Preparation and Characterization of Low-Cost Ceramic Membrane Coated with Chitosan: Application to the Ultrafine Filtration of Cr(VI)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Ceramic Membrane (CM)
2.3. Preparation of the Chitosan-Coated Ceramic Membranes (CCCMs)
2.4. Characterization of CMs and CCCMs
2.5. Ultrafine Filtration of Chromium(VI)
3. Results
3.1. Properties of CMs Prepared at Different Sintering Temperatures and CCCMs
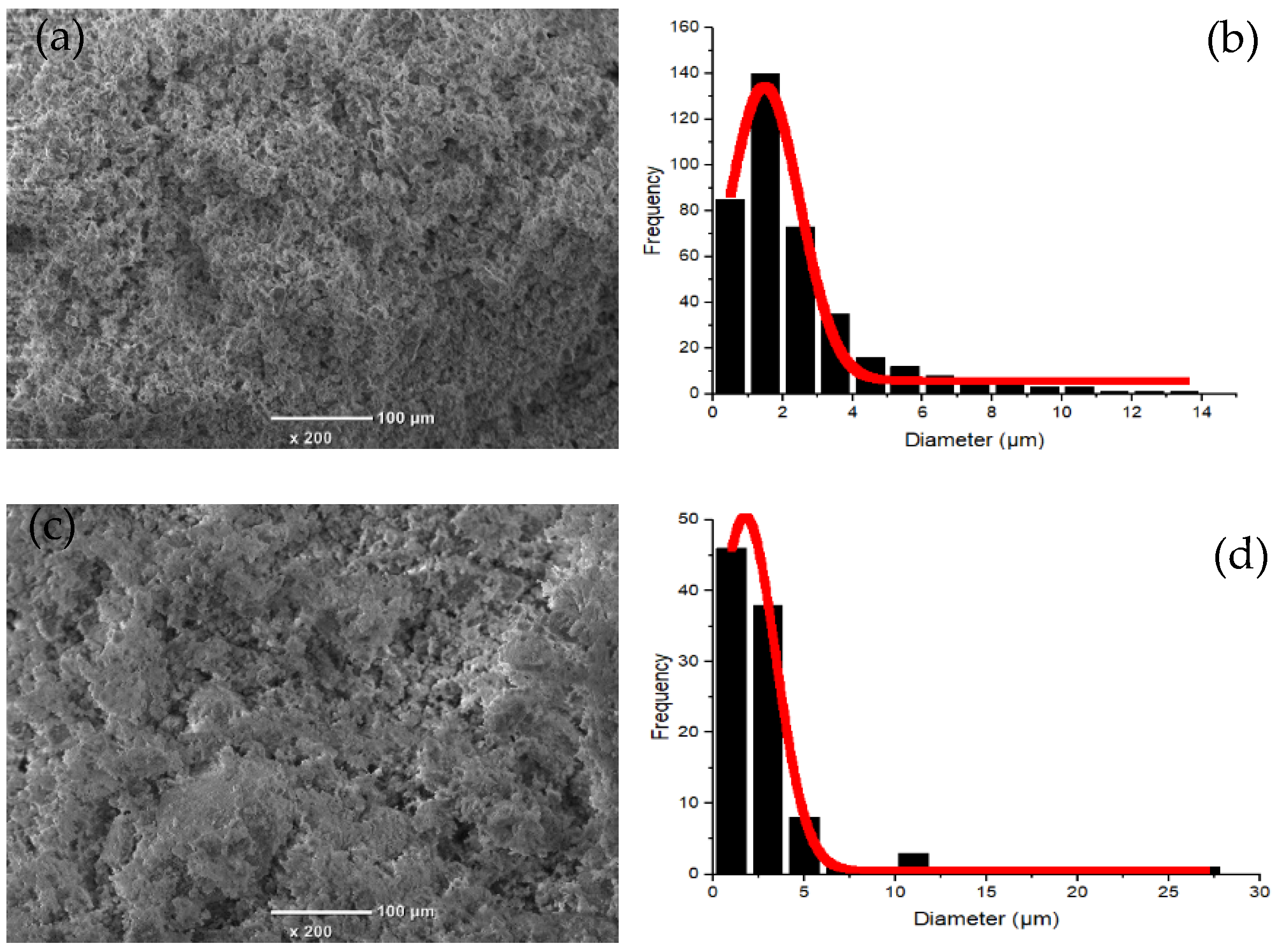
3.1.1. Characterization of Kaolin and Clay
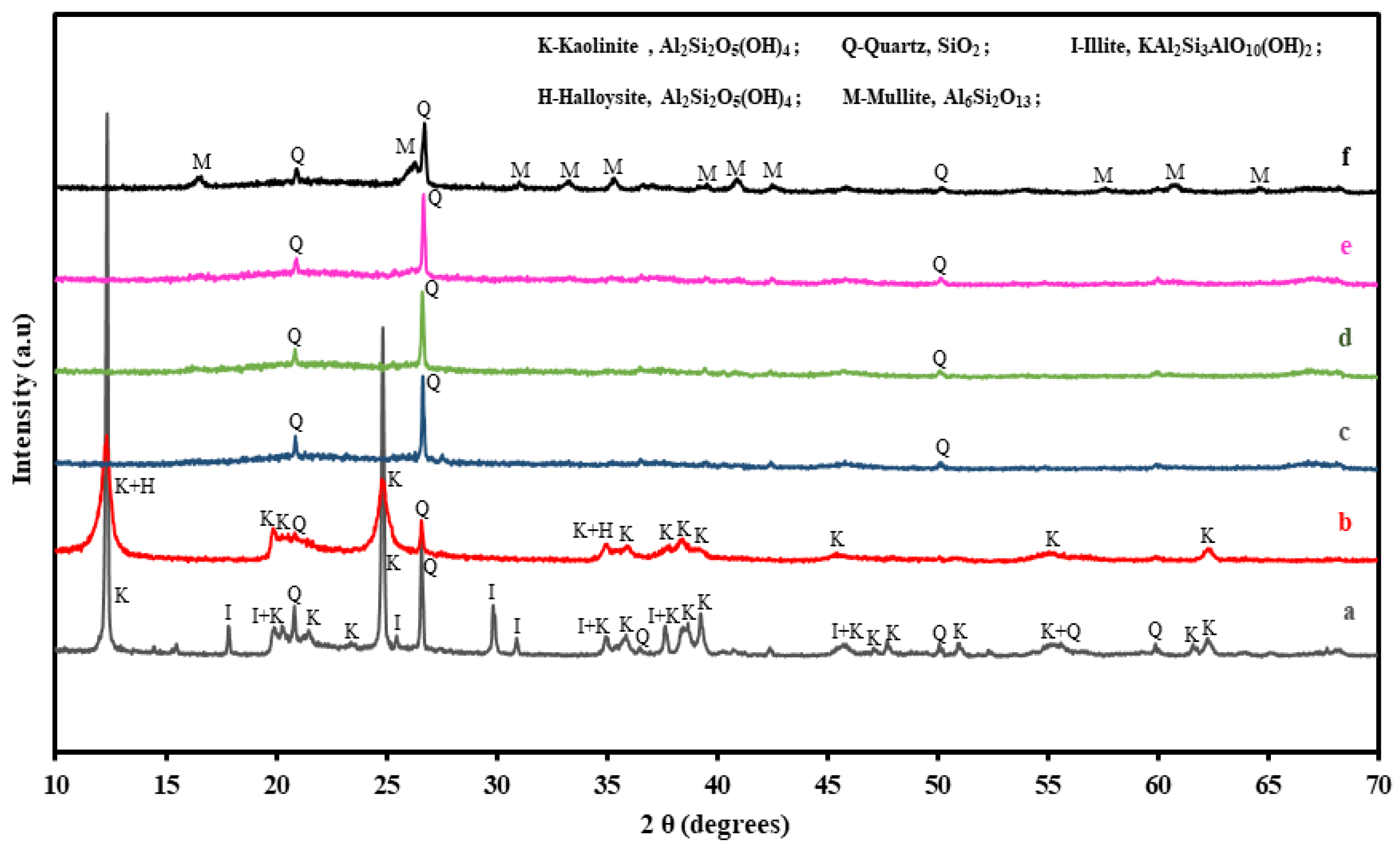
3.1.2. XRD Analysis
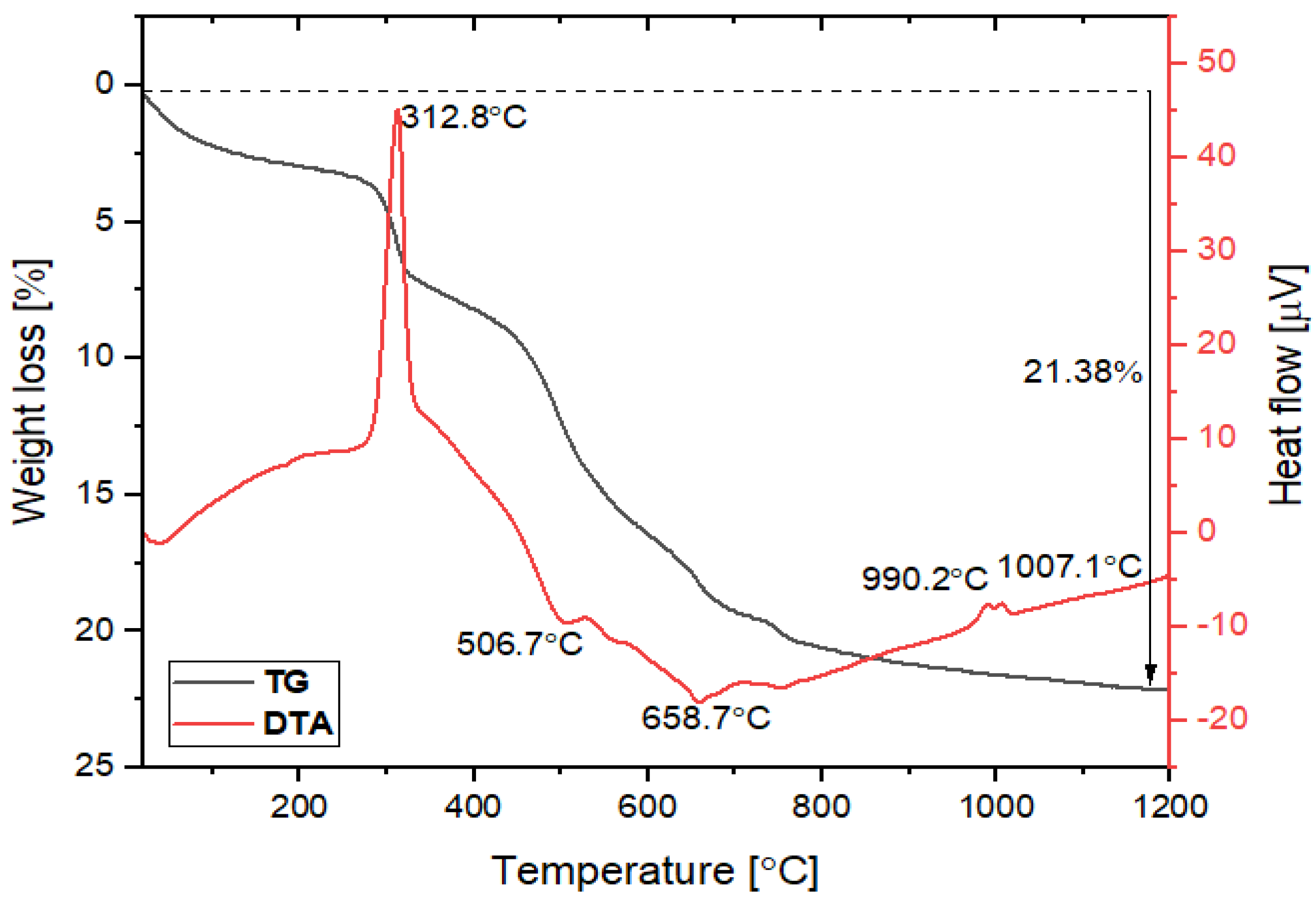
3.1.3. TG-DTA Analysis
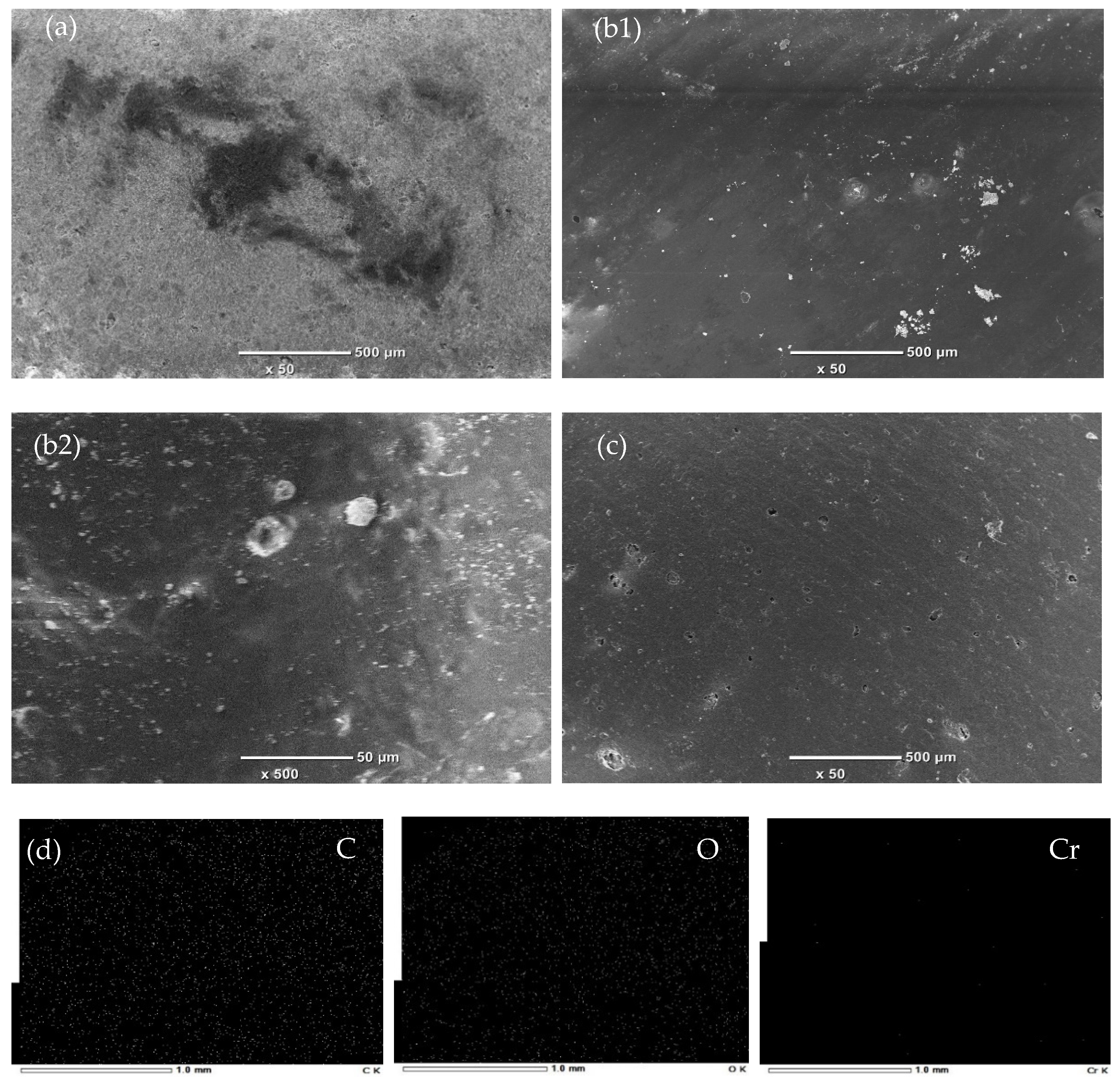
3.1.4. SEM-EDS Analysis
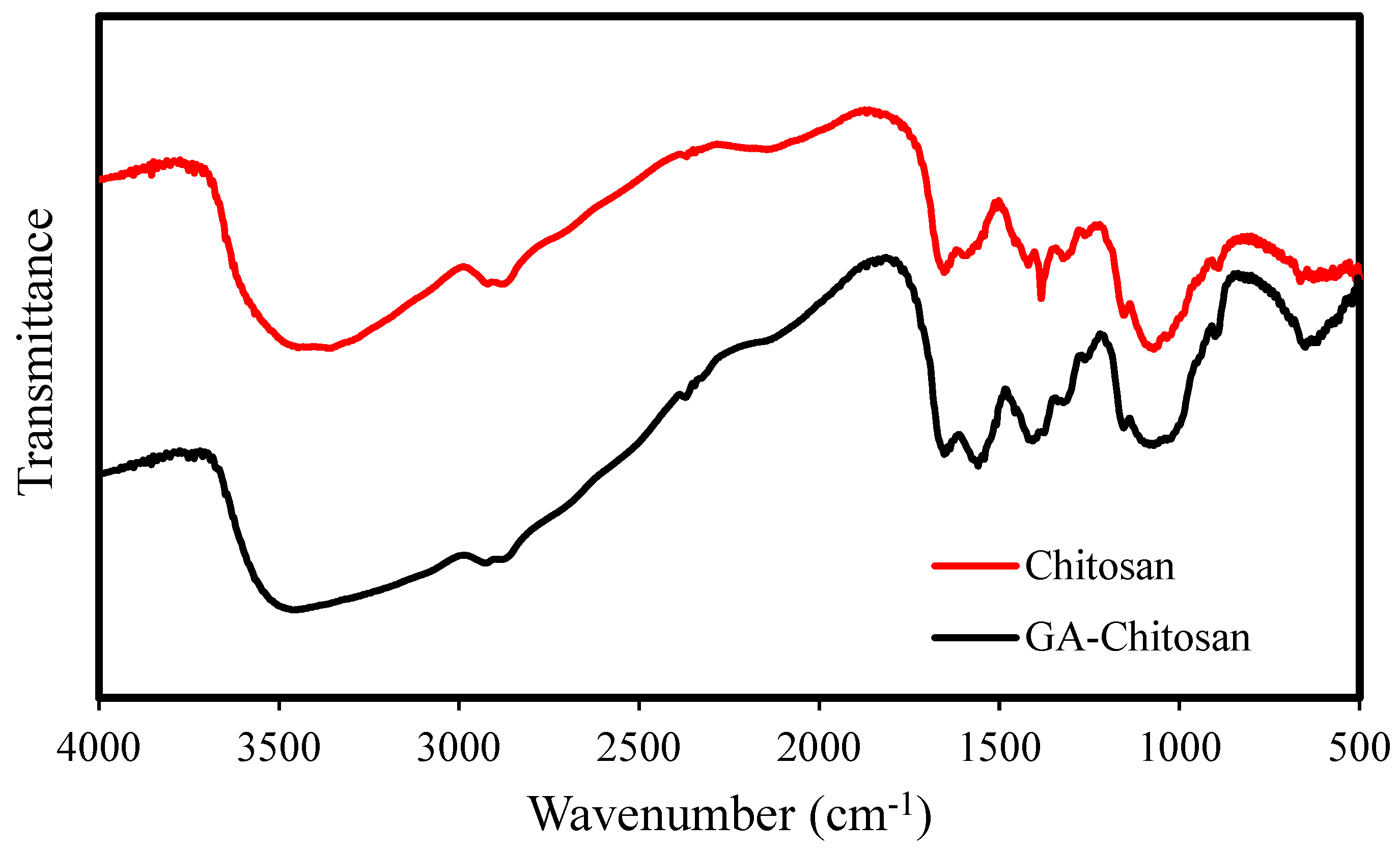
3.1.5. FT-IR Analysis
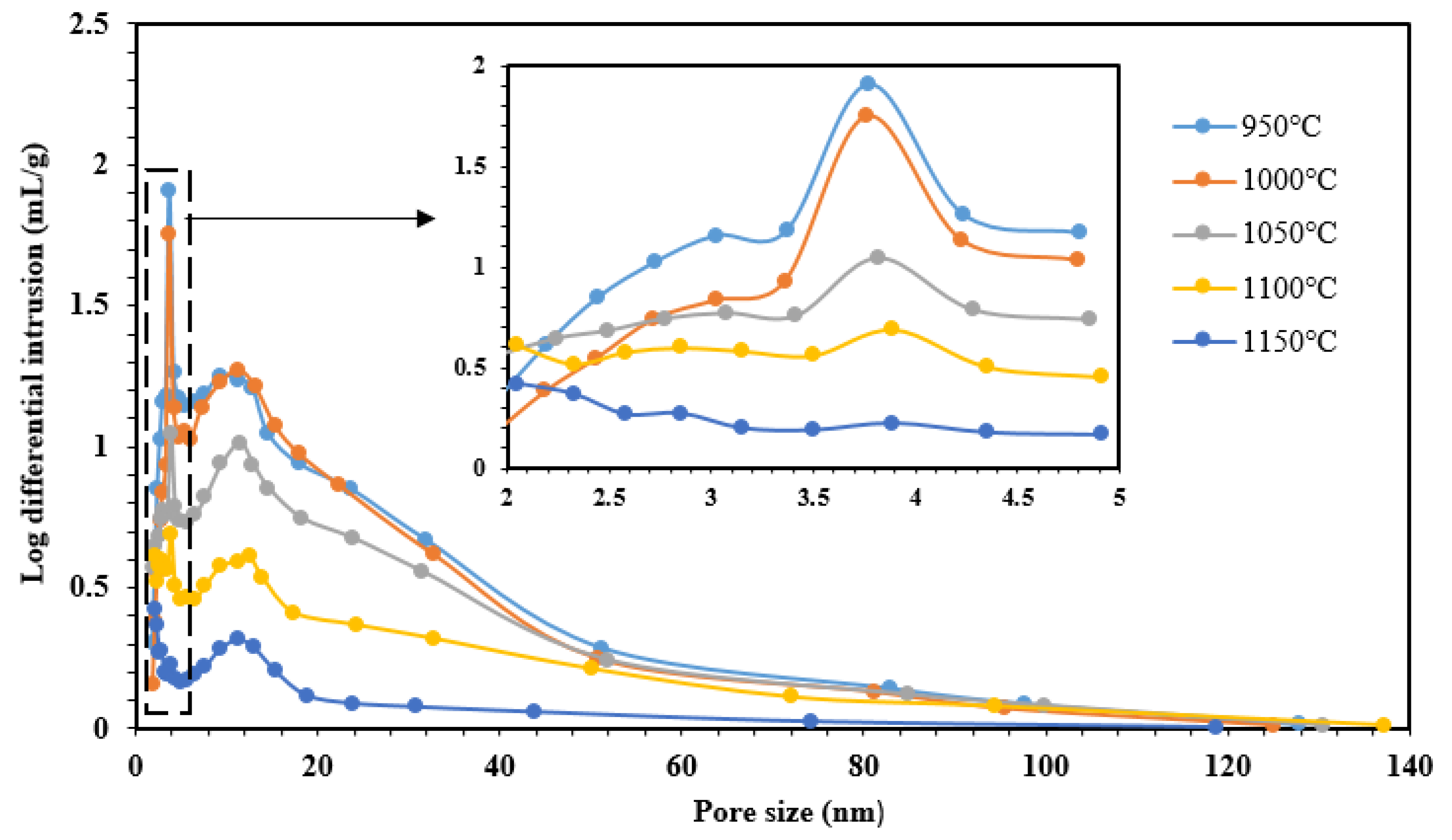
3.1.6. BET Analysis
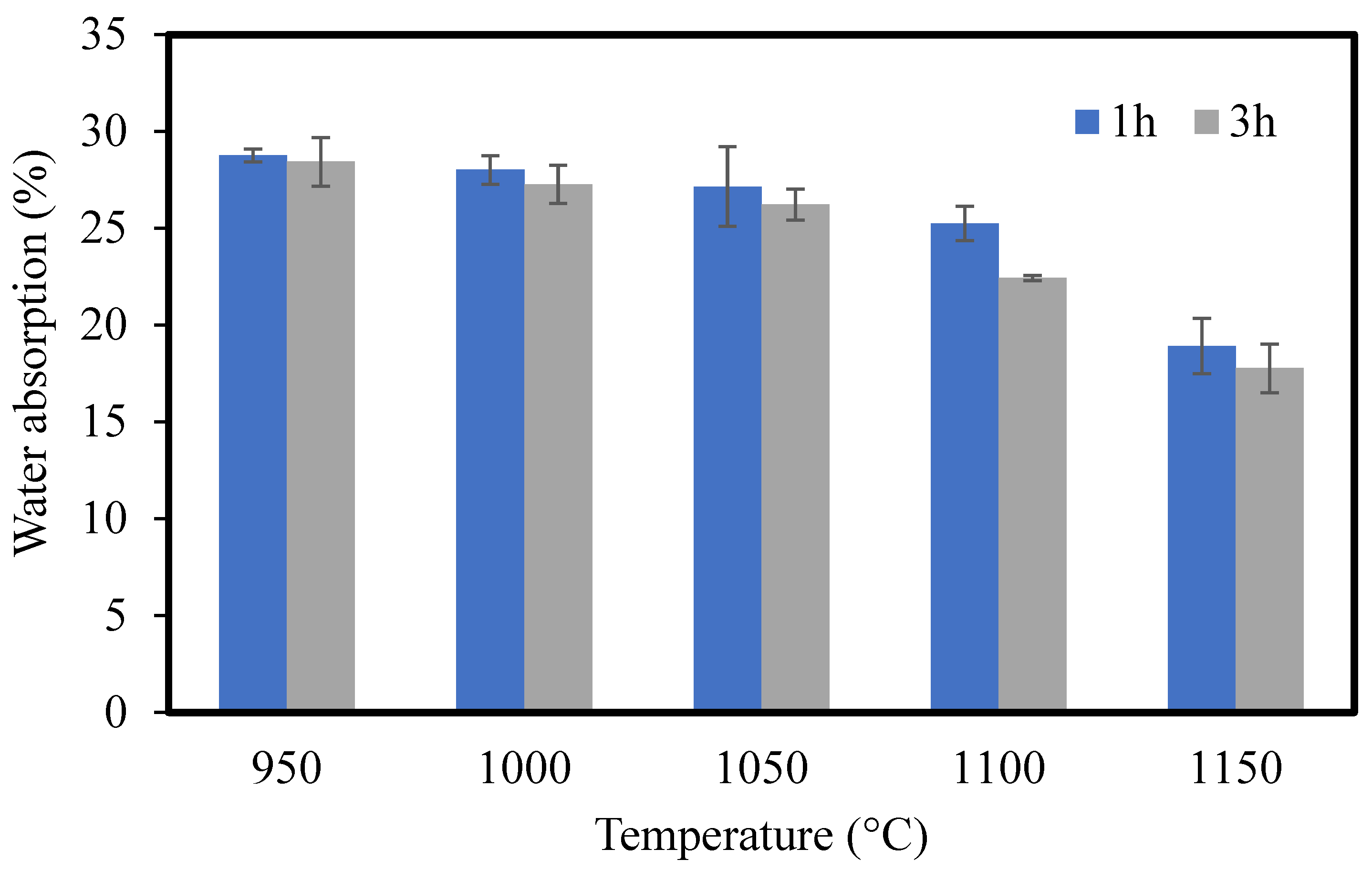
3.1.7. Water Absorption
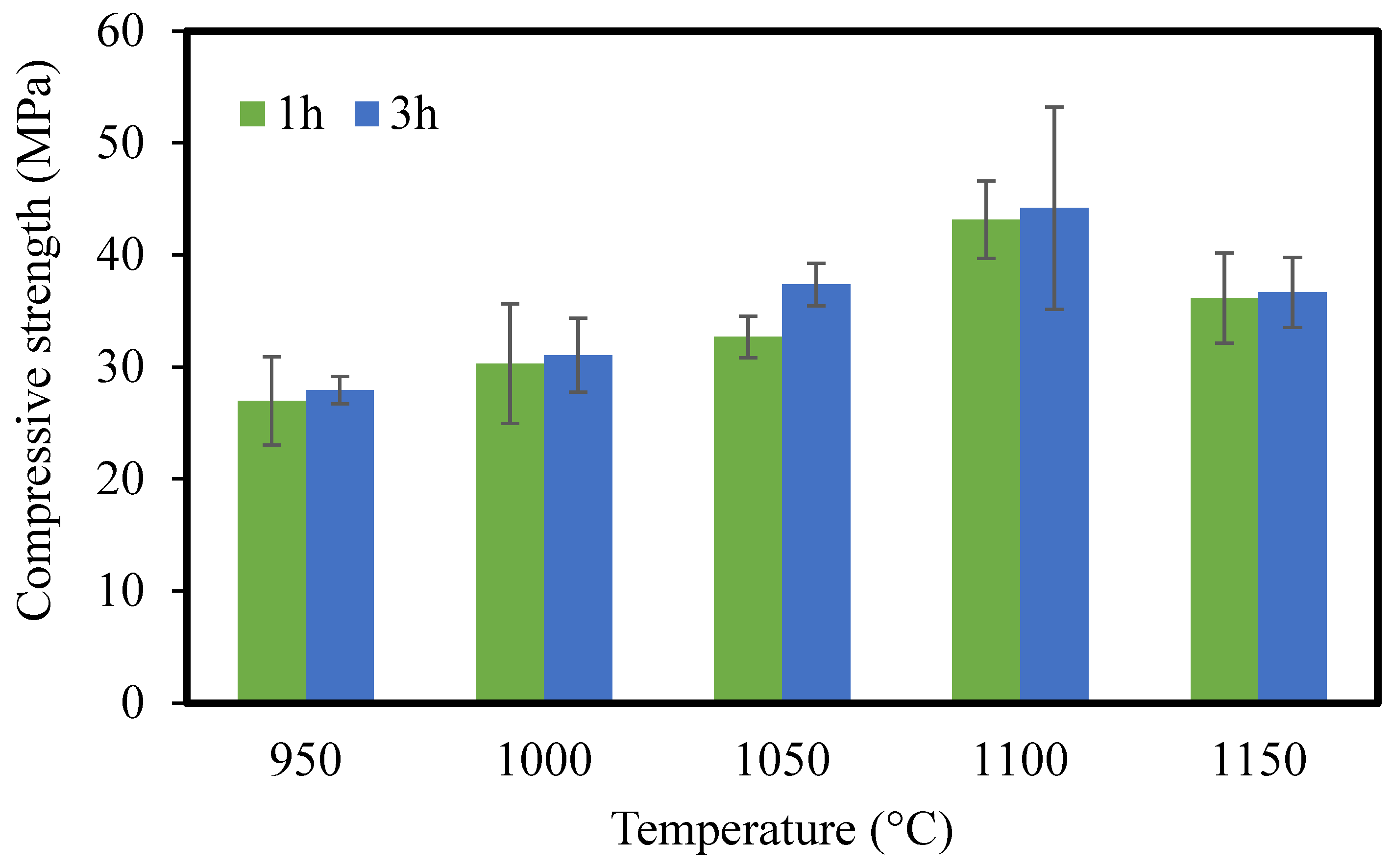
3.1.8. Mechanical Strength
3.2. Ultrafine Filtration of Chromium(VI)
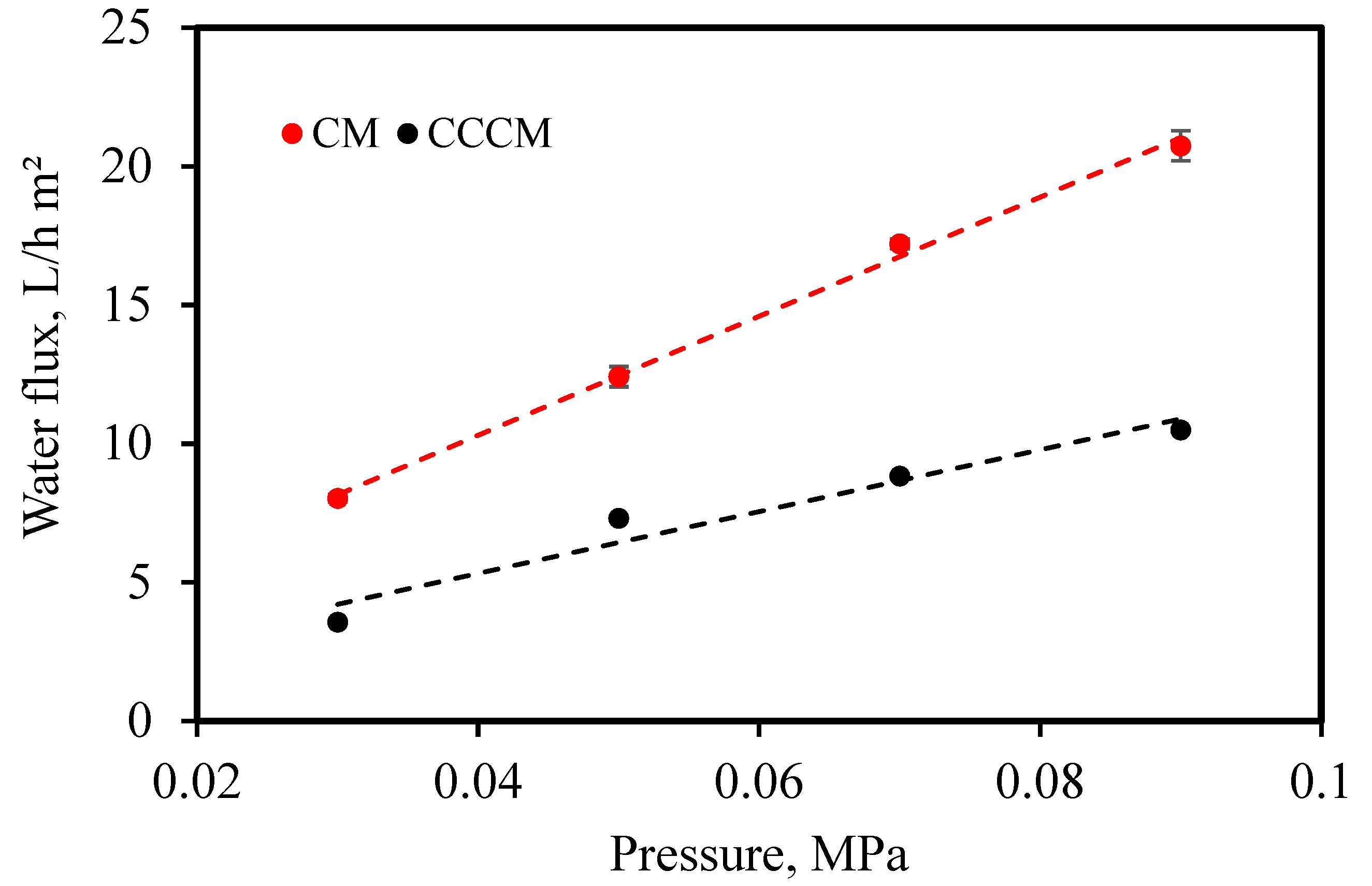
3.2.1. Water Flux
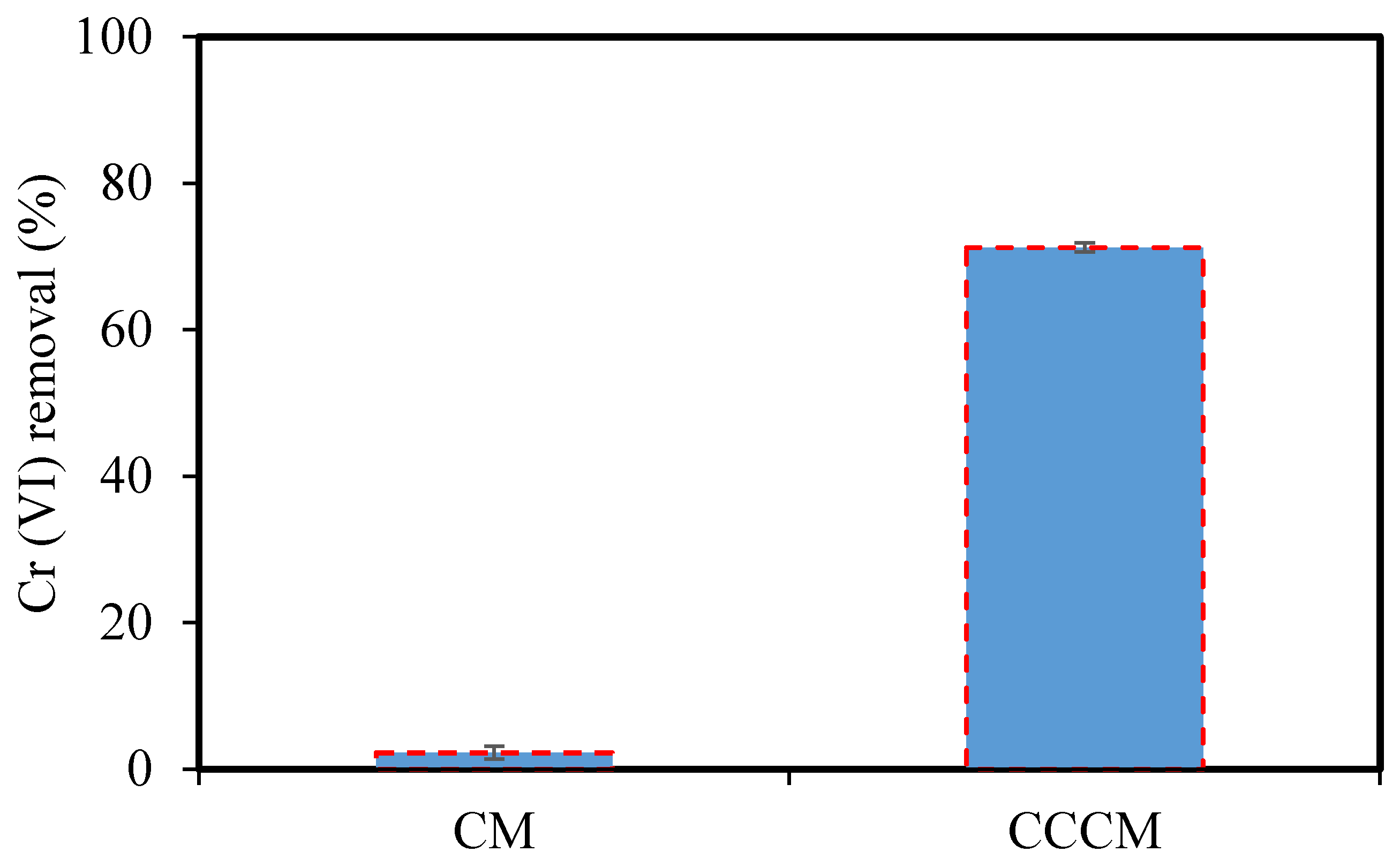
3.2.2. The Performance of CM and CCCM in the Removal of Cr(VI)
3.2.3. Effect of Feed pH
3.2.4. The Mechanism of Cr(VI) Removal by CCCM
3.2.5. Comparison with Other Composite Membranes
4. Conclusions
- CMs were sintered at different temperatures ranging from 950 to 1150 °C for 1 and 3 h. The optimal sintering temperature and time were considered to be 1000 °C and 3 h, respectively. The optimal ceramic membrane showed water absorption of 27.27%, a compressive strength of 31.05 MPa, and pure water flux of 20.74 L/h*m2. The results of the experiment show that the sintering process has an effect on the microstructure and properties of the membranes.
- Chitosan crosslinked with glutaraldehyde was coated on the surface of the selected CM. The CCCM provided a pore size of 16.24 nm and was used for the removal of Cr(VI). The highest removal of Cr(VI) reached 71.25% at pH 3.
