Preparation of Porous Silicate Cement Membranes via a One-Step Water-Based Hot–Dry Casting Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
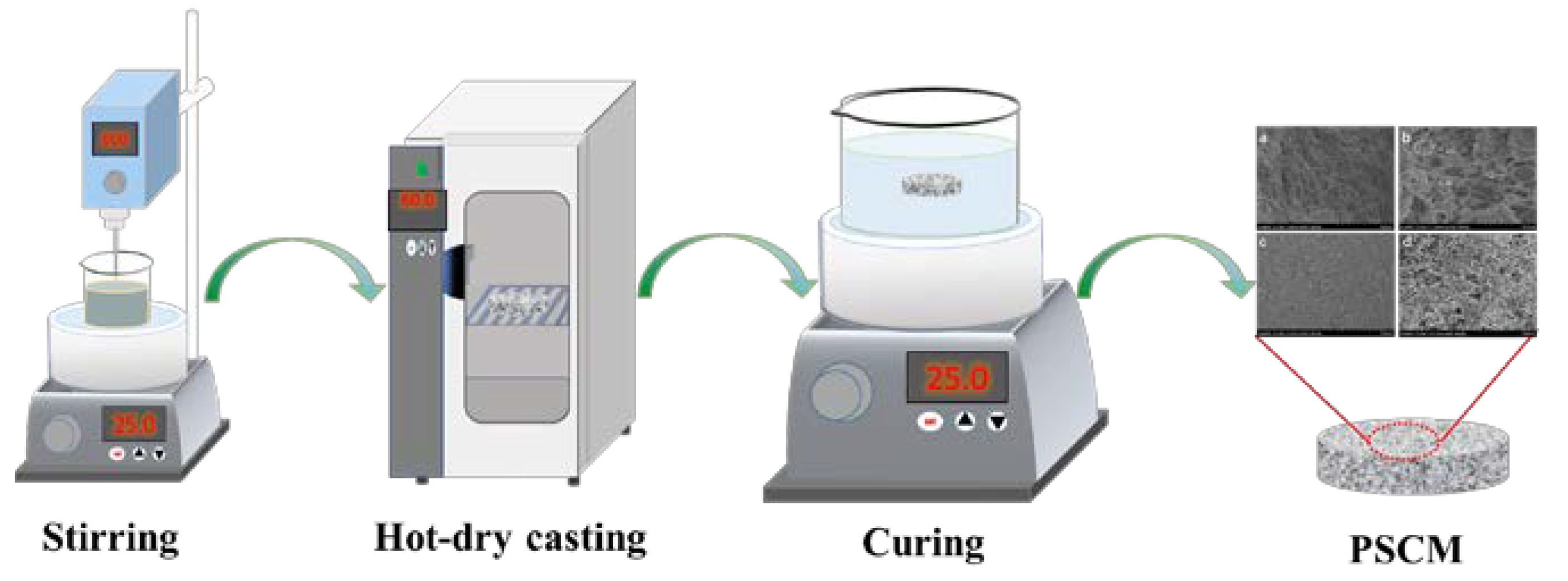
2.2. Preparation of PSCMs
2.3. Characterizations of PSCMs
2.4. Membrane Performance Measurements
3. Results and Discussion
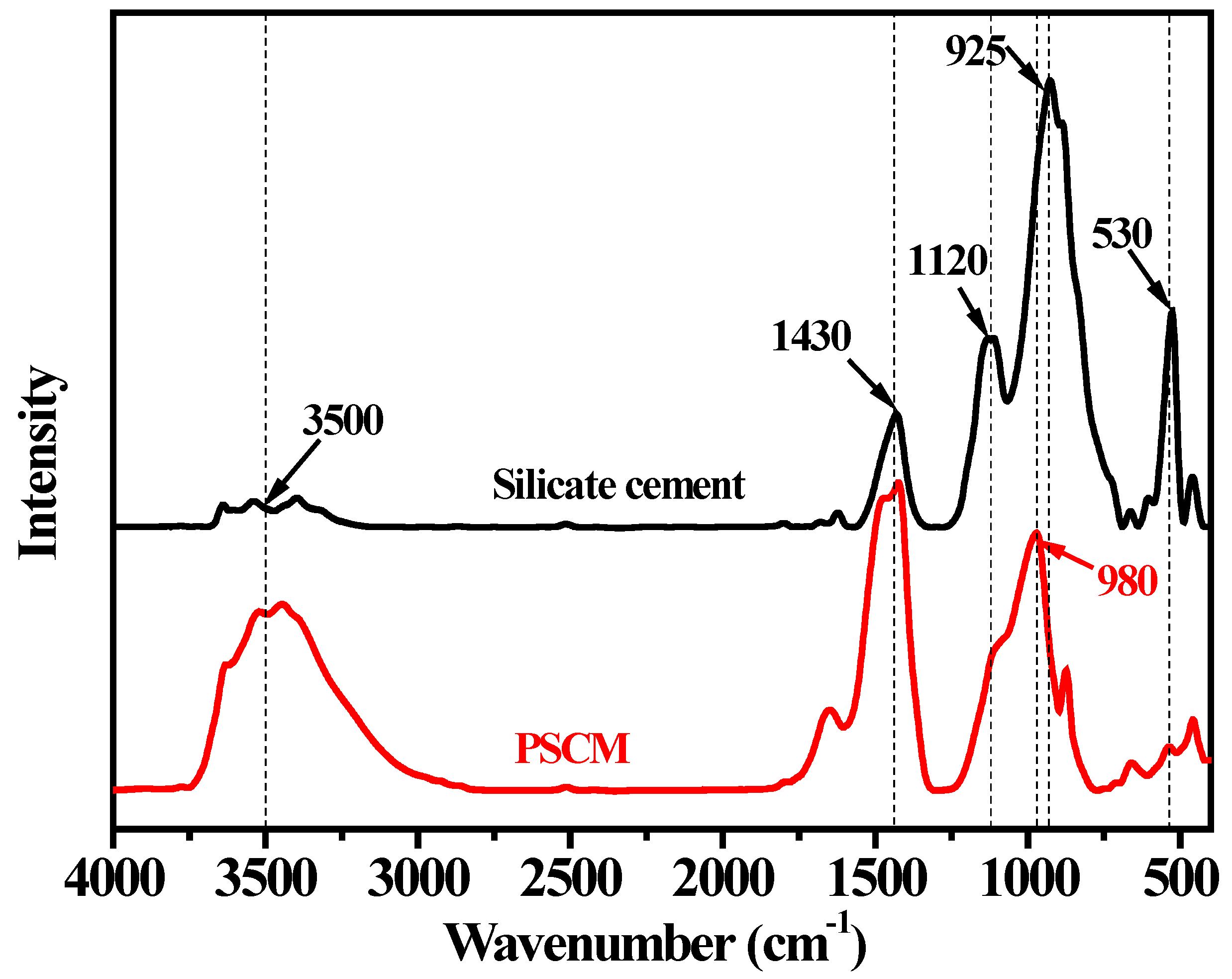
3.1. Chemical Structure Analysis
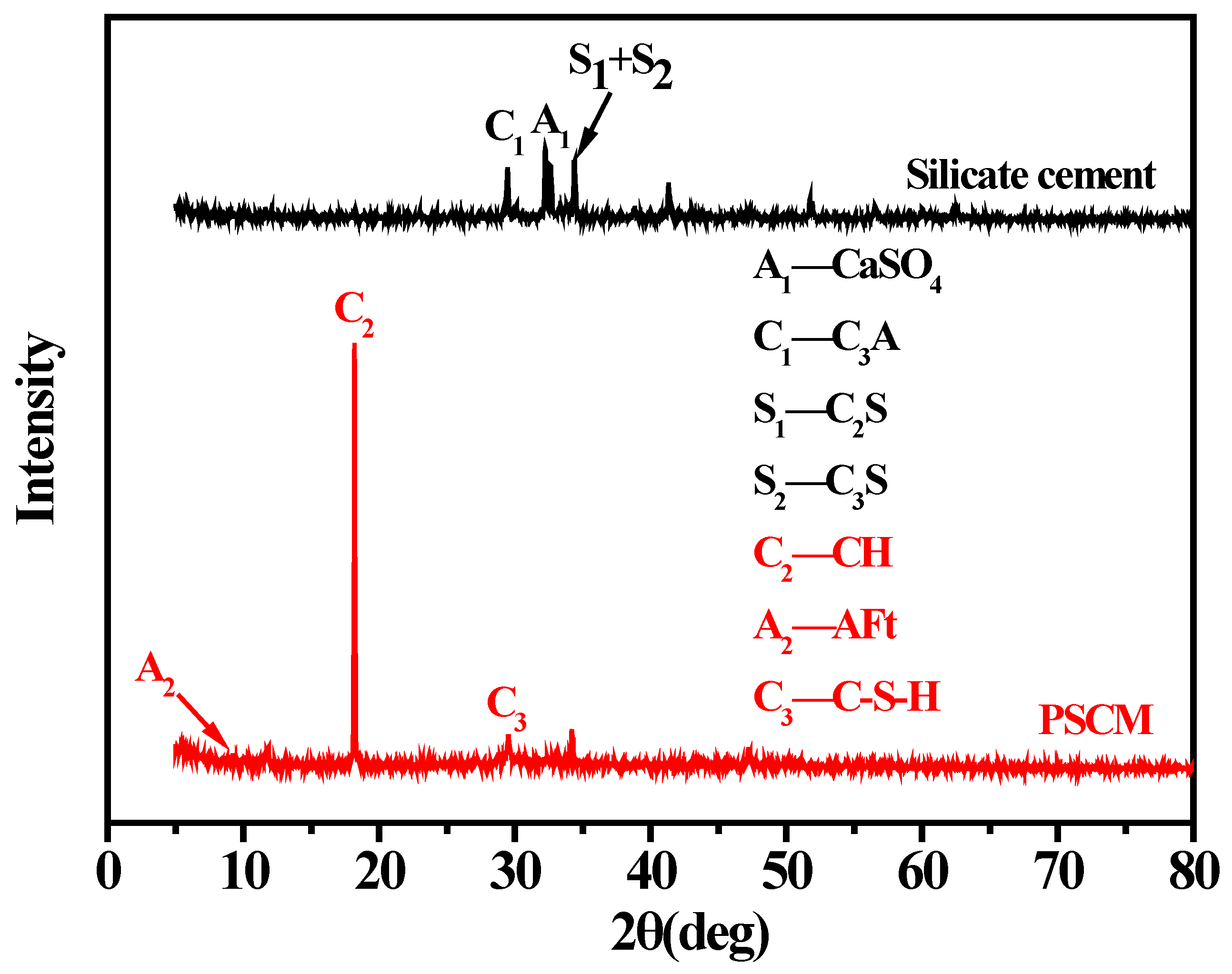
3.2. Phase Analysis
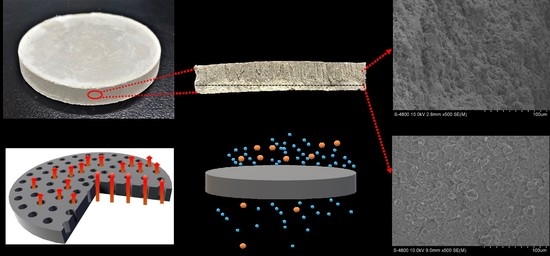
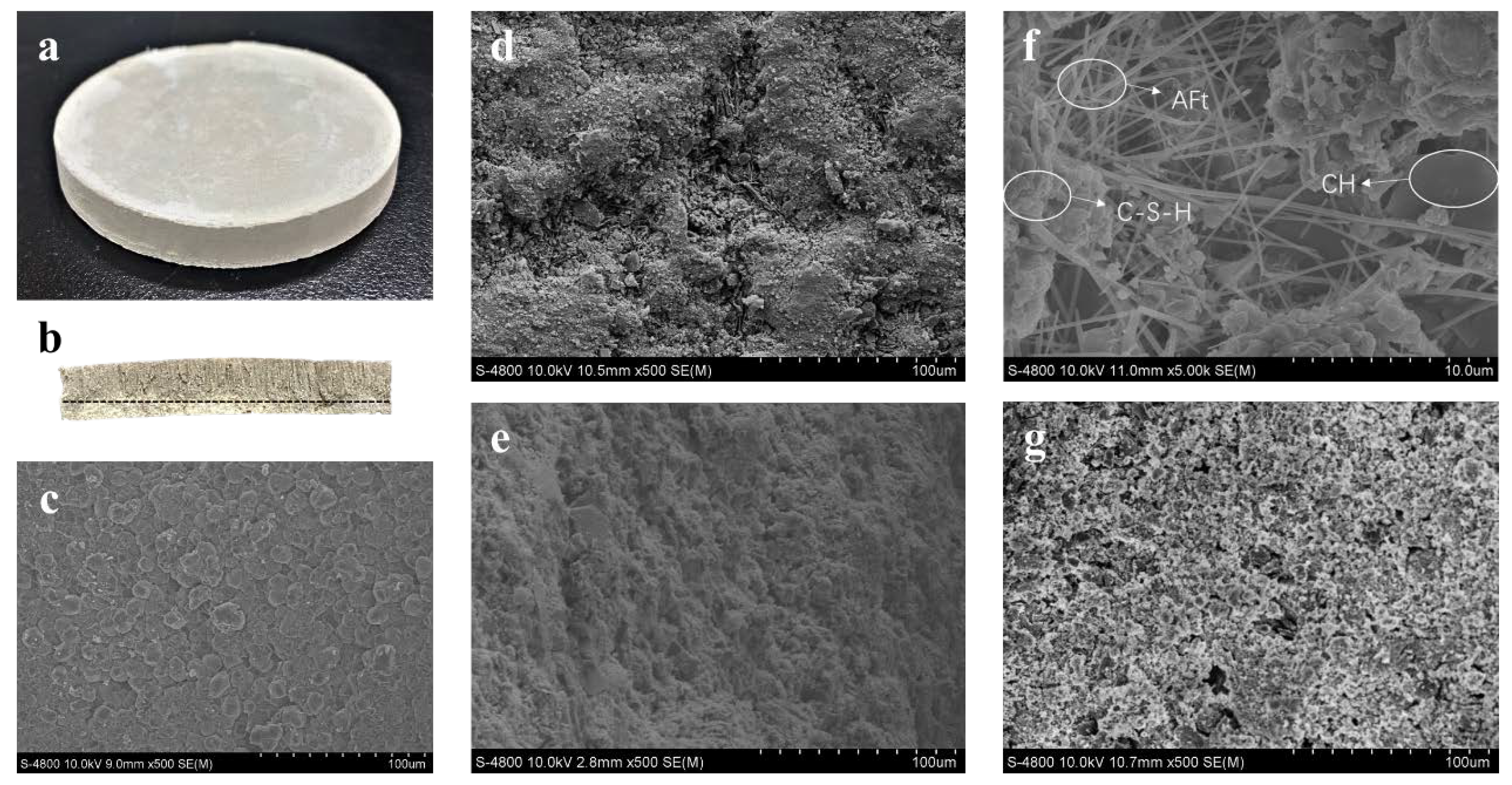
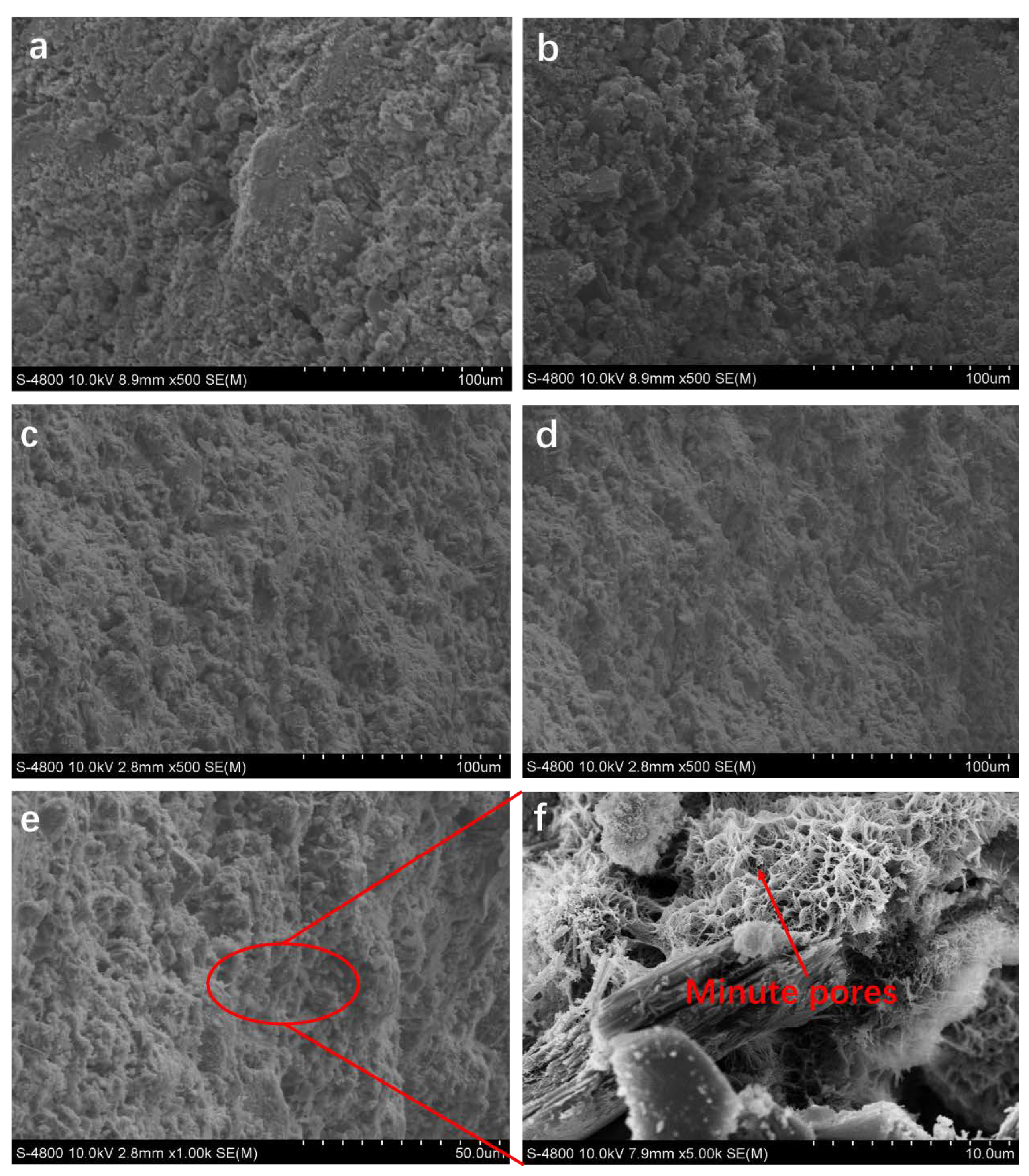
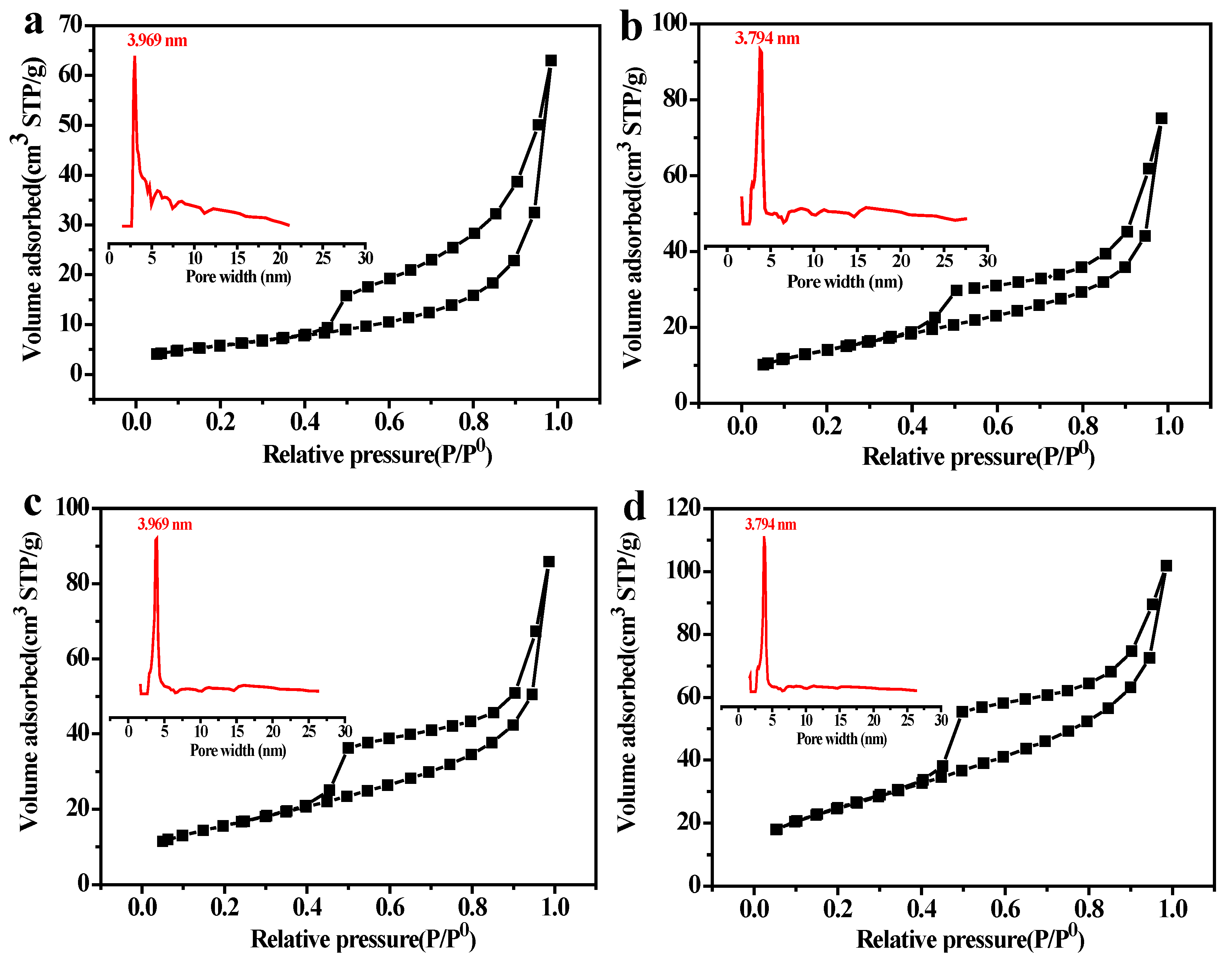
3.3. Micro-Morphological Characteristics
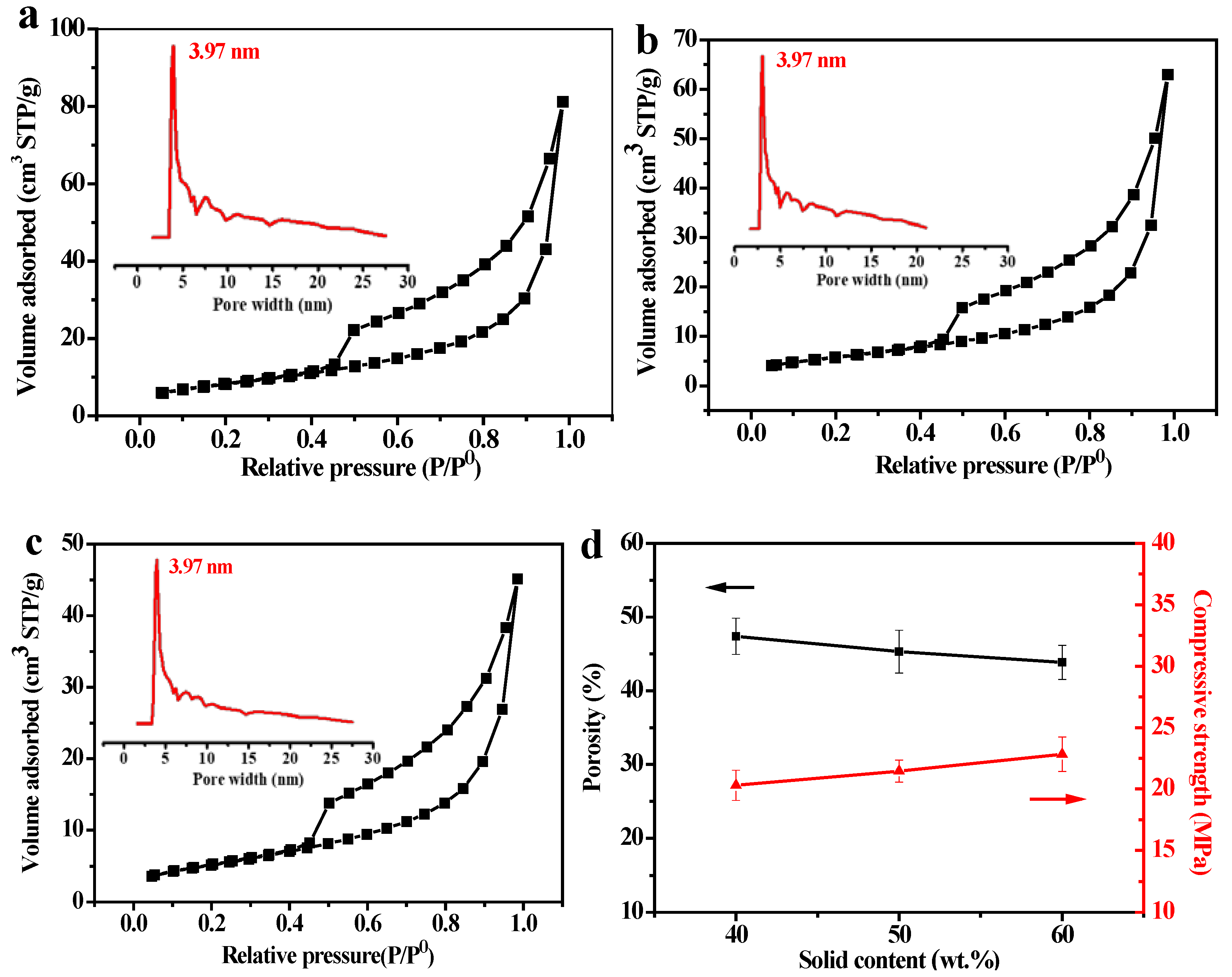
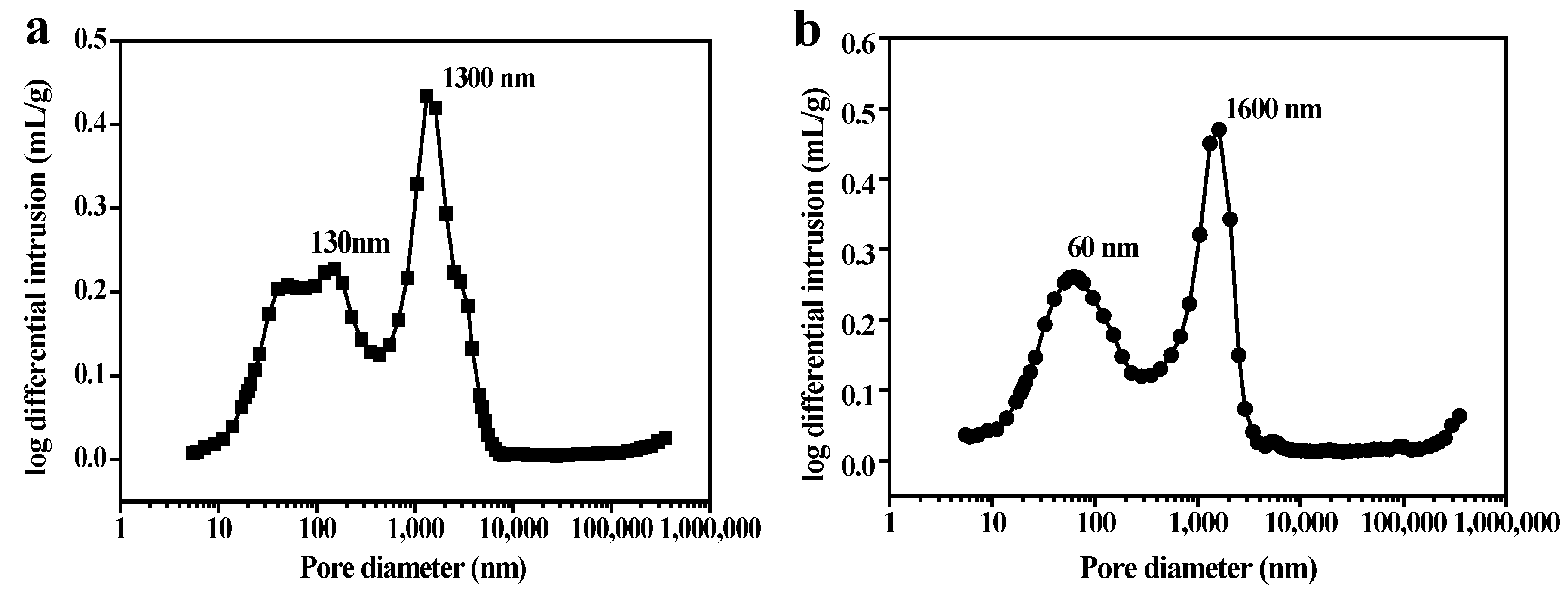
3.4. Affect of Solid Content on the PSCM
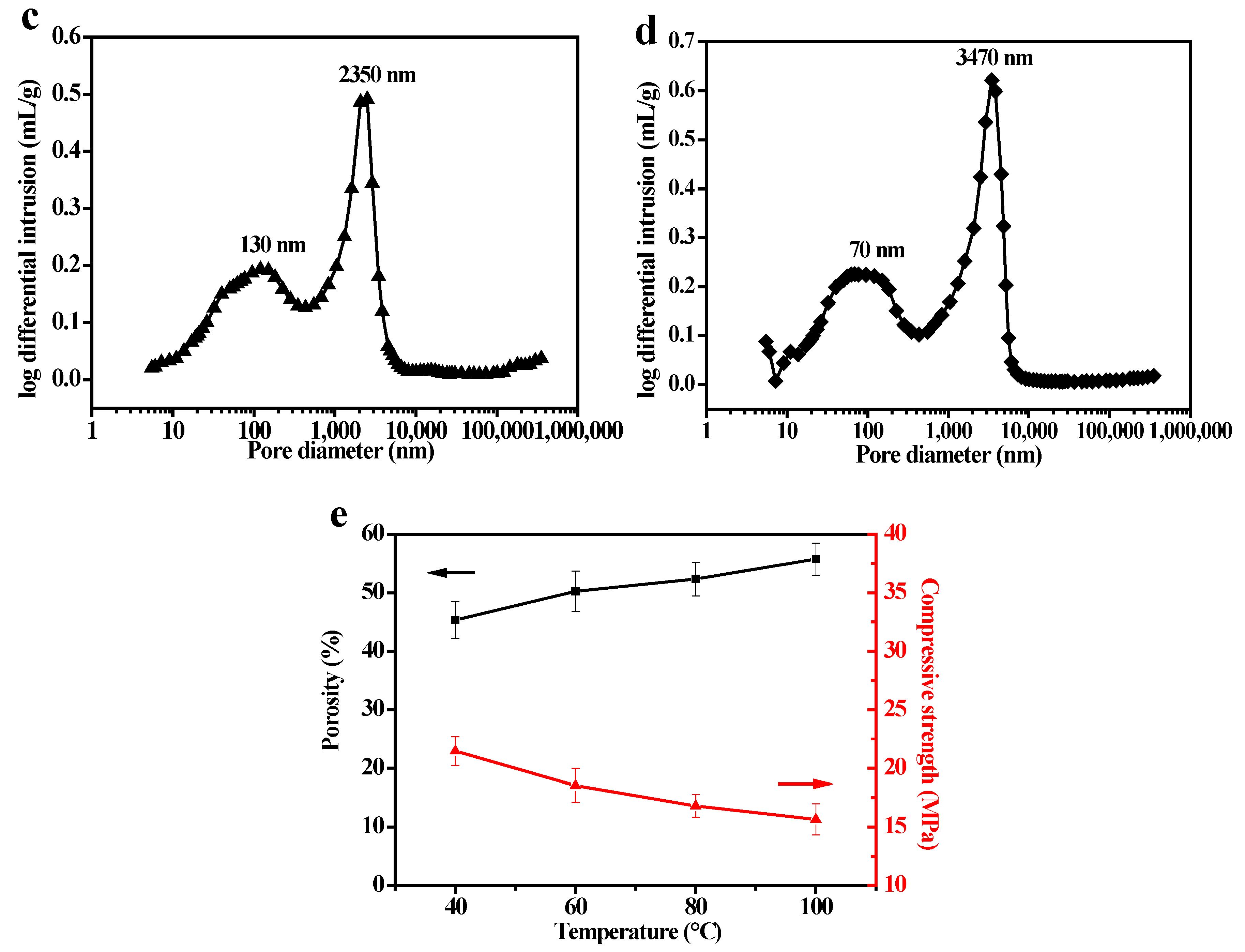
3.5. Affect of the Hot–Dry Temperature on the PSCM
3.6. Performance Test of the PSCMs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, W.; Gaggl, M.; Gluth, G.J.; Behrendt, F. Gas separation using porous cement membrane. J. Environ. Sci. 2014, 26, 140–146. [Google Scholar] [CrossRef]
- Ahmad, N.A.; Goh, P.S.; Yogarathinam, L.T.; Zulhairun, A.K.; Ismail, A.F. Current advances in membrane technologies for produced water desalination. Desalination 2020, 493, 114643. [Google Scholar] [CrossRef]
