Treatment of Tuna Cooking Juice via Ceramic Ultrafiltration Membrane: Optimization Using Response Surface Methodology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tuna Cooking Juices Collection
2.2. Ultrafiltration Process
2.3. Analytical Methods
2.4. Experimental Design Methodology
2.5. Investigation of Fouling Mechanism
2.6. Fouling Resistance Abilities and Membrane Regeneration
3. Results and Discussion
3.1. Wastewater UF
3.2. Protein Removal Response
3.3. Permeate Flux Response
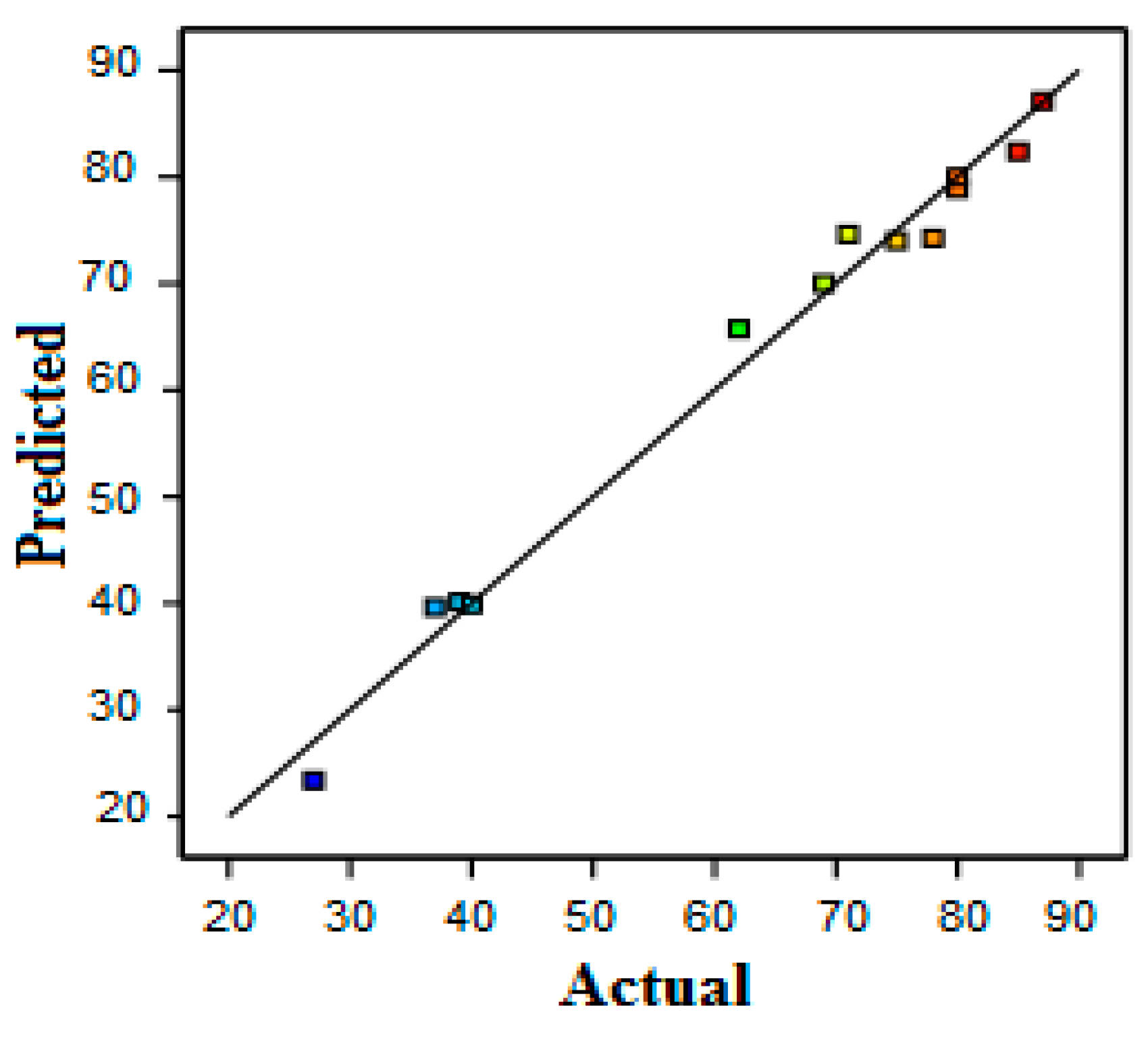
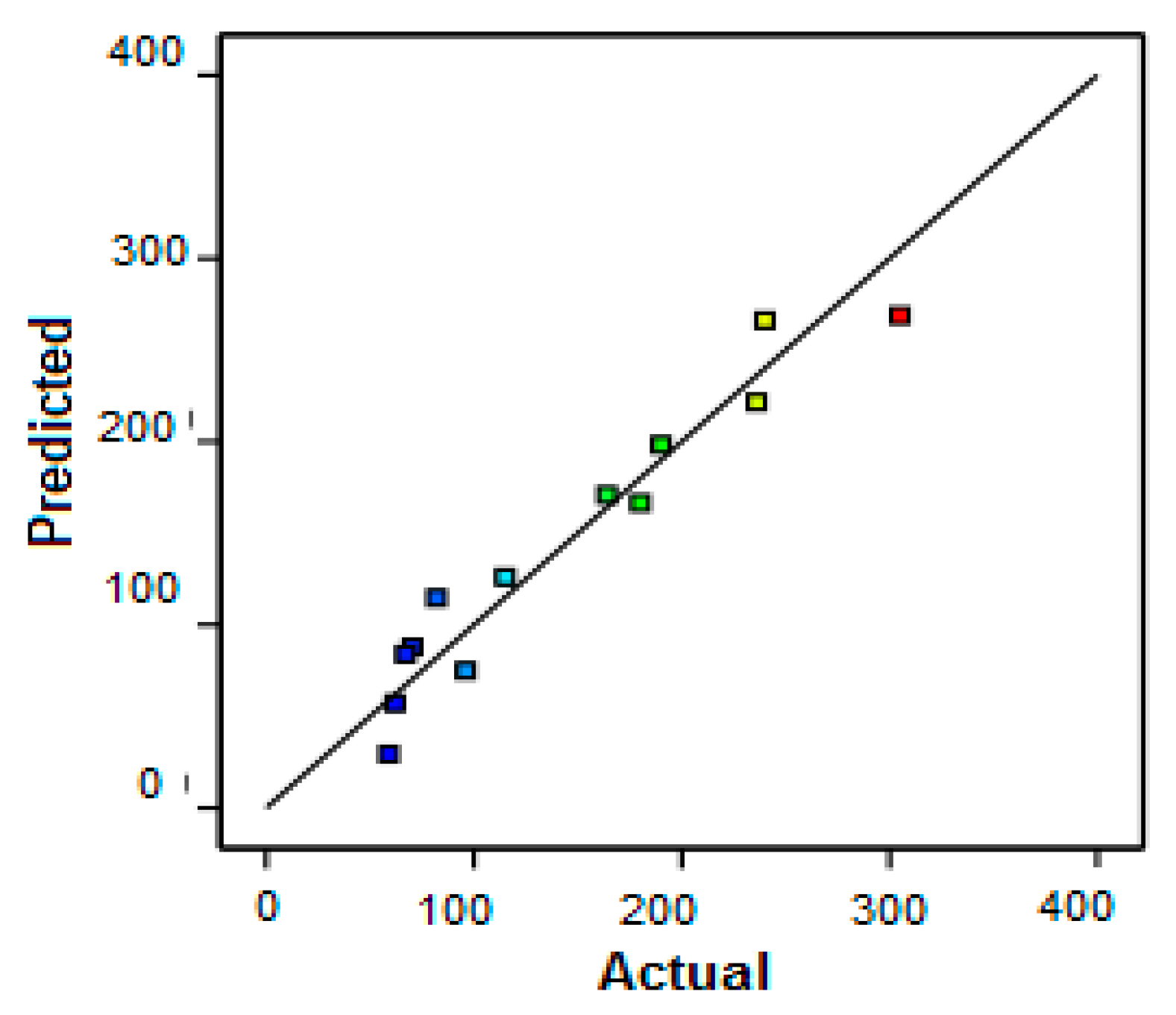
3.4. Optimization of Permeate Flux and Protein Removal
3.5. Global Mass Balance
3.6. Application of Polarization Concentration Model
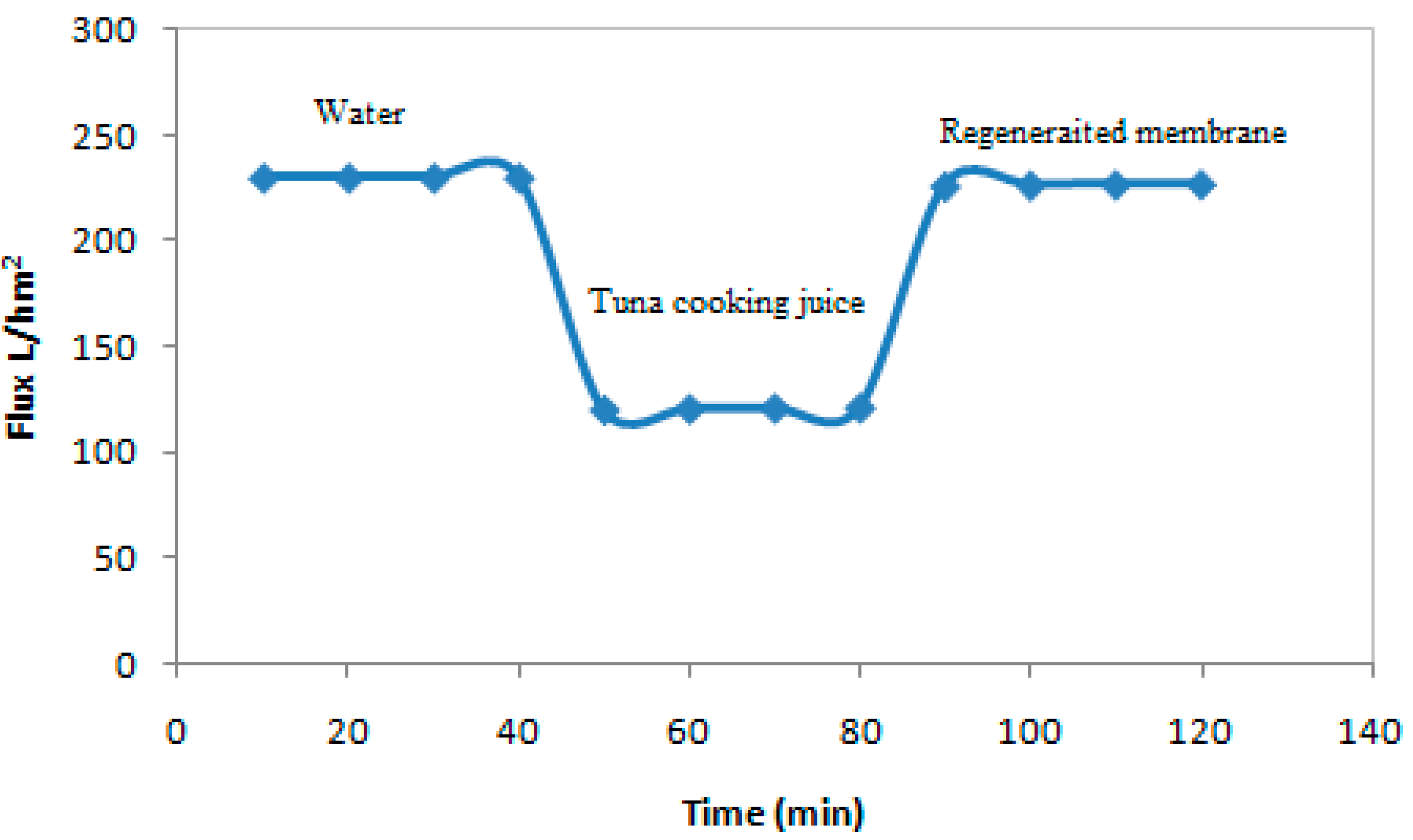
3.7. Antifouling and Cleaning Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saidi, S.; Ben Amar, R. Valorisation of tuna processing waste biomass for recovery of functional and antioxidant peptides using enzymatic hydrolysis and membrane fractionation process. Environ. Sci. Pollut. Res. 2016, 23, 21070–21085. [Google Scholar] [CrossRef] [PubMed]
- Walhaa, K.; Ben Amar, R.; Masséb, A.; Bourseaub, P.; Cardinald, M.; Cornetd, J.; Proste, C.; Jaouenb, P. Aromas potentiality of tuna cooking juice concentrated by nanofiltration. LWT Food Sci. Technol. 2011, 44, 153–157. [Google Scholar] [CrossRef] [Green Version]
- Cros, S.; Lignot, B.; Razafintsalama, C.; Jaouen, P.; Bourseau, P. Electrodialysis desalination and reverse osmosis concentration of an industrial mussel juice: Process impact on pollution reduction and aroma quality. J. Food Sci. 2004, 69, 435–442. [Google Scholar] [CrossRef]
- Jao, C.L.; Ko, W.C. Utilization of cooking juice of young tuna processed into canned tuna as condiments: Effects of enzymatic hydrolysis and membrane treatment. Fish. Sci. 2002, 68, 1344–1351. [Google Scholar] [CrossRef]
