Thin Film Composite Polyamide Reverse Osmosis Membrane Technology towards a Circular Economy
Abstract
:1. Introduction
2. Manufacturing and Usage Phase
2.1. Current RO Membranes and Modules
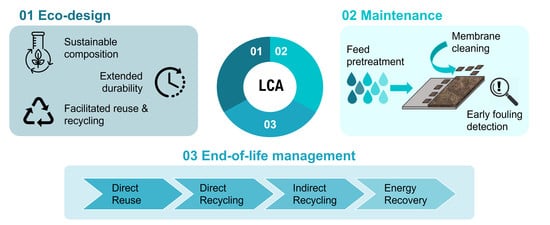
2.2. Implementation of Eco-Design Principles in RO Module Manufacturing
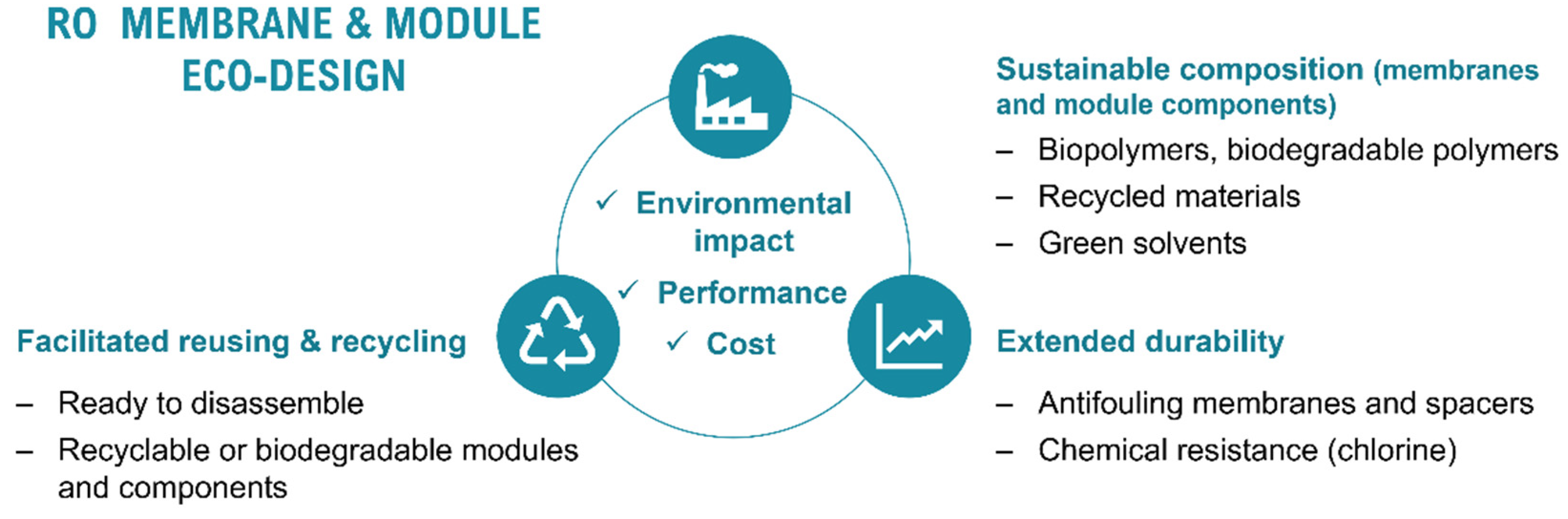
2.2.1. Sustainable Composition
2.2.2. Extended Durability
2.2.3. Facilitated Reuse and Recycling
2.3. Fouling Prevention and Mitigation during the Usage Phase
2.3.1. Feed Pre-Treatment Technologies
2.3.2. Early Fouling Detection Methods
2.3.3. Membrane Cleaning
3. End-of-Life Membrane Management
3.1. Direct Reuse
3.2. Direct Recycling
3.3. Indirect Recycling
3.3.1. Recycling Flat Sheet Membranes
3.3.2. Recycling Other Module Components
3.4. Energy Recovery
4. Life Cycle Thinking and Life Cycle Assessment
5. Future Outlook and Perspectives
- Biopolymers, recycled materials, and green solvents have an essential role in future sustainable polymer and membrane science. Accordingly, research in those fields is rapidly growing. Even if a considerable number of works have been devoted to the preparation of biobased membranes at a laboratory scale, the larger-scale implementation of biobased membranes, long-term studies, and the LCA of the technologies are yet to be explored. In addition, most of the literature on biobased membrane manufacturing is focused on MF, UF, NF, or pervaporation membranes, while a far smaller number of studies is dedicated to the preparation of RO membranes. Likewise, due to the vast development of current PA TFC RO membranes, performance trade-offs of novel biobased membranes could be expected. Thus, there is still a long way for research in this area to reach the implementation of high-performance biobased RO membrane manufacturing on a large scale.
- Extending the life service time of products is a fundamental concept of the circular economy. Enhancing fouling and chlorine resistance have been identified as the main conditions to extend the service time of current PA TFC RO membranes. Despite the large number of scientific papers focused on the synthesis and modification of PA TFC RO membranes to impair greater fouling and chlorine resistance, a lack of large-scale implementation examples along with long-term stability studies has been identified, hence manifesting a gap between the academic research directions and industrial practical needs. Future works should be devoted to increasing the technology readiness level of long-lasting membrane manufacturing and modification strategies.
- Apart from the membranes, RO module materials design (58% of module weight) should also approach sustainability criteria. Therefore, the replacement or modification of current petroleum-based polymers will be required to improve the biodegradability, reusability, and recyclability of the modules and module materials. However, scarce references in this area can be found in the literature, and thus this research line should be further explored. It has been identified that the current fibreglass casing of the modules increases the labour costs associated with module disassembling, which could limit the implementation of indirect recycling strategies. Furthermore, to date, there are few recycling alternatives for fibreglass materials, mostly related to their use as filler additives. In addition, its high inorganic content (silica glass) with respect to the carbon ratio, means that the material is less suitable as feedstock in waste-to-fuel technologies. Thus, the modification or replacement of the fibreglass casing should be addressed in future RO module design.
- RO membrane reuse and direct recycling technologies have been demonstrated to be technically, economically, and environmentally feasible, and their technological readiness level has already approached the pilot scale validation. Future efforts in these lines should be dedicated to attempting the industrial implementation of validated technologies.
- Recently developed indirect recycling strategies can markedly broaden the applications of recycled membranes and simultaneously enable the individual recycling of plastic components of the RO module, resulting in potential environmental benefits. Up to now, several indirect recycling strategies have been technically validated at a laboratory scale. However, future research should address the potential scalability of each alternative in terms of economic competitiveness and environmental potential. Meanwhile, technological advances in plastic waste recycling (i.e., chemical recycling) would allow the recovery of monomers and other valuable compounds from plastic materials. Thus, advances in this research line (i.e., plastic waste recycling) would favour the potential implementation of indirect recycling strategies.
- Membrane reuse and recycling alternatives are presently technically feasible, have demonstrated economic competitiveness, and could help greatly to reduce the environmental footprint associated with RO membrane-based separation technologies. These alternatives have a promising future in the water and wastewater treatment market. The main limitations encountered for the implementation of membrane reuse and recycling technologies are related to the actual low cost of landfilling, difficulties to bridge the gap between research and industry stakeholders, and the social rejection of second-generation products. However, according to the objectives of the European Commission, increasingly restrictive legislation on unsustainable waste management practices is expected, and among other criteria, a prospective rise in landfilling taxation could be expected. This situation would mean that reuse, recycling and recovering alternatives are even more economically attractive in the near future, thus facilitating their industrial implementation. The future implementation of membrane reuse and recycling technologies would bring several economic, social, and environmental benefits, such as the implementation of low-cost second-generation membranes for the production of high-quality water (e.g., wastewater treatment, desalination).
- Considering the actual situation of the energy sector, waste-to-fuel technologies are increasingly compelling to reduce the volume of waste while producing local energy. Nevertheless, the emissions and residues produced by those alternatives should be carefully evaluated.
