It was previously reported that pristine GO membranes are completely impermeable to all gasses and liquids except water vapor due to their unique nanochannel structure [
3]. The major inner-membrane structures that dominate the nanochannels are inter-layer galleries, intra-layer frameworks, and vacancy defects [
9,
12]. These nanochannels are more generally spaces in the membrane through which molecules can permeate, rather than well-defined tubes that can be experimentally visualized and quantified. The inter-layer and intra-layers structures play an important role in the stacking of the GO nanosheets within the membrane. This stacking affects the nanochannel size, which in turn affects the membrane stability and permeability. The enlarging of the GO nanochannel by increasing either interlayer or intra-layer spacing can accelerate the water flow or permit larger vapor molecular to pass through the membrane. It was previously reported that for membranes modified with cations of the same valency, a larger interlayer spacing results in greater water permeation if the intra-layer spacing change is negligible [
19]. Multivalent metal cation modification is effective at both improving the aqueous stability of the GO membrane and increasing the interplanar distance, but this modification does not always result in increased water permeation [
15,
19]. Because it is difficult to experimentally measure the intra-sheet spacing, previous studies focused on understanding the effects of the interplanar (inter-layer) distance on the nanochannel structure and the membrane stability [
9,
20,
21,
22,
23]. In our previous study and as demonstrated in
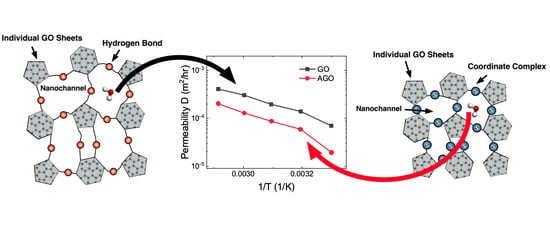
Figure 1, we determined that modification of the GO membrane with Al
3+ via oxidation of Al foil resulted in an increase in the membrane inter-layer distance, which was previously reported, and in a decrease in the membrane intra-layer spacing, which is not often discussed [
24]. In the studies described below, we related the changes in the inter- and intra-layer distances upon Al
3+ modification of the GO membrane to the membrane properties as well as stability in and permeability to different polar solvents.
3.1. Solution and Membrane Characterization
The GO solution in the absence of Al
3+ is a liquid crystal. Its rheological-related properties such as viscosity determine the applicable fabrication methods, which may include electrospraying, wet/dry spinning, inkjet printing techniques, etc. [
30,
31,
32,
33]. Unlike the unmodified GO solution, the AGO solution exhibited a “muddy” appearance (
Figure S2), which was initially considered to be due to the formation of a gel. To fully understand the “muddy” appearance of the AGO solution, the viscosity of the unmodified GO and AGO solutions (
Figure S3) was measured. Both the GO and AGO solutions exhibited a typical and similar pseudoplastic behavior, or so-called viscoelastic behavior, which means the viscosity (η) decreases and the shear stress (τ) increases with the shearing rate. Thus, the solution viscosity cannot explain the “muddy” appearance of the AGO solution. Instead, it is likely that the change in consistency of the sheet solution upon the addition of the Al foil was due to interactions of the Al
3+ cations in solution with the OFGs on the GO sheet surfaces.
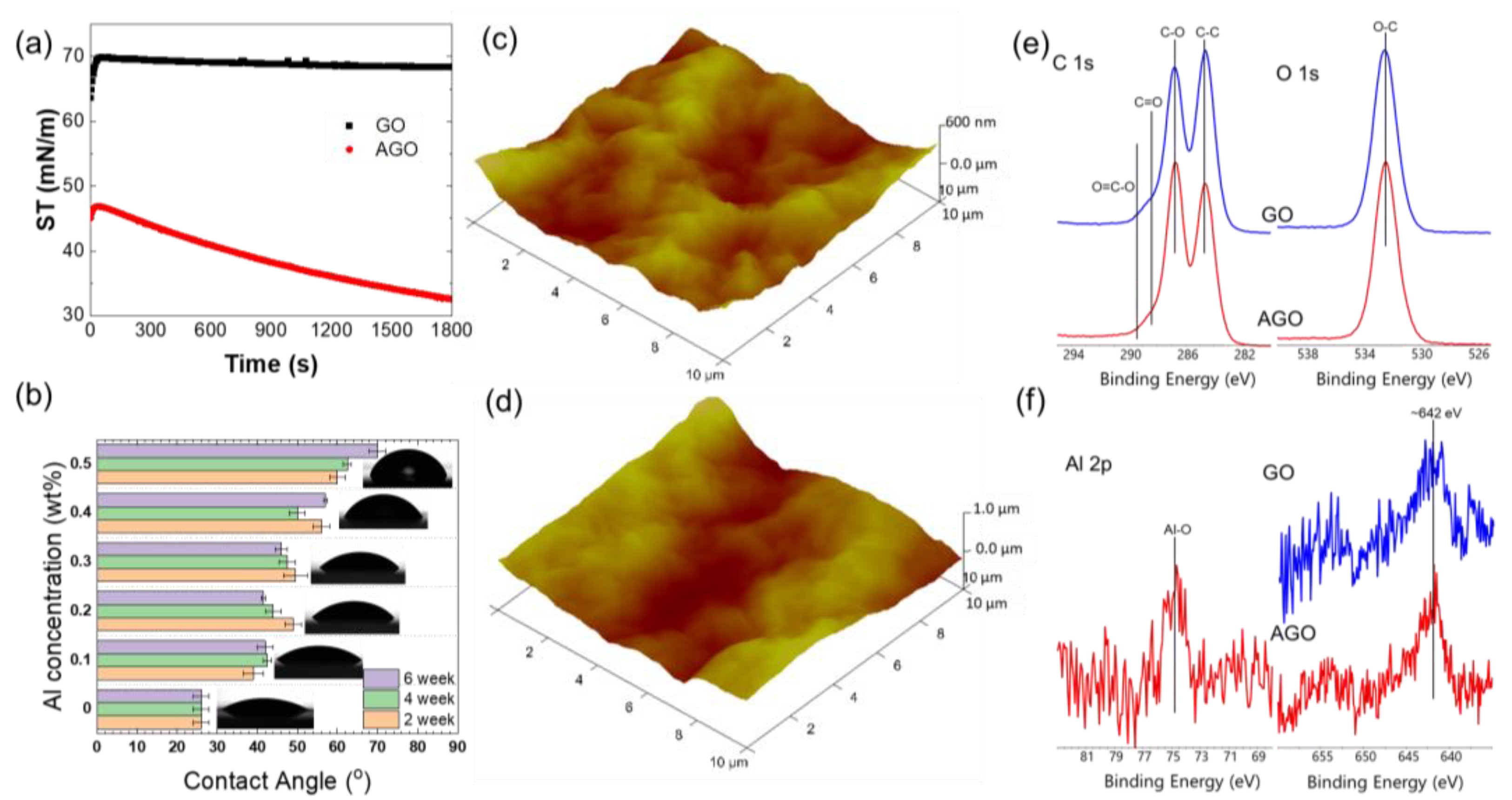
The interactions between Al
