Targeting the Structural Integrity of Extracellular Vesicles via Nano Electrospray Gas-Phase Electrophoretic Mobility Molecular Analysis (nES GEMMA)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. EV Enrichment
2.2.1. Enrichment of Monocytic EVs (mEVs)
2.2.2. Enrichment of MSC-Derived EVs (mscEVs)
2.2.3. Enrichment of EVs from Red Blood Cells (RBCs)
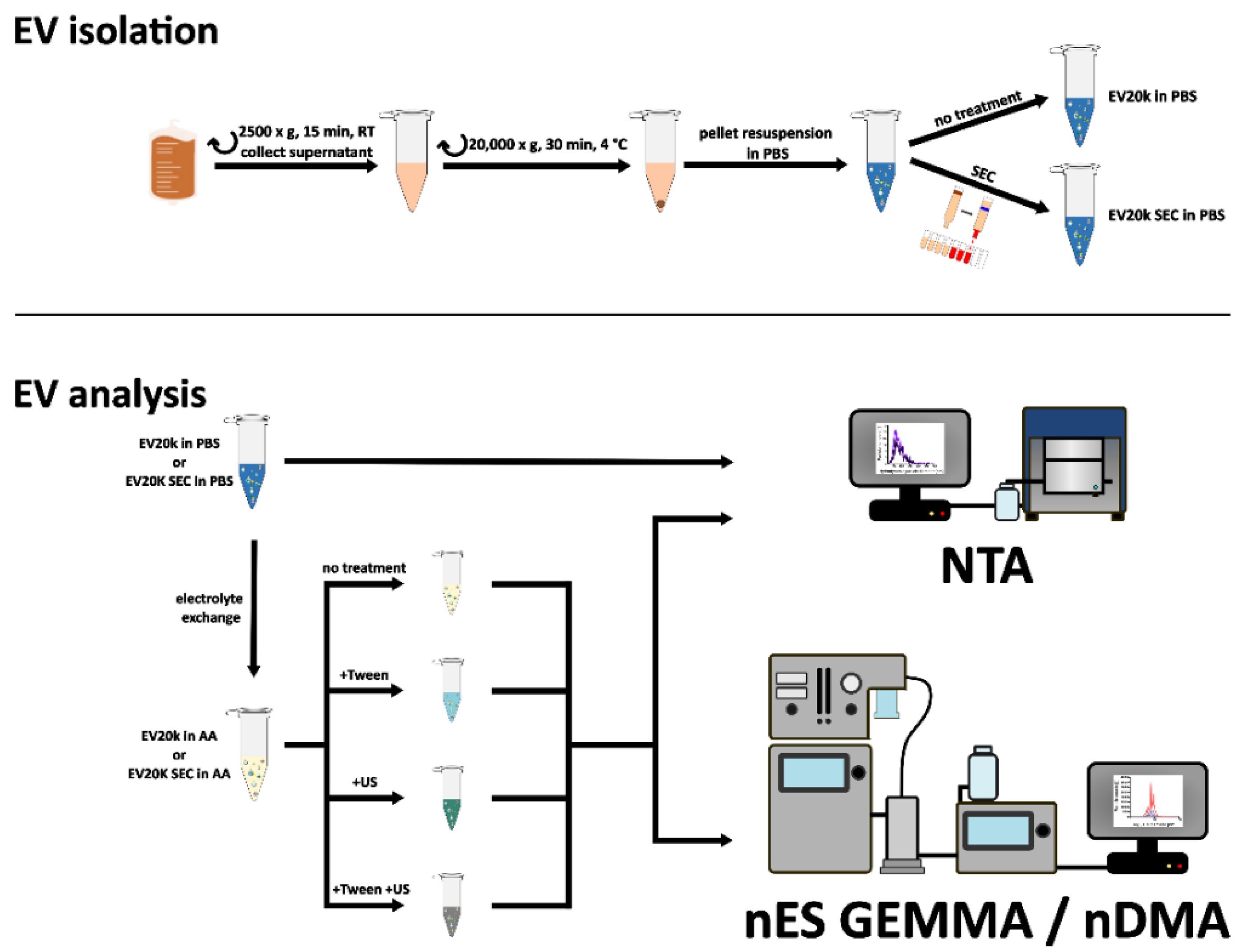
2.3. Isolation of EVs
2.4. Size Exclusion Chromatography (SEC)
2.5. Flow Cytometric Characterization of Blood-Derived EVs
2.6. nES GEMMA Measurements
2.7. Nanoparticle Tracking Analysis (NTA)
2.8. EV Disruption
3. Results and Discussion
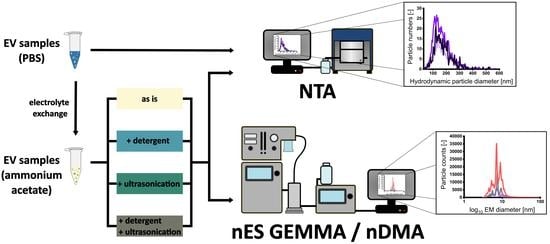
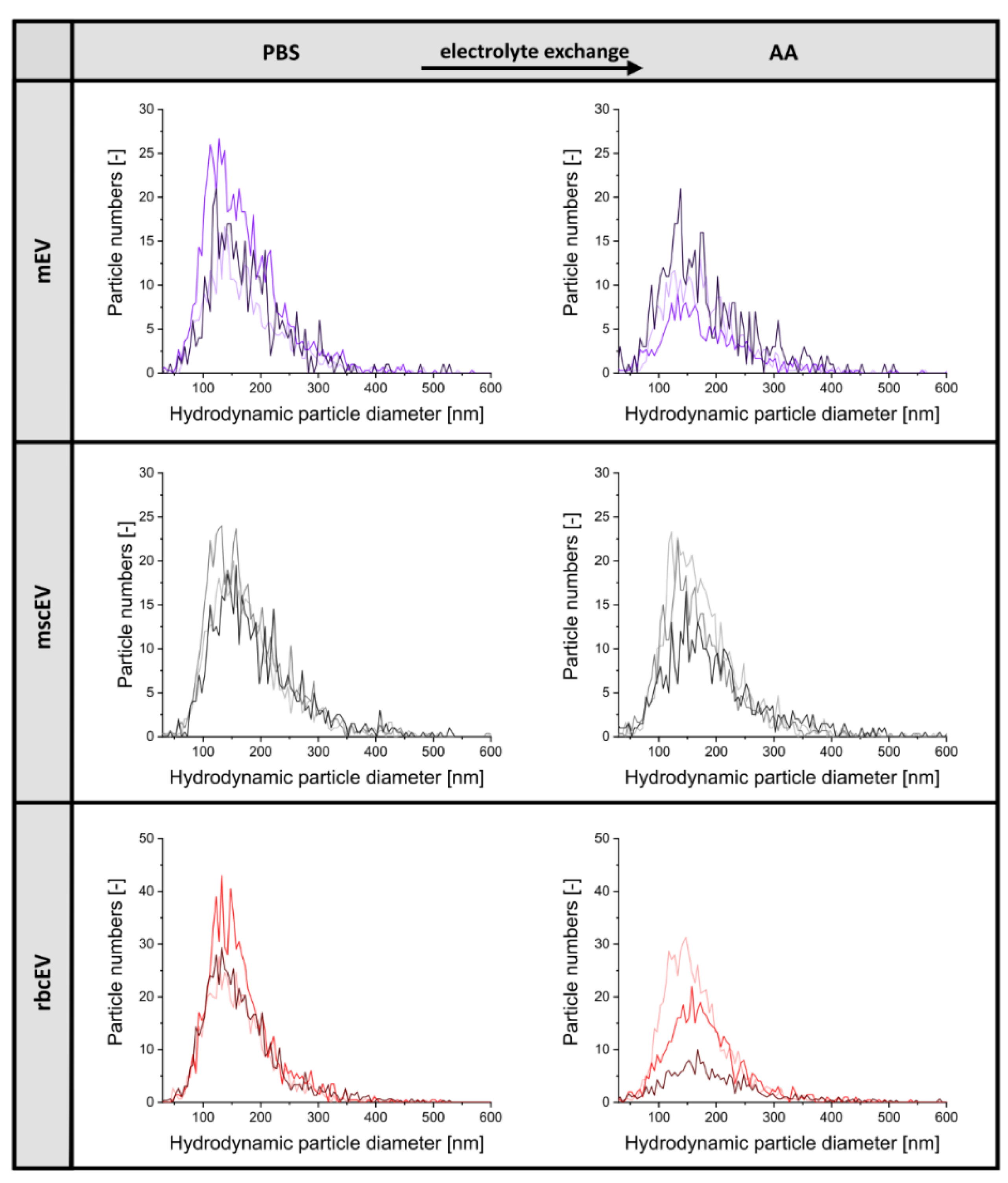
3.1. Sample Preparation of EV Material from Various Cellular Sources for Subsequent nES GEMMA Analysis
3.2. Gas-Phase Electrophoresis of EV-Containing Samples
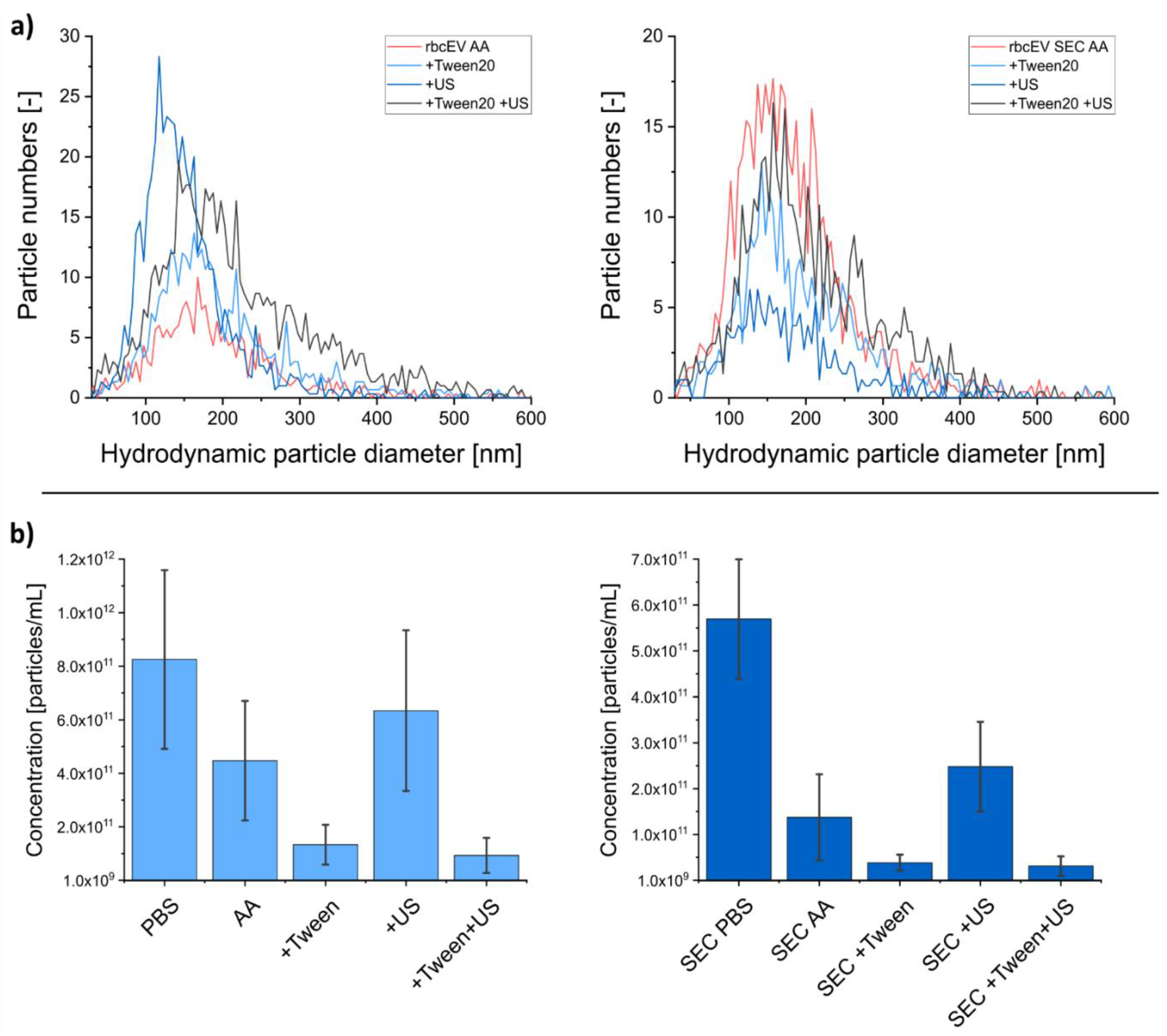
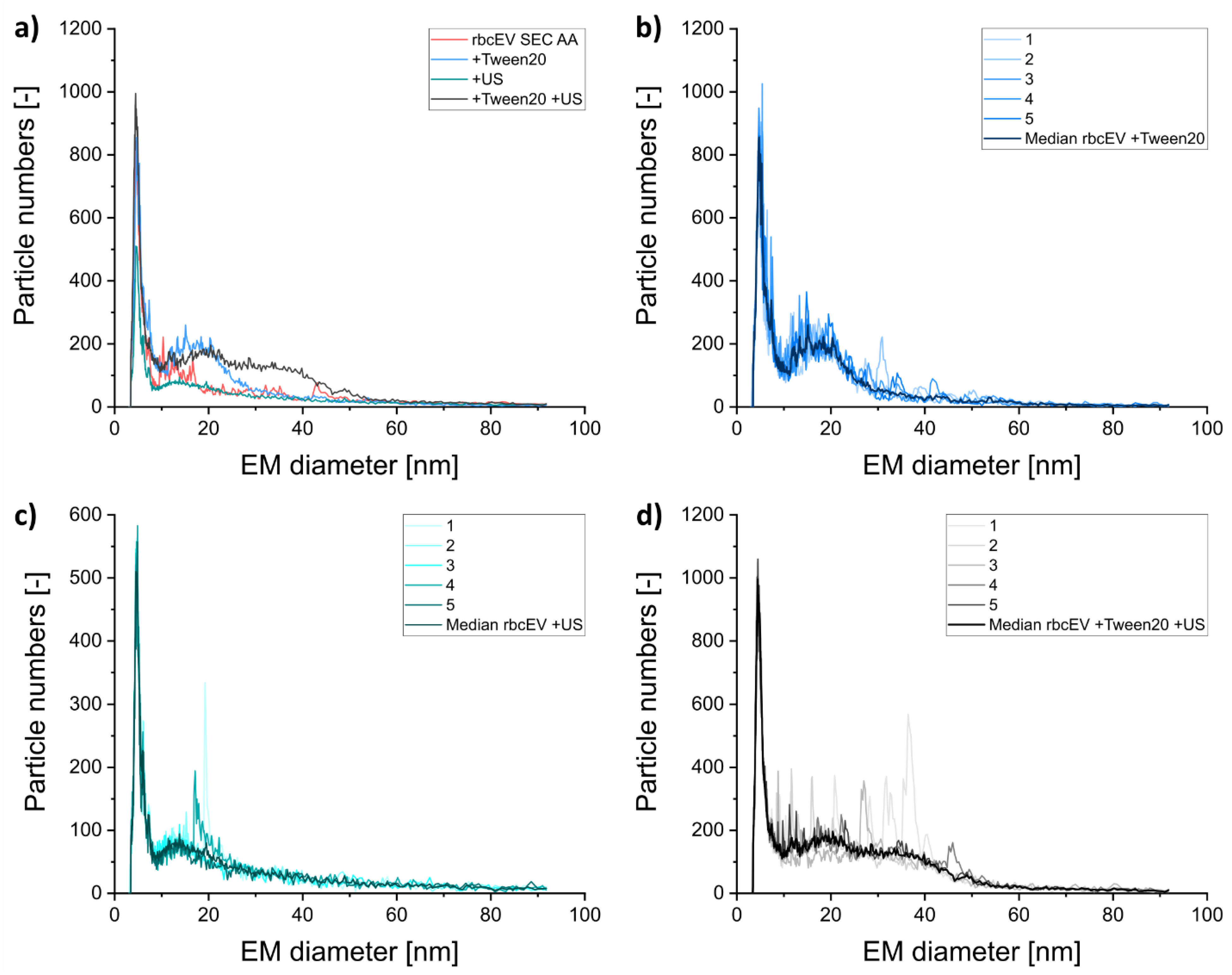
3.3. Including Further EV Purification Steps in Sample Pretreatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 2016, 30, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Colombo, E.; Borgiani, B.; Verderio, C.; Furlan, R. Microvesicles: Novel biomarkers for neurological disorders. Front. Physiol. 2012, 3, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Thery, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Schatz, D.; Rosenwasser, S.; Malitsky, S.; Wolf, S.G.; Feldmesser, E.; Vardi, A. Communication via extracellular vesicles enhances viral infection of a cosmopolitan alga. Nat. Microbiol. 2017, 2, 1485–1492. [Google Scholar] [CrossRef]
- Mehanny, M.; Lehr, C.M.; Fuhrmann, G. Extracellular vesicles as antigen carriers for novel vaccination avenues. Adv. Drug Deliv. Rev. 2021, 173, 164–180. [Google Scholar] [CrossRef]
- Armstrong, J.P.; Holme, M.N.; Stevens, M.M. Re-Engineering Extracellular Vesicles as Smart Nanoscale Therapeutics. ACS Nano 2017, 11, 69–83. [Google Scholar] [CrossRef]
- Armstrong, J.P.K.; Stevens, M.M. Strategic design of extracellular vesicle drug delivery systems. Adv. Drug Deliv. Rev. 2018, 130, 12–16. [Google Scholar] [CrossRef]