- Zhang, T.; Li, C.; Sun, S. Effect of Temperature on Oil–Water Separations Using Membranes in Horizontal Separators. Membranes 2022, 12, 232. [Google Scholar] [CrossRef]
- Ravi, J.; Othman, M.H.D.; Matsuura, T.; Bilad, M.R.I.; El-badawy, T.H.; Aziz, F.; Ismail, A.F.; Rahman, M.A.; Jaafar, J. Polymeric membranes for desalination using membrane distillation: A review. Desalination 2020, 490, 114530. [Google Scholar] [CrossRef]
- Goh, P.; Ismail, A. Chemically functionalized polyamide thin film composite membranes: The art of chemistry. Desalination 2020, 495, 114655. [Google Scholar] [CrossRef]
- Jia, Y.; Guan, K.; Zhang, L.; Lin, Y.; Shen, Q.; Zhang, P.; Matsuyama, H. Enabling polyketone membrane with underwater superoleophobicity via a hydrogel-based modification for high-efficiency oil-in-water emulsion separation. J. Membr. Sci. 2020, 618, 118705. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, X.; Lan, Y.; Liu, L.; Gao, C. In situ metal-polyphenol interfacial assembly tailored superwetting PES/SPES/MPN membranes for oil-in-water emulsion separation. J. Membr. Sci. 2020, 615, 118566. [Google Scholar] [CrossRef]
- Gu, K.; Pang, S.; Yang, B.; Ji, Y.; Zhou, Y.; Gao, C. Polyethyleneimine/4,4′-Bis(chloromethyl)-1,1′-biphenyl nanofiltration membrane for metal ions removal in acid wastewater. J. Membr. Sci. 2020, 614, 118497. [Google Scholar]