3+ and the GO sheets is supported by surface tension measurements of the GO and AGO solutions. These surface tension measurements provide information about the extent of adsorption of a species in solution to the air–water interface. If the surface tension of a solution does not decrease much relative to that of the neat air–water interface (72 mN/m), then the species in the solution is not highly surface active. If the surface tension of a solution significantly decreases relative to that of the neat air–water interface, then the species is surface active. The average surface tension vs. time data were collected for both GO and AGO solutions using a pendant drop tensiometer and are summarized in
Figure 2a. In the absence of Al
3+, the surface tension of the GO solution initially increased from approximately 60 mN/m to approximately 70 mN/m over the course of around one minute. After this rapid increase, the surface tension did not significantly change over time. The fact that the surface tension is similar to the neat air–water interface suggests that GO sheets are not highly surface active and likely desorbed from the interface over the first minute of interface formation. This result is consistent with the surface structure of GO, in which the negatively charged OFGs cause the material to be highly water-soluble. In the presence of Al
3+, the surface tension of the AGO solution showed a small initial increase followed by a decrease to approximately 30 mN/m over the course of 1800 s. The decrease in surface tension over time indicates that AGO sheets continually adsorb species to the air–water interface, potentially forming multiple layers through OFG-Al
3+ interactions. The surface tension data suggest that the addition of Al
3+ can impact the self-assembling process of the GO sheets at the air–water interface, which acts to increase the hydrophobicity of the sheets and decreases their solubility in water.
Our previous report showed that releasing Al
3+ in the GO solution via the oxidation of Al foil is a slow process and results in an increase in the solution pH [
24]. The protonation of the OFGs is highly sensitive to the solution pH, which in turn affects the extent of crosslinking between the OFGs and Al
3+. Thus, it is likely that the hydrophobicity of the AGO membrane is impacted by both the Al
3+ concentration and the time at which the GO solution is exposed to the Al foil before membrane formation. The relative hydrophobicities of GO membranes formed from solutions that were exposed to Al foil for varying times were determined from contact angle measurements, as presented in
Figure 2b. Here, the contact angles of ultrapure water droplets with membranes casted on the glass slide surfaces were used to estimate the hydrophobicity of the GO and AGO membranes. As shown in
Figure 2b, the contact angle of the GO membrane increased with the Al
3+ concentration, which indicates an increase in the hydrophobicity of AGO membranes when compared to the GO membranes. The increase in sheet hydrophobicity, as observed in the surface tension and contact angle measurements, is consistent with the interaction of the positively charged Al
3+ cations with the negatively charged OFGs on the sheet surfaces, either through electrostatic screening or chelation. Additionally, the increase in the density of wrinkles formed with the addition of Al
3+ can further increase the hydrophobicity of the GO membranes [
24].