- The LCA can estimate the potential sustainability of a technology, identify hot spots, and help in the decision-making process. The results on EoL RO management prioritisation are in good agreement with the waste hierarchy. In this sense, cascade open loop reuse, recycling, and recovering processes are recommended to enable several lifespans of RO elements. In addition, the exploitation of machine learning and artificial intelligence algorithms could revolutionise several sectors and science disciplines, including EoL membrane management processes, allowing for an inexpensive and rapid decision-making process to disclose the most adequate EoL membrane valorisation route, among others.
Author Contributions
Funding
Conflicts of Interest
References
- UNU-INWEH; UN-ESCAP. Water Security & The Global Water Agenda. The UN-Water Analytical Brief; United Nations University: Hamilton, ON, Canada, 2013. [Google Scholar]
- Abu-zeid, M.; Shiklomanov, I.A. Water Resources as a Challenge of the Twenty-First Century; World Meteorological Organization: Geneva, Switzerland, 2003; ISBN 92-63-10969-9. [Google Scholar]
- Ibrahim, Y.; Ismail, R.A.; Ogungbenro, A.; Pankratz, T.; Banat, F.; Arafat, H.A. The sociopolitical factors impacting the adoption and proliferation of desalination: A critical review. Desalination 2021, 498, 114798. [Google Scholar] [CrossRef]
- Van der Bruggen, B. Membrane Technology. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 1–47. ISBN 9780471238966. [Google Scholar]
- Jones, E.; Qadir, M.; van Vliet, M.T.H.; Smakhtin, V.; Kang, S. The state of desalination and brine production: A global outlook. Sci. Total Environ. 2019, 657, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Hanft, S. Major Reverse Osmosis System Components for Water Treatment: The Global Market; BCC Research: Wellesley, MA, USA, 2020. [Google Scholar]
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef]
- Senán-Salinas, J.; Blanco, A.; García-Pacheco, R.; Landaburu-Aguirre, J.; García-Calvo, E. Prospective Life Cycle Assessment and economic analysis of direct recycling of end-of-life reverse osmosis membranes based on Geographic Information Systems. J. Clean. Prod. 2021, 282, 124400. [Google Scholar] [CrossRef]
- Landaburu-Aguirre, J.; García-Pacheco, R.; Molina, S.; Rodríguez-Sáez, L.; Rabadán, J.; García-Calvo, E. Fouling prevention, preparing for re-use and membrane recycling. Towards circular economy in RO desalination. Desalination 2016, 393, 16–30. [Google Scholar] [CrossRef]
- Kirchherr, J.; Reike, D.; Hekkert, M. Conceptualizing the circular economy: An analysis of 114 definitions. Resour. Conserv. Recycl. 2017, 127, 221–232. [Google Scholar] [CrossRef]
- European Comission Directive 2008/98/EC of the European parliament and of the council of 19 November 2008 on waste and repealing certain directives. Off. J. Eur. Union 2008, L 312, 3–30.
- EC Directive 2018/851 amending Directive 2008/98/EC on Waste Framework. Off. J. Eur. Union 2018, L-150, 109–140.
- Fetting, C. The European Green Deal; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- European Commission. For a Cleaner and More Competitive Europe; Circular Economy Action Plan; European Commission (EC): Brussels, Belgium, 2020; pp. 1–28. [Google Scholar] [CrossRef]
- Lu, X.; Elimelech, M. Fabrication of desalination membranes by interfacial polymerization: History, current efforts, and future directions. Chem. Soc. Rev. 2021, 50, 6290–6307. [Google Scholar] [CrossRef]
- Rana, D.; Matsuura, T.; Kassim, M.; Ismail, A. Reverse osmosis membrane. In Handbook of Membrane Separations. Chemical, Pharmaceutical, Food, and Biotechnological Applications; CRC Press: Boca Raton, FL, USA, 2015; pp. 35–52. ISBN 9781466555587. [Google Scholar]
- Ng, Z.C.; Lau, W.J.; Matsuura, T.; Ismail, A.F. Thin film nanocomposite RO membranes: Review on fabrication techniques and impacts of nanofiller characteristics on membrane properties. Chem. Eng. Res. Des. 2021, 165, 81–105. [Google Scholar] [CrossRef]
- Nunes, S.P.; Culfaz-Emecen, P.Z.; Ramon, G.Z.; Visser, T.; Koops, G.H.; Jin, W.; Ulbricht, M. Thinking the future of membranes: Perspectives for advanced and new membrane materials and manufacturing processes. J. Membr. Sci. 2020, 598, 117761. [Google Scholar] [CrossRef]
- Razali, M.; Kim, J.F.; Attfield, M.; Budd, P.M.; Drioli, E.; Lee, Y.M.; Szekely, G. Sustainable wastewater treatment and recycling in membrane manufacturing. Green Chem. 2015, 17, 5196–5205. [Google Scholar] [CrossRef]
- Schwinge, J.; Neal, P.R.; Wiley, D.E.; Fletcher, D.F.; Fane, A.G. Spiral wound modules and spacers: Review and analysis. J. Membr. Sci. 2004, 242, 129–153. [Google Scholar] [CrossRef]
- Lawler, W.; Bradford-Hartke, Z.; Cran, M.J.; Duke, M.; Leslie, G.; Ladewig, B.P.; Le-Clech, P. Towards new opportunities for reuse, recycling and disposal of used reverse osmosis membranes. Desalination 2012, 299, 103–112. [Google Scholar] [CrossRef]
- European Comission Directive 2009/125/EC of the European Parliament and of the council of 21 October 2009 establishing a framework for the setting of ecodesign requirements for energy-related products. Off. J. Eur. Union 2009, 285, 10–34. [CrossRef]
- Prézélus, F.; Chabni, D.; Barna, L.; Guigui, C.; Remigy, J.C. A metrics-based approach to preparing sustainable membranes: Application to ultrafiltration. Green Chem. 2019, 21, 4457–4469. [Google Scholar] [CrossRef]
- Kim, D.; Kim, I.C.; Kyun, Y.N.; Myung, S. Novel bio-based polymer membranes fabricated from isosorbide-incorporated poly(arylene ether)s for water treatment. Eur. Polym. J. 2020, 136, 109931. [Google Scholar] [CrossRef]
- Galiano, F.; Ghanim, A.H.; Rashid, K.T.; Marino, T.; Simone, S.; Alsalhy, Q.F.; Figoli, A. Preparation and characterization of green polylactic acid (PLA) membranes for organic/organic separation by pervaporation. Clean Technol. Environ. Policy 2019, 21, 109–120. [Google Scholar] [CrossRef]