- The preparation of ceramic membranes from ultrafine powders shows that these raw materials have a significant impact on the main properties of ceramic membranes. All the characterization results showed that the membrane can be a very competitive candidate for wastewater treatment.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Znad, H.; Awual, M.R.; Martini, S. The Utilization of Algae and Seaweed Biomass for Bioremediation of Heavy Metal-Contaminated Wastewater. Molecules 2022, 27, 1275. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Dai, M.; Yang, F.; Ali, I.; Naz, I.; Peng, C. Remediation of Chromium (VI) from Groundwater by Metal-Based Biochar under Anaerobic Conditions. Water 2022, 14, 894. [Google Scholar] [CrossRef]
- Fathy, N.A.; El-Wakeel, S.T.; Abd El-Latif, R.R. Biosorption and desorption studies on chromium(VI) by novel biosorbents of raw rutin and rutin resin. J. Environ. Chem. Eng. 2015, 3, 1137–1145. [Google Scholar] [CrossRef]
- Mouiya, M.; Abourriche, A.; Bouazizi, A.; Benhammou, A.; El Hafiane, Y.; Abouliatim, Y.; Nibou, L.; Oumam, M.; Ouammou, M.; Smith, A.; et al. Flat ceramic microfiltration membrane based on natural clay and Moroccan phosphate for desalination and industrial wastewater treatment. Desalination 2018, 427, 42–50. [Google Scholar] [CrossRef]
- Kumar, V.R.; Ghoshal, K.A.; Pugazhenthi, G. Elaboration of novel tubular ceramic membrane from inexpensive raw materials by extrusion method and its performance in microfiltration of synthetic oily wastewater treatment. J. Membr. Sci. 2015, 490, 92–102. [Google Scholar] [CrossRef]
- Elanchezhiyan, S.S.; Karthikeyan, P.; Rathinam, K.; Hasmath Farzana, M.; Park, C.M. Magnetic kaolinite immobilized chitosan beads for the removal of Pb(II) and Cd(II) ions from an aqueous environment. Carbohydr. Polym. 2021, 261, 117892. [Google Scholar] [CrossRef]
- Zewail, T.M.; Yousef, N.S. Kinetic study of heavy metal ions removal by ion exchange in batch conical air spouted bed. Alex. Eng. J. 2015, 54, 83–90. [Google Scholar] [CrossRef]
- Teh, C.Y.; Budiman, P.M.; Shak, K.P.Y.; Wu, T.Y. Recent Advancement of Coagulation–Flocculation and Its Application in Wastewater Treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. [Google Scholar] [CrossRef]