The interactions between Al
3+ and the OFGs not only affect membrane hydrophobicity, but also the presence of membrane features such as wrinkles, shrinkage, and cracks. Specifically, it was observed that the muddy-like AGO solutions with an Al wt.% of greater than 0.3 wt.% formed membranes that, upon drying, demonstrated a significant decrease in diameter and excessive cracking. Significant membrane shrinking and cracking was not observed for membranes formed from less muddy-like AGO solutions with an Al wt.% of less than 0.3 wt.% (
Figure S4). The decrease in the membrane diameter with an increase in the Al wt.% supports the previous conclusion that the addition of Al
3+ decreases the intra-sheet spacing [
24].
The “brick-and-mortar” stacking configuration of laminated GO sheets introduces mixed interactions among the basal planes and edges, which lead to ‘peak and valley’ undulations in the membrane structure. The accumulation of these undulations results in wrinkles, which increases the surface roughness of the membrane [
1]. Our previous report shows that Al
3+ modification via Al foil oxidation causes more wrinkles to form on both GO sheets and membranes, which can lead to an increase in surface roughness [
24]. Stronger interactions between Al
3+ and the OFGs on GO sheet surfaces can increase the ‘peak and valley’ undulations and thus cause more wrinkles in the AGO membrane compared to the GO membrane. Three-dimensional AFM images of the GO (
Figure 2c) and AGO (
Figure 2d) membranes can be used to estimate the average surface roughness (RA) of as-fabricated GO and AGO membranes. According to the average RA value collected from five AFM images of the GO and AGO membranes, the AGO membrane had an RA roughness value of 98nm, while the value of pure GO membrane was 87nm, which is consistent with the greater density of wrinkles in the AGO membrane when compared to the GO membrane [
24].
To better understand the effect of Al
3+ on the chemical composition of the dried GO membranes, XPS data were collected and are shown in
Figure 2e,f. XPS is a surface-sensitive technique that can be used to effectively determine the elemental composition and chemical environment of a GO membrane within the first few nanometers of its surface.
Figure 2e, which provides the overall surface composition of the GO and AGO membranes, demonstrates that carbon and oxygen comprised more than 98 at. % of the surface. Moreover, the C/O ratio varied between 2.3 and 2.6 for all GO and AGO membranes studied. These results are consistent with the presence of hydroxyl, epoxy, and carboxyl groups on the membrane surfaces, as expected. All membranes showed peaks due to small amounts of N and S, while only the AGO membranes showed a small peak due to the presence of Al near 75 eV (
Figure 2e). As shown in
Figure 2f, the primary C 1s peaks at 284.5 eV and 286.8 eV confirm the presence of C–C (or C−C/C=C) and C-O (C−O/C−N, C−O−C) functional groups, respectively.
Figure 2f also shows two small C 1s peaks at 288.8 eV and 289.4 eV, which indicate the presence of O-C=O and C=O functional groups. The primary O 1s peak at 532.3 eV is attributed to the presence of OFGs within the GO membrane. Importantly, the introduction of Al
3+ to the GO membrane resulted in a decrease in the C-C peak area and an increase in the C-O peak area in the C 1s spectra. This result can be attributed to the coordination between Al
3+ and epoxide groups that underwent ring opening reactions.
3.2. Membrane Stability
The instability of GO membranes in aqueous environments is a long-standing problem for applications that require GO membranes to be used in polar solvents, which is due to the chemical structure of the GO sheets that compose the membranes [
10,
11,
12]. Specifically, the hydrophilic OFGs on GO sheets cause both the sheets and laminated membranes to become hydrophilic and hold a negative surface potential. The electrostatic repulsions between negatively charged GO sheets within the membranes can overcome the van der Waals attractions and hydrogen bonding interactions between sheets in the aqueous environment, especially in the presence of shear force and sonication energy [
12]. The low contact angle of unmodified GO indicates the high tendency of GO sheets to be solvated by water molecules after becoming fully soaked, which further decreases the extent of interactions between adjacent GO sheets within the GO membrane.