- Havlik, M.; Marchetti-Deschmann, M.; Friedbacher, G.; Messner, P.; Winkler, W.; Perez-Burgos, L.; Tauer, C.; Allmaier, G. Development of a bio-analytical strategy for characterization of vaccine particles combining SEC and nanoES GEMMA. Analyst 2014, 139, 1412–1419. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gamez-Valero, A.; Monguio-Tortajada, M.; Carreras-Planella, L.; Franquesa, M.; Beyer, K.; Borras, F.E. Size-Exclusion Chromatography-based isolation minimally alters Extracellular Vesicles’ characteristics compared to precipitating agents. Sci. Rep. 2016, 6, 33641–33649. [Google Scholar] [CrossRef]
- Gyorgy, B.; Szabo, T.G.; Pasztoi, M.; Pal, Z.; Misjak, P.; Aradi, B.; Laszlo, V.; Pallinger, E.; Pap, E.; Kittel, A.; et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell Mol. Life Sci. 2011, 68, 2667–2688. [Google Scholar] [CrossRef]
- Dragovic, R.A.; Gardiner, C.; Brooks, A.S.; Tannetta, D.S.; Ferguson, D.J.; Hole, P.; Carr, B.; Redman, C.W.; Harris, A.L.; Dobson, P.J.; et al. Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis. Nanomedicine 2011, 7, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, C.; Ferreira, Y.J.; Dragovic, R.A.; Redman, C.W.; Sargent, I.L. Extracellular vesicle sizing and enumeration by nanoparticle tracking analysis. J. Extracell. Vesicles 2013, 2, 19671. [Google Scholar] [CrossRef]
- van der Pol, E.; Coumans, F.A.; Grootemaat, A.E.; Gardiner, C.; Sargent, I.L.; Harrison, P.; Sturk, A.; van Leeuwen, T.G.; Nieuwland, R. Particle size distribution of exosomes and microvesicles determined by transmission electron microscopy, flow cytometry, nanoparticle tracking analysis, and resistive pulse sensing. J. Thromb. Haemost. 2014, 12, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- van der Pol, E.; Hoekstra, A.G.; Sturk, A.; Otto, C.; van Leeuwen, T.G.; Nieuwland, R. Optical and non-optical methods for detection and characterization of microparticles and exosomes. J. Thromb. Haemost. 2010, 8, 2596–2607. [Google Scholar] [CrossRef]
