Nitrogen Recovery from Landfill Leachate Using Lab- and Pilot-Scale Membrane Contactors: Research into Fouling Development and Membrane Characterization Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feed Properties
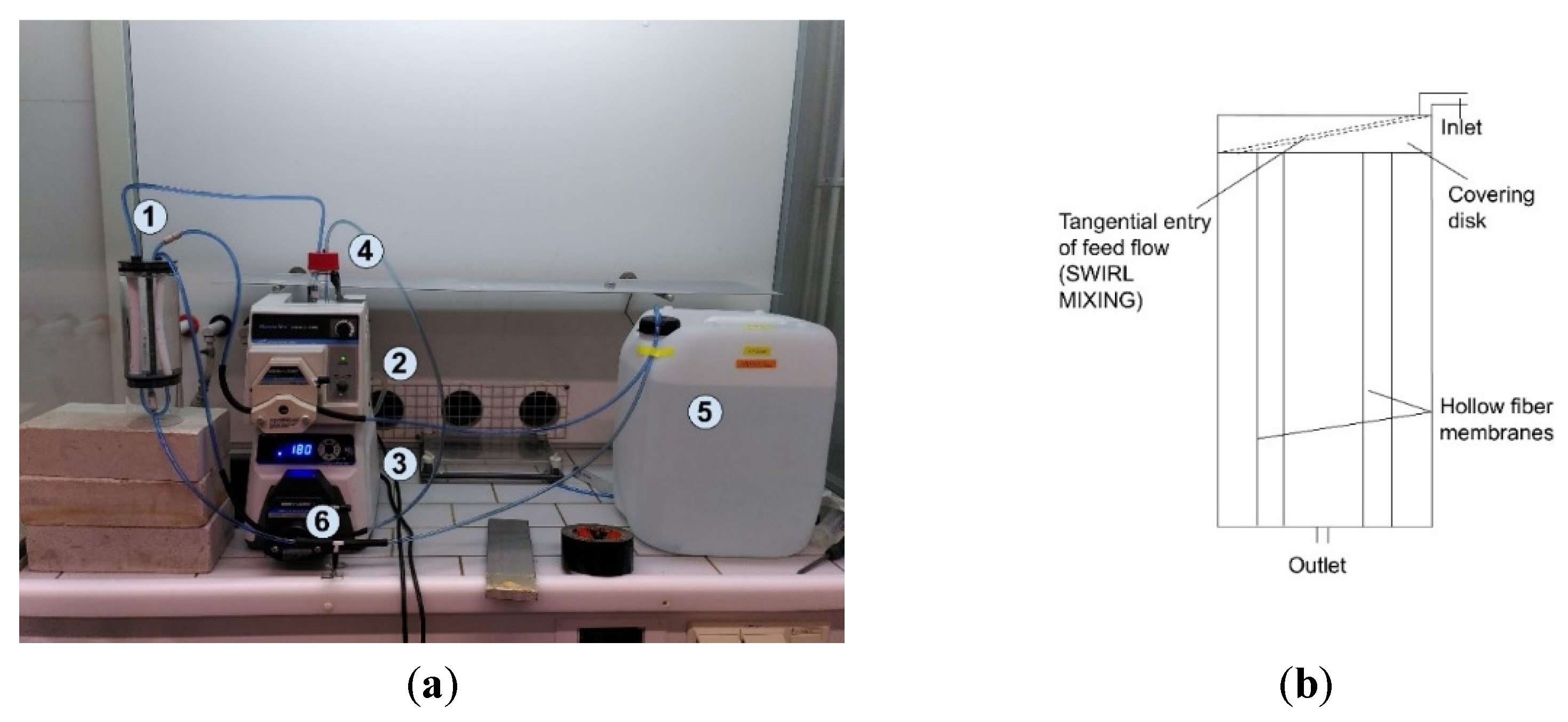
2.2. Experimental Lab Set-Up
2.3. Experimental Lab Procedure