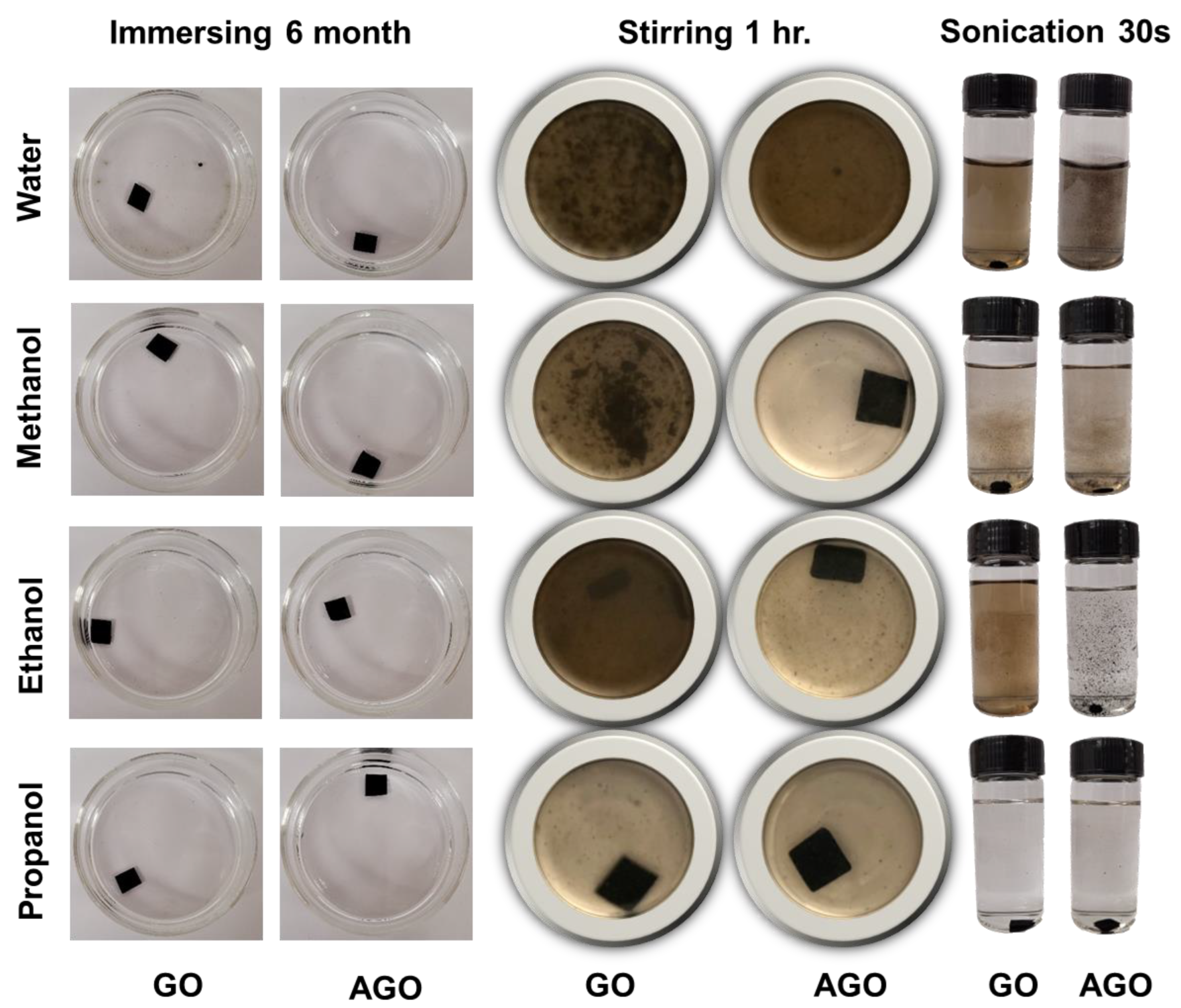
In this study, the stabilities of GO and AGO membranes in water and three different polar solvents were assessed under three different degrees of agitation including soaking, stirring, and sonication. The results of this study are illustrated in the digital camera photos shown in
Figure 3 and
Figures S5–S8. Compared to GO membranes typically prepared through the vacuum filtration process, GO membranes prepared with layer-by-layer self-assembly demonstrate better stability due to the greater number of hydrogen bonds formed between individual GO sheets during the slow-drying process [
2,
3]. Specifically, previous studies demonstrated that GO membranes formed via vacuum filtration are only stable in water for up to an hour after soaking [
2]. The results here show that the as-fabricated unmodified GO membranes are stable and maintain their original morphologies after being soaked in pure water as well as methanol, ethanol, and propanol for more than 6 months (
Figure 3, column 1;
Figure S5) [
24].
However, agitation of the membranes decreased their stability over time. Under a sonication frequency of 60 Hz, the GO membranes in water, methanol, and ethanol disintegrated within 30 s of sonication(
Figure 3, column 5), while the GO membranes in propanol maintained their integrity during 60 s of sonication (
Figure S6). Agitation of the membranes via stirring (
Figure 3, column 3), which is more representative of a filtration working environment, resulted in GO membranes that were less stable than those soaked in the solvents with no agitation, but more stable than those subjected to sonication. This result is consistent with the fact that the shear force generated by stirring is much lower than the shear force generated through sonication. During stirring, the disintegration of the GO membrane in water exhibited an edge-to-center (intra-layer) disassembly pattern rather than a layer-by-layer (inter-layer) disassembly pattern when the time of stirring increased. The complete dispersion of the unmodified GO membrane in water required about 96 h of stirring. The rate of membrane agitation, and thus rate of disintegration, was adjusted by increasing the size of the stir bar. Because the solvent volume and sample vial used remained the same, the increase in the stir bar size resulted in collisions between the stir bar and membrane during each stir bar rotation. For GO membranes in water, membrane disintegration became visible after 20 min of stirring with a larger stir bar, with complete dispersion occurring within 1 h (
Figure S7). Here, the rate of membrane disintegration was much faster than for membranes agitated by a smaller stir bar because the membrane was exposed to both the force of the shear stress and the impact of the stir bar (
Figures S7 and S8).