- Tomietto, P.; Loulergue, P.; Paugam, L.; Audic, J.L. Biobased polyhydroxyalkanoate (PHA) membranes: Structure/performances relationship. Sep. Purif. Technol. 2020, 252, 117419. [Google Scholar] [CrossRef]
- Urbina, L.; Guaresti, O.; Requies, J.; Gabilondo, N.; Eceiza, A.; Corcuera, M.A.; Retegi, A. Design of reusable novel membranes based on bacterial cellulose and chitosan for the filtration of copper in wastewaters. Carbohydr. Polym. 2018, 193, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, J.; Ma, L.; Shi, X.; Li, L. Biomimetic preparation of a polycaprolactone membrane with a hierarchical structure as a highly efficient oil-water separator. J. Mater. Chem. A 2019, 7, 24532–24542. [Google Scholar] [CrossRef]
- Galiano, F.; Briceño, K.; Marino, T.; Molino, A.; Christensen, K.V.; Figoli, A. Advances in biopolymer-based membrane preparation and applications. J. Membr. Sci. 2018, 564, 562–586. [Google Scholar] [CrossRef]
- Yang, T.; Zall, R.R. Chitosan membranes for reverse osmosis application. J. Food Sci. 1984, 49, 91–93. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A natural biopolymer with a wide and varied range of applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef]
- Hardian, R.; Cywar, R.M.; Chen, E.Y.-X.; Szekely, G. Sustainable nanofiltration membranes based on biosourced fully recyclable polyesters and green solvents. J. Membr. Sci. Lett. 2022, 2, 100016. [Google Scholar] [CrossRef]
- Le Phuong, H.A.; Izzati Ayob, N.A.; Blanford, C.F.; Mohammad Rawi, N.F.; Szekely, G. Nonwoven membrane supports from renewable resources: Bamboo fiber reinforced poly(lactic acid) composites. ACS Sustain. Chem. Eng. 2019, 7, 11885–11893. [Google Scholar] [CrossRef]
- Ndruru, S.T.C.L.; Pramono, E.; Wahyuningrum, D.; Bundjali, B.; Arcana, I.M. Preparation and characterization of biopolymer blend electrolyte membranes based on derived celluloses for lithium-ion batteries separator. Bull. Mater. Sci. 2021, 44, 104. [Google Scholar] [CrossRef]
- Hardian, R.; Alammar, A.; Holtzl, T.; Szekely, G. Fabrication of sustainable organic solvent nanofiltration membranes using cellulose–chitosan biopolymer blends. J. Membr. Sci. 2022, 658, 120743. [Google Scholar] [CrossRef]
- Chen, X.; Li, W.; Shao, Z.; Zhong, W.; Yu, T. Separation of alcohol-water mixture by pervaporation through a novel natural polymer blend membrane-chitosan/silk fibroin blend membrane. J. Appl. Polym. Sci. 1999, 73, 975–980. [Google Scholar] [CrossRef]
- Shafiq, M.; Sabir, A.; Islam, A.; Khan, S.M.; Hussain, S.N.; Butt, M.T.Z.; Jamil, T. Development and performance characteristics of silane crosslinked poly(vinyl alcohol)/chitosan membranes for reverse osmosis. J. Ind. Eng. Chem. 2017, 48, 99–107. [Google Scholar] [CrossRef]
- Al-Shaeli, M.; Al-Juboori, R.A.; Al Aani, S.; Ladewig, B.P.; Hilal, N. Natural and recycled materials for sustainable membrane modification: Recent trends and prospects. Sci. Total Environ. 2022, 838, 156014. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.; Kumar, A. Biobased technologies for the efficient extraction of biopolymers from waste biomass. Bioprocess Biosyst. Eng. 2019, 42, 1893–1901. [Google Scholar] [CrossRef]
- Alammar, A.; Hardian, R.; Szekely, G. Upcycling agricultural waste into membranes: From date seed biomass to oil and solvent-resistant nanofiltration. Green Chem. 2022, 24, 365–374. [Google Scholar] [CrossRef]
- Li, H.; Zhong, Q.; Sun, Q.; Xiang, B.; Li, J. Upcycling Waste Pine nut Shell Membrane for Highly Efficient Separation of Crude Oil-in-Water Emulsion. Langmuir 2022, 38, 3493–3500. [Google Scholar] [CrossRef]
- Cavalcante, J.; Hardian, R.; Szekely, G. Antipathogenic upcycling of face mask waste into separation materials using green solvents. Sustain. Mater. Technol. 2022, 32, e00448. [Google Scholar] [CrossRef]
- Kwak, J.I.; An, Y.J. Post COVID-19 pandemic: Biofragmentation and soil ecotoxicological effects of microplastics derived from face masks. J. Hazard. Mater. 2021, 416, 126169. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Han, H.; Wang, T.; Li, J.; Tang, C.Y.; Wang, Z. Fouling is the beginning: Upcycling biopolymer-fouled substrates for fabricating high-permeance thin-film composite polyamide membranes. Green Chem. 2021, 23, 1013–1025. [Google Scholar] [CrossRef]
- Kim, D.; Nunes, S.P. Green solvents for membrane manufacture: Recent trends and perspectives. Curr. Opin. Green Sustain. Chem. 2021, 28, 100427. [Google Scholar] [CrossRef]
- Vinoth Kumar, R.; Kumar Ghoshal, A.; Pugazhenthi, G. Elaboration of novel tubular ceramic membrane from inexpensive raw materials by extrusion method and its performance in microfiltration of synthetic oily wastewater treatment. J. Membr. Sci. 2015, 490, 92–102. [Google Scholar] [CrossRef]
- Huertas, R.M.; Fraga, M.C.; Crespo, J.G.; Pereira, V.J. Solvent-free process for the development of photocatalytic membranes. Molecules 2019, 24, 4481. [Google Scholar] [CrossRef]
- Kamp, J.; Emonds, S.; Borowec, J.; Restrepo Toro, M.A.; Wessling, M. On the organic solvent free preparation of ultrafiltration and nanofiltration membranes using polyelectrolyte complexation in an all aqueous phase inversion process. J. Membr. Sci. 2021, 618, 118632. [Google Scholar] [CrossRef]
- Marino, T.; Blasi, E.; Tornaghi, S.; Di Nicolò, E.; Figoli, A. Polyethersulfone membranes prepared with Rhodiasolv® Polarclean as water soluble green solvent. J. Membr. Sci. 2018, 549, 192–204. [Google Scholar] [CrossRef]
- Kim, D.; Livazovic, S.; Falca, G.; Nunes, S.P. Oil-water separation using membranes manufactured from cellulose/ionic liquid solutions. ACS Sustain. Chem. Eng. 2019, 7, 5649–5659. [Google Scholar] [CrossRef]
