Ordered Domain (Raft) Formation in Asymmetric Vesicles and Its Induction upon Loss of Lipid Asymmetry in Artificial and Natural Membranes
Abstract
:1. Membrane Lipid Asymmetry and Loss of Asymmetry
2. Preparation of Asymmetric Lipid Vesicles with Cyclodextrins
3. Stability of Lipid Asymmetry in Artificial Lipid Vesicles
4. Membrane Physical State and Domain Formation
5. Range of Domain Properties in Asymmetric Membranes
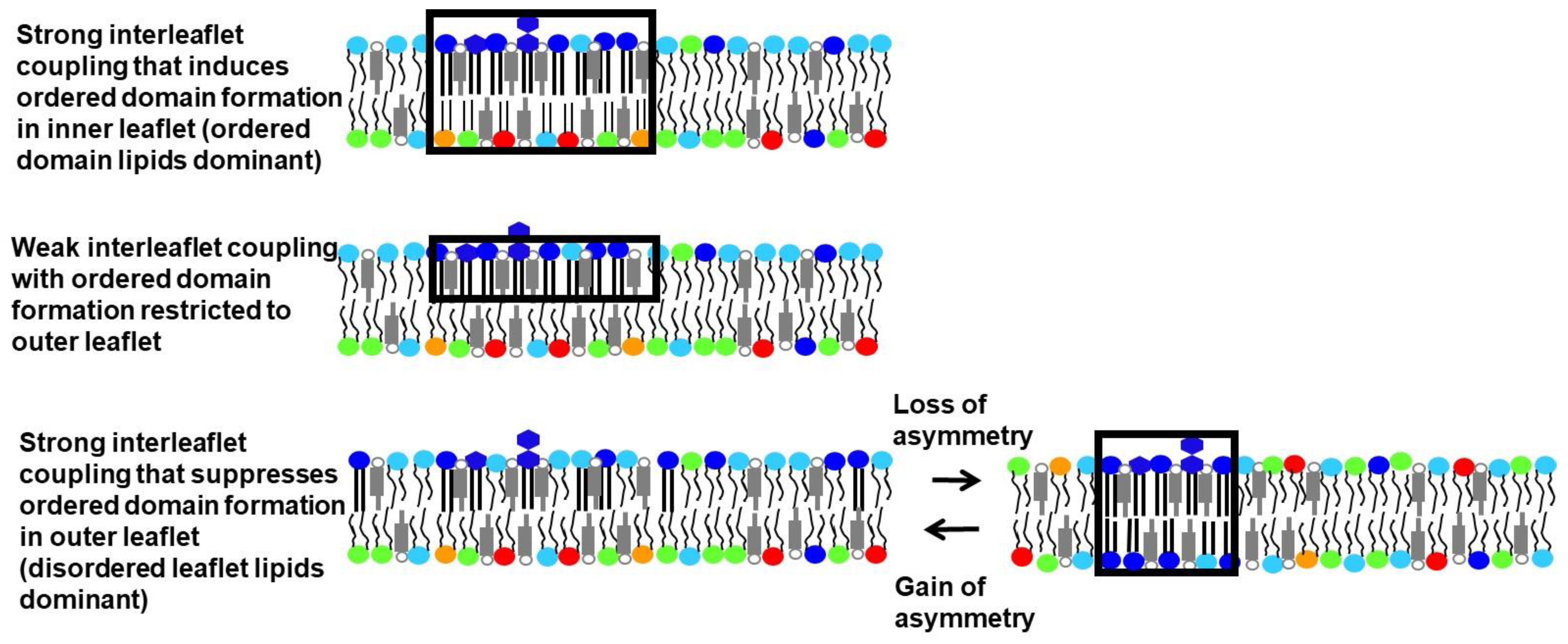
6. Experimental Studies of Interleaflet Coupling in Asymmetric Vesicles without Sterol
7. Experimental Studies in Asymmetric Vesicles in the Presence of Sterol: Induction of Ordered Domains Due to Interleaflet Coupling
8. Experimental Studies in the Presence of Sterol: Suppression of Ordered Domain Formation Due to Interleaflet Coupling
9. Effect of Differences between the Amount of Inner and Outer Leaflet Lipid upon Domain Formation
10. Domain Formation in Plasma Membrane Preparations
11. Association of Loss of Asymmetry with Ordered Domain Formation in Plasma Membranes
12. Implications of Asymmetry Loss Due to Detergent Addition for Interpretation of Detergent Resistant Membranes Isolated from Cells
13. Conclusions
Funding
Conflicts of Interest
References
- Lorent, J.H.; Levental, K.R.; Ganesan, L.; Rivera-Longsworth, G.; Sezgin, E.; Doktorova, M.; Lyman, E.; Levental, I. Plasma membranes are asymmetric in lipid unsaturation, packing and protein shape. Nat. Chem. Biol. 2020, 16, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Kim, J.; Huang, Z.; Clair, J.R.S.; Brown, D.A.; London, E. Efficient replacement of plasma membrane outer leaflet phospholipids and sphingolipids in cells with exogenous lipids. Proc. Natl. Acad. Sci. USA 2016, 113, 14025–14030. [Google Scholar] [CrossRef] [PubMed]
- Verkleij, A.J.; Zwaal, R.F.; Roelofsen, B.; Comfurius, P.; Kastelijn, D.; van Deenen, L.L. The asymmetric distribution of phospholipids in the human red cell membrane. A combined study using phospholipases and freeze-etch electron microscopy. Biochim. Biophys. Acta 1973, 323, 178–193. [Google Scholar] [CrossRef]
- Clarke, R.; Hossain, K.; Cao, K. Physiological roles of transverse lipid asymmetry of animal membranes. Biochim. Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183382. [Google Scholar] [CrossRef]
- Doktorova, M.; Symons, J.L.; Levental, I. Structural and functional consequences of reversible lipid asymmetry in living membranes. Nat. Chem. Biol. 2020, 16, 1321–1330. [Google Scholar] [CrossRef]
- Mercer, J.; Helenius, A. Vaccinia Virus Uses Macropinocytosis and Apoptotic Mimicry to Enter Host Cells. Science 2008, 320, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Mercer, J.; Helenius, A. Apoptotic mimicry: Phosphatidylserine-mediated macropinocytosis of vaccinia virus. Ann. N. Y. Acad. Sci. 2010, 1209, 49–55. [Google Scholar] [CrossRef]