As seen in
Figure 3, the stability of the unmodified GO membrane decreased with decreasing solvent hydrophobicity, such that the membrane was most stable in propanol and least stable in water. The instability of the GO membrane in aqueous solution results from the hydration of OFGs that are both exposed on the surface of the membrane and embedded in the membrane interior [
34]. Specifically, hydrogen bonding, ion-dipole, and dipole–dipole interactions between water molecules and hydrophilic OFGs on the surface of the GO membrane can overcome the relatively weak van der Waals and hydrogen bonding interactions that hold the surface-exposed sheets together. Additionally, the size of a water molecule is likely smaller than the inter-sheet lamellar distance, or intra-sheet framework space, and so it can permeate into the membrane interior. Here, interactions between water molecules and OFGs located in the membrane interior can increase the inter-sheet distances, thereby weakening the π-π interactions that hold individual sheets together in the membrane. The increase in membrane stability with increasing solvent hydrophobicity is likely due to both a decrease in the extent of interactions between the solvent alcohol groups and surface-exposed membrane OFGs and an inability to permeate into the membrane interior due to the increasing molecular size beyond that of the critical lamellar distance.
Previous work demonstrated that the water stability AGO membranes formed via pre-coordination followed by vacuum filtration is only improved under soaking conditions for up to a few hours [
14,
22]. In our study, AGO membranes that were formed via pre-coordination followed by a slow layer-by-layer self-assembly process remained stable under soaking conditions in water, methanol, ethanol, and propanol for more than 6 months (
Figure S5). Thus, to assess the effect of Al
3+ modification on GO membrane stability, the responses of the as-prepared GO and AGO membranes to stirring and sonication in the different polar solvents were compared (
Figure 3 and
Figures S6–S8). These results show that when the membranes are agitated by both stirring and sonication, the AGO membranes show a better stability and survive for longer than the unmodified GO membranes in all four different polar solvents.
However, consistent with previous studies of GO membranes formed with vacuum filtration, improvements in membrane stability were not dramatic when GO membranes were modified with Al
3+ via the pre-coordination method [
2]. As seen in
Figures S6–S8, the AGO membranes still disintegrated in water, methanol, and ethanol upon increasing the time of stirring and sonication. Both GO and AGO membranes showed good stability in propanol and did not disintegrate after 60 s of sonication. Moreover, the process by which the GO and AGO membranes dissolved in pure water under stirring and sonication were observed to be quite different. In water, the GO membrane disintegrated uniformly into a well-dispersed brown solution with no visible particles or flakes, as observed by the eye. Under the same conditions as the GO membrane, the AGO membrane disintegrated non-uniformly into large black flakes consisting of AGO sheet aggregates that could be seen by eye. In the water solution, the disintegrated AGO membrane existed as large black colored flakes. After sonication and storage, these flakes persisted in water, methanol, and ethanol (
Figure S6). The persistence of the AGO flakes compared to the uniformly dispersed GO membranes further demonstrates that Al
3+ modification increases the stability of GO membranes in polar solvents. This increased membrane stability was achieved by replacing inter-sheet hydrogen bonds with cation-to-OFGs coordinate covalent bonds, cation-π interactions, and electrostatic interactions. In addition to decreasing membrane hydrophilicity by screening the negative charges on the membrane surface, these interactions are overall stronger than inter-sheet hydrogen bonds. Both factors are critical for increasing the stability of GO membranes in polar solvents.
It is likely that the limited stability improvement of the AGO membrane is in part due to its reduced water permeability. The AGO membranes modified with Al
3+ from Al foil exhibited increasing membrane wrinkling, shrinking, and cracking after increasing the Al wt.% in the GO solution (
Figure S4). The shrinking of the AGO membrane supports our previous report [
24], which showed a decrease in the intra-sheet spacing after increasing the Al
3+ wt.%. This membrane diameter decrease is consistent with stronger intra-layer interactions and increased membrane stability. However, the highly wrinkled and contracted AGO membrane exhibited swelling after being fully soaked in water, suggesting that the membrane is permeable to water (
Figure S9). The fact that water can intercalate into the membrane interior likely prevents the membrane from being completely stable in water under all agitation conditions. The extent of AGO membrane swelling compared to the extent of GO membrane swelling in ultrapure water could not be reliably quantified (
Figure S10); therefore, it was difficult to assess the effect of Al
3+ modification on membrane permeability purely from soaking in water.
In summary, the unmodified GO membrane prepared using the slow layer-by-layer self-assembling process can maintain its integrity when soaked in water, methanol, ethanol, and propanol, but will severely disintegrate and disperse into water, methanol, or ethanol within 30 s of vigorous sonication or 2 h of stirring. The stability of GO in methanol and ethanol is better than that in water, while the best stability is observed in propanol. Overall, AGO membranes demonstrated enhanced stability in water, methanol, and ethanol, but this improvement was limited.