- Zhang, K.; Yang, K.; Chen, Y.; Hu, Y. Ionic and pH responsive thin film composite hollow fiber nanofiltration membrane for molecular separation. Desalination 2020, 496, 114709. [Google Scholar] [CrossRef]
- Zou, D.; Fan, W.; Xu, J.; Drioli, E.; Chen, X.; Qiu, M.; Fan, Y. One-step engineering of low-cost kaolin/fly ash ceramic membranes for efficient separation of oil-water emulsions. J. Membr. Sci. 2020, 621, 118954. [Google Scholar] [CrossRef]
- Schnittger, J.; McCutcheon, J.; Hoyer, T.; Weyd, M.; Fischer, G.; Puhlfürß, P.; Halisch, M.; Voigt, I.; Lerch, A. Hydrophobic ceramic membranes in MD processes—Impact of material selection and layer characteristics. J. Membr. Sci. 2020, 618, 118678. [Google Scholar] [CrossRef]
- Xie, B.; Zhou, C.; Sang, L.; Ma, X.; Zhang, J. Preparation and characterization of microbubbles with a porous ceramic membrane. Chem. Eng. Process. Process Intensif. 2020, 159, 108213. [Google Scholar] [CrossRef]
- Kong, X.; Xu, P.; Fu, K.; Gong, D.; Chen, X.; Qiu, M.; Fan, Y. Critical gas velocity of hydrophobic ceramic membrane contactors for SO2 absorption. Chem. Eng. Sci. 2020, 231, 116327. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.; Chen, H.; Huang, J.; Fu, H. Water vapor capture using microporous ceramic membrane. Desalination 2020, 482, 114405. [Google Scholar] [CrossRef]