- Tran, T.-K.; Chiu, K.-F.; Lin, C.-Y.; Leu, H.-J. Electrochemical treatment of wastewater: Selectivity of the heavy metals removal process. Int. J. Hydrog. Energy 2017, 42, 27741–27748. [Google Scholar] [CrossRef]
- He, Z.; Lyu, Z.; Gu, Q.; Zhang, L.; Wang, J. Ceramic-based membranes for water and wastewater treatment. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123513. [Google Scholar] [CrossRef]
- Kayvani, F.A.; McKay, G.; Buekenhoudt, A.; Al Sulaiti, H.; Motmans, F.; Khraisheh, M.; Atieh, M. Inorganic Membranes: Preparation and Application for Water Treatment and Desalination. Materials 2018, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Hubadillah, S.K.; Othman, M.H.D.; Matsuura, T.; Ismail, A.F.; Rahman, M.A.; Harun, Z.; Jaafar, J.; Nomura, M. Fabrications and applications of low cost ceramic membrane from kaolin: A comprehensive review. Ceram. Int. 2018, 44, 4538–4560. [Google Scholar] [CrossRef]
- Monash, P.; Pugazhenthi, G. Development of Ceramic Supports Derived from Low-Cost Raw Materials for Membrane Applications and its Optimization Based on Sintering Temperature. Int. J. Appl. Ceram. Technol. 2011, 8, 227–238. [Google Scholar] [CrossRef]
- De Oliveira Henriques, J.D.; Pedrassani, M.W.; Klitzke, W.; Mariano, A.B.; Vargas, J.V.C.; Vieira, R.B. Thermal treatment of clay-based ceramic membranes for microfiltration of Acutodesmus obliquus. Appl. Clay Sci. 2017, 150, 217–224. [Google Scholar] [CrossRef]
- Harabi, A.; Zenikheri, F.; Boudaira, B.; Bouzerara, F.; Guechi, A.; Foughali, L. A new and economic approach to fabricate resistant porous membrane supports using kaolin and CaCO3. J. Eur. Ceram. Soc. 2014, 34, 1329–1340. [Google Scholar] [CrossRef]
- Almandoz, M.C.; Pagliero, C.L.; Ochoa, N.A.; Marchese, J. Composite ceramic membranes from natural aluminosilicates for microfiltration applications. Ceram. Int. 2015, 41, 5621–5633. [Google Scholar] [CrossRef]