3.3. Membrane Permeability
In this study, the membrane permeability of both GO and AGO to water, methanol, ethanol, and propanol vapor was assessed by measuring the loss of solvent mass from a bottle that was capped with the membrane of interest (
Figure S1). From the loss of mass, the flux,
J, was calculated using Equation (2), and subsequently the diffusion coefficient,
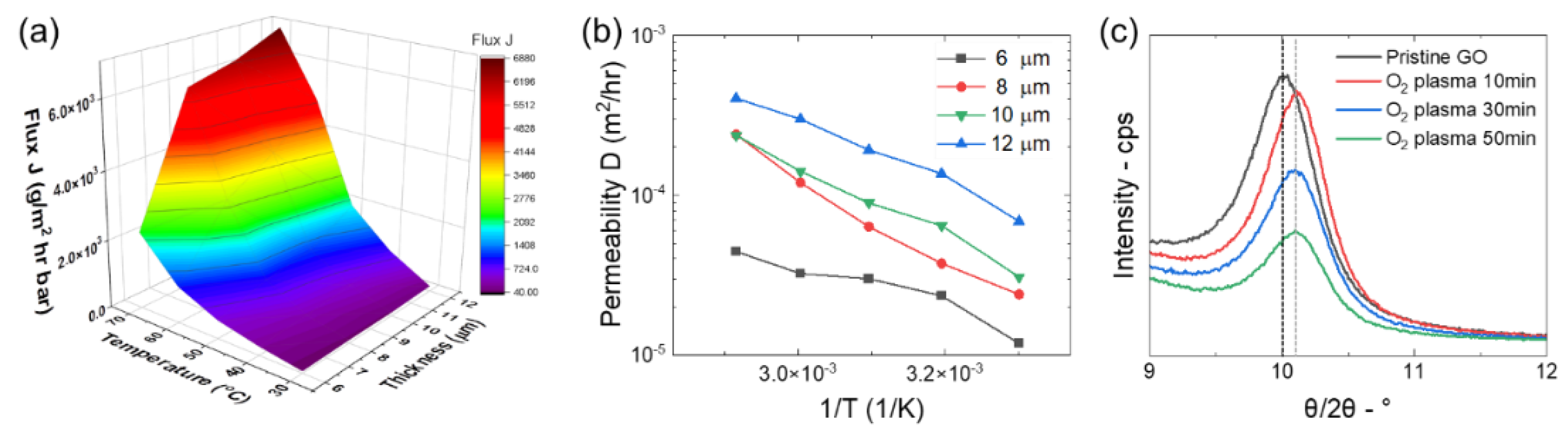
D, was derived using Equation (3), which was equated to the membrane permeability. The flux of water vapor passing through membranes with thicknesses ranging from 6 µM to 12 µM and at temperatures ranging from 30 to 70 °C was calculated first (
Figure 4a). We were unable to measure the permeability of thinner membranes due to their fragility and thicker membranes due to their extensive wrinkling and inability to properly seal the permeability set-up. It is expected that as the temperature increases, the flux of water vapor through the membrane will increase due to an increase in the kinetic energy, and thus speed, of the vapor molecules passing through the membrane. Moreover, as membrane thickness increases, it is expected from Fick’s Law that the flux of water vapor through the membrane will decrease due to an increase in the amount of time the molecules can spend in the thicker membrane. As expected, the flux of water vapor through the GO membrane increased with an increasing temperature; however, the flux also increased with increasing membrane thickness. The flux data were used to calculate the permeability,
D, for membranes at each thickness and temperature (
Figure 4b). These data indicate that the permeability also increased with increasing temperatures and membrane thickness.
The increase in permeability with increasing membrane thickness was unexpected, and likely resulted from how the individual GO sheets were structured differently at the surface of the membrane compared to the interior of the membrane during drying in the layer-by-layer self-assembly process. As suggested in the surface tension data and observed during the experiment, GO sheets accumulated at the air–water interface and dried first from the surface to the interior during the self-assembly process. To explore the effect of membrane thickness on the inter-sheet structure at the surface of the GO membrane, XRD spectra of GO membranes were collected with varying thicknesses (
Figure S11). These results reveal no obvious trend in the average interplanar distance of GO membranes with different thicknesses. Thus, it is likely that the surface structure of the membrane does not depend on the membrane thickness. However, it is possible that the membrane surface structure is significantly different than the structure of the membrane interior, which could affect the flux of water through membranes with different thicknesses.
To determine the difference of the inter-sheet structure between the GO membrane surface and GO membrane interior, XRD spectra of membranes were collected before and after exposure to oxygen plasma for varying times (
Figure 4c). Here, a radio-frequency oxygen plasma at the processing power of 60 W from 10 to 60 min was applied to remove the surface of the GO membrane before each XRD scan. The resultant spectra show a slight shift of the peak from 10.01°, corresponding to an average interlayer distance of 8.826 nm, to 10.11°, corresponding to an average interlayer distance of 8.739 nm. The decrease in the average inter-sheet distance after removal of the surface suggests that the sheets near the membrane surface are somewhat more densely packed in the vertical direction than sheets in the membrane interior. The formation of the densely packed membrane interior occurs during the membrane drying process. It is likely that the GO sheets that assemble at the air–water interface form a permeation layer that inhibits the evaporation of water molecules from the bulk solution and slows down the drying process. This can result in a uniformly assembled membrane interior structure through which water molecules have to slowly permeate before reaching the surface.