- Liu, Y.-P.; Qi, X.-H.; Li, L.; Zhang, S.-H.; Bi, T. MOF-derived PPy/carbon-coated copper sulfide ceramic nanocomposite as high-performance electrode for supercapacitor. Ceram. Int. 2019, 45, 17216–17223. [Google Scholar] [CrossRef]
- Yin, N.; Wang, K.; Wang, L.; Li, Z. Amino-functionalized MOFs combining ceramic membrane ultrafiltration for Pb (II) removal. Chem. Eng. J. 2016, 306, 619–628. [Google Scholar] [CrossRef]
- Li, K. Ceramic Membranes for Separation and Reaction; John Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Anis, S.F.; Lalia, B.S.; Hashaikeh, R.; Hilal, N. Titanium coating on ultrafiltration inorganic membranes for fouling control. Sep. Purif. Technol. 2021, 282, 119997. [Google Scholar] [CrossRef]
- Goh, P.; Ismail, A. A review on inorganic membranes for desalination and wastewater treatment. Desalination 2018, 434, 60–80. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, H.; Wang, Y.; Song, G.; Zhang, L. Industrial application of ceramic ultrafiltration membrane in cold-rolling emulsion wastewater treatment. Sep. Purif. Technol. 2022, 289, 120724. [Google Scholar] [CrossRef]