- Nguyen Thi, H.Y.; Kim, S.; Duy Nguyen, B.T.; Lim, D.; Kumar, S.; Lee, H.; Szekely, G.; Kim, J.F. Closing the Sustainable Life Cycle Loop of Membrane Technology via a Cellulose Biomass Platform. ACS Sustain. Chem. Eng. 2022, 10, 2532–2544. [Google Scholar] [CrossRef]
- Mariën, H.; Bellings, L.; Hermans, S.; Vankelecom, I.F.J. Sustainable process for the preparation of high-performance thin-film composite membranes using ionic liquids as the reaction medium. ChemSusChem 2016, 9, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Coutinho de Paula, E.; Amaral, M.C.S. Extending the life-cycle of reverse osmosis membranes: A review. Waste Manag. Res. 2017, 35, 456–470. [Google Scholar] [CrossRef]
- Shin, D.H.; Kim, N.; Lee, Y.T. Modification to the polyamide TFC RO membranes for improvement of chlorine-resistance. J. Membr. Sci. 2011, 376, 302–311. [Google Scholar] [CrossRef]
- Jiang, S.; Li, Y.; Ladewig, B.P. A review of reverse osmosis membrane fouling and control strategies. Sci. Total Environ. 2017, 595, 567–583. [Google Scholar] [CrossRef]
- Asadollahi, M.; Bastani, D.; Musavi, S.A. Enhancement of surface properties and performance of reverse osmosis membranes after surface modification: A review. Desalination 2017, 420, 330–383. [Google Scholar] [CrossRef]
- Khoo, Y.S.; Lau, W.J.; Liang, Y.Y.; Yusof, N.; Fauzi Ismail, A. Surface modification of PA layer of TFC membranes: Does it effective for performance improvement? J. Ind. Eng. Chem. 2021, 102, 271–292. [Google Scholar] [CrossRef]
- Choudhury, R.R.; Gohil, J.M.; Mohanty, S.; Nayak, S.K. Antifouling, fouling release and antimicrobial materials for surface modification of reverse osmosis and nanofiltration membranes. J. Mater. Chem. A 2018, 6, 313–333. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Kasemset, S.; Lee, A.; Miller, D.J.; Freeman, B.D.; Sharma, M.M. Effect of polydopamine deposition conditions on fouling resistance, physical properties, and permeation properties of reverse osmosis membranes in oil/water separation. J. Membr. Sci. 2013, 425–426, 208–216. [Google Scholar] [CrossRef]
- McCloskey, B.D.; Park, H.B.; Ju, H.; Rowe, B.W.; Miller, D.J.; Chun, B.J.; Kin, K.; Freeman, B.D. Influence of polydopamine deposition conditions on pure water flux and foulant adhesion resistance of reverse osmosis, ultrafiltration and microfiltration membranes. Polymer 2010, 51, 3472–3485. [Google Scholar] [CrossRef]
- McCloskey, B.D.; Park, H.B.; Ju, H.; Rowe, B.W.; Miller, D.J.; Freeman, B.D. A bioinspired fouling-resistant surface modification for water purification membranes. J. Membr. Sci. 2012, 413–414, 82–90. [Google Scholar] [CrossRef]
- Dong, C.; Wang, Z.; Wu, J.; Wang, Y.; Wang, J.; Wang, S. A green strategy to immobilize silver nanoparticles onto reverse osmosis membrane for enhanced anti-biofouling property. Desalination 2017, 401, 32–41. [Google Scholar] [CrossRef]
- Liu, T.; Chen, D.; Yang, F.; Chen, J.; Cao, Y.; Xiang, M.; Kang, J.; Xu, R. Enhancing the permeability and anti-fouling properties of a polyamide thin-film composite reverse osmosis membrane: Via surface grafting of l-lysine. RSC Adv. 2019, 9, 20044–20052. [Google Scholar] [CrossRef]
- Hirsch, U.; Ruehl, M.; Teuscher, N.; Heilmann, A. Antifouling coatings via plasma polymerization and atom transfer radical polymerization on thin film composite membranes for reverse osmosis. Appl. Surf. Sci. 2018, 436, 207–216. [Google Scholar] [CrossRef]
- Huang, H.; Lin, S.; Zhang, L.; Hou, L. Chlorine-resistant polyamide reverse osmosis membrane with monitorable and regenerative sacrificial layers. ACS Appl. Mater. Interfaces 2017, 9, 10214–10223. [Google Scholar] [CrossRef]
- Nor Akalili, A.; Pei Sean, G.; Chun Wong, K.; Chinedu Mamah, S.; Fauzi Ismail, A.; Karim Zuhalirun, A. Accelerated spraying-assisted layer by layer assembly of polyethyleneimine/titania nanosheet on thin film composite membrane for reverse osmosis desalination. Desalination 2022, 529, 115645. [Google Scholar] [CrossRef]
- Choi, W.; Choi, J.; Bang, J.; Lee, J.H. Layer-by-layer assembly of graphene oxide nanosheets on polyamide membranes for durable reverse-osmosis applications. ACS Appl. Mater. Interfaces 2013, 5, 12510–12519. [Google Scholar] [CrossRef] [PubMed]
- Torre-Celeizabal, A.; Garea, A.; Casado-Coterillo, C. Chitosan: Polyvinyl alcohol based mixed matrix sustainable coatings for reusing composite membranes in water treatment: Fouling characterization. Chem. Eng. J. Adv. 2022, 9, 100236. [Google Scholar] [CrossRef]
- Zhao, D.L.; Zhao, Q.; Lin, H.; Chen, S.B.; Chung, T.S. Pressure-assisted polydopamine modification of thin-film composite reverse osmosis membranes for enhanced desalination and antifouling performance. Desalination 2022, 530, 115671. [Google Scholar] [CrossRef]
- Chae, H.; Lee, J.; Lee, C.; Kim, I.; Park, P. Graphene oxide-embedded thin- film composite reverse osmosis membrane with high flux, anti-biofouling, and chlorine resistance. J. Membr. Sci. 2015, 483, 128–135. [Google Scholar] [CrossRef]
- Reis, R.; Dumée, L.F.; Tardy, B.L.; Dagastine, R.; Orbell, J.D.; Schutz, J.A.; Duke, M.C. Towards Enhanced Performance Thin-film Composite Membranes via Surface Plasma Modification. Sci. Rep. 2016, 6, 29206. [Google Scholar] [CrossRef]
- Juhn, I.; Greenberg, A.R.; Khare, V.P. Synthesis and characterization of interfacially polymerized polyamide thin films. Desalination 2006, 191, 279–290. [Google Scholar] [CrossRef]
- Zhao, H.; Qiu, S.; Wu, L.; Zhang, L.; Chen, H.; Gao, C. Improving the performance of polyamide reverse osmosis membrane by incorporation of modi fi ed multi-walled carbon nanotubes. J. Membr. Sci. 2014, 450, 249–256. [Google Scholar] [CrossRef]
- Mayyahi, A. Al TiO2 polyamide thin film nanocomposite reverses osmosis membrane for water desalination. Membranes 2018, 8, 66. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, P.; Jiang, C.; DuChanois, R.M.; Zhang, X.; Elimelech, M. High performance polyester reverse osmosis desalination membrane with chlorine resistance. Nat. Sustain. 2021, 4, 138–146. [Google Scholar] [CrossRef]