- Malik, N.; Bulasara, V.K.; Basu, S. Preparation of novel porous ceramic microfiltration membranes from fly ash, kaolin and dolomite mixtures. Ceram. Int. 2020, 46, 6889–6898. [Google Scholar] [CrossRef]
- Adam, M.R.; Salleh, N.M.; Othman, M.H.D.; Matsuura, T.; Ali, M.H.; Puteh, M.H.; Ismail, A.F.; Rahman, M.A.; Jaafar, J. The adsorptive removal of chromium (VI) in aqueous solution by novel natural zeolite based hollow fibre ceramic membrane. J. Environ. Manag. 2018, 224, 252–262. [Google Scholar] [CrossRef]
- Jeong, Y.; Lee, S.; Hong, S.; Park, C. Preparation, characterization and application of low-cost pyrophyllite-alumina composite ceramic membranes for treating low-strength domestic wastewater. J. Membr. Sci. 2017, 536, 108–115. [Google Scholar] [CrossRef]
- Ha, J.-H.; Lee, J.; Song, I.-H.; Lee, S.-H. The effects of diatomite addition on the pore characteristics of a pyrophyllite support layer. Ceram. Int. 2015, 41, 9542–9548. [Google Scholar] [CrossRef]
- Abdullayev, A.; Bekheet, M.; Hanaor, D.; Gurlo, A. Materials and Applications for Low-Cost Ceramic Membranes. Membranes 2019, 9, 105. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Zou, D.; Xu, J.; Chen, X.; Qiu, M.; Fan, Y. Enhanced Performance of Fly Ash-Based Supports for Low-Cost Ceramic Membranes with the Addition of Bauxite. Membranes 2021, 11, 711. [Google Scholar] [CrossRef]
- Raychaudhuri, A.; Behera, M. Ceramic membrane modified with rice husk ash for application in microbial fuel cells. Electrochim. Acta 2020, 363, 137261. [Google Scholar] [CrossRef]
- Nandi, B.K.; Uppaluri, R.; Purkait, M.K. Preparation and characterization of low cost ceramic membranes for micro-filtration applications. Appl. Clay Sci. 2008, 42, 102–110. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Rahman, M.A.; Ismail, A.F.; Jaafar, J. Preparation and characterization of inexpensive kaolin hollow fibre membrane (KHFM) prepared using phase inversion/sintering technique for the efficient separation of real oily wastewater. Arab. J. Chem. 2020, 13, 2349–2367. [Google Scholar] [CrossRef]
- Lorente-Ayza, M.M.; Orts, M.J.; Pérez-Herranz, V.; Mestre, S. Role of starch characteristics in the properties of low-cost ceramic membranes. J. Eur. Ceram. Soc. 2015, 35, 2333–2341. [Google Scholar] [CrossRef]