- Usman, J.; Othman, M.H.D.; Ismail, A.F.; Rahman, M.A.; Jaafar, J.; Raji, Y.O.; Gbadamosi, A.O.; El Badawy, T.H.; Said, K.A.M. An overview of superhydrophobic ceramic membrane surface modification for oil-water separation. J. Mater. Res. Technol. 2021, 12, 643–667. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, Z.; Ma, J.; Xu, S.; Wu, Z. Development of an Electrochemical Ceramic Membrane Filtration System for Efficient Contaminant Removal from Waters. Environ. Sci. Technol. 2018, 52, 4117–4126. [Google Scholar] [CrossRef]
- Asif, M.B.; Zhang, Z. Ceramic membrane technology for water and wastewater treatment: A critical review of performance, full-scale applications, membrane fouling and prospects. Chem. Eng. J. 2021, 418, 129481. [Google Scholar] [CrossRef]
- Kayvani Fard, A.; McKay, G.; Buekenhoudt, A.; Al Sulaiti, H.; Motmans, F.; Khraisheh, M.; Atieh, M. Inorganic Membranes: Preparation and Application for Water Treatment and Desalination. Materials 2018, 11, 74. [Google Scholar] [CrossRef]
- Li, C.; Sun, W.; Lu, Z.; Ao, X.; Li, S. Ceramic nanocomposite membranes and membrane fouling: A review. Water Res. 2020, 175, 115674. [Google Scholar] [CrossRef]
- Tang, S.; Huang, J.; Duan, L.; Yu, P.; Chen, E. A review on fractal footprint of cement-based materials. Powder Technol. 2020, 370, 237–250. [Google Scholar] [CrossRef]
