Nannochloropsis sp. Biorefinery: Recovery of Soluble Protein by Membrane Ultrafiltration/Diafiltration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
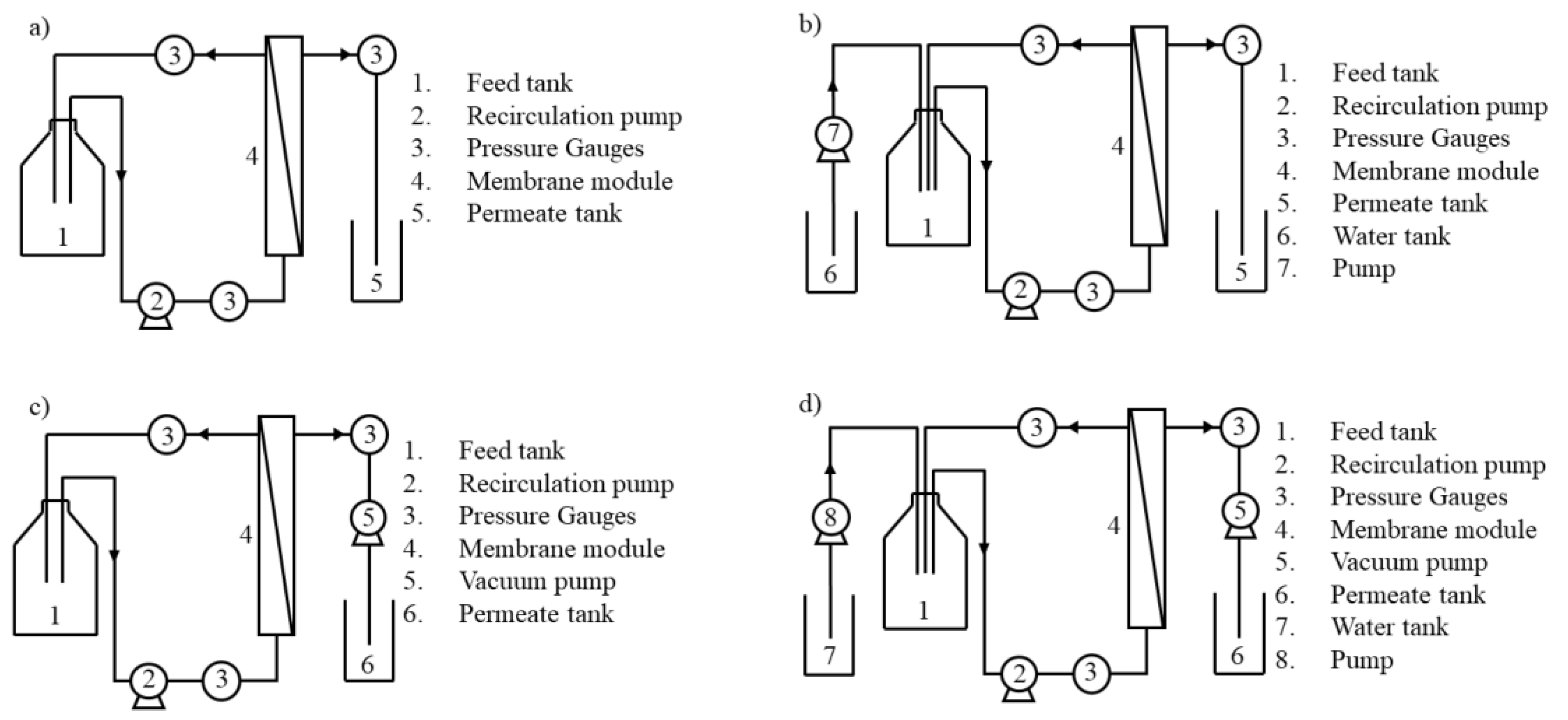
2.2. Membrane Processing
2.3. Membrane Cleaning
2.4. Dry Weight and Salinity Quantification
2.5. Protein Quantification
2.6. Total Lipids Quantification
2.7. Chlorophyll-a Quantification
3. Results and Discussion
3.1. Nannochloropsis sp. Supernatant Characterization
3.2. Ultrafiltration/Diafiltration
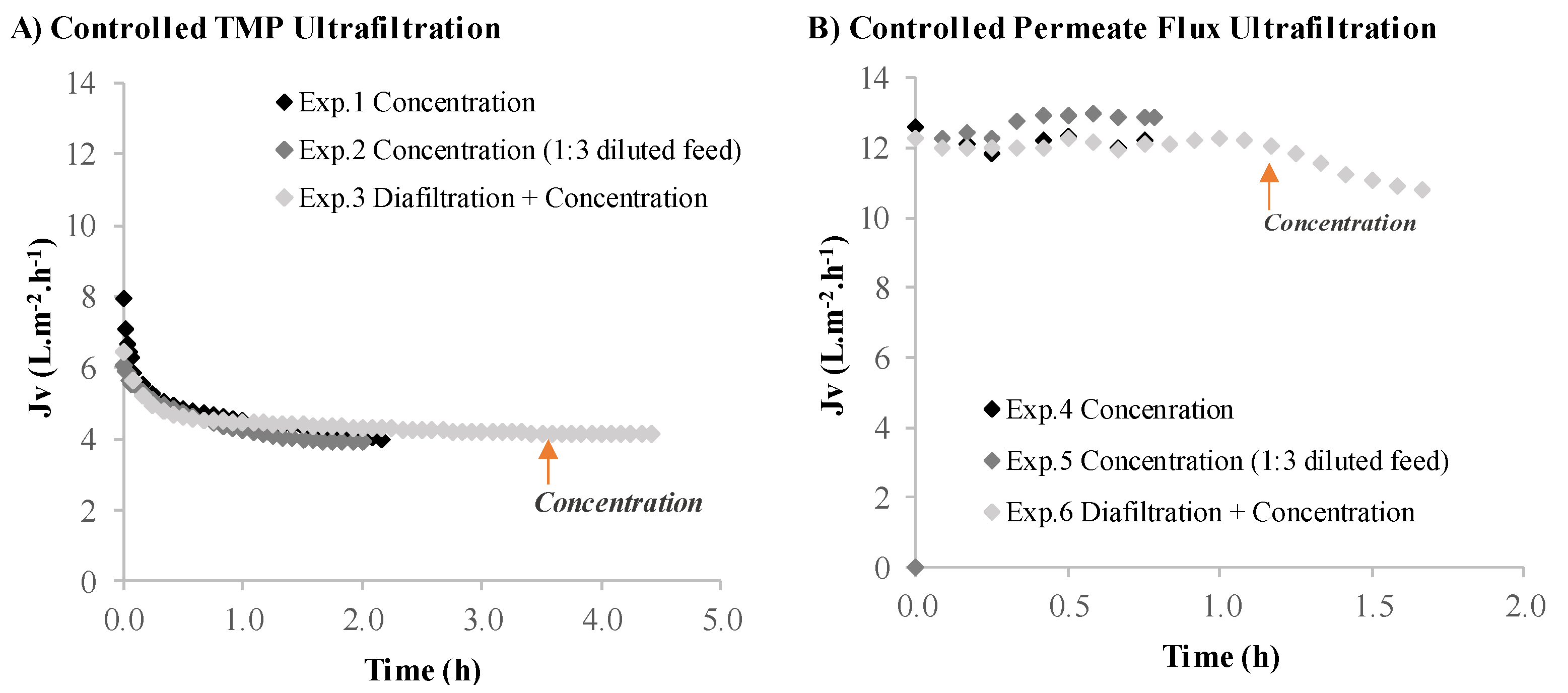
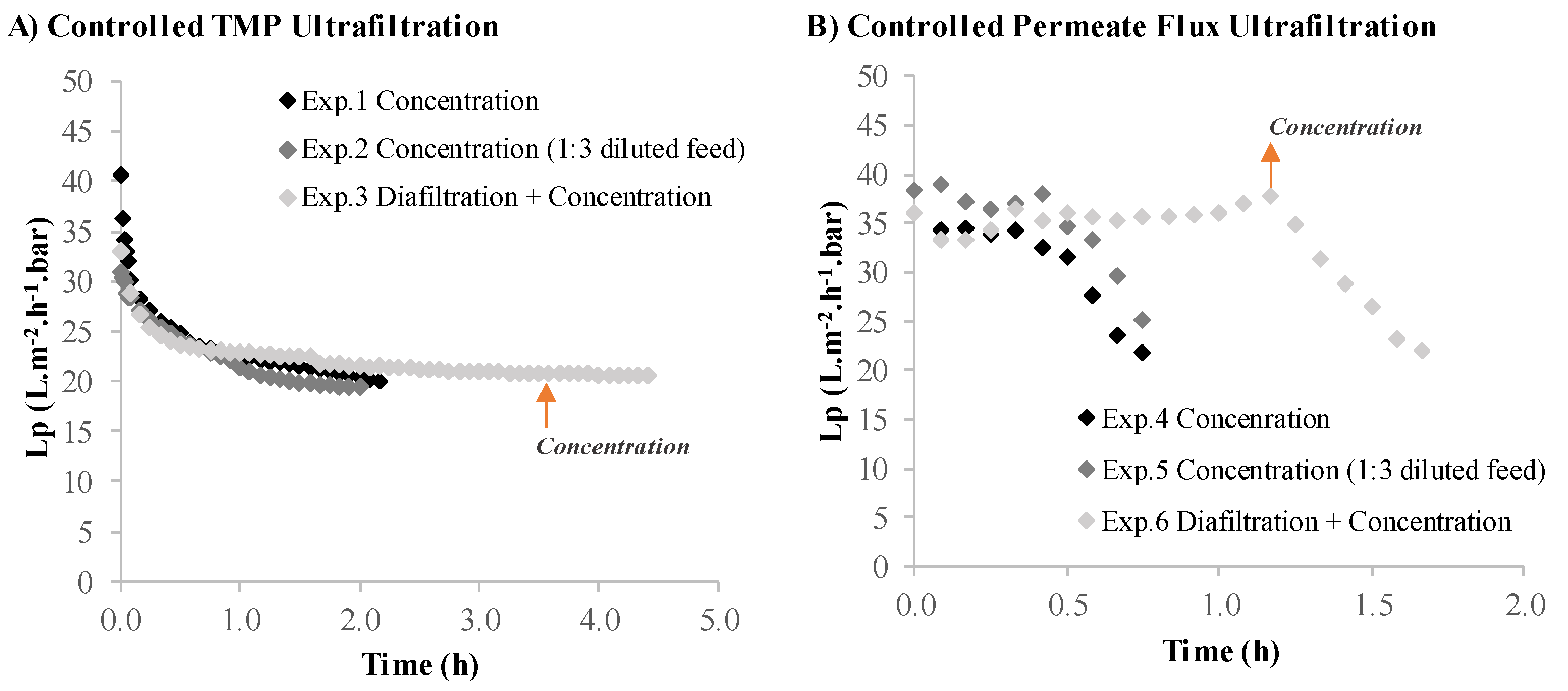
3.2.1. Volumetric Flux and Permeance
3.2.2. Soluble Protein Recovery and Permeate Characterization
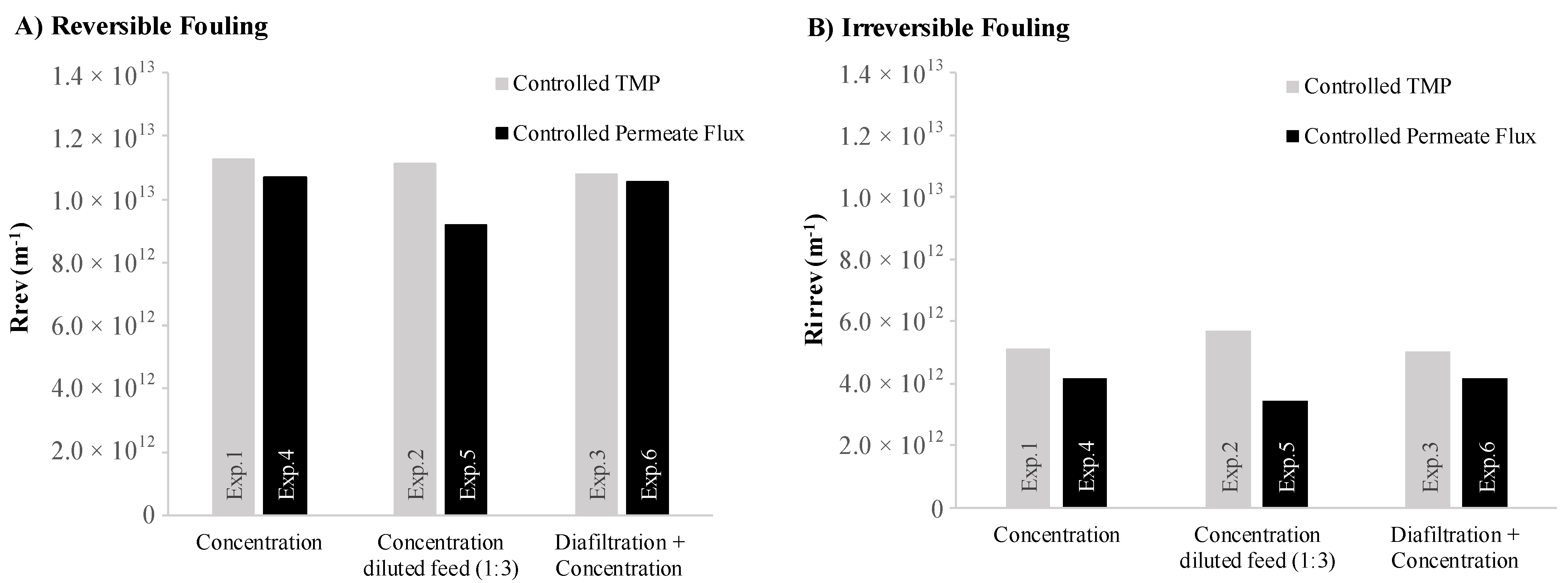
3.2.3. Fouling and Membrane Cleaning
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fradique, M.; Batista, A.P.; Nunes, M.C.; Gouveia, L.; Bandarra, N.M.; Raymundo, A. Incorporation of Chlorella vulgaris and Spirulina maxima biomass in pasta products. Part 1: Preparation and evaluation. J. Sci. Food Agric. 2010, 90, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.A.; Guldhe, A.; Gupta, S.K.; Rawat, I.; Bux, F. Improving the feasibility of aquaculture feed by using microalgae. Environ. Sci. Pollut. Res. 2021, 28, 43234–43257. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Samborska, K.; Lee, C.C.; Tomas, M.; Capanoglu, E.; Tarhan, Ö.; Taze, B.; Jafari, S.M. Phycocyanin, a super functional ingredient from algae; properties, purification characterization, and applications. Int. J. Biol. Macromol. 2021, 193, 2320–2331. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Waghmare, A.G.; Salve, M.K.; LeBlanc, J.G.; Arya, S.S. Concentration and characterization of microalgae proteins from Chlorella pyrenoidosa. Bioresour. Bioprocess. 2016, 3, 16. [Google Scholar] [CrossRef] [Green Version]