- Richard, A.S.; Zhang, A.; Park, S.J.; Farzan, M.; Zong, M.; Choe, H. Virion-associated phosphatidylethanolamine promotes TIM1-mediated infection by Ebola, dengue, and West Nile viruses. Proc. Natl. Acad. Sci. USA 2015, 112, 14682–14687. [Google Scholar] [CrossRef]
- Niekamp, P.; Scharte, F.; Sokoya, T.; Vittadello, L.; Kim, Y.; Deng, Y.; Südhoff, E.; Hilderink, A.; Imlau, M.; Clarke, C.J.; et al. Ca2+-activated sphingomyelin scrambling and turnover mediate ESCRT-independent lysosomal repair. Nat. Commun. 2022, 13, 1875. [Google Scholar] [CrossRef]
- Enoki, T.A.; Feigenson, G.W. Asymmetric Bilayers by Hemifusion: Method and Leaflet Behaviors. Biophys. J. 2019, 117, 1037–1050. [Google Scholar] [CrossRef]
- London, E. Membrane Structure-Function Insights from Asymmetric Lipid Vesicles. Acc. Chem. Res. 2019, 52, 2382–2391. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K. Development of Artificial Cell Models Using Microfluidic Technology and Synthetic Biology. Micromachines 2020, 11, 559. [Google Scholar] [CrossRef] [PubMed]
- Arriaga, L.R.; Huang, Y.; Kim, S.-H.; Aragones, J.L.; Ziblat, R.; Koehler, S.A.; Weitz, D.A. Single-step assembly of asymmetric vesicles. Lab A Chip 2019, 19, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, D.; Geier, B.; Pabst, G. Asymmetric Lipid Membranes: Towards More Realistic Model Systems. Membranes 2015, 5, 180–196. [Google Scholar] [CrossRef]
- Cheng, H.-T.; Megha; London, E. Preparation and properties of asymmetric vesicles that mimic cell membranes: Effect upon lipid raft formation and transmembrane helix orientation. J. Biol. Chem. 2011, 286, 29441. [Google Scholar] [CrossRef]
- Cheng, H.-T.; London, E. Preparation and Properties of Asymmetric Large Unilamellar Vesicles: Interleaflet Coupling in Asymmetric Vesicles Is Dependent on Temperature but Not Curvature. Biophys. J. 2011, 100, 2671–2678. [Google Scholar] [CrossRef]
- Heberle, F.A.; Marquardt, D.; Doktorova, M.; Geier, B.; Standaert, R.F.; Heftberger, P.; Kollmitzer, B.; Nickels, J.D.; Dick, R.A.; Feigenson, G.W.; et al. Subnanometer structure of an asymmetric model membrane: Interleaflet coupling influences domain properties. Langmuir 2016, 32, 5195–5200. [Google Scholar] [CrossRef]
- Lin, Q.; London, E. Preparation of Artificial Plasma Membrane Mimicking Vesicles with Lipid Asymmetry. PLoS ONE 2014, 9, e87903. [Google Scholar] [CrossRef]
- Li, G.; Kakuda, S.; Suresh, P.; Canals, D.; Salamone, S.; London, E. Replacing plasma membrane outer leaflet lipids with exogenous lipid without damaging membrane integrity. PLoS ONE 2019, 14, e0223572. [Google Scholar] [CrossRef]
- Suresh, P.; Miller, W.T.; London, E. Phospholipid exchange shows insulin receptor activity is supported by both the propensity to form wide bilayers and ordered raft domains. J. Biol. Chem. 2021, 297, 101010. [Google Scholar] [CrossRef]