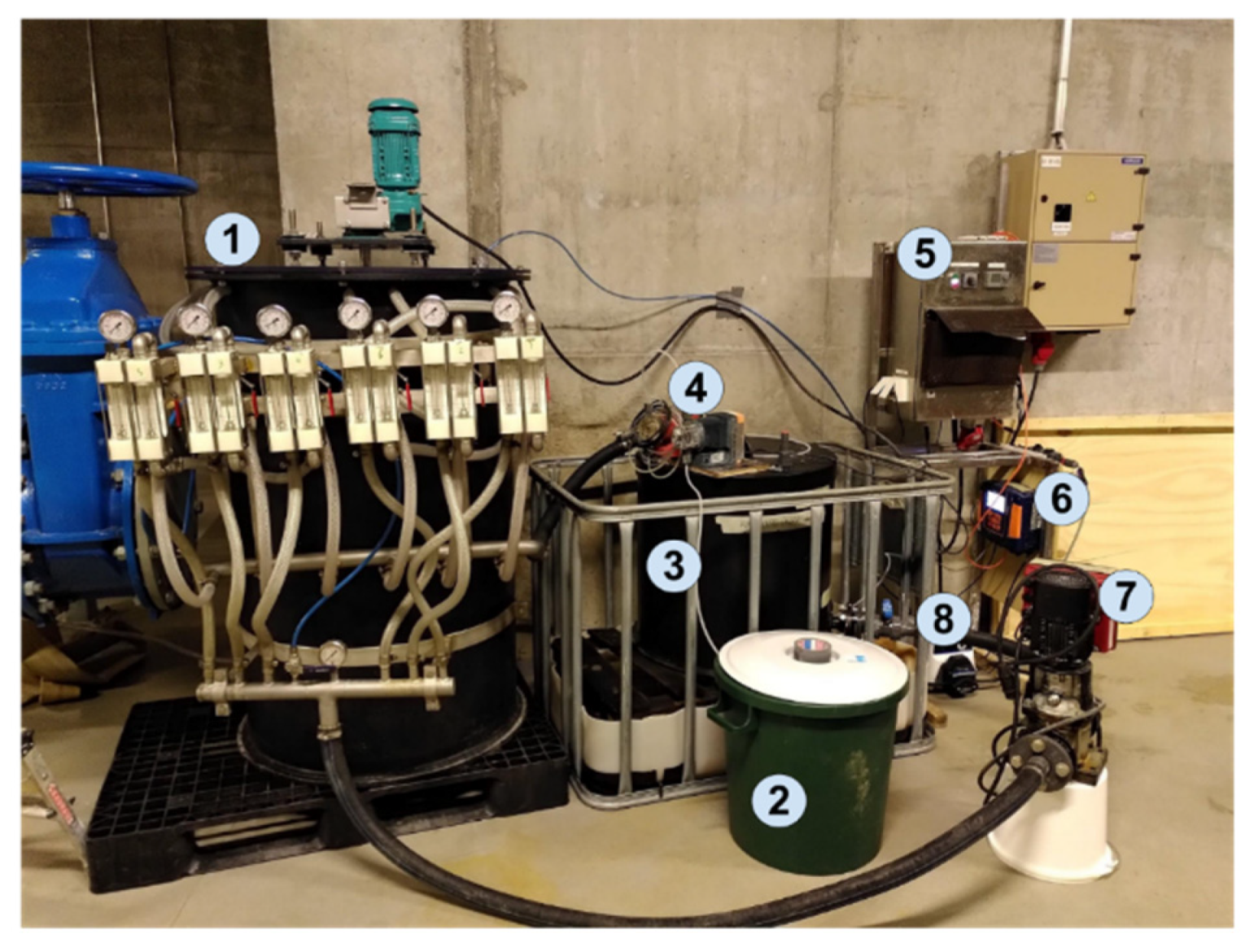
2.4. Pilot-Scale System Description
2.5. Pilot Test Procedure
- Inlet: after the pH adjustment and before entering the reactor;
- Bulk: inside the reactor, at around a 0.5 m depth from the top;
- Outlet: after the treatment and before discharging into the sewer;
- Acid: taken from the acid side.