- Lorente-Ayza, M.M.; Sánchez, E.; Sanz, V.; Mestre, S. Influence of starch content on the properties of low-cost microfiltration ceramic membranes. Ceram. Int. 2015, 41, 13064–13073. [Google Scholar] [CrossRef]
- El Batouti, M.; Al-Harby, N.F.; Elewa, M.M. A Review on Promising Membrane Technology Approaches for Heavy Metal Removal from Water and Wastewater to Solve Water Crisis. Water 2021, 13, 3241. [Google Scholar] [CrossRef]
- Xia, L.; Ren, J.; Weyd, M.; McCutcheon, J.R. Ceramic-supported thin film composite membrane for organic solvent nanofiltration. J. Membr. Sci. 2018, 563, 857–863. [Google Scholar] [CrossRef]
- Peyravi, M.; Rahimpour, A.; Jahanshahi, M. Thin film composite membranes with modified polysulfone supports for organic solvent nanofiltration. J. Membr. Sci. 2012, 423–424, 225–237. [Google Scholar] [CrossRef]
- Jana, S.; Purkait, M.K.; Mohanty, K. Clay supported polyvinyl acetate coated composite membrane by modified dip coating method: Application for the purification of lysozyme from chicken egg white. J. Membr. Sci. 2011, 382, 243–251. [Google Scholar] [CrossRef]
- Apriyanti, E.; Hadiyanto; Wijayanto, W. Preparation and Characterization of Volcanic Ash-chitosan Composite Ceramic Membrane for Clean Water Production. J. Environ. Sci. Technol. 2018, 11, 112–118. [Google Scholar] [CrossRef]
- Mukhtar, M.; Fényes, E.; Bartos, C.; Zeeshan, M.; Ambrus, R. Chitosan biopolymer, its derivatives and potential applications in nano-therapeutics: A comprehensive review. Eur. Polym. J. 2021, 160, 110767. [Google Scholar] [CrossRef]
- Nayab, S.S.; Abbas, M.A.; Mushtaq, S.; Niazi, B.K.; Batool, M.; Shehnaz, G.; Ahmad, N.; Ahmad, N.M. Anti-Foulant Ultrafiltration Polymer Composite Membranes Incorporated with Composite Activated Carbon/Chitosan and Activated Carbon/Thiolated Chitosan with Enhanced Hydrophilicity. Membranes 2021, 11, 827. [Google Scholar] [CrossRef]