- Benelhadj, S.; Gharsallaoui, A.; Degraeve, P.; Attia, H.; Ghorbel, D. Effect of pH on the functional properties of Arthrospira (Spirulina) platensis protein isolate. Food Chem. 2016, 194, 1056–1063. [Google Scholar] [CrossRef]
- Ba, F.; Ursu, A.V.; Laroche, C.; Djelveh, G. Haematococcuspluvialis soluble proteins: Extraction, characterization, concentration/fractionation and emulsifying properties. Bioresour. Technol. 2016, 200, 147–152. [Google Scholar] [CrossRef]
- Waghmare, A.G.; Salve, M.K.; LeBlanc, J.G.; Arya, S.S. Concentration and characterization of microalgae proteins from Chlorella pyrenoidosa. Bioresour. Bioprocess. 2016, 3, 16. [Google Scholar] [CrossRef] [Green Version]
- Desai, R.K.; Streefland, M.; Wijffels, R.H.; Eppink, H.M.M. Extraction and stability of selected proteins in ionic liquid based aqueous two phase systems. Green Chem. 2014, 16, 2670–2679. [Google Scholar] [CrossRef]
- Balti, R.; Le Balc’h, R.; Brodu, N.; Gilbert, M.; Le Gouic, B.; Le Gall, S.; Sinquin, C.; Massé, A. Concentration and purification of Porphyridiumcruentum exopolysaccharides by membrane filtration at various cross-flow velocities. Process Biochem. 2018, 74, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Balti, R.; Brodu, N.; Zhang, J.; Amzil, Z.; Drouin, D.; Séchet, V.; Massé, A. Pre-purification by membrane filtration of paralytic shellfish toxins from Alexandriumminutum dinoflagellate. Sep. Purif. Technol. 2019, 210, 152–158. [Google Scholar] [CrossRef] [Green Version]
- Zaouk, L.; Massé, A.; Bourseau, P.; Taha, S.; Rabiller-Baudry, M.; Jubeau, S.; Teychené, B.; Pruvost, J.; Jaouen, P. Filterability of exopolysaccharides solutions from the red microalga Porphyridiumcruentum by tangential filtration on a polymeric membrane. Environ. Technol. 2020, 41, 1167–1184. [Google Scholar] [CrossRef] [PubMed]