- Morani, M.; Mai, T.D.; Krupova, Z.; Defrenaix, P.; Multia, E.; Riekkola, M.L.; Taverna, M. Electrokinetic characterization of extracellular vesicles with capillary electrophoresis: A new tool for their identification and quantification. Anal. Chim. Acta 2020, 1128, 42–51. [Google Scholar] [CrossRef]
- Piotrowska, M.; Ciura, K.; Zalewska, M.; Dawid, M.; Correia, B.; Sawicka, P.; Lewczuk, B.; Kasprzyk, J.; Sola, L.; Piekoszewski, W.; et al. Capillary zone electrophoresis of bacterial extracellular vesicles: A proof of concept. J. Chromatogr. A 2020, 1621, 461047–461055. [Google Scholar] [CrossRef]
- Kaufman, S.L.; Skogen, J.W.; Dorman, F.D.; Zarrin, F.; Lewis, K.C. Macromolecule analysis based on electrophoretic mobility in air: Globular proteins. Anal. Chem. 1996, 68, 1895–1904. [Google Scholar] [CrossRef]
- Allmaier, G.; Weiss, V.U.; Engel, N.Y.; Marchetti-Deschmann, M.; Szymanski, W.W. Soft X-ray Radiation Applied in the Analysis of Intact Viruses and Antibodies by Means of Nano Electrospray Differential Mobility Analysis. Nato. Sci. Peace Sec. A 2017, 149–157. [Google Scholar]
- Kallinger, P.; Szymanski, W.W. Experimental determination of the steady-state charging probabilities and particle size conservation in non-radioactive and radioactive bipolar aerosol chargers in the size range of 5–40 nm. J. Nanopart. Res. 2015, 17, 171–182. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weiss, V.U.; Frank, J.; Piplits, K.; Szymanski, W.W.; Allmaier, G. Bipolar Corona Discharge-Based Charge Equilibration for Nano Electrospray Gas-Phase Electrophoretic Mobility Molecular Analysis of Bio- and Polymer Nanoparticles. Anal. Chem. 2020, 92, 8665–8669. [Google Scholar] [CrossRef]
- Flagan, R.C. History of electrical aerosol measurements. Aerosol. Sci. Tech. 1998, 28, 301–380. [Google Scholar] [CrossRef]
- Chen, D.R.; Pui, D.Y.H.; Hummes, D.; Fissan, H.; Quant, F.R.; Sem, G.J. Design and evaluation of a nanometer aerosol differential mobility analyzer (Nano-DMA). J. Aerosol. Sci. 1998, 29, 497–509. [Google Scholar] [CrossRef]