- Suresh, P.; London, E. Using cyclodextrin-induced lipid substitution to study membrane lipid and ordered membrane domain (raft) function in cells. Biochim. Biophys. Acta (BBA)-Biomembr. 2021, 1864, 183774. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, D.; Heberle, F.A.; Miti, T.; Eicher, B.; London, E.; Katsaras, J.; Pabst, G. 1H NMR shows slow phospholipid flip-flop in gel and fluid bilayers. Langmuir 2017, 33, 3731–3741. [Google Scholar] [CrossRef]
- Son, M.; London, E. The dependence of lipid asymmetry upon phosphatidylcholine acyl chain structure. J. Lipid Res. 2013, 54, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Son, M.; London, E. The dependence of lipid asymmetry upon polar headgroup structure. J. Lipid Res. 2013, 54, 3385–3393. [Google Scholar] [CrossRef] [PubMed]
- Leventis, R.; Silvius, J.R. Use of Cyclodextrins to Monitor Transbilayer Movement and Differential Lipid Affinities of Cholesterol. Biophys. J. 2001, 81, 2257–2267. [Google Scholar] [CrossRef]
- Liu, J.; Conboy, J.C. 1,2-Diacyl-Phosphatidylcholine Flip-Flop Measured Directly by Sum-Frequency Vibrational Spectroscopy. Biophys. J. 2005, 89, 2522–2532. [Google Scholar] [CrossRef]
- Nakao, H.; Nakano, M. Flip-Flop Promotion Mechanisms by Model Transmembrane Peptides. Chem. Pharm. Bull. 2022, 70, 519–523. [Google Scholar] [CrossRef]
- LeBarron, J.; London, E. Effect of lipid composition and amino acid sequence upon transmembrane peptide-accelerated lipid transleaflet diffusion (flip-flop). Biochim. Biophys. Acta (BBA)-Biomembr. 2016, 1858, 1812–1820. [Google Scholar] [CrossRef]
- Doktorova, M.; Heberle, F.A.; Marquardt, D.; Rusinova, R.; Sanford, R.L.; Peyear, T.A.; Katsaras, J.; Feigenson, G.W.; Weinstein, H.; Andersen, O.S. Gramicidin Increases Lipid Flip-Flop in Symmetric and Asymmetric Lipid Vesicles. Biophys. J. 2019, 116, 860–873. [Google Scholar] [CrossRef]
- Nguyen, M.H.L.; DiPasquale, M.; Rickeard, B.W.; Doktorova, M.; Heberle, F.A.; Scott, H.L.; Barrera, F.N.; Taylor, G.; Collier, C.P.; Stanley, C.B.; et al. Peptide-Induced Lipid Flip-Flop in Asymmetric Liposomes Measured by Small Angle Neutron Scattering. Langmuir 2019, 35, 11735–11744. [Google Scholar] [CrossRef]
- Koynova, R.; Caffrey, M. Phases and phase transitions of the phosphatidylcholines. Biochim. Biophys. Acta (BBA)-Rev. Biomembr. 1998, 1376, 91–145. [Google Scholar] [CrossRef]
- Yano, Y.; Hanashima, S.; Tsuchikawa, H.; Yasuda, T.; Slotte, J.P.; London, E.; Murata, M. Sphingomyelins and ent-Sphingomyelins Form Homophilic Nano-Subdomains within Liquid Ordered Domains. Biophys. J. 2020, 119, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Matsumori, N.; Kinoshita, M.; London, E. Molecular substructure of the liquid-ordered phase formed by sphingomyelin and cholesterol: Sphingomyelin clusters forming nano-subdomains are a characteristic feature. Biophys. Rev. 2022, 14, 655–678. [Google Scholar] [CrossRef]