- de Brito, J.; Kurda, R. The past and future of sustainable concrete: A critical review and new strategies on cement-based materials. J. Clean. Prod. 2020, 281, 123558. [Google Scholar] [CrossRef]
- Li, F.; Liu, J. An experimental investigation of hydration mechanism of cement with silicane. Constr. Build. Mater. 2018, 166, 684–693. [Google Scholar] [CrossRef]
- Shafie, A.H.; An, W.; Hejazi SA, H.; Sawada, J.A.; Kuznicki, S.M. Natural zeolite-based cement composite membranes for H2/CO2 separation. Sep. Purif. Technol. 2012, 88, 24–28. [Google Scholar] [CrossRef]
- Dong, S.; Zhu, W.; Gao, X.; Wang, Z.; Wang, L.; Wang, X.; Gao, C. Preparation of tubular hierarchically porous silicate cement compacts via a tert-butyl alcohol (TBA)-based freeze casting method. Chem. Eng. J. 2016, 295, 530–541. [Google Scholar] [CrossRef]
- Dong, S.; Wang, L.; Gao, X.; Zhu, W.; Wang, Z.; Ma, Z.; Gao, C. Freeze casting of novel porous silicate cement supports using tert-butyl alcohol-water binary crystals as template: Microstructure, strength and permeability. J. Membr. Sci. 2017, 541, 143–152. [Google Scholar] [CrossRef]
- Dong, S.; Gao, X.; Ma, Z.; Wang, X.; Gao, C. Ice-templated porous silicate cement with hierarchical porosity. Mater. Lett. 2018, 217, 292–295. [Google Scholar] [CrossRef]