- Petrosino, F.; Wickramasinghe, S.; Pal, U. Computational Modeling in studying phase change materials. J. Phase Change Mater. 2021, 1. [Google Scholar] [CrossRef]
- Bourseau, P.; Massé, A.; Cros, S.; Vandanjon, L.; Jaouen, P. Recovery of aroma compounds from seafood cooking juices by membrane processes. J. Food Eng. 2014, 128, 157–166. [Google Scholar] [CrossRef]
- Balti, R.; Zayoud, N.; Hubert, F.; Beaulieu, L.; Massé, A. Fractionation of Arthrospira platensis (Spirulina) water soluble proteins by membrane diafiltration. Sep. Purif. Technol. 2021, 256, 117756. [Google Scholar] [CrossRef]
- Aloulou, H.; Attia, A.; Aloulou, W.; Chakraborty, S.; Baklouti, L.; Dammak, L.; Amar, R.B. Statistical Simulation, a Tool for the Process Optimization of Oily Wastewater by Crossflow Ultrafiltration. Membranes 2022, 12, 676. [Google Scholar] [CrossRef]
- Suresh, K.; Pugazhenthi, G.; Uppaluri, R. Fly ash based ceramic microfiltration membranes for oil-water emulsion treatment: Parametric optimization using response surface methodology. J. Water Process. Eng. 2016, 13, 27–43. [Google Scholar] [CrossRef]
- Belgada, A.; Charik, F.Z.; Achiou, B.; NtambweKambuyi, T.; AlamiYounssi, S.; Beniazza, R.; Dani, A.; Benhid, R.; Ouammou, M. Optimization of phosphate/kaolinite microfiltration membrane using Box–Behnken design for treatment of industrial wastewater. J. Environ. Chem. Eng. 2021, 9, 104972. [Google Scholar] [CrossRef]
- Aloulou, W.; Aloulou, H.; Ben Amar, R. Low-cost composite ultrafiltration membrane made from TiO2 and nanocomposite clay materials over zeolite support for oily wastewater purification and heavy metals removal. Desal. Wat. Treat. 2022, 246, 166–173. [Google Scholar] [CrossRef]
