] [CrossRef]
- Bounaceur, R. Membrane processes for post-combustion carbon dioxide capture: A parametric study. Energy 2006, 31, 2220–2234. [Google Scholar] [CrossRef]
- Favre, E. Carbon dioxide recovery from post-combustion processes: Can gas permeation membranes compete with absorption? J. Membr. Sci. 2007, 294, 50–59. [Google Scholar] [CrossRef]
- Belaissaoui, B.; Willson, D.; Favre, E. Membrane gas separations and postcombustion carbon dioxide capture: Parametric sensitivity and process integration strategies. Chem. Eng. J. 2012, 211–212, 122–132. [Google Scholar] [CrossRef]
- Moll, D.J.; Burmester, A.F.; Young, T.C.; Mcreynolds, K.B.; Clark, J.E.; Hotz, C.Z.; Wessling, R.A.; Quarderer, G.J.; Lacher, R.M. Gas Separations Using Glassy Polymer Membranes at Sub-Ambient Temperatures. Patent WO1992/13628A2, 20 August 1992. [Google Scholar]
- Zhao, L.; Riensche, E.; Blum, L.; Stolten, D. Multi-stage gas separation membrane processes used in post-combustion capture: Energetic and economic analyses. J. Membr. Sci. 2010, 359, 160–172. [Google Scholar] [CrossRef]
- Ramasubramanian, K.; Verweij, H.; Ho, W.S.W. Membrane processes for carbon capture from coal-fired power plant flue gas: A modeling and cost study. J. Membr. Sci. 2012, 421–422, 299–310. [Google Scholar] [CrossRef]
- Giordano, L.; Roizard, D.; Bounaceur, R.; Favre, E. Evaluating the effects of CO2 capture benchmarks on efficiency and costs of membrane systems for postcombustion capture: A parametric simulation study. Int. J. Greenh. Gas Control 2017, 63, 449–461. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Z.; Qiao, Z.; Wu, H.; Dong, S.; Zhao, S.; Wang, J. Post-combustion CO2 capture with membrane process: Practical membrane performance and appropriate pressure. J. Membr. Sci. 2019, 581, 195–213. [Google Scholar] [CrossRef]
- He, X.; Chen, D.; Liang, Z.; Yang, F. Insight and Comparison of Energy-efficient Membrane Processes for CO2 Capture from Flue Gases in Power Plant and Energy-intensive Industry. Carbon Capture Sci. Technol. 2022, 2, 100020. [Google Scholar] [CrossRef]
- Janakiram, S.; Lindbråthen, A.; Ansaloni, L.; Peters, T.; Deng, L. Two-stage membrane cascades for post-combustion CO2 capture using facilitated transport membranes: Importance on sequence of membrane types. Int. J. Greenh. Gas Control 2022, 119, 103698. [Google Scholar] [CrossRef]
- Yang, D.; Wang, Z.; Wang, J.; Wang, S. Potential of two-stage membrane system with recycle stream for CO2 capture from postcombustion gas. Energy Fuels 2009, 23, 4755–4762. [Google Scholar] [CrossRef]
- Low, B.T.; Zhao, L.; Merkel, T.C.; Weber, M.; Stolten, D. A parametric study of the impact of membrane materials and process operating conditions on carbon capture from humidified flue gas. J. Membr. Sci. 2013, 431, 139–155. [Google Scholar] [CrossRef]
- Pfister, M.; Belaissaoui, B.; Favre, E. Membrane gas separation processes from wet postcombustion flue gases for carbon capture and use: A critical reassessment. Ind. Eng. Chem. Res. 2017, 56, 591–602. [Google Scholar] [CrossRef]
- Castel, C.; Bounaceur, R.; Favre, E. Engineering of membrane gas separation processes: State of the art and prospects. J. Membr. Sci. Res. 2020, 6, 295–303. [Google Scholar]
- Bozorg, M.; Addis, B.; Piccialli, V.; Ramírez-Santos, A.; Castel, C.; Pinnau, I.; Favre, E. Polymeric membrane materials for nitrogen production from air: A process synthesis study. Chem. Eng. Sci. 2019, 207, 1196–1213. [Google Scholar] [CrossRef]
- Lee, S.; Binns, M.; Kim, J.K. Automated process design and optimization of membrane-based CO2 capture for a coal-based power plant. J. Membr. Sci. 2018, 563, 820–834. [Google Scholar] [CrossRef]
- Kentish, S. 110th Anniversary: Process Developments in Carbon Dioxide Capture Using Membrane Technology. Ind. Eng. Chem. Res. 2019, 58, 12868–12875. [Google Scholar] [CrossRef]
- Sholl, D.S.; Lively, R.P. Exemplar mixtures for studying complex mixture effects in practical chemical separations. JACS Au 2022, 2, 322–327. [Google Scholar] [CrossRef]
- Favre, E. CO2/N2 reverse selective gas separation membranes: Technological opportunities and scientific challenges. Ind. Eng. Chem. Res. 2009, 48, 3700–3701. [Google Scholar] [CrossRef]
- Hussain, A.; Farrukh, S.; Minhas, F.T. Two-Stage Membrane System for Post-combustion CO2 Capture Application. Energy Fuels 2015, 29, 6664–6669. [Google Scholar] [CrossRef]
- McKee, R. CO2 removal: Membrane plus amine. Hydrocarb. Process. 1991, 70, 63–65. [Google Scholar]