- Elzey, S.; Tsai, D.H.; Yu, L.L.; Winchester, M.R.; Kelley, M.E.; Hackley, V.A. Real-time size discrimination and elemental analysis of gold nanoparticles using ES-DMA coupled to ICP-MS. Anal. Bioanal. Chem. 2013, 405, 2279–2288. [Google Scholar] [CrossRef]
- Carazzone, C.; Raml, R.; Pergantis, S.A. Nanoelectrospray ion mobility spectrometry online with inductively coupled plasma-mass spectrometry for sizing large proteins, DNA, and nanoparticles. Anal. Chem. 2008, 80, 5812–5818. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.B.; Orf, G.S.; Reisch, S.; Harrington, L.B.; Prado, M.; Blankenship, R.E.; Biswas, P. Characterization and deposition of various light-harvesting antenna complexes by electrospray atomization. Anal. Bioanal. Chem. 2012, 404, 2329–2338. [Google Scholar] [CrossRef]
- Bacher, G.; Szymanski, W.W.; Kaufman, S.L.; Zollner, P.; Blaas, D.; Allmaier, G. Charge-reduced nano electrospray ionization combined with differential mobility analysis of peptides, proteins, glycoproteins, noncovalent protein complexes and viruses. J. Mass Spectrom. 2001, 36, 1038–1052. [Google Scholar] [CrossRef]
- Weiss, V.U.; Balantic, K.; Pittenauer, E.; Tripisciano, C.; Friedbacher, G.; Weber, V.; Marchetti-Deschmann, M.; Allmaier, G. Nano electrospray differential mobility analysis based size-selection of liposomes and very-low density lipoprotein particles for offline hyphenation to MALDI mass spectrometry. J. Pharmaceut. Biomed. 2020, 179, 112998–113005. [Google Scholar] [CrossRef]
- Weiss, V.U.; Urey, C.; Gondikas, A.; Golesne, M.; Friedbacher, G.; von der Kammer, F.; Hofmann, T.; Andersson, R.; Marko-Varga, G.; Marchetti-Deschmann, M.; et al. Nano electrospray gas-phase electrophoretic mobility molecular analysis (nES GEMMA) of liposomes: Applicability of the technique for nano vesicle batch control. Analyst 2016, 141, 6042–6050. [Google Scholar] [CrossRef]
- Weiss, V.U.; Wieland, K.; Schwaighofer, A.; Lendl, B.; Allmaier, G. Native Nano-electrospray Differential Mobility Analyzer (nES GEMMA) Enables Size Selection of Liposomal Nanocarriers Combined with Subsequent Direct Spectroscopic Analysis. Anal. Chem. 2019, 91, 3860–3868. [Google Scholar] [CrossRef] [PubMed]