- Petruzielo, R.S.; Heberle, F.A.; Drazba, P.; Katsaras, J.; Feigenson, G.W. Phase behavior and domain size in sphingomyelin-containing lipid bilayers. Biochim. Biophys. Acta (BBA)-Biomembr. 2013, 1828, 1302–1313. [Google Scholar] [CrossRef] [PubMed]
- Enoki, T.A.; Wu, J.; Heberle, F.A.; Feigenson, G.W. Dataset of asymmetric giant unilamellar vesicles prepared via hemifusion: Observation of anti-alignment of domains and modulated phases in asymmetric bilayers. Data Brief 2021, 35, 106927. [Google Scholar] [CrossRef]
- Chiantia, S.; London, E. Acyl Chain Length and Saturation Modulate Interleaflet Coupling in Asymmetric Bilayers: Effects on Dynamics and Structural Order. Biophys. J. 2012, 103, 2311–2319. [Google Scholar] [CrossRef]
- Eicher, B.; Marquardt, D.; Heberle, F.; Letofsky-Papst, I.; Rechberger, G.N.; Appavou, M.-S.; Katsaras, J.; Pabst, G. Intrinsic Curvature-Mediated Transbilayer Coupling in Asymmetric Lipid Vesicles. Biophys. J. 2018, 114, 146–157. [Google Scholar] [CrossRef]
- Xu, X.; London, E. The Effect of Sterol Structure on Membrane Lipid Domains Reveals How Cholesterol Can Induce Lipid Domain Formation. Biochemistry 2000, 39, 843–849. [Google Scholar] [CrossRef]
- Xu, X.; Bittman, R.; Duportail, G.; Heissler, D.; Vilcheze, C.; London, E. Effect of the structure of natural sterols and sphingolipids on the formation of ordered sphingolipid/sterol domains (rafts). Comparison of cholesterol to plant, fungal, and disease-associated sterols and comparison of sphingomye-lin, cerebrosides, and ceramide. J. Biol. Chem. 2001, 276, 33540–33546. [Google Scholar] [CrossRef]
- Wang, J.; Megha; London, E. Relationship between Sterol/Steroid Structure and Participation in Ordered Lipid Domains (Lipid Rafts): Implications for Lipid Raft Structure and Function. Biochemistry 2004, 43, 1010–1018. [Google Scholar] [CrossRef]
- Megha; Bakht, O.; London, E. Cholesterol precursors stabilize ordinary and ceramide-rich ordered lipid domains (lipid rafts) to different degrees. Implications for the Bloch hypothesis and sterol biosynthesis disorders. J. Biol. Chem. 2006, 281, 21903–21913. [Google Scholar] [CrossRef] [PubMed]
- Clair, J.W.S.; London, E. Effect of sterol structure on ordered membrane domain (raft) stability in symmetric and asymmetric vesicles. Biochim. Biophys. Acta (BBA)-Biomembr. 2019, 1861, 1112–1122. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Kiessling, V.; Tamm, L.K. Coupling of Cholesterol-Rich Lipid Phases in Asymmetric Bilayers. Biochemistry 2008, 47, 2190–2198. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.D.; Keller, S.L. Tuning lipid mixtures to induce or suppress domain formation across leaflets of unsupported asymmetric bilayers. Proc. Natl. Acad. Sci. USA 2008, 105, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; London, E. Lipid Structure and Composition Control Consequences of Interleaflet Coupling in Asymmetric Vesicles. Biophys. J. 2018, 115, 664–678. [Google Scholar] [CrossRef]