- Haidari, A.H.; Heijman, S.G.J.; van der Meer, W.G.J. Optimal design of spacers in reverse osmosis. Sep. Purif. Technol. 2018, 192, 441–456. [Google Scholar] [CrossRef]
- Schwinge, J.; Wiley, D.E.; Fane, A.G. Novel spacer design improves observed flux. J. Membr. Sci. 2004, 229, 53–61. [Google Scholar] [CrossRef]
- Koo, J.W.; Ho, J.S.; Tan, Y.Z.; Tan, W.S.; An, J.; Zhang, Y.; Chua, C.K.; Chong, T.H. Fouling mitigation in reverse osmosis processes with 3D printed sinusoidal spacers. Water Res. 2021, 207, 117818. [Google Scholar] [CrossRef] [PubMed]
- Kerdi, S.; Qamar, A.; Alpatova, A.; Vrouwenvelder, J.S.; Ghaffour, N. Membrane filtration performance enhancement and biofouling mitigation using symmetric spacers with helical filaments. Desalination 2020, 484, 114454. [Google Scholar] [CrossRef]
- Natalia, F.; Aguilar-Sánchez, A.; Mathew, A.P. 3D-printable biopolymer-based materials for water treatment: A review. Chem. Eng. J. 2022, 430, 132964. [Google Scholar] [CrossRef]
- Anis, S.F.; Hashaikeh, R.; Hilal, N. Reverse osmosis pretreatment technologies and future trends: A comprehensive review. Desalination 2019, 452, 159–195. [Google Scholar] [CrossRef]
- Al-Abri, M.; Al-Ghafri, B.; Bora, T.; Dobretsov, S.; Dutta, J.; Castelletto, S.; Rosa, L.; Boretti, A. Chlorination disadvantages and alternative routes for biofouling control in reverse osmosis desalination. NPJ Clean Water 2019, 2, 2. [Google Scholar] [CrossRef]
- Abada, B.; Safarik, J.; Ishida, K.P.; Chellam, S. Surface characterization of end-of-life reverse osmosis membranes from a full-scale advanced water reuse facility: Combined role of bioorganic materials and silicon on chemically irreversible fouling. J. Membr. Sci. 2022, 653, 120511. [Google Scholar] [CrossRef]
- Matin, A.; Laoui, T.; Falath, W.; Farooque, M. Fouling control in reverse osmosis for water desalination & reuse: Current practices & emerging environment-friendly technologies. Sci. Total Environ. 2021, 765, 142721. [Google Scholar] [CrossRef]
- García-Pacheco, R.; Landaburu-Aguirre, J.; Terrero-Rodríguez, P.; Campos, E.; Molina-Serrano, F.; Rabadán, J.; Zarzo, D.; García-Calvo, E. Validation of recycled membranes for treating brackish water at pilot scale. Desalination 2018, 433, 199–208. [Google Scholar] [CrossRef]
- Sim, L.N.; Chong, T.H.; Taheri, A.H.; Krantz, W.B.; Fane, A.G.; Sim, S.T. V A review of fouling indices and monitoring techniques for reverse osmosis. Desalination 2018, 434, 169–188. [Google Scholar] [CrossRef]
- Mangal, M.N.; Salinas-Rodriguez, S.G.; Dusseldorp, J.; Kemperman, A.J.B.; Schippers, J.C.; Kennedy, M.D.; van der Meer, W.G.J. Effectiveness of antiscalants in preventing calcium phosphate scaling in reverse osmosis applications. J. Membr. Sci. 2021, 623, 119090. [Google Scholar] [CrossRef]
- Mangal, M.N.; Yangali-Quintanilla, V.A.; Salinas-Rodriguez, S.G.; Dusseldorp, J.; Blankert, B.; Kemperman, A.J.B.; Schippers, J.C.; Kennedy, M.D.; van der Meer, W.G.J. Application of a smart dosing pump algorithm in identifying real-time optimum dose of antiscalant in reverse osmosis systems. J. Membr. Sci. 2022, 658, 120717. [Google Scholar] [CrossRef]
- Salinas-Rodriguez, S.G.; Amy, G.L.; Schippers, J.C.; Kennedy, M.D. The modified fouling index ultrafiltration constant flux for assessing particulate/colloidal fouling of RO systems. Desalination 2015, 365, 79–91. [Google Scholar] [CrossRef]
- Abushaban, A.; Salinas-Rodriguez, S.G.; Philibert, M.; Le Bouille, L.; Necibi, M.C.; Chehbouni, A. Biofouling potential indicators to assess pretreatment and mitigate biofouling in SWRO membranes: A short review. Desalination 2022, 527, 115543. [Google Scholar] [CrossRef]
- Shin, J.; Nuang, L.; Webster, R.D.; Viswanath, B.; Coster, H.G.L.; Fane, A.G. Monitoring fouling behavior of reverse osmosis membranes using electrical impedance spectroscopy: A field trial study. Desalination 2017, 407, 75–84. [Google Scholar] [CrossRef]
- An, G.; Lin, J.; Li, J.; Li, X.; Jian, X. Non-invasive measurement of membrane scaling and cleaning in spiral-wound reverse osmosis modules by ultrasonic time-domain reflectometry with sound intensity calculation. Desalination 2011, 283, 3–9. [Google Scholar] [CrossRef]
- Pawlowski, S.; Galinha, C.F.; Crespo, J.G.; Velizarov, S. 2D fluorescence spectroscopy for monitoring ion-exchange membrane based technologies—Reverse electrodialysis (RED). Water Res. 2016, 88, 184–198. [Google Scholar] [CrossRef]
- García-Pacheco, R.; Lawler, W.; Landaburu-Aguirre, J.; Gacría-Calvo, E.; Le-Clech, P. End-of-life membranes: Challenges and opportunities. In Comprehensive Membrane Science and Engineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–19. ISBN 978-0-08-093250-7. [Google Scholar]
- Feng, D.; Van Deventer, J.S.J.; Aldrich, C. Ultrasonic defouling of reverse osmosis membranes used to treat wastewater effluents. Sep. Purif. Technol. 2006, 50, 318–323. [Google Scholar] [CrossRef]
- Jiang, W.; Xu, X.; Lin, L.; Wang, H.; Shaw, R.; Lucero, D. A pilot study of an electromagnetic field for control of reverse osmosis membrane fouling and scaling during brackish groundwater desalination. Water 2019, 11, 1015. [Google Scholar] [CrossRef]
- Madaenl, S.S.; Mohamamdi, T.; Kazemi, M. Chemical cleaning of reverse osmosis membranes. Desalination 2001, 134, 77–82. [Google Scholar] [CrossRef]
- García-Pacheco, R.; Landaburu-Aguirre, J.; Molina, S.; Rodríguez-Sáez, L.; Teli, S.B.; García-Calvo, E. Transformation of end-of-life RO membranes into NF and UF membranes: Evaluation of membrane performance. J. Membr. Sci. 2015, 495, 305–315. [Google Scholar] [CrossRef]
- Lawler, W.; Alvarez-gaitan, J.; Leslie, G.; Le-clech, P. Comparative life cycle assessment of end-of-life options for reverse osmosis membranes. Desalination 2015, 357, 45–54. [Google Scholar] [CrossRef]