- Bousbih, S.; Belhadj Ammar, R.; Ben Amar, R.; Dammak, L.; Darragi, F.; Selmane, E. Synthesis and Evaluation of Asymmetric Mesoporous PTFE/Clay Composite Membranes for Textile Wastewater Treatment. Membranes 2021, 11, 850. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, C.M. Response Surfaces Methodology: Process and Product Optimization Using Designed Experiments; Wiley: New York, NY, USA, 1995. [Google Scholar]
- Martí-Calatayud, M.C.; Vincent-Vela, M.C.; Alvarez-Blanco, S.; Lora-García, J.; Bergantiños-Rodríguez, E. Analysis and optimization of the influence of operating conditions in the ultrafiltration of macromolecules using a response surface methodological approach. Chem. Eng. J. 2010, 156, 337e346. [Google Scholar] [CrossRef]
- Francesco, P.; Coppola, G.; Curcio, S. Modeling aspects in simulation of phase change materials used for thermal regulation of buildings. J. Phase Change Mater. 2021, 1. [Google Scholar] [CrossRef]
- Sablani, S.S.; Goosena, M.F.A.; Al-Belush, R.; Wilf, M. Concentration polarization in ultrafiltration and reverse osmosis: A critical review. Desalination 2001, 141, 269–289. [Google Scholar] [CrossRef]
- Blatt, W.; Dravid, A.; Michaels, A.S.; Nelsen, L.; Flinn, J.E. Membrane Science and Technology; Plenum: New York, NY, USA, 1970; pp. 47–91. [Google Scholar]
- Giacobbo, A.; Moura Bernardes, A.; João, M.; Rosa, F.; Norberta de Pinho, M. Concentration Polarization in Ultrafiltration/Nanofiltration for the Recovery of Polyphenols from Winery Wastewaters. Membranes 2018, 8, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beqqoura, D.; Derouicha, G.; Taanaouia, W.; Essatea, A.; Ouammoua, M.; AlamiYounssia, S.; Bennazhaa, J.; Jason, A.; Mama El Rhaz, C. Development of composite ultrafiltration membrane made of PmPD/PVA layer deposited on ceramic pozzolan/micronized phosphate support and its application for congo red dye removal. Desal. Wat. Treat. 2021, 240, 152–164. [Google Scholar] [CrossRef]