- Lin, Q.; London, E. Ordered Raft Domains Induced by Outer Leaflet Sphingomyelin in Cholesterol-Rich Asymmetric Vesicles. Biophys. J. 2015, 108, 2212–2222. [Google Scholar] [CrossRef] [PubMed]
- Enoki, T.A.; Feigenson, G.W. Improving our picture of the plasma membrane: Rafts induce ordered domains in a simplified model cytoplasmic leaflet. Biochim. Biophys. Acta (BBA)-Biomembr. 2022, 1864, 183995. [Google Scholar] [CrossRef]
- Pathak, P.; London, E. The Effect of Membrane Lipid Composition on the Formation of Lipid Ultrananodomains. Biophys. J. 2015, 109, 1630–1638. [Google Scholar] [CrossRef]
- Visco, I.; Chiantia, S.; Schwille, P. Asymmetric supported lipid bilayer formation via methyl-beta-cyclodextrin mediated lipid exchange: Influence of asymmetry on lipid dynamics and phase behavior. Langmuir 2014, 30, 7475–7484. [Google Scholar] [CrossRef]
- Clair, J.W.S.; Kakuda, S.; London, E. Induction of Ordered Lipid Raft Domain Formation by Loss of Lipid Asymmetry. Biophys. J. 2020, 119, 483–492. [Google Scholar] [CrossRef]
- Varma, M.; Deserno, M. Distribution of Cholesterol in Asymmetric Membranes Driven by Composition and Differential Stress. Biophys. J. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Foley, S.L.; Hossein, A.; Deserno, M. Fluid-gel coexistence in lipid membranes under differential stress. Biophys. J. 2022, 121, 2997–3009. [Google Scholar] [CrossRef] [PubMed]
- Hossein, A.; Deserno, M. Spontaneous Curvature, Differential Stress, and Bending Modulus of Asymmetric Lipid Membranes. Biophys. J. 2019, 118, 624–642. [Google Scholar] [CrossRef]
- Li, B.; London, E. Preparation and Drug Entrapment Properties of Asymmetric Liposomes Containing Cationic and Anionic Lipids. Langmuir 2020, 36, 12521–12531. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, T.; Hammond, A.T.; Sengupta, P.; Hess, S.T.; Holowka, D.A.; Baird, B.A.; Webb, W.W. Large-scale fluid/fluid phase separation of proteins and lipids in giant plasma membrane vesicles. Proc. Natl. Acad. Sci. USA 2007, 104, 3165–3170. [Google Scholar] [CrossRef]
- Levental, I.; Grzybek, M.; Simons, K. Raft domains of variable properties and compositions in plasma membrane vesicles. Proc. Natl. Acad. Sci. USA 2011, 108, 11411–11416. [Google Scholar] [CrossRef]
- Sezgin, E.; Kaiser, H.J.; Baumgart, T.; Schwille, P.; Simons, K.; Levental, I. Elucidating membrane structure and protein behavior using giant plasma membrane vesicles. Nat. protoc. 2012, 7, 1042–1051. [Google Scholar] [CrossRef]
- Li, G.; Wang, Q.; Kakuda, S.; London, E. Nanodomains can persist at physiologic temperature in plasma membrane vesicles and be modulated by altering cell lipids. J. Lipid Res. 2020, 61, 758–766. [Google Scholar] [CrossRef]
- Keller, H.; Lorizate, M.; Schwille, P. PI(4,5)P2 Degradation Promotes the Formation of Cytoskeleton-Free Model Membrane Systems. ChemPhysChem 2009, 10, 2805–2812. [Google Scholar] [CrossRef]