- Weiss, V.U.; Pogan, R.; Zoratto, S.; Bond, K.M.; Boulanger, P.; Jarrold, M.F.; Lyktey, N.; Pahl, D.; Puffler, N.; Schelhaas, M.; et al. Virus-like particle size and molecular weight/mass determination applying gas-phase electrophoresis (native nES GEMMA). Anal. Bioanal. Chem. 2019, 411, 5951–5962. [Google Scholar] [CrossRef] [PubMed]
- Zoratto, S.; Weiss, V.U.; Friedbacher, G.; Buengener, C.; Pletzenauer, R.; Foettinger-Vacha, A.; Graninger, M.; Allmaier, G. Adeno-associated Virus Virus-like Particle Characterization via Orthogonal Methods: Nanoelectrospray Differential Mobility Analysis, Asymmetric Flow Field-Flow Fractionation, and Atomic Force Microscopy. ACS Omega 2021, 6, 16428–16437. [Google Scholar] [CrossRef] [PubMed]
- Chernyshev, V.S.; Rachamadugu, R.; Tseng, Y.H.; Belnap, D.M.; Jia, Y.; Branch, K.J.; Butterfield, A.E.; Pease, L.F., 3rd; Bernard, P.S.; Skliar, M. Size and shape characterization of hydrated and desiccated exosomes. Anal. Bioanal. Chem. 2015, 407, 3285–3301. [Google Scholar] [CrossRef]
- Brennan, K.; Martin, K.; FitzGerald, S.P.; O’Sullivan, J.; Wu, Y.; Blanco, A.; Richardson, C.; Mc Gee, M.M. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci. Rep. 2020, 10, 1039–1051. [Google Scholar] [CrossRef]
- Fendl, B.; Weiss, R.; Eichhorn, T.; Spittler, A.; Fischer, M.B.; Weber, V. Storage of human whole blood, but not isolated monocytes, preserves the distribution of monocyte subsets. Biochem. Biophys. Res. Commun. 2019, 517, 709–714. [Google Scholar] [CrossRef]
- Osteikoetxea, X.; Sodar, B.; Nemeth, A.; Szabo-Taylor, K.; Paloczi, K.; Vukman, K.V.; Tamasi, V.; Balogh, A.; Kittel, A.; Pallinger, E.; et al. Differential detergent sensitivity of extracellular vesicle subpopulations. Org. Biomol. Chem. 2015, 13, 9775–9782. [Google Scholar] [CrossRef]