- Fernández, E.; Benito, J.M.; Pazos, C.; Coca, J. Ceramic membrane ultrafiltration of anionic and nonionic surfactant solutions. J. Membr. Sci. 2005, 246, 1–6. [Google Scholar] [CrossRef]
- Shahrin, R.; Bobko, C.P. Micropillar compression investigation of size effect on microscale strength and failure mechanism of Calcium-Silicate-Hydrates (C-S-H) in cement paste. Cem. Concr. Res. 2019, 125, 105863. [Google Scholar] [CrossRef]
- Kurihara, R.; Maruyama, I. Effects of heating and drying on the strength and stiffness of high-early-strength Portland cement pastes. Cem. Concr. Compos. 2020, 106, 103455. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, J.; Li, K.; Pu, H.; Meng, X.; Zhang, H.; Liu, K. Effects of steam on the compressive strength and microstructure of cement paste cured under alternating ultrahigh temperature. Cem. Concr. Compos. 2020, 112, 103681. [Google Scholar] [CrossRef]
- Lorenzoni, R.; Paciornik, S.; Silva, F. Characterization by microcomputed tomography of class G oil well cement paste exposed to elevated temperatures. J. Pet. Sci. Eng. 2019, 175, 896–904. [Google Scholar] [CrossRef]
- Ivanets, A.I.; Rat, A.I.; Azarova, T.A.; Azarov, S.M.; Al-Khowaiter, S.H.; Al-Harbi, O. Preparation and properties of microfiltration membranes based on natural crystalline SiO2. Ceram. Int. 2014, 40, 12343–12351. [Google Scholar] [CrossRef]