- El-Fadel, M.; Findikakis, A.N.; Leckie, J.O. Environmental impacts of solid waste landfilling. J. Environ. Manag. 1997, 50, 1–25. [Google Scholar] [CrossRef]
- Yang, Z.; Lü, F.; Zhang, H.; Wang, W.; Shao, L.; Ye, J.; He, P. Is incineration the terminator of plastics and microplastics? J. Hazard. Mater. 2021, 401, 123429. [Google Scholar] [CrossRef]
- Krook, J.; Svensson, N.; Eklund, M. Landfill mining: A critical review of two decades of research. Waste Manag. 2012, 32, 513–520. [Google Scholar] [CrossRef]
- Tavares, T.; Tavares, J.; León-Zerpa, F.A.; Peñate-Suárez, B.; Ramos-Martín, A. Assessment of Processes to Increase the Useful Life and the Reuse of Reverse Osmosis Elements in Cape Verde and Macaronesia. Membranes 2022, 12, 613. [Google Scholar] [CrossRef]
- Lejarazu-Larrañaga, A.; Ortiz, J.M.; Molina, S.; Pawlowski, S.; Galinha, C.F.; Otero, V.; García-Calvo, E.; Velizarov, S.; Crespo, J.G. Nitrate removal by Donnan dialysis and anion-exchange membrane bioreactor using upcycled end-of-life reverse osmosis membranes. Membranes 2022, 12, 101. [Google Scholar] [CrossRef]
- MemEOL Test. Available online: http://www.desalwiki.che.unsw.edu.au/w/index.php/MemEOL_Test (accessed on 26 May 2022).
- García-Pacheco, R.; Comas, J.; Le-Clech, P.; Saló Grau, J.; Brugués i Pujolràs, R. REMapp Decision-Making Tool. Find Your Alternative Solution for a Sustainable End-of-Life Reverse Osmosis Membrane Management. Available online: https://remapp.icra.cat/#/ (accessed on 26 May 2022).
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef]
- Muñoz, S.; Rogalla, F.; Icaran, P.; Pérez, C.; Simón, F.X. Life + Remembrane: End-of-Life recovery of reverse osmosis membranes. FuturEnviro 2014, 25–29. [Google Scholar]
- Awaleh, M.O.; Ahmed, M.M.; Soubaneh, Y.D.; Hoch, F.B.; Bouh, S.M.; Dirieh, E.S. Wastewater reclamation using discarded reverse osmosis membranes for reuse in irrigation in Djibouti, an arid country. Water Sci. Technol. 2013, 67, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.J.; Jiménez, V.; Trujillo, O.; Veza, J. Reuse of reverse osmosis membranes in advanced wastewater treatment. Desalination 2002, 150, 219–225. [Google Scholar] [CrossRef]
- Veza, J.M.; Rodriguez-Gonzalez, J.J. Second use for old reverse osmosis membranes: Wastewater treatment. Desalination 2003, 157, 65–72. [Google Scholar] [CrossRef]
- Ambrosi, A.; Tessaro, I.C. Study on potassium permanganate chemical treatment of discarded reverse osmosis membranes aiming their reuse. Sep. Sci. Technol. 2013, 48, 1537–1543. [Google Scholar] [CrossRef]
- De Paula, E.C.; Gomes, J.C.L.; Amaral, M.C.S. Recycling of end-of-life reverse osmosis membranes by oxidative treatment: A technical evaluation. Water Sci. Technol. 2017, 76, 605–622. [Google Scholar] [CrossRef] [PubMed]
- Lawler, W.; Antony, A.; Cran, M.; Duke, M.; Leslie, G.; Le-Clech, P. Production and characterisation of UF membranes by chemical conversion of used RO membranes. J. Membr. Sci. 2016, 447, 203–211. [Google Scholar] [CrossRef]
- Pontié, M. Old RO membranes: Solutions for reuse. Desalin. Water Treat. 2015, 53, 1492–1498. [Google Scholar] [CrossRef]
- Senán-Salinas, J.; García-Pacheco, R.; Landaburu-Aguirre, J.; García-Calvo, E. Recycling of end-of-life reverse osmosis membranes: Comparative LCA and cost-effectiveness analysis at pilot scale. Resour. Conserv. Recycl. 2019, 150, 104423. [Google Scholar] [CrossRef]
- Van Der Bruggen, B.; Vandecasteele, C.; Van Gestel, T.; Doyenb, W.; Leysenb, R. Review of Pressure-Driven Membrane Processes. Environ. Prog. 2003, 22, 46–56. [Google Scholar] [CrossRef]
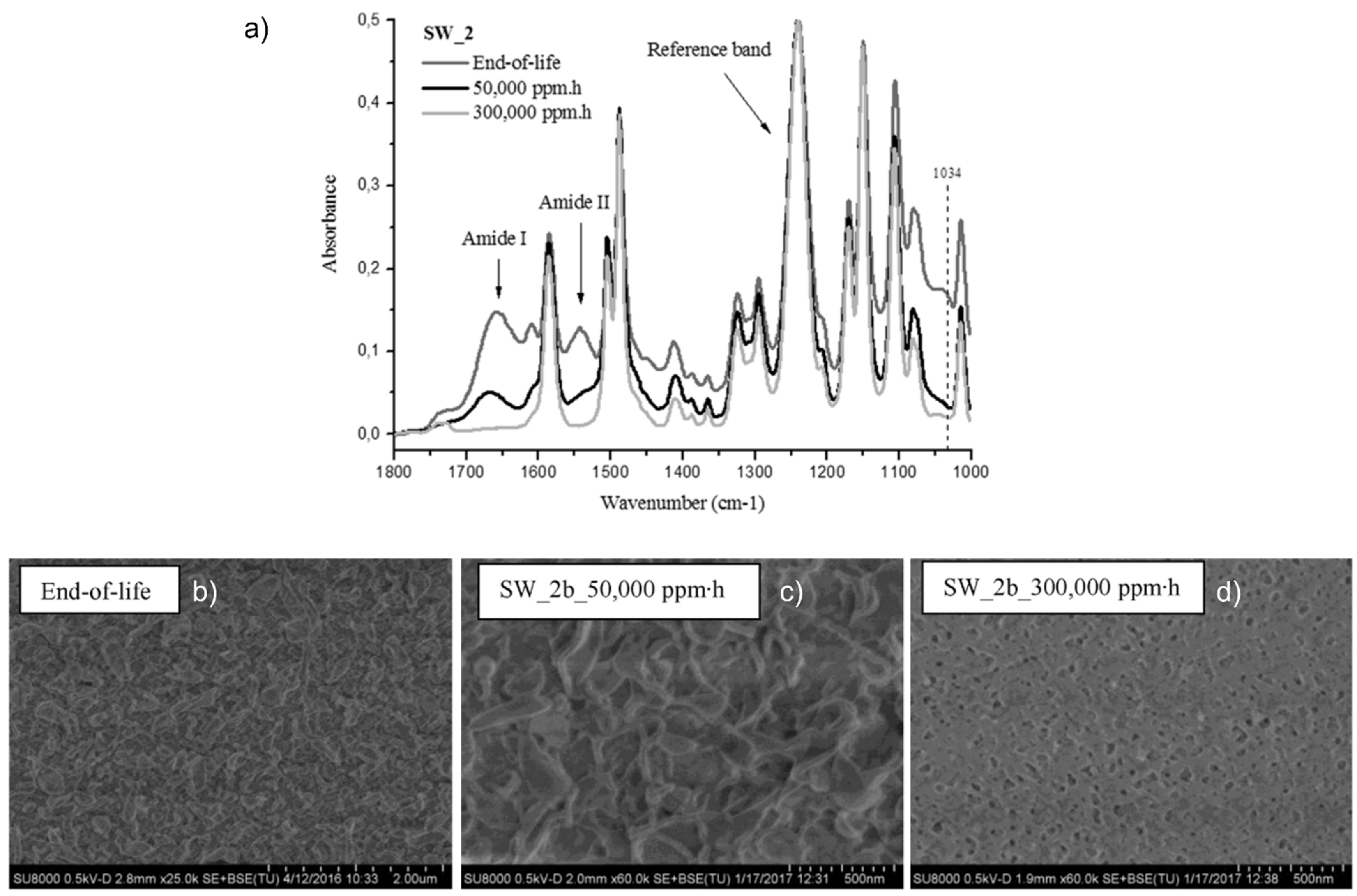
- Molina, S.; Landaburu-Aguirre, J.; Rodríguez-Sáez, L.; García-Pacheco, R.; de la Campa, J.G.; García-Calvo, E. Effect of sodium hypochlorite exposure on polysulfone recycled UF membranes and their surface characterization. Polym. Degrad. Stab. 2018, 150, 46–56. [Google Scholar] [CrossRef]
- Campos Pozuelo, E.; Terrero Rodríguez, P.; Zarzo Martínez, D.; Molina Serrano, F.J.; Calzada Garzón, M.; García Pacheco, R.; Molina Martínez, S.; Rodríguez Sáez, L.; Rabadán, F.J.; Landaburu Aguirre, J.; et al. Transformation of Spiral Wound Polyamide Membranes after Its Industrial Lifespan. Spanish Patent PCT/EP2016/30931, 8 July 2016. [Google Scholar]
- Seibel, F.I.; Otávio, G.; Giubel, M.; Barbosa Brião, V.; Shabani, M.; Pontié, M. End-of-life reverse osmosis membranes: Recycle procedure and its applications for the treatment of brackish and surface water. J. Appl. Res. Water Wastewater 2021, 8, 77–87. [Google Scholar] [CrossRef]
- García-Pacheco, R.; Li, Q.; Comas, J.; Taylor, R.A.; Le-Clech, P. Novel housing designs for nanofiltration and ultrafiltration gravity-driven recycled membrane-based systems. Sci. Total Environ. 2020, 767, 144181. [Google Scholar] [CrossRef] [PubMed]
- Khaless, K.; Achiou, B.; Boulif, R.; Benhida, R. Recycling of spent reverse osmosis membranes for second use in the clarification of wet-process phosphoric acid. Minerals 2021, 11, 637. [Google Scholar] [CrossRef]
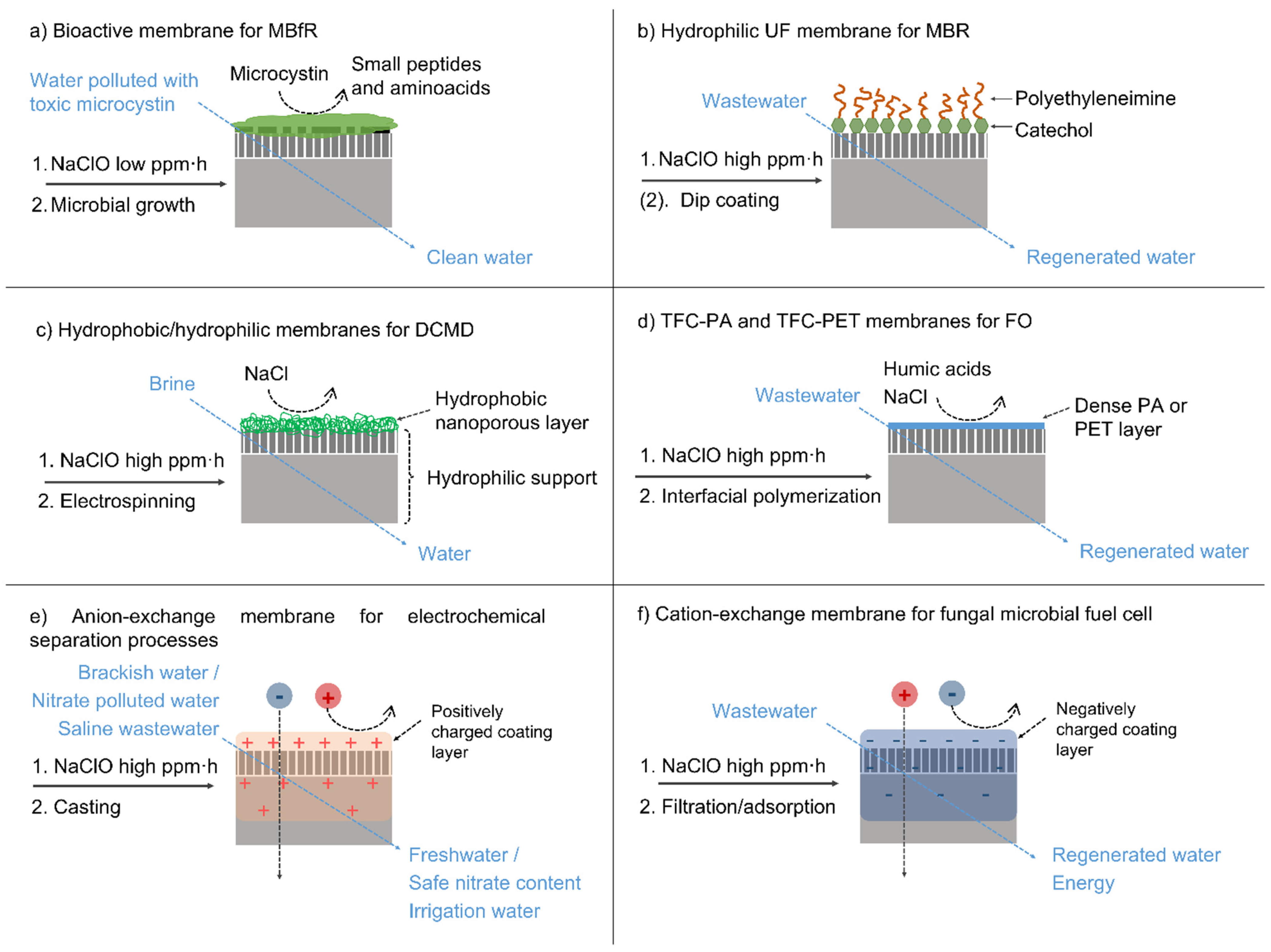
- Morón-López, J.; Nieto-Reyes, L.; Senán-Salinas, J.; Molina, S.; El-Shehawy, R. Recycled desalination membranes as a support material for biofilm development: A new approach for microcystin removal during water treatment. Sci. Total Environ. 2019, 647, 785–793. [Google Scholar] [CrossRef]
- Morón-López, J.; Nieto-Reyes, L.; Molina, S.; Lezcano, M.Á. Exploring microcystin-degrading bacteria thriving on recycled membranes during a cyanobacterial bloom. Sci. Total Environ. 2020, 736, 139672. [Google Scholar] [CrossRef]
- Morón-López, J.; Nieto-Reyes, L.; Aguado, S.; El-Shehawy, R.; Molina, S. Recycling of end-of-life reverse osmosis membranes for membrane biofilms reactors (MBfRs). Effect of chlorination on the membrane surface and gas permeability. Chemosphere 2019, 231, 103–112. [Google Scholar] [CrossRef]