- Deville, S.; Berckmans, P.; Van Hoof, R.; Lambrichts, I.; Salvati, A.; Nelissen, I. Comparison of extracellular vesicle isolation and storage methods using high-sensitivity flow cytometry. PLoS ONE 2021, 16, e0245835. [Google Scholar] [CrossRef]
- Tripisciano, C.; Weiss, R.; George, S.K.L.; Fischer, M.B.; Weber, V. Extracellular Vesicles Derived from Platelets, Red Blood Cells, and Monocyte-Like Cells Differ Regarding Their Ability to Induce Factor XII-Dependent Thrombin Generation. Front. Cell Dev. Biol. 2020, 8, 298–308. [Google Scholar] [CrossRef]
- Almeria, C.; Weiss, R.; Roy, M.; Tripisciano, C.; Kasper, C.; Weber, V.; Egger, D. Hypoxia Conditioned Mesenchymal Stem Cell-Derived Extracellular Vesicles Induce Increased Vascular Tube Formation In Vitro. Front. Bioeng. Biotechnol. 2019, 7, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Kitka, D.; Mihály, J.; Fraikin, J.L.; Beke-Somfai, T.; Varga, Z. Detection and phenotyping of extracellular vesicles by size exclusion chromatography coupled with on-line fluorescence detection. Sci. Rep. 2019, 9, 19868–19874. [Google Scholar] [CrossRef] [PubMed]
- Tripisciano, C.; Weiss, R.; Eichhorn, T.; Spittler, A.; Heuser, T.; Fischer, M.B.; Weber, V. Different Potential of Extracellular Vesicles to Support Thrombin Generation: Contributions of Phosphatidylserine, Tissue Factor, and Cellular Origin. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.; Gröger, M.; Rauscher, S.; Fendl, B.; Eichhorn, T.; Fischer, M.B.; Spittler, A.; Weber, V. Differential Interaction of Platelet-Derived Extracellular Vesicles with Leukocyte Subsets in Human Whole Blood. Sci. Rep. 2018, 8, 6598–6608. [Google Scholar] [CrossRef] [PubMed]
- George, S.K.L.L.; Weiss, R.; Semak, V.; Fendl, B.; Weiss, V.U.; Steinberger, S.; Allmaier, G.; Tripisciano, C.; Weber, V. Comparative Analysis of Platelet-Derived Extracellular Vesicles Using Flow Cytometry and Nanoparticle Tracking Analysis. Int. J. Mol. Sci. 2021, 22, 3839. [Google Scholar] [CrossRef] [PubMed]
- Tycova, A.; Prikryl, J.; Foret, F. Reproducible preparation of nanospray tips for capillary electrophoresis coupled to mass spectrometry using 3D printed grinding device. Electrophoresis 2016, 37, 924–930. [Google Scholar] [CrossRef]