- Ali, N.; Khan, A.; Malik, S.; Badshah, S.; Bilal, M.; Iqbal, H.M.N. Chitosan-based green sorbent material for cations removal from an aqueous environment. J. Environ. Chem. Eng. 2020, 8, 104064. [Google Scholar] [CrossRef]
- Bui, T.H.; Lee, W.; Jeon, S.-B.; Kim, K.-W.; Lee, Y. Enhanced Gold(III) adsorption using glutaraldehyde-crosslinked chitosan beads: Effect of crosslinking degree on adsorption selectivity, capacity, and mechanism. Sep. Purif. Technol. 2020, 248, 116989. [Google Scholar] [CrossRef]
- Islam, N.; Wang, H.; Maqbool, F.; Ferro, V. In Vitro Enzymatic Digestibility of Glutaraldehyde-Crosslinked Chitosan Nanoparticles in Lysozyme Solution and Their Applicability in Pulmonary Drug Delivery. Molecules 2019, 24, 1271. [Google Scholar] [CrossRef]
- Chen, M.; Shang, R.; Sberna, P.M.; Luiten-Olieman, M.W.J.; Rietveld, L.C.; Heijman, S.G.J. Highly permeable silicon carbide-alumina ultrafiltration membranes for oil-in-water filtration produced with low-pressure chemical vapor deposition. Sep. Purif. Technol. 2020, 253, 117496. [Google Scholar] [CrossRef]
- Chen, L.; Guan, K.; Zhu, W.; Peng, C.; Wu, J. Preparation and mechanism analysis of high performance ceramic membrane by spray coating. RSC Adv. 2018, 8, 39884–39892. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, Y.; Chang, Q.; Liu, F.; Wang, Y.; Rao, J. Preparation of a High-Performance Porous Ceramic Membrane by a Two-Step Coating Method and One-Step Sintering. Appl. Sci. 2018, 9, 52. [Google Scholar] [CrossRef] [Green Version]
- Bouazizi, A.; Breida, M.; Karim, A.; Achiou, B.; Ouammou, M.; Calvo, J.I.; Aaddane, A.; Khiat, K.; Younssi, S.A. Development of a new TiO 2 ultrafiltration membrane on flat ceramic support made from natural bentonite and micronized phosphate and applied for dye removal. Ceram. Int. 2017, 43, 1479–1487. [Google Scholar] [CrossRef]
- Merlet, R.B.; Pizzoccaro-Zilamy, M.-A.; Nijmeijer, A.; Winnubst, L. Hybrid ceramic membranes for organic solvent nanofiltration: State-of-the-art and challenges. J. Membr. Sci. 2020, 599, 117839. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, W.; Guan, K.; Peng, C.; Wu, J. Preparation of high permeable alumina ceramic membrane with good separation performance via UV curing technique. RSC Adv. 2018, 8, 13567–13577. [Google Scholar] [CrossRef] [PubMed]