- Morón-López, J.; Molina, S. Optimization of recycled-membrane biofilm reactor (R-MBfR) as a sustainable biological treatment for microcystins removal. Biochem. Eng. J. 2020, 153, 107422. [Google Scholar] [CrossRef]
- Rodríguez-Sáez, L.; Patsios, S.I.; Senán-Salinas, J.; Landaburu-Aguirre, J.; Molina, S.; García-Calvo, E. A novel application of recycled ultrafiltration membranes in an aerobic membrane bioreactor (aMBR): A proof-of-concept study. Membranes 2022, 12, 218. [Google Scholar] [CrossRef]
- Rodríguez-Sáez, L.; Landaburu-Aguirre, J.; Molina, S.; García-Payo, M.C.; García-Calvo, E. Study of surface modification of recycled ultrafiltration membranes using statistical design of experiments. Surf. Interfaces 2021, 23, 100978. [Google Scholar] [CrossRef]
- Contreras-Martínez, J.; García-Payo, C.; Arribas, P.; Rodríguez-Sáez, L.; Lejarazu-Larrañaga, A.; García-Calvo, E.; Khayet, M. Recycled reverse osmosis membranes for forward osmosis technology. Desalination 2022, 519, 115312. [Google Scholar] [CrossRef]
- Lejarazu-Larrañaga, A.; Molina, S.; Ortiz, J.M.; Navarro, R.; García-Calvo, E. Circular economy in membrane technology: Using end-of-life reverse osmosis modules for preparation of recycled anion exchange membranes and validation in electrodialysis. J. Membr. Sci. 2020, 593, 117423. [Google Scholar] [CrossRef]
- Lejarazu-Larrañaga, A.; Molina, S.; Ortiz, J.M.; Riccardelli, G.; García-Calvo, E. Influence of acid/base activation treatment in the performance of recycled electromembrane for fresh water production by electrodialysis. Chemosphere 2020, 248, 126027. [Google Scholar] [CrossRef] [PubMed]
- Lejarazu-Larrañaga, A.; Ortiz, J.M.; Molina, S.; Zhao, Y.; García-Calvo, E. Nitrate-selective anion exchange membranes prepared using discarded reverse osmosis membranes as support. Membranes 2020, 10, 377. [Google Scholar] [CrossRef] [PubMed]
- Pompa-Pernía, A.; Molina, S.; Lejarazu-Larrañaga, A.; Landaburu-Aguirre, J.; García-Calvo, E. Validation of recycled nanofiltration and anion-exchange membranes for the treatment of urban wastewater for crop irrigation. Membranes 2022, 12, 746. [Google Scholar] [CrossRef] [PubMed]
- Somrani, A.; Shabani, M.; Mohamed, Z.; Ghaffour, N.; Seibel, F.; Briao, V.B.; Pontié, M. Transforming an end-of-life reverse osmosis membrane in a cationic exchange membrane and its application in a fungal microbial fuel cell. Ionics 2021, 27, 3169–3184. [Google Scholar] [CrossRef]
- Prince, C.; Cran, M.; LeCleche, P. Reuse and recycling of used desalination membranes. In Proceedings of the OzWater’11, Adelaide, Australia, 9–11 May 2011. [Google Scholar]
- Contreras-Martínez, J.; García-Payo, C.; Khayet, M. Electrospun nanostructured membrane engineering using reverse osmosis recycled modules: Membrane distillation application. Nanomaterials 2021, 11, 1601. [Google Scholar] [CrossRef] [PubMed]
- Shuaib, N.A.; Mativenga, P.T. Energy demand in mechanical recycling of glass fibre reinforced thermoset plastic composites. J. Clean. Prod. 2016, 120, 198–206. [Google Scholar] [CrossRef]
- Fazli, A.; Rodrigue, D. Waste rubber recycling: A review on the evolution and properties of thermoplastic elastomers. Materials 2020, 13, 782. [Google Scholar] [CrossRef]
- Valentín, L.J.; Pérez-Aparicio, R.; Fernandez-Torres, A.; Posadas, P.; Herrero, R.; Salamanca, F.M.; Navarro, R.; Saiz-Rodríguez, L. Advanced characterization of recycled rubber from end-of-life tires. Rubber Chem. Technol. 2020, 93, 683–703. [Google Scholar] [CrossRef]
- Guddeti, R.R.; Knight, R.; Grossmann, E.D. Depolymerization of polypropylene in an induction-coupled plasma (ICP) reactor. Ind. Eng. Chem. Res. 2000, 39, 1171–1176. [Google Scholar] [CrossRef]
- Zefirov, V.V.; Elmanovich, I.V.; Stakhanov, A.I.; Pavlov, A.A.; Stakhanova, S.V.; Kharitonova, E.P.; Gallyamov, M.O. A new look at the chemical recycling of polypropylene: Thermal oxidative destruction in aqueous oxygen-enriched medium. Polymers 2022, 14, 744. [Google Scholar] [CrossRef] [PubMed]
- Dios Caputto, M.D.; Navarro, R.; Valentín, J.L.; Marcos-Fernández, Á. Chemical upcycling of poly(ethylene terephthalate) waste: Moving to a circular model. J. Polym. Sci. 2022, 1–15. [Google Scholar] [CrossRef]
- Souza dos Passos, J.; Glasius, M.; Biller, P. Screening of common synthetic polymers for depolymerization by subcritical hydrothermal liquefaction. Process Saf. Environ. Prot. 2020, 139, 371–379. [Google Scholar] [CrossRef]
- Oliveux, G.; Bailleul, J.L.; Salle, E.L.G. La Chemical recycling of glass fibre reinforced composites using subcritical water. Compos. Part A Appl. Sci. Manuf. 2012, 43, 1809–1818. [Google Scholar] [CrossRef]
- Liang, L.; Veksha, A.; Mohamed Amrad, M.Z.B.; Snyder, S.A.; Lisak, G. Upcycling of exhausted reverse osmosis membranes into value-added pyrolysis products and carbon dots. J. Hazard. Mater. 2021, 419, 126472. [Google Scholar] [CrossRef]
- Wu, H.; Yao, C.; Li, C.; Miao, M.; Zhong, Y.; Lu, Y.; Liu, T. Review of application and innovation of geotextiles in geotechnical engineering. Materials 2020, 13, 1774. [Google Scholar] [CrossRef]
- Rahimi, A.R.; Garciá, J.M. Chemical recycling of waste plastics for new materials production. Nat. Rev. Chem. 2017, 1, 46. [Google Scholar] [CrossRef]
- Garcia, J.M.; Robertson, M.L. The future of plastics recycling. Science 2017, 358, 870–872. [Google Scholar] [CrossRef]
- Antelava, A.; Damilos, S.; Hafeez, S.; Manos, G.; Al-Salem, S.M.; Sharma, B.K.; Kohli, K.; Constantinou, A. Plastic Solid Waste (PSW) in the context of Life Cycle Assessment (LCA) and sustainable management. Environ. Manag. 2019, 64, 230–244. [Google Scholar] [CrossRef]
- Satiada, M.A.; Calderon, A. Comparative analysis of existing waste-to-energy reference plants for municipal solid waste. Clean. Environ. Syst. 2021, 3, 100063. [Google Scholar] [CrossRef]