- Steinberger, S.; George, S.K.; Laukova, L.; Weiss, R.; Tripisciano, C.; Birner-Gruenberger, R.; Weber, V.; Allmaier, G.; Weiss, V.U. A possible role of gas-phase electrophoretic mobility molecular analysis (nES GEMMA) in extracellular vesicle research. Anal. Bioanal. Chem. 2021, 413, 7341–7352. [Google Scholar] [CrossRef]
- Weiss, V.U.; Kerul, L.; Kallinger, P.; Szymanski, W.W.; Marchetti-Deschmann, M.; Allmaier, G. Liquid phase separation of proteins based on electrophoretic effects in an electrospray setup during sample introduction into a gas-phase electrophoretic mobility molecular analyzer (CE-GEMMA/CE-ES-DMA). Anal. Chim. Acta. 2014, 841, 91–98. [Google Scholar] [CrossRef]
- Brown, B.A.; Zeng, X.Y.; Todd, A.R.; Barnes, L.F.; Winstone, J.M.A.; Trinidad, J.C.; Novotny, M.V.; Jarrold, M.F.; Clemmer, D.E. Charge Detection Mass Spectrometry Measurements of Exosomes and other Extracellular Particles Enriched from Bovine Milk. Anal. Chem. 2020, 92, 3285–3292. [Google Scholar] [CrossRef]


| Sample | Protein Concentration (µg/mL) | EV Concentration (EVs/µL) | CD90+ EVs (% of all EVs) | CD73+ EVs (% of all EVs) | CD90+ CD73+ EVs (% of all EVs) |
|---|---|---|---|---|---|
| mscEVs | 846 ± 117 | 1.64 ± 0.61 × 106 | 46 ± 3 | 31 ± 9 | 27 ± 7 |
| Sample | Protein concentration (µg/mL) | EV concentration (EVs/µL) | CD45+ EVs (% of all EVs) | ||
| mEVs | 864 ± 176 | 2.15 ± 2.19 × 106 | 41 ± 6 | ||
| Sample | Protein concentration (µg/mL) | EV concentration (EVs/µL) | CD235a+ EVs (% of all EVs) | ||
| rbcEVs | 2486 ± 1129 | 4.80 ± 0.50 × 106 | 83 ± 6 |
| Sample | Concentration (Particles/mL) | Hydrodynamic Particle Diameter (nm) X50 | Recovery (%) |
|---|---|---|---|
| mscEV PBS | 4.35 ± 2.25 × 1011 | 163.7 ± 13.6 | - |
| mscEV AA | 7.63 ± 6.16 × 1010 | 162.5 ± 16.1 | 17.5 |
| mEV PBS | 4.88 ± 1.77 × 1011 | 156.1 ± 14.8 | - |
| mEV AA | 1.03 ± 0.61 × 1011 | 162.3 ± 19.5 | 21.1 |
| rbcEV PBS | 8.26 ± 3.34 × 1011 | 152.8 ± 12.1 | - |
| rbcEV AA | 4.48 ± 2.24 × 1011 | 163.1 ± 22.1 | 54.3 |