- Ahmad, T.; Guria, C.; Mandal, A. Optimal synthesis and operation of low-cost polyvinyl chloride/bentonite ultrafiltration membranes for the purification of oilfield produced water. J. Memb. Sci. 2018, 564, 859–877. [Google Scholar] [CrossRef]
- Kouakou Eric, A.; Gouesse, B.; Briton, H.; Donatien Ehouman, A.; Adouby, K.; Drogui, P. Modelling COD Removal from Slaughterhouse Wastewater by Electrocoagulation Using Response Surface Methodology. J. Chem. Eng. 2021, 9, 154–161. [Google Scholar] [CrossRef]
- Joglekar, A.; May, A. Product excellence through design of experiments. Cereal Foods World 1987, 32, 8. [Google Scholar]
- Veréb, G.; Kassai, P.; Santos, E.N.; Arthanareeswaran, G.; Hodúr, C.; László, Z. Intensification of the ultrafiltration of real oil-contaminated (produced) water with pre ozonation and/or with TiO2, TiO2/CNT nanomaterial-coated membrane surfaces. Environ. Sci. Pollut. Res. 2020, 27, 22195–22205. [Google Scholar] [CrossRef] [Green Version]
- Lavanya, C.; Soontarapa, K.; Jyothi, M.S.; Geetha Balakrishna, R. Environmental friendly and cost effective caramel for congo red removal, high flux, and fouling resistance of polysulfone membranes. Sep. Purif. Technol. 2019, 211, 348–358. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Chandel, A.K.; Taherzadeh, M.; Bhattacharjee, C. Enhance the bioethanol production from the extracted fermentable sugar by sonication. J. Phase Chang. Mater. 2022, 2, 48–61. [Google Scholar] [CrossRef]
- Gharsallah, A.; Tahri, N.; Duplay, J.; Ben Amar, R. Performances of NF and RO applied in combined system and separately for the treatment and recycling of biologically pretreated real textile wastewater. J. Phase Change Mater. 2022, 2, 24–47. [Google Scholar] [CrossRef]