- Lafarga, T. Effect of microalgal biomass incorporation into foods: Nutritional and sensorial attributes of the end products. Algal Res. 2019, 41, 101566. [Google Scholar] [CrossRef]
- Schüler, L.; Greque de Morais, E.; Trovão, M.; Machado, A.; Carvalho, B.; Carneiro, M.; Maia, I.; Soares, M.; Duarte, P.; Barros, A.; et al. Isolation and Characterization of Novel Chlorella Vulgaris Mutants with Low Chlorophyll and Improved Protein Contents for Food Applications. Front. Bioeng. Biotechnol. 2020, 8, 469. [Google Scholar] [CrossRef]
- Qazi, W.M.; Ballance, S.; Uhlen, A.K.; Kousoulaki, K.; Haugen, J.E.; Rieder, A. Protein enrichment of wheat bread with the marine green microalgae Tetraselmis chuii—Impact on dough rheology and bread quality. LWT 2021, 143, 111115. [Google Scholar] [CrossRef]
- Camacho-Rodríguez, J.; Cerón-García, M.C.; Fernández-Sevilla, J.M.; Molina-Grima, E. The influence of culture conditions on biomass and high value product generation by Nannochloropsis gaditana in aquaculture. Algal Res. 2015, 11, 63–73. [Google Scholar] [CrossRef]
- Hulatt, C.J.; Wijffels, R.H.; Bolla, S.; Kiron, V. Production of fatty acids and protein by nannochloropsis in flat-plate photobioreactors. PLoS ONE 2017, 12, e0170440. [Google Scholar] [CrossRef]
- Valente, L.M.P.; Custódio, M.; Batista, S.; Fernandes, H.; Kiron, V. Defatted microalgae (Nannochloropsis sp.) from biorefinery as a potential feed protein source to replace fishmeal in European sea bass diets. Fish Physiol. Biochem. 2019, 45, 1067–1081. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, L.; Ebert, S.; Hinrichs, J.; Weiss, J. Production of protein-rich extracts from disrupted microalgae cells: Impact of solvent treatment and lyophilization. Algal Res. 2018, 36, 67–76. [Google Scholar] [CrossRef]