| Components | CaO | SiO2 | Fe2O3 | Al2O3 | MgO |
|---|---|---|---|---|---|
| Content (wt. %) | 62.47 | 22.15 | 5.73 | 4.03 | 3.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Wang, X.; Yuan, H.; Sang, S.; Xu, H.; Huang, Y.; Gao, C.; Gao, X. Preparation of Porous Silicate Cement Membranes via a One-Step Water-Based Hot–Dry Casting Method. Membranes 2022, 12, 838. https://doi.org/10.3390/membranes12090838
Sun Z, Wang X, Yuan H, Sang S, Xu H, Huang Y, Gao C, Gao X. Preparation of Porous Silicate Cement Membranes via a One-Step Water-Based Hot–Dry Casting Method. Membranes. 2022; 12(9):838. https://doi.org/10.3390/membranes12090838
Chicago/Turabian StyleSun, Zhantong, Xiaojuan Wang, Haifeng Yuan, Shizhong Sang, Huacheng Xu, Yijun Huang, Congjie Gao, and Xueli Gao. 2022. "Preparation of Porous Silicate Cement Membranes via a One-Step Water-Based Hot–Dry Casting Method" Membranes 12, no. 9: 838. https://doi.org/10.3390/membranes12090838
APA StyleSun, Z., Wang, X., Yuan, H., Sang, S., Xu, H., Huang, Y., Gao, C., & Gao, X. (2022). Preparation of Porous Silicate Cement Membranes via a One-Step Water-Based Hot–Dry Casting Method. Membranes, 12(9), 838. https://doi.org/10.3390/membranes12090838