- Dasgupta, J.; Mondal, D.; Chakraborty, S.; Sikder, J.; Curcio, S.; Arafat, H.A. Nanofiltration based water reclamation from tannery effluent following coagulation pretreatment. Ecotoxicol. Environ. Saf. 2015, 121, 22–30. [Google Scholar] [CrossRef] [PubMed]










| Parameters | Unity | Sample 1 | Sample 2 | Sample 3 |
|---|---|---|---|---|
| pH | - | 7.14 ± 0.2 | 8.12 ± 0.2 | 6.9 ± 0.2 |
| Conductivity | mS/cm | 53.6 ± 0.4 | 52.8 ± 0.4 | 49.6 ± 0.4 |
| Turbidity | NTU | 1525 ± 100 | 1266 ± 100 | 1269 ± 100 |
| Protein | g/L | 6.32 ± 1 | 7.28 ± 1 | 8.4 ± 1 |
| Variables | Factor Levels | |||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Input factors | ||||
| VCF | X1 | 1.03 | 2.6 | 4.25 |
| T (°C) | X2 | 20 | 40 | 60 |
| ΔP (bar) | X3 | 2 | 3.5 | 7 |
| Run | Input Factors | Responses | |||
|---|---|---|---|---|---|
| VCF | T (°C) | ΔP (bar) | R Protein (%) | Permeate Flux (L/h·m²) | |
| 1 | 4.25 | 20 | 3.5 | 27 | 59 |
| 2 | 2.64 | 60 | 2 | 78 | 236 |
| 3 | 2.64 | 60 | 5 | 75 | 305 |
| 4 | 2.64 | 20 | 5 | 62 | 115 |
| 5 | 4.25 | 40 | 5 | 40 | 67 |
| 6 | 4.25 | 40 | 2 | 37 | 62 |
| 7 | 1.03 | 20 | 3.5 | 80 | 96 |
| 8 | 1.03 | 40 | 5 | 85 | 190 |
| 9 | 2.64 | 40 | 3.5 | 80 | 164 |
| 10 | 1.03 | 60 | 3.5 | 71 | 240 |
| 11 | 4.25 | 60 | 3.5 | 39 | 70.4 |
| 12 | 2.64 | 20 | 2 | 69 | 82 |
| 13 | 1.03 | 40 | 2 | 87 | 180 |
| b0 | b1 | b2 | b3 | b12 | b13 | b23 | b11 | b22 | b33 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Protein | 80 | −22.5 | 3.125 | −1.125 | 5.25 | 1.25 | 1 | −17.25 | −8.5 | −0.5 |
| p-values | 0.001 | 0.1705 | 0.5642 | 0.1228 | 0.6467 | 0.7119 | 0.0131 | 0.0797 | 0.8877 | |
| Flux | 170.914 | −55.95 | 62.425 | 14.625 | −33.15 | −1.25 | 9 | −56.2929 | 11.8571 | |