- Basso, S.; Simionato, D.; Gerotto, C.; Segalla, A.; Giacometti, G.M.; Morosinotto, T. Characterization of the photosynthetic apparatus of the Eustigmatophycean Nannochloropsis gaditana: Evidence of convergent evolution in the supramolecular organization of photosystem I. Biochim. Biophys. Acta-Bioenerg. 2014, 1837, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Hibberd, D.J. Notes on the taxonomy and nomenclature of the algal classes Eustigmatophyceae and Tribophyceae (synonym Xanthophyceae). Bot. J. Linn. Soc. 1981, 82, 93–119. [Google Scholar] [CrossRef]
- Cecchin, M.; Berteotti, S.; Paltrinieri, S.; Vigliante, I.; Iadarola, B.; Giovannone, B.; Maffei, M.E.; Delledonne, M.; Ballottari, M. Improved lipid productivity in Nannochloropsis gaditana in nitrogen-replete conditions by selection of pale green mutants. Biotechnol. Biofuels 2020, 13, 78. [Google Scholar] [CrossRef]
- Chua, E.T.; Schenk, P.M. A biorefinery for Nannochloropsis: Induction, harvesting, and extraction of EPA-rich oil and high-value protein. Bioresour. Technol. 2017, 244, 1416–1424. [Google Scholar] [CrossRef] [Green Version]
- Scholz, M.J.; Weiss, T.L.; Jinkerson, R.E.; Jing, J.; Roth, R.; Goodenough, U.; Posewitz, M.C.; Gerken, H.G. Ultrastructure and Composition of the Nannochloropsis gaditana Cell Wall. Eukaryot. Cell 2014, 13, 1450–1464. [Google Scholar] [CrossRef] [Green Version]
- Dunker, S.; Wilhelm, C. Cell wall structure of coccoid green algae as an important trade-offbetween biotic interference mechanisms and multidimensional cell growth. Front. Microbiol. 2018, 9, 719. [Google Scholar] [CrossRef]
- Allard, B.; Templier, J. Comparison of neutral lipid profile of various trilaminar outer cell wall (TLS)-containing microalgae with emphasis on algaenan occurrence. Phytochemistry 2000, 54, 369–380. [Google Scholar] [CrossRef]