It is expected that as the membrane thickness increases and the interior structure rather than the surface structure dominates the properties of the membrane, permeability decreases due to the decrease in the inter-sheet distance in the membrane interior. However, the permeation channel is controlled by the inter-sheet distance, the intra-sheet spacing, and vacancy defects on the GO sheet. Specifically, and as we show in our AGO studies below, an increase in the inter-sheet distance does not increase the membrane permeability due to the decreased intra-sheet spacing. The finding that the permeation coefficient increases with membrane thickness and larger inter-sheet distances between sheets near the surface than in the interior supports our hypothesis that the inter-sheet distance does not dominate the permeation channel of the GO membrane. However, the intra-sheet structure difference between the surface and the interior of the membrane is unclear. Moreover, it is also unclear if the ambient oxidation of the surface during the week-long drying process occurs, which could also affect the nanochannel structure.
Previous reports revealed that metal cation-decorated GO membranes exhibited selective permeation to organic solvents [
27], which is consistent with the results presented here. To determine the effect of Al
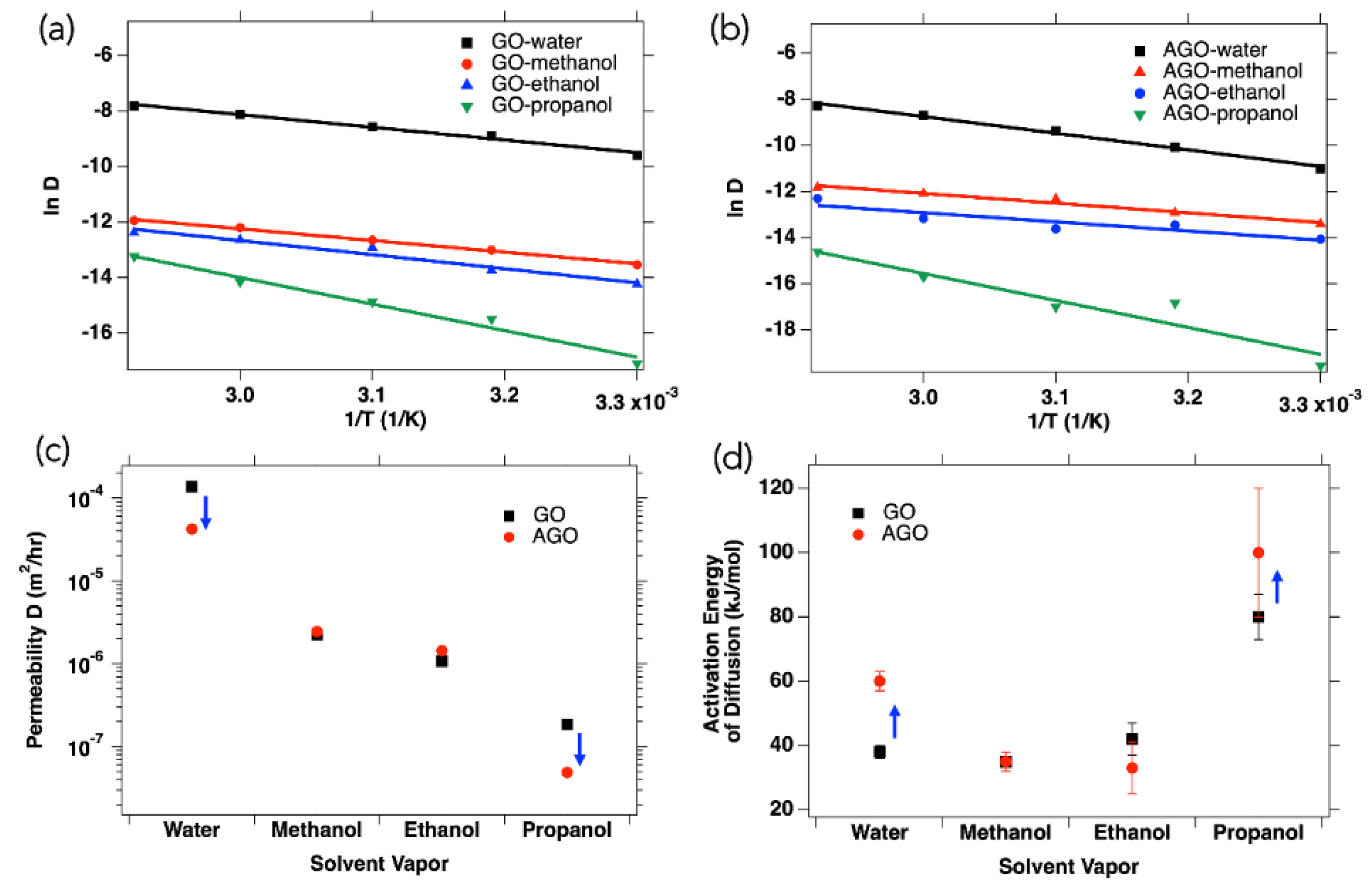
3+ modification on membrane permeability, the permeabilities of 12 μm thick GO and AGO membranes to four different polar solvents at temperatures ranging from 30 °C to 70 °C were compared (
Figure 5). A summary of the temperature-dependent data is provided in
Figure S12. Here, the temperature dependent permeability data for the GO membrane (
Figure 5a) and the AGO membrane (
Figure 5b) were plotted according to the Arrhenius equation (Equation (4)) to better understand the kinetic parameters affecting GO and AGO membrane permeability to different polar solvents.