| p-values | 0.0135 | 0.0092 | 0.3319 | 0.1518 | 0.95 | 0.6563 | 0.0659 | 0.6247 |
| COD (mg/L) | Salinity (g/L) | Protein (g/L) | |
|---|---|---|---|
| Before ultrafiltration (Raw juice cooking) | 24,250 | 37.4 | 6.32 |
| After ultrafiltration | 1750 (93%) | 31 (17%) | 1.3 (80%) |
| VCF | CiVi (g/L) | CpVp + CcVc | Error (%) |
|---|---|---|---|
| 1.03 | 123.76 | 122.67 | 1.09 |
| 1.54 | 123.76 | 122.76 | 1.26 |
| 2.6 | 123.76 | 95.51 | 28.25 |
| 3.77 | 123.76 | 88.35 | 33.47 |
| 4.26 | 123.76 | 87.5 | 36.26 |
| VCF | Cc (g/L) | Log Cc | Flux (L/h·m2) |
|---|---|---|---|
| 1.03 | 7.4 | 2 | 339 |
| 1.54 | 8.5 | 2.14 | 314 |
| 2.6 | 10.73 | 2.37 | 305 |
| 3.77 | 11.3 | 2.42 | 235 |
| 4.26 | 11.8 | 2.47 | 182 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aloulou, W.; Aloulou, H.; Attia, A.; Chakraborty, S.; Ben Amar, R. Treatment of Tuna Cooking Juice via Ceramic Ultrafiltration Membrane: Optimization Using Response Surface Methodology. Membranes 2022, 12, 813. https://doi.org/10.3390/membranes12080813
Aloulou W, Aloulou H, Attia A, Chakraborty S, Ben Amar R. Treatment of Tuna Cooking Juice via Ceramic Ultrafiltration Membrane: Optimization Using Response Surface Methodology. Membranes. 2022; 12(8):813. https://doi.org/10.3390/membranes12080813
Chicago/Turabian StyleAloulou, Wala, Hajer Aloulou, Afef Attia, Sudip Chakraborty, and Raja Ben Amar. 2022. "Treatment of Tuna Cooking Juice via Ceramic Ultrafiltration Membrane: Optimization Using Response Surface Methodology" Membranes 12, no. 8: 813. https://doi.org/10.3390/membranes12080813
APA StyleAloulou, W., Aloulou, H., Attia, A., Chakraborty, S., & Ben Amar, R. (2022). Treatment of Tuna Cooking Juice via Ceramic Ultrafiltration Membrane: Optimization Using Response Surface Methodology. Membranes, 12(8), 813. https://doi.org/10.3390/membranes12080813