- Gong, Y.; Guo, X.; Wan, X.; Liang, Z.; Jiang, M. Triacylglycerol accumulation and change in fatty acid content of four marine oleaginous microalgae under nutrient limitation and at different culture ages. J. Basic Microbiol. 2013, 53, 29–36. [Google Scholar] [CrossRef]
- Niggli, V. Structural properties of lipid-binding sites in cytoskeletal proteins. Trends Biochem. Sci. 2001, 26, 604–611. [Google Scholar] [CrossRef]
- Cavonius, L.R.; Albers, E.; Undeland, I. pH-shift processing of Nannochloropsis oculata microalgal biomass to obtain a protein-enriched food or feed ingredient. Algal Res. 2015, 11, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Gerardo, M.L.; Oatley-Radcliffe, D.L.; Lovitt, R.W. Integration of membrane technology in microalgae biorefineries. J. Membr. Sci. 2014, 464, 86–99. [Google Scholar] [CrossRef]
- Crespo, J.G.; Brazinha, C. Membrane processing: Natural antioxidants from winemaking by-products. Filtr. Sep. 2010, 47, 32–35. [Google Scholar] [CrossRef]
- Ursu, A.V.; Marcati, A.; Sayd, T.; Sante-Lhoutellier, V.; Djelveh, G.; Michaud, P. Extraction, fractionation and functional properties of proteins from the microalgae Chlorella vulgaris. Bioresour. Technol. 2014, 157, 134–139. [Google Scholar] [CrossRef]
- Safi, C.; Olivieri, G.; Campos, R.P.; Engelen-Smit, N.; Mulder, W.J.; Van den Broek, L.A.M.; Sijtsma, L. Biorefinery of microalgal soluble proteins by sequential processing and membrane filtration. Bioresour. Technol. 2016, 225, 151–158. [Google Scholar] [CrossRef] [Green Version]
- Miller, D.J.; Kasemset, S.; Paul, D.R.; Freeman, B.D. Comparison of membrane fouling at constant flux and constant transmembrane pressure conditions. J. Membr. Sci. 2014, 454, 505–515. [Google Scholar] [CrossRef]