- Pontié, M.; Awad, S.; Tazerout, M.; Chaouachi, O.; Chaouachi, B. Recycling and energy recovery solutions of end-of-life reverse osmosis (RO) membrane materials: A sustainable approach. Desalination 2017, 423, 30–40. [Google Scholar] [CrossRef]
- European Comission Joint Research Centre. Supporting Environmentally Sound Decisions for Waste Management. Technical Guide to Life Cycle Thinking (LCT) and Life Cycle Assessment (LCA) for Waste Experts and LCA Practitioners; European Comission Joint Research Centre: Ispra, Italy, 2011. [Google Scholar]
- Conesa, J.A.; Ortuño, N.; Palmer, D. Estimation of Industrial Emissions during Pyrolysis and Combustion of Different Wastes Using Laboratory Data. Sci. Rep. 2020, 10, 6750. [Google Scholar] [CrossRef] [PubMed]
- Hospido, A.; Sanchez, I.; Rodriguez-garcia, G.; Iglesias, A.; Buntner, D.; Reif, R.; Moreira, M.T.; Feijoo, G. Are all membrane reactors equal from an environmental point of view? Desalination 2012, 285, 263–270. [Google Scholar] [CrossRef]
- Muñoz, I.; Fernández-Alba, A.R. Reducing the environmental impacts of reverse osmosis desalination by using brackish groundwater resources. Water Res. 2008, 42, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Bhakar, V.; Kumar, D.N.S.H.; Sai, N.K.; Sangwan, K.S.; Raghuvanshi, S. Life Cycle Assessment of Filtration Systems of Reverse Osmosis Units: A Case Study of a University Campus. Procedia CIRP 2016, 40, 268–273. [Google Scholar] [CrossRef]
- Holloway, R.W.; Miller-Robbie, L.; Patel, M.; Stokes, J.R.; Munakata-Marr, J.; Dadakis, J.; Cath, T.Y. Life-cycle assessment of two potable water reuse technologies: MF/RO/UV-AOP treatment and hybrid osmotic membrane bioreactors. J. Membr. Sci. 2016, 507, 165–178. [Google Scholar] [CrossRef]
- Zhou, J.; Chang, V.W.C.; Fane, A.G. Environmental life cycle assessment of brackish water reverse osmosis desalination for different electricity production models. Energy Environ. Sci. 2011, 4, 2267–2278. [Google Scholar] [CrossRef]
- Galinha, C.F.; Crespo, J.G. From black box to machine learning: A journey through membrane process modelling. Membranes 2021, 11, 574. [Google Scholar] [CrossRef]
- Senán-Salinas, J.; Landaburu-Aguirre, J.; Contreras-Martinez, J.; García-Calvo, E. Life Cycle Assessment application for emerging membrane recycling technologies: From reverse osmosis into forward osmosis. Resour. Conserv. Recycl. 2022, 179, 106075. [Google Scholar] [CrossRef]
- Senán-Salinas, J.; Blanco, A.; Landaburu-Aguirre, J.; García-Pacheco, R.; Garcia-Calvo, E. LCA-GIS results of prospective analysis of an end-of-life reverse osmosis direct recycling plant within Segurs’s Watershed (Spain). Mendeley Data 2020, 1. [Google Scholar] [CrossRef]
- Senán-salinas, J.; Landaburu-aguirre, J. Data of the life cycle impact assessment and cost analysis of prospective direct recycling of end-of-life reverse osmosis membrane at full scale. Data Brief 2020, 33, 106487. [Google Scholar] [CrossRef] [PubMed]







| Reverse Osmosis (RO) | Nanofiltration (NF) | Ultrafiltration (UF) | |
|---|---|---|---|
| Pore size (µm) | <0.001 | 0.01–0.001 | 0.1–0.01 |
| Hydraulic permeability (L m−2 h−1 bar−1) | 0.05–1.5 | 1.5–30 | 10–1000 |
| Working pressure (bar) | 20–50 | 3–20 | 0.1–5 |
| Separation mechanism | Solution-diffusion model | Sieving and charge effect | Sieving effect |
| Rejection capacity | Monovalent salts. | Multivalent salts, small organic compounds | Macromolecules, bacteria, viruses |
| Type of Processing | EoL Component | Processing Method | Recycled Product | Ref. |
|---|---|---|---|---|
| Direct valorisation | Feed and permeate spacers | Cleaned with water and disinfected |
| [116,136] |
| [137] | |||
| Feed spacer | Cleaned with water and disinfected |
| [137] | |
| [131,132] | |||
| Mechanical recycling | Thermoplastics (PP, PET, ABS) | Sorting, shredding, melting, and extruding into new products. |
| [108] |
| [131] | |||
| Thermosets composites (fibreglass and rubber) | Downsizing by shredding, and granulation to obtain a powder. |
| [138] | |
| [139,140] | |||
| Chemical recycling | PP |
|
| [141] |
|
| [142] | ||
| PET |
|
| [143] | |
| ABS | Hydrothermal liquefaction in an alkaline environment. | An oil product composed of oligomers requiring further upgrading. | [144] | |
| Fibreglass | Chemolysis using subcritical water as a solvent. | Glass fibres and resin monomers | [145] | |
| Rubber | Devulcanisation by chemical, evulcarmo-mechanical, microwave, or ultrasound processes. | Virgin raw material to be revulcanised into rubber | [139,140] | |
| RO module | H2O2-assisted hydrothermal method (pyrolysis) | Oil and gas for fuel and chemical feedstock. Char as carbon precursor for fabricating functional carbon dots. | [146] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lejarazu-Larrañaga, A.; Landaburu-Aguirre, J.; Senán-Salinas, J.; Ortiz, J.M.; Molina, S. Thin Film Composite Polyamide Reverse Osmosis Membrane Technology towards a Circular Economy. Membranes 2022, 12, 864. https://doi.org/10.3390/membranes12090864
Lejarazu-Larrañaga A, Landaburu-Aguirre J, Senán-Salinas J, Ortiz JM, Molina S. Thin Film Composite Polyamide Reverse Osmosis Membrane Technology towards a Circular Economy. Membranes. 2022; 12(9):864. https://doi.org/10.3390/membranes12090864
Chicago/Turabian StyleLejarazu-Larrañaga, Amaia, Junkal Landaburu-Aguirre, Jorge Senán-Salinas, Juan Manuel Ortiz, and Serena Molina. 2022. "Thin Film Composite Polyamide Reverse Osmosis Membrane Technology towards a Circular Economy" Membranes 12, no. 9: 864. https://doi.org/10.3390/membranes12090864
APA StyleLejarazu-Larrañaga, A., Landaburu-Aguirre, J., Senán-Salinas, J., Ortiz, J. M., & Molina, S. (2022). Thin Film Composite Polyamide Reverse Osmosis Membrane Technology towards a Circular Economy. Membranes, 12(9), 864. https://doi.org/10.3390/membranes12090864