2.6. Analysis
3. Results and Discussion
3.1. Effect of Membrane Characterization on NH3 Recovery and Transfer Rate
3.2. Ammonia Recovery from Landfill Leachate: Lab Scale vs. Pilot Scale
3.3. Fouling Development and Cleaning Effects
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agriculture Organization of the United Nations (FAO). World Fertilizer Trends and Outlook to 2020; Agriculture Organization of the United Nations (FAO): Rome, Italy, 2017. [Google Scholar]
- Fertilizers Europe. 2018/19 Overview; Fertilizers Europe: Brussels, Belgium, 2019. [Google Scholar]
- Cherkasov, N.; Ibhadon, A.O.; Fitzpatrick, P. A Review of the Existing and Alternative Methods for Greener Nitrogen Fixation. Chem. Eng. Process. 2015, 90, 24–33. [Google Scholar] [CrossRef]
- Xie, M.; Shon, H.K.; Gray, S.R.; Elimelech, M. Membrane-Based Processes for Wastewater Nutrient Recovery: Technology, Challenges, and Future Direction. Water Res. 2016, 89, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Van der Hoek, J.P.; Duijff, R.; Reinstra, O. Nitrogen Recovery from Wastewater: Possibilities, Competition with Other Resources, and Adaptation Pathways. Sustainability 2018, 10, 4605. [Google Scholar] [CrossRef]
- Maktabifard, M.; Zaborowska, E.; Makinia, J. Achieving Energy Neutrality in Wastewater Treatment Plants through Energy Savings and Enhancing Renewable Energy Production. Rev. Environ. Sci. Biotechnol. 2018, 17, 655–689. [Google Scholar] [CrossRef]
- Snip, L. Quantifying the Greenhouse Gas Emissions of Wastewater Treatment Plants; Wageningen University: Wageningen, The Netherlands, 2010. [Google Scholar]
- Zhang, C.; Ding, W.; Zeng, X.; Xu, X. Recovery of Ammonia Nitrogen from Landfill Leachate Using a Biopolar Membrane Equipped Electrodialysis System. Water Sci. Technol. 2020, 82, 1758–1770. [Google Scholar] [CrossRef] [PubMed]
- SMART-Plant Recover Nutrients. Available online: https://www.smart-plant.eu/index.php/services/recover-nutrients (accessed on 1 August 2022).
- Morales, N.; Boehler, M.A.; Buettner, S.; Liebi, C.; Siegrist, H. Recovery of N and P from Urine by Struvite Precipitation Followed by Combined Stripping with Digester Sludge Liquid at Full Scale. Water 2013, 5, 1262–1278. [Google Scholar] [CrossRef]
- Rossi, L.; Reuna, S.; Fred, T.; Heinonen, M. RAVITA Technology–New Innovation for Combined Phosphorus and Nitrogen Recovery. Water Sci. Technol. 2018, 78, 2511–2517. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Hlavínek, P.; Raboni, M. Physico-Chemical Technologies for Nitrogen Removal from Wastewaters: A Review. Rev. Ambiente Agua 2015, 10, 481–498. [Google Scholar]
- Darestani, M.; Haigh, V.; Couperthwaite, S.J.; Millar, G.J.; Nghiem, L.D. Hollow Fibre Membrane Contactors for Ammonia Recovery: Current Status and Future Developments. J. Environ. Chem. Eng. 2017, 5, 1349–1359. [Google Scholar] [CrossRef]
- Mehta, C.M.; Khunjar, W.O.; Nguyen, V.; Tait, S.; Batstone, D.J. Technologies to Recover Nutrients from Waste Streams: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 385–427. [Google Scholar] [CrossRef]
- Robles, Á.; Aguado, D.; Barat, R.; Borrás, L.; Bouzas, A.; Giménez, J.B.; Martí, N.; Ribes, J.; Ruano, M.V.; Serralta, J. New Frontiers from Removal to Recycling of Nitrogen and Phosphorus from Wastewater in the Circular Economy. Bioresour. Technol. 2020, 300, 122673. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Nawaz, T.; Beaudry, J. Nitrogen and Phosphorus Recovery from Wastewater. Curr. Pollut. Rep. 2015, 1, 155–166. [Google Scholar] [CrossRef]