- Field, R.W.; Wu, D.; Howell, J.A.; Gupta, B.B. Critical flux concept for microfiltration fouling. J. Membr. Sci. 1995, 100, 259–272. [Google Scholar] [CrossRef]
- Monte, J.; Bernardo, J.; Sá, M.; Parreira, C.; Galinha, C.F.; Costa, L.; Casanovas, C.; Brazinha, C.; Crespo, J.G. Development of an integrated process of membrane filtration for harvesting carotenoid-rich Dunaliella salina at laboratory and pilot scales. Sep. Purif. Technol. 2020, 233, 116021. [Google Scholar] [CrossRef]
- Serra, M.; Weng, V.; Coelhoso, I.M.; Alves, V.D.; Brazinha, C. Purification of arabinoxylans from corn fiber and preparation of bioactive films for food packaging. Membranes 2020, 10, 95. [Google Scholar] [CrossRef]
- Teuling, E.; Wierenga, P.A.; Schrama, J.W.; Gruppen, H. Comparison of Protein Extracts from Various Unicellular Green Sources. J. Agric. Food Chem. 2017, 65, 7989–8002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheryan, M. Ultrafiltration and Microfiltration Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1998; ISBN 1566765986. [Google Scholar]
- Tian, J.Y.; Chen, Z.L.; Yang, Y.L.; Liang, H.; Nan, J.; Li, G.B. Consecutive chemical cleaning of fouled PVC membrane using NaOH and ethanol during ultrafiltration of river water. Water Res. 2010, 44, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.M. The bicinchoninic acid (BCA) assay for protein quantitation. In The Protein Protocols Handbook; Walk, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2009; Volume 32, pp. 5–8. [Google Scholar] [CrossRef]
- Gomes, D. Extraction and Characterization of Microalgae Proteins from the Extremophile Dunaliella. Master’s Thesis, Universidade de Lisboa, Lisbon, Portugal, 2017. [Google Scholar]