- Gray, E.M.; Díaz-Vázquez, G.; Veatch, S.L. Growth Conditions and Cell Cycle Phase Modulate Phase Transition Temperatures in RBL-2H3 Derived Plasma Membrane Vesicles. PLoS ONE 2015, 10, e0137741. [Google Scholar] [CrossRef] [PubMed]
- Kakuda, S.; Suresh, P.; Li, G.; London, E. Loss of plasma membrane lipid asymmetry can induce ordered domain (raft) formation. J. Lipid Res. 2021, 63, 100155. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.A.; Rose, J.K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 1992, 68, 533–544. [Google Scholar] [CrossRef]
- Brown, D.A.; London, E. Structure of detergent-resistant membrane domains: Does phase separation occur in biological membranes? Biochem. Biophys. Res. Commun. 1997, 240, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.N.; Brown, D.A.; London, E. On the origin of sphingolipid/cholesterol-rich detergent-insoluble cell membranes: Physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model membranes. Biochemistry 1997, 36, 10944–10953. [Google Scholar] [CrossRef]
- Heerklotz, H. Triton Promotes Domain Formation in Lipid Raft Mixtures. Biophys. J. 2002, 83, 2693–2701. [Google Scholar] [CrossRef]
- Pathak, P.; London, E. Measurement of Lipid Nanodomain (Raft) Formation and Size in Sphingomyelin/POPC/Cholesterol Vesicles Shows TX-100 and Transmembrane Helices Increase Domain Size by Coalescing Preexisting Nanodomains But Do Not Induce Domain Formation. Biophys. J. 2011, 101, 2417–2425. [Google Scholar] [CrossRef]
- Dietel, L.; Kalie, L.; Heerklotz, H. Lipid Scrambling Induced by Membrane-Active Substances. Biophys. J. 2020, 119, 767–779. [Google Scholar] [CrossRef]
- Pantaler, E.; Kamp, D.; Haest, C.W. Acceleration of phospholipid flip-flop in the erythrocyte membrane by detergents differing in polar head group and alkyl chain length. Biochim. Biophys. Acta (BBA)-Biomembr. 2000, 1509, 397–408. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
London, E. Ordered Domain (Raft) Formation in Asymmetric Vesicles and Its Induction upon Loss of Lipid Asymmetry in Artificial and Natural Membranes. Membranes 2022, 12, 870. https://doi.org/10.3390/membranes12090870
London E. Ordered Domain (Raft) Formation in Asymmetric Vesicles and Its Induction upon Loss of Lipid Asymmetry in Artificial and Natural Membranes. Membranes. 2022; 12(9):870. https://doi.org/10.3390/membranes12090870
Chicago/Turabian StyleLondon, Erwin. 2022. "Ordered Domain (Raft) Formation in Asymmetric Vesicles and Its Induction upon Loss of Lipid Asymmetry in Artificial and Natural Membranes" Membranes 12, no. 9: 870. https://doi.org/10.3390/membranes12090870
APA StyleLondon, E. (2022). Ordered Domain (Raft) Formation in Asymmetric Vesicles and Its Induction upon Loss of Lipid Asymmetry in Artificial and Natural Membranes. Membranes, 12(9), 870. https://doi.org/10.3390/membranes12090870