- Sridhar, S.; Bee, S.; Bhargava, S. Membrane-Based Gas Separation: Principle, Applications and Future Potential. Chem. Eng. Dig 2014, 1, 1–25. [Google Scholar]
- Tang, H.; Zhang, Y.; Wang, F.; Zhang, H.; Guo, Y. Long-Term Stability of Polytetrafluoroethylene (PTFE) Hollow Fiber Membranes for CO2 Capture. Energy Fuels 2016, 30, 492–503. [Google Scholar] [CrossRef]
- Kunz, A.; Mukhtar, S. Hydrophobic Membrane Technology for Ammonia Extraction from Wastewaters. Eng. Agricola 2016, 36, 377–386. [Google Scholar] [CrossRef]
- Hasanoğlu, A.; Romero, J.; Pérez, B.; Plaza, A. Ammonia Removal from Wastewater Streams through Membrane Contactors: Experimental and Theoretical Analysis of Operation Parameters and Configuration. Chem. Eng. J. 2010, 160, 530–537. [Google Scholar] [CrossRef]
- Matassa, S.; Batstone, D.J.; Hülsen, T.; Schnoor, J.; Verstraete, W. Can Direct Conversion of Used Nitrogen to New Feed and Protein Help Feed the World? Environ. Sci. Technol. 2015, 49, 5247–5254. [Google Scholar] [CrossRef]
- Noriega-Hevia, G.; Serralta, J.; Borrás, L.; Seco, A.; Ferrer, J. Nitrogen Recovery Using a Membrane Contactor: Modelling Nitrogen and PH Evolution. J. Environ. Chem. Eng. 2020, 8, 103880. [Google Scholar] [CrossRef]
- Norddahl, B.; Horn, V.G.; Larsson, M.; Du Preez, J.H.; Christensen, K. A Membrane Contactor for Ammonia Stripping, Pilot Scale Experience and Modeling. Desalination 2006, 199, 172–174. [Google Scholar] [CrossRef]
- Zhu, Z.; Hao, Z.; Shen, Z.; Chen, J. Modified Modeling of the Effect of PH and Viscosity on the Mass Transfer in Hydrophobic Hollow Fiber Membrane Contactors. J. Membr. Sci. 2005, 250, 269–276. [Google Scholar] [CrossRef]
- Amaral, M.C.; Magalhães, N.C.; Moravia, W.G.; Ferreira, C.D. Ammonia Recovery from Landfill Leachate Using Hydrophobic Membrane Contactors. Water Sci. Technol. 2016, 74, 2177–2184. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qin, Y.; Cui, D.; Cai, T.; Hao, X.; Wang, Y. Removal/Recovery of Ammonia from Landfill Leachate by Supported Gas Membrane-Based Separation Process. Chin. J. Environ. Eng. 2014, 8, 612–618. [Google Scholar]
- Uzkurt Kaljunen, J.; Al-Juboori, R.A.; Mikola, A.; Righetto, I.; Konola, I. Newly Developed Membrane Contactor-Based N and P Recovery Process: Pilot-Scale Field Experiments and Cost Analysis. J. Clean. Prod. 2021, 281, 125288. [Google Scholar] [CrossRef]
- Righetto, I.; Al-Juboori, R.A.; Kaljunen, J.U.; Mikola, A. Multipurpose Treatment of Landfill Leachate Using Natural Coagulants–Pretreatment for Nutrient Recovery and Removal of Heavy Metals and Micropollutants. J. Environ. Chem. Eng. 2021, 9, 105213. [Google Scholar] [CrossRef]
- Kaljunen, J. Nitrogen Harvesting from Liquid Waste Streams Using Hydrophobic Gas Permeable Membranes. Master’s Thesis, Aalto University, Aalto, Finland, 2018. [Google Scholar]
- Lauterböck, B.; Moder, K.; Germ, T.; Fuchs, W. Impact of Characteristic Membrane Parameters on the Transfer Rate of Ammonia in Membrane Contactor Application. Sep. Purif. Technol. 2013, 116, 327–334. [Google Scholar] [CrossRef]
- Chang, Y.S.; Ooi, B.S.; Ahmad, A.L.; Leo, C.P.; Low, S.C. Vacuum Membrane Distillation for Desalination: Scaling Phenomena of Brackish Water at Elevated Temperature. Sep. Purif. Technol. 2021, 254, 117572. [Google Scholar] [CrossRef]
- Iversen, S.B.; Bhatia, V.K.; Dam-Johansen, K.; Jonsson, G. Characterization of Microporous Membranes for Use in Membrane Contactors. J. Membr. Sci 1997, 130, 205–217. [Google Scholar] [CrossRef]