| rbcEV + Tween | 1.34 ± 0.74 × 1011 | 175.7 ± 28.8 | 16.3 |
| rbcEV + US | 6.35 ± 3.00 × 1011 | 142.1 ± 14.5 | 76.9 * |
| rbcEV + Tween +US | 9.38 ± 6.58 × 1010 | 205.4 ± 37.1 | 11.4 |
| rbcEV SEC PBS | 5.70 ± 1.30 × 1011 | 155.7 ± 9.3 | - |
| rbcEV SEC AA | 1.38 ± 0.94 × 1011 | 166.3 ± 12.8 | 24.3 |
| rbcEV SEC + Tween | 3.95 ± 1.75 × 1010 | 177.9 ± 23.4 | 6.9 |
| rbcEV SEC + US | 2.49 ± 0.98 × 1011 | 158.4 ± 24.2 | 43.7 * |
| rbcEV SEC + Tween + US | 3.22 ± 2.11 × 1010 | 199.1 ± 37.6 | 5.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steinberger, S.; Karuthedom George, S.; Lauková, L.; Weiss, R.; Tripisciano, C.; Marchetti-Deschmann, M.; Weber, V.; Allmaier, G.; Weiss, V.U. Targeting the Structural Integrity of Extracellular Vesicles via Nano Electrospray Gas-Phase Electrophoretic Mobility Molecular Analysis (nES GEMMA). Membranes 2022, 12, 872. https://doi.org/10.3390/membranes12090872
Steinberger S, Karuthedom George S, Lauková L, Weiss R, Tripisciano C, Marchetti-Deschmann M, Weber V, Allmaier G, Weiss VU. Targeting the Structural Integrity of Extracellular Vesicles via Nano Electrospray Gas-Phase Electrophoretic Mobility Molecular Analysis (nES GEMMA). Membranes. 2022; 12(9):872. https://doi.org/10.3390/membranes12090872
Chicago/Turabian StyleSteinberger, Stephanie, Sobha Karuthedom George, Lucia Lauková, René Weiss, Carla Tripisciano, Martina Marchetti-Deschmann, Viktoria Weber, Günter Allmaier, and Victor U. Weiss. 2022. "Targeting the Structural Integrity of Extracellular Vesicles via Nano Electrospray Gas-Phase Electrophoretic Mobility Molecular Analysis (nES GEMMA)" Membranes 12, no. 9: 872. https://doi.org/10.3390/membranes12090872
APA StyleSteinberger, S., Karuthedom George, S., Lauková, L., Weiss, R., Tripisciano, C., Marchetti-Deschmann, M., Weber, V., Allmaier, G., & Weiss, V. U. (2022). Targeting the Structural Integrity of Extracellular Vesicles via Nano Electrospray Gas-Phase Electrophoretic Mobility Molecular Analysis (nES GEMMA). Membranes, 12(9), 872. https://doi.org/10.3390/membranes12090872