Here,
D° is the pre-exponential factor,
Qd is the energy of activation of diffusion,
T is the absolute temperature in Kelvin, and
R (8.314 J/mol K) is the gas constant. As expected, the permeability of both GO and AGO to all solvents increased with increased temperature. The GO membrane has a water vapor flux of 68 ± 5 g/m
2h at 30 °C, which is consistent with other reports [
11]. It is impressive that the flux of water vapor across the 12 µm thick GO membranes can go up to 600 g/m
2h at 70 °C, which is almost the same as the vaporization rate of water at the same temperature.
At all temperatures studied, both membranes showed an increase in permeability with a decreasing molecular size, such that the 12 µm thick GO and AGO membranes were most permeable to water and least permeable to propanol. Without the effect of hydration by water vapor present in the atmosphere, the GO membrane permeabilities to methanol, ethanol, and propanol (
Figure 5c) were 1.32 × 10
−6, 6.57 × 10
−7, and 3.76 × 10
−8 m
2/h, respectively, at 30 °C, which were much lower that the water vapor permeability at the same temperature (6.85 × 10
−6 m
2/h). This result is consistent with the stability studies, which showed that membranes were most stable in propanol and least stable in water under vigorous stirring and sonication. A similar explanation to that of the the stability data can be used for the permeability data. Specifically, the GO membrane was most permeable to water because it is likely that the nanochannels are larger than the size of a water molecule. As the molecular size increases relative to the size of the nanochannels, the vapor becomes less permeable to the membrane. Another possible effect that was previously reported is the effect of water vapor present in the atmosphere on membrane permeability [
11,
27]. In this case, intercalating water molecules in GO-based membranes either block or impede the alcohol molecules from passing through the membranes. As seen in
Figure 5c, Al
3+ modification decreased membrane permeability to both water and propanol vapor, while having little effect on permeability to methanol and ethanol vapor. At 30 °C, the permeability to water decreased to 1.65 × 10
−6 m
2/h (76% decrease) and the permeability to propanol decreased to 3.20 × 10
−9 m
2/h (91% decrease).
To see if the change in membrane permeability to different solvents was related to the activation energy of diffusion, the lnD vs. 1/T data in
Figure 5a,b were fit to Equation (4). The activation energies, which can be used to quantify the energy barrier, that the vapor molecule must overcome to diffuse through the GO or AGO membrane are reported in
Figure 5d. From these results, it can be seen that upon Al
3+ modification of the GO membrane, the activation energy of water vapor diffusion increased from 38 ± 2 kJ/mol to 60 ± 3 kJ/mol, the activation energy of propanol diffusion increased from 80 ± 7 kJ/mol to 100 ± 20 kJ/mol, and the activation energies for methanol and ethanol diffusion did not significantly change. Thus, it was possible to relate a decrease in membrane permeability to an increase in the activation energy of diffusion for a given solvent through the membrane.
The decrease in GO membrane permeability to water, and the corresponding increase in the activation energy of diffusion, upon Al
3+ modification, is consistent with our previous studies of GO and AGO membranes formed via the layer-by-layer self-assembly method [
24]. Our studies demonstrated that modification via post-cation intercalation leads to GO membranes that possess controllable thicknesses and interlayer spacings. Specifically, it was discovered that crosslinking Al
3+ to the OFGs in the GO membrane resulted in an increase in the inter-layer distance and a decrease in intra-layer spacing. The intra-layer framework of the nanochannels impacted the water vapor flow more than the inter-layer gallery, which resulted in both longer and narrower nanochannels than those in unmodified membranes as shown in
Figure 1. Moreover, the surface tension and contact angle data presented here indicate that Al
3+ modification increases the hydrophobicity of the GO membrane due to interactions between the cations and the negatively charged OFGs. Both a decrease in intra-layer spacing and an increase in membrane hydrophobicity can decrease membrane permeability to water vapor. In the first case, the narrowing of the nanochannels due to Al
3+ crosslinking can block water molecules from permeating through the membrane. In the second case, the edge-to-edge coordination of Al
3+ with the carboxylate groups on the edges of the GO sheets can inhibit permeability by consuming the OFGs of GO sheets such that water molecules cannot interact with these hydrophilic groups. For methanol, ethanol, and propanol, which are all more hydrophobic than water, it is likely that the increase in membrane hydrophobicity upon Al
3+ modification does not greatly affect GO membrane permeability to these solvent vapors. For propanol, however, it is likely that the decrease in intra-layer spacing greatly reduces the GO membrane permeability to this relatively large vapor molecule, whereas no significant effect is observed in the GO membrane permeability to the smaller methanol and ethanol vapor molecules upon Al
3+ modification.