- Johnson, R.A.; Nguyen, M.H. Understanding Membrane Distillation and Osmotic Distillation; John Wiley & Sons: Hoboken, NJ, USA, 2017; ISBN 0-470-12216-1. [Google Scholar]
- Rivera, F.; Muñoz, R.; Prádanos, P.; Hernández, A.; Palacio, L. A Systematic Study of Ammonia Recovery from Anaerobic Digestate Using Membrane-Based Separation. Membranes 2022, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, D.; Chen, Y.; Gao, B.; Wang, Z. Scaling Control of Forward Osmosis-Membrane Distillation (FO-MD) Integrated Process for Pre-Treated Landfill Leachate Treatment. Desalination 2021, 520, 115342. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Z.; Gao, L.; Gray, S.; Xie, Z. Study of MOF Incorporated Dual Layer Membrane with Enhanced Removal of Ammonia and Per-/Poly-Fluoroalkyl Substances (PFAS) in Landfill Leachate Treatment. Sci. Total Environ. 2022, 806, 151207. [Google Scholar] [CrossRef]
- Zoungrana, A.; Zengin, İ.H.; Türk, O.K.; Çakmakcı, M. Ammoniacal Nitrogen Reclamation by Membrane Distillation from High Ammonia Polluted Solutions. Chem. Pap. 2020, 74, 1903–1915. [Google Scholar] [CrossRef]
- Chen, L.; Li, F.; He, F.; Mao, Y.; Chen, Z.; Wang, Y.; Cai, Z. Membrane Distillation Combined with Electrocoagulation and Electrooxidation for the Treatment of Landfill Leachate Concentrate. Sep. Purif. Technol. 2022, 291, 120936. [Google Scholar] [CrossRef]
- Xue, Y.; Zhao, H.; Ge, L.; Chen, Z.; Dang, Y.; Sun, D. Comparison of the Performance of Waste Leachate Treatment in Submerged and Recirculated Membrane Bioreactors. Int. Biodeterior. Biodegrad. 2015, 102, 73–80. [Google Scholar] [CrossRef]











| Constituents | Concentration (mg/L) |
|---|---|
| Total nitrogen | 1019.0 |
| Total phosphorous | 7.7 |
| Total organic carbon | 1189.0 |
| Suspended solids | 20.0 |
| Volatile suspended solids | 25.0 |
| Ammonia | 969.0 |
| pH | 7.9 |
| Membrane Sample | Contact Angle (°) | Mean Roughness, Ra (nm) | Root Mean Square Roughness, Rq (nm) |
|---|---|---|---|
| Virgin | 123 ± 0.07 | 360 ± 11 | 451 ± 15 |
| Ämmässou pilot | 121 ± 0.60 | 435 ± 18 | 557 ± 24 |
| Ämmässou lab—untreated leachate | 119 ± 0.3 | 383 ± 09 | 483 ± 10 |
| Ämmässou lab—treated leachate | 121 ± 0.05 | 361 ± 22 | 465 ± 15 |
| Ämmässou lab—cleaned with UV + H2O2 | 120 ± 0.06 | 338 ± 13 | 418 ± 19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Righetto, I.; Al-Juboori, R.A.; Kaljunen, J.U.; Huynh, N.; Mikola, A. Nitrogen Recovery from Landfill Leachate Using Lab- and Pilot-Scale Membrane Contactors: Research into Fouling Development and Membrane Characterization Effects. Membranes 2022, 12, 837. https://doi.org/10.3390/membranes12090837
Righetto I, Al-Juboori RA, Kaljunen JU, Huynh N, Mikola A. Nitrogen Recovery from Landfill Leachate Using Lab- and Pilot-Scale Membrane Contactors: Research into Fouling Development and Membrane Characterization Effects. Membranes. 2022; 12(9):837. https://doi.org/10.3390/membranes12090837
Chicago/Turabian StyleRighetto, Ilaria, Raed A. Al-Juboori, Juho Uzkurt Kaljunen, Ngoc Huynh, and Anna Mikola. 2022. "Nitrogen Recovery from Landfill Leachate Using Lab- and Pilot-Scale Membrane Contactors: Research into Fouling Development and Membrane Characterization Effects" Membranes 12, no. 9: 837. https://doi.org/10.3390/membranes12090837
APA StyleRighetto, I., Al-Juboori, R. A., Kaljunen, J. U., Huynh, N., & Mikola, A. (2022). Nitrogen Recovery from Landfill Leachate Using Lab- and Pilot-Scale Membrane Contactors: Research into Fouling Development and Membrane Characterization Effects. Membranes, 12(9), 837. https://doi.org/10.3390/membranes12090837