- Ryckebosch, E.; Muylaert, K.; Foubert, I. Optimization of an analytical procedure for extraction of lipids from microalgae. J. Am. Oil Chem. Soc. 2012, 89, 189–198. [Google Scholar] [CrossRef]
- Ritchie, R.J. Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth. Res. 2006, 89, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Bogatcheva, N.V.; Machado, R.F. Cytoskeletal Proteins, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128012383. [Google Scholar]
- Crespo, J.P.S.G.; Trotin, M.; Hough, D.; Howell, J.A. Use of fluorescence labelling to monitor protein fractionation by ultrafiltration under controlled permeate flux. J. Membr. Sci. 1999, 155, 209–230. [Google Scholar] [CrossRef]
- Miller, D.J.; Paul, D.R.; Freeman, B.D. A crossflow filtration system for constant permeate flux membrane fouling characterization. Rev. Sci. Instrum. 2013, 84, 035003. [Google Scholar] [CrossRef] [PubMed]
- Balti, R.; Zayoud, N.; Hubert, F.; Beaulieu, L.; Massé, A. Fractionation of Arthrospira platensis (Spirulina) water soluble proteins by membrane diafiltration. Sep. Purif. Technol. 2021, 256, 117756. [Google Scholar] [CrossRef]
- Gifuni, I.; Lavenant, L.; Pruvost, J.; Masse, A. Recovery of microalgal protein by three-steps membrane filtration: Advancements and feasibility. Algal Res. 2020, 51, 102082. [Google Scholar] [CrossRef]
- Vyas, H.K.; Bennett, R.J.; Marshall, A.D. Performance of crossflow microfiltration during constant transmembrane pressure and constant flux operations. Int. Dairy J. 2002, 12, 473–479. [Google Scholar] [CrossRef]
 ) and Exp.6 concentration step (
) and Exp.6 concentration step (  )) with protein recovery (%) against process time (h); Exp.3—diafiltration under controlled TMP; Exp.6—diafiltration under controlled permeate flux.