- Pagana, A.E.; Sklari, S.D.; Kikkinides, E.S.; Zaspalis, V.T. Combined adsorption–permeation membrane process for the removal of chromium (III) ions from contaminated water. J. Membr. Sci. 2011, 367, 319–324. [Google Scholar] [CrossRef]
- Li, B.; Shan, C.-L.; Zhou, Q.; Fang, Y.; Wang, Y.-L.; Xu, F.; Han, L.-R.; Ibrahim, M.; Guo, L.-B.; Xie, G.-L.; et al. Synthesis, Characterization, and Antibacterial Activity of Cross-Linked Chitosan-Glutaraldehyde. Mar. Drugs 2013, 11, 1534–1552. [Google Scholar] [CrossRef]
- Du, J.; Xiao, X.; Ai, D.; Liu, J.; Qiu, L.; Chen, Y.; Zhu, K.; Wang, L. Fabrication, Characterization and Drainage Capacity of Single-Channel Porous Alumina Ceramic Membrane Tube. Membranes 2022, 12, 390. [Google Scholar] [CrossRef]
- Ghouil, B.; Harabi, A.; Bouzerara, F.; Boudaira, B.; Guechi, A.; Demir, M.M.; Figoli, A. Development and characterization of tubular composite ceramic membranes using natural alumino-silicates for microfiltration applications. Mater. Charact. 2015, 103, 18–27. [Google Scholar] [CrossRef]
- Valadi, F.M.; Shahsavari, S.; Akbarzadeh, E.; Gholami, M.R. Preparation of new MOF-808/chitosan composite for Cr(VI) adsorption from aqueous solution: Experimental and DFT study. Carbohydr. Polym. 2022, 288, 119383. [Google Scholar] [CrossRef]
- Omer, A.M.; Abd El-Monaem, E.M.; Eltaweil, A.S. Novel reusable amine-functionalized cellulose acetate beads impregnated aminated graphene oxide for adsorptive removal of hexavalent chromium ions. Int. J. Biol. Macromol. 2022, 208, 925–934. [Google Scholar] [CrossRef]
- Mishima, K.; Du, X.; Sekiguchi, S.; Kano, N. Experimental and Theoretical Studies on the Adsorption and Desorption Mechanisms of Chromate Ions on Cross-Linked Chitosan. J. Funct. Biomater. 2017, 8, 51. [Google Scholar] [CrossRef] [Green Version]
- Basaran, G.; Kavak, D.; Dizge, N.; Asci, Y.; Solener, M.; Ozbey, B. Comparative study of the removal of nickel(II) and chromium(VI) heavy metals from metal plating wastewater by two nanofiltration membranes. Desalination Water Treat. 2015, 57, 21870–21880. [Google Scholar] [CrossRef]