- Weller, S.; Steiner, W.A. Separation of Gases by Fractional Permeation through Membranes. J. Appl. Phys. 1950, 21, 279–283. [Google Scholar] [CrossRef]
- Kaldis, S.P.; Kapantaidakis, G.C.; Sakellaropoulos, G.P. Simulation of multicomponent gas separation in a hollow fiber membrane by orthogonal collocation—hydrogen recovery from refinery gases. J. Membr. Sci. 2000, 173, 61–71. [Google Scholar] [CrossRef]
- Bounaceur, R.; Berger, E.; Pfister, M.; Santos, A.A.R.; Favre, E. Rigorous variable permeability modelling and process simulation for the design of polymeric membrane gas separation units: MEMSIC simulation tool. J. Membr. Sci. 2017, 523, 77–91. [Google Scholar] [CrossRef]
- Lüdtke, O.; Behling, R.-D.; Ohlrogge, K. Concentration polarization in gas permeation. J. Membr. Sci. 1998, 146, 145–157. [Google Scholar] [CrossRef]
- Brinkmann, T.; Pohlmann, J.; Bram, M.; Zhao, L.; Tota, A.; Escalona, N.J.; de Graaff, M.; Stolten, D. Investigating the influence of the pressure distribution in a membrane module on the cascaded membrane system for post-combustion capture. Int. J. Greenh. Gas Control 2015, 39, 194–204. [Google Scholar] [CrossRef]
- Wilcox, J. An electro-swing approach. Nat. Energy 2020, 5, 121–122. [Google Scholar] [CrossRef]
- Castel, C.; Favre, E. Membrane separations and energy efficiency. J. Membr. Sci. 2018, 548, 345–355. [Google Scholar] [CrossRef]
- Agrawal, R. Separations: Perspective of a process developer/designer. AIChE J. 2001, 47, 967. [Google Scholar] [CrossRef]
- Favre, E. Specialty Grand Challenges in Separation Processes. Frontiers in Chemical. Engineering 2020, 2, e44. [Google Scholar] [CrossRef]
- Freeman, B.; Hao, P.; Baker, R.; Kniep, J.; Chen, E.; Ding, J.; Zhang, Y.; Rochelle, G.T. Hybrid Membrane-absorption CO2 Capture Process. Energy Procedia 2014, 63, 605–613. [Google Scholar] [CrossRef]
- Castel, C.; Bounaceur, R.; Favre, E. Membrane Processes for Direct Carbon Dioxide Capture from Air: Possibilities and Limitations. Front. Chem. Eng. 2021, 3, 668867. [Google Scholar] [CrossRef]
| Source | % CO2 (xIN) | Other Compounds |
|---|---|---|
| Power plant (coal) | 12–15 | N2 (O2) |
| Power plant (gas) | 4–5 | N2 (O2) |
| Steel | 5–20 | CO, N2, H2 |
| Cement | 20–30 | N2 (O2) |
| Petrochemicals | 10–30 | N2 |
| Waste incineration | 5–15 | N2 (O2) |
| Biomass boilers | 5–15 | N2 (O2) |
| Biogas | 40–60 | CH4 |
| Air (DAC) | 4 × 10−4 | N2, O2 |
| Material Type | CO2/N2 Selectivity (–) | CO2 Permeance (GPU *) | Reference |
|---|---|---|---|
| Early studies (dense polymers) | |||
| PPO | 19 | 375 | [15,16] |
| PI | 43 | 100 | |
| Current commercially available polymeric membranes | |||
| Polaris (MTR) | 50 | 2200 | [19] |
| Polyactive (Hereon) | 46 | 1450 | [20] |
| High-performance non-commercially available materials | |||
| Polymers | 78 | 3000 | [18] |
| Silica | 50 | 900 | [21] |
| Zeolite | 69–170 | 2100–4000 | [22,23,24] |
| Carbon molecular sieve | 25 | 9000 | [25] |
| Graphene | 30 | 10 000 | [26] |
| Chemical separation mechanism | |||
| Fixed site carrier | 165 | 1450 | [27,28] |
| Liquid membrane (LM) | 140 | 3000 | [29,30] |
| Enzymatic LM | 788 | 2600 | [31] |
| Hydroxide ceramic ** | 1000 | 250 | [32,33] |
| Molecular Mechanism | Temperature Range | Absorption Process | Membrane Process |
|---|---|---|---|
| Physical | −10–60 °C | Ethylene glycol based solvents (Selexol) | Ethyleneglycol-based dense polymers |
| Chemical | 40–120 °C | Amine solvents | Amine-based reacting membranes FSCM, LM |
| High-temperature chemical | >200 °C | Hot carbonate | High-temperature carbonate-based membranes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Favre, E. Membrane Separation Processes and Post-Combustion Carbon Capture: State of the Art and Prospects. Membranes 2022, 12, 884. https://doi.org/10.3390/membranes12090884
Favre E. Membrane Separation Processes and Post-Combustion Carbon Capture: State of the Art and Prospects. Membranes. 2022; 12(9):884. https://doi.org/10.3390/membranes12090884
Chicago/Turabian StyleFavre, Eric. 2022. "Membrane Separation Processes and Post-Combustion Carbon Capture: State of the Art and Prospects" Membranes 12, no. 9: 884. https://doi.org/10.3390/membranes12090884
APA StyleFavre, E. (2022). Membrane Separation Processes and Post-Combustion Carbon Capture: State of the Art and Prospects. Membranes, 12(9), 884. https://doi.org/10.3390/membranes12090884