)) with protein recovery (%) against process time (h); Exp.3—diafiltration under controlled TMP; Exp.6—diafiltration under controlled permeate flux.
 ) and Exp.6 concentration step (
) and Exp.6 concentration step (  )) with protein recovery (%) against process time (h); Exp.3—diafiltration under controlled TMP; Exp.6—diafiltration under controlled permeate flux.
)) with protein recovery (%) against process time (h); Exp.3—diafiltration under controlled TMP; Exp.6—diafiltration under controlled permeate flux.
| Experiment | Total Feed Concentration (gSFDW·L−1) | Operation Mode | Operating Conditions | TMP (bar) | Jv (L·m−2·h−1) |
|---|---|---|---|---|---|
| 1 | 30 | Concentration | Controlled transmembrane pressure | 0.2 | |
| 2 | 10 | Concentration | 0.2 | ||
| 3 | 30 | Diafiltration + Concentration | 0.2 | ||
| 4 | 30 | Concentration | Controlled permeate flux | 12 | |
| 5 | 10 | Concentration | 12 | ||
| 6 | 30 | Diafiltration + Concentration | 12 |
| Total protein (g·L−1) | 8.77 ± 0.37 |
| Soluble protein (g·L−1) | 7.28 ± 0.58 |
| Insoluble protein (g·L−1) | 1.48 ± 0.27 |
| Total lipids (g·L−1) | 4.37 ± 0.60 |
| Chlorophyll-a (g·L−1) | 0.08 ± 0.02 |
| (A) Controlled TMP Experiments | |||
| Exp.1 Concentration | Exp.2 Concentration (1:3 Diluted Feed) | Exp.3 Diafiltration + Concentration | |
| Soluble Protein Recovery (%) | 35.0 ± 0.4 | 40.0 ± 1.5 | 58.0 ± 2.0 |
| Soluble Protein Rejection (%) | 57.0 ± 0.5 | 50.0 ± 1.8 | 37.0 ± 2.1 |
| (B) Controlled Permeate Flux Experiments | |||
| Exp.4 Concentration | Exp.5 Concentration (1:3 Diluted Feed) | Exp.6 Diafiltration + Concentration | |
| Soluble Protein Recovery (%) | 44.0 ± 4.1 | 66.0 ± 1.9 | 78.0 ± 0.4 |
| Soluble Protein Rejection (%) | 49.0 ± 4.2 | 26.0 ± 2.3 | 19.0 ± 0.4 |
| (A) Controlled TMP Experiments | |||
| Exp.1 Concentration | Exp.2 Concentration (1:3 Diluted Feed) | Exp.3 Diafiltration + Concentration | |
| INFeed (g) | 15.62 ± 0.20 | 5.40 ± 0.10 | 7.30 ± 0.06 |
| OUTPermeate (g) | 5.49 ± 0.13 | 2.16 ± 0.12 | 4.26 ± 0.18 |
| OUTRetentate (g) | 3.53 ± 0.05 | 1.33 ± 0.02 | 0.34 ± 0.02 |
| ƩOUTPermeate+Retentate (g) | 9.02 ± 0.18 | 3.49 ± 0.14 | 4.60 ± 0.20 |
| OUTAdsorbed (g) (*) | 6.60 ± 0.02 | 1.91 ± 0.04 | 2.71 ± 0.14 |
| Protein Loss (%) | 42.27 ± 0.44 | 35.37 ± 1.47 | 37.05 ± 2.01 |
| Proteins Adsorbed (%) | 42.27 ± 0.44 | 35.37 ± 1.47 | 37.05 ± 2.01 |
| (B) Controlled Permeate Flux Experiments | |||
| Exp.4 Concentration | Exp.5 Concentration (1:3 Diluted Feed) | Exp.6 Diafiltration + Concentration | |
| INFeed (g) | 12.80 ± 0.60 | 5.52 ± 0.18 | 10.35 ± 0.03 |
| OUTPermeate (g) | 5.62 ± 0.83 | 3.40 ± 0.01 | 7.96 ± 0.04 |
| OUTRetentate (g) | 2.45 ± 0.03 | 1.41 ± 0.01 | 1.40 ± 0.03 |
| ƩOUTPermeate+Retentate (g) | 8.07 ± 0.86 | 4.81 ± 0.01 | 9.58 ± 0.07 |
| OUTAdsorbed (g) (*) | 4.73 ± 0.26 | 0.71 ± 0.17 | 0.77 ± 0.04 |
| OUTAdsorbed (%) | 36.95 ± 2.71 | 12.93 ± 2.61 | 7.42± 0.40 |
| (A) Non-Diluted Feed Experiments | |||||
| Permeate | |||||
| Controlled TMP | Controlled Permeate Flux | ||||
| Feed | Exp.1 Concentration | Exp.3 Diafiltration + Concentration | Exp.4 Concentration | Exp.6 Diafiltration + Concentration | |
| Insoluble Proteins (g·L−1) | 1.48 ± 0.27 | ND (1) | ND (1) | ND (1) | ND (1) |
| Total Lipids (g·L−1) | 4.37 ± 0.61 | 0.47 ± 0.05 | 0.61 ± 0.05 | 0.47 ± 0.05 | 0.50 ± 0.05 |
| Chlorophyll-a (g·L−1) | 0.08 ± 0.02 | ND (2) | ND (2) | ND (2) | ND (2) |
| (B) Diluted Feed (1:3) Experiments | |||||
| Permeate | |||||
| Controlled TMP | Controlled Permeate Flux | ||||
| Feed | Exp.2 Concentration | Exp.5 Concentration | |||
| Insoluble Proteins (g·L−1) | 0.50 ± 0.13 | ND (1) | ND (1) | ||
| Total Lipids (g·L−1) | 1.53 ± 0.13 | 0.20 ± 0.05 | 0.18 ± 0.05 | ||
| Chlorophyll-a (g·L−1) | 0.04 ± 0.01 | ND (2) | ND (2) | ||
| (A) Controlled TMP Experiments | ||||
| Exp.1 | Exp.2 | Exp.3 | ||
| Concentration | Concentration (1:3 Diluted Feed) | Diafiltration | Diafiltration + Concentration | |
| Lpw,i [L/(h·m2·bar)] | 219.6 | 227.3 | 218.7 | 218.7 |
| Lpw,f [L/(h·m2·bar)] | 53.1 | 49.2 | - | 53.8 |
| Lpw Loss (%) | 76% | 78% | - | 75% |
| Lpf [L/(h·m2·bar)] | 19.9 | 19.5 | 21.0 * | 20.6 |
| (B) Controlled Permeate Flux Experiments | ||||
| Exp.4 | Exp.5 | Exp.6 | ||
| Concentration | Concentration (1:3 Diluted Feed) | Diafiltration | Diafiltration + Concentration | |
| Lpw,i [L/(h·m2·bar)] | 221.8 | 219.8 | 221.7 | 221.7 |
| Lpw,f [L/(h·m2·bar)] | 62.1 | 70.81 | - | 62.4 |
| Lpw Loss (%) | 72% | 68% | - | 72% |
| Lpf [L/(h·m2·bar)] | 21.8 | 25.2 | 37.0 * | 22.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, C.; Santos, E.T.; Costa, L.; Brazinha, C.; Saraiva, P.; Crespo, J.G. Nannochloropsis sp. Biorefinery: Recovery of Soluble Protein by Membrane Ultrafiltration/Diafiltration. Membranes 2022, 12, 401. https://doi.org/10.3390/membranes12040401
Ribeiro C, Santos ET, Costa L, Brazinha C, Saraiva P, Crespo JG. Nannochloropsis sp. Biorefinery: Recovery of Soluble Protein by Membrane Ultrafiltration/Diafiltration. Membranes. 2022; 12(4):401. https://doi.org/10.3390/membranes12040401
Chicago/Turabian StyleRibeiro, Cláudia, Edgar T. Santos, Luís Costa, Carla Brazinha, Pedro Saraiva, and João G. Crespo. 2022. "Nannochloropsis sp. Biorefinery: Recovery of Soluble Protein by Membrane Ultrafiltration/Diafiltration" Membranes 12, no. 4: 401. https://doi.org/10.3390/membranes12040401
APA StyleRibeiro, C., Santos, E. T., Costa, L., Brazinha, C., Saraiva, P., & Crespo, J. G. (2022). Nannochloropsis sp. Biorefinery: Recovery of Soluble Protein by Membrane Ultrafiltration/Diafiltration. Membranes, 12(4), 401. https://doi.org/10.3390/membranes12040401