- Choudhury, P.; Mondal, P.; Majumdar, S.; Saha, S.; Sahoo, G.C. Preparation of ceramic ultrafiltration membrane using green synthesized CuO nanoparticles for chromium (VI) removal and optimization by response surface methodology. J. Clean. Prod. 2018, 203, 511–520. [Google Scholar] [CrossRef]
- Vasanth, D.; Pugazhenthi, G.; Uppaluri, R. Biomass assisted microfiltration of chromium(VI) using Baker’s yeast by ceramic membrane prepared from low cost raw materials. Desalination 2012, 285, 239–244. [Google Scholar] [CrossRef]

| Components | Wt.% | |
|---|---|---|
| Dry Basis | Wet Basis | |
| Clay | 50 | 38.46 |
| Kaolin | 45 | 34.62 |
| Starch | 5 | 3.85 |
| Water | - | 23.07 |
| Elements | Wt.% | |
|---|---|---|
| Kaolin | Clay | |
| Si | 54.12 | 54.51 |
| Al | 38.92 | 34.61 |
| Ca | 0.27 | 0.71 |
| K | 3.94 | 1.69 |
| Mg | 2.28 | 1.84 |
| Fe | 0.47 | 6.64 |
| Sample | Pore Volume | Pore Size (nm) | |
|---|---|---|---|
| CM | 11.3 | 0.0478 | 16.9 |
| CCCM | 12.1 | 0.0494 | 16.2 |
| Membrane | Pressure (MPa) | Feed pH | Feed Concentration (mg/L) | Removal Efficiency (%) | References |
|---|---|---|---|---|---|
| Natural zeolite HFCM | 0.1 | 4 | 40 | 44 | [18] |
| NF270 | 3 | 10 | 60 ± 3 | 95 | [51] |
| NF90 | 3 | 10 | 60 ± 3 | 96 | [51] |
| Clay-alumina ceramic CuO | 0.3 | 6.8 | 5 | 88 | [52] |
| UF membrane | |||||
| Ceramic MF membrane | 0.207 | 1 | 100 | 94 | [53] |
| (baker’s yeast biomass) | |||||
| CCCM | 0.09 | 3 | 50 | 71 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bat-Amgalan, M.; Miyamoto, N.; Kano, N.; Yunden, G.; Kim, H.-J. Preparation and Characterization of Low-Cost Ceramic Membrane Coated with Chitosan: Application to the Ultrafine Filtration of Cr(VI). Membranes 2022, 12, 835. https://doi.org/10.3390/membranes12090835
Bat-Amgalan M, Miyamoto N, Kano N, Yunden G, Kim H-J. Preparation and Characterization of Low-Cost Ceramic Membrane Coated with Chitosan: Application to the Ultrafine Filtration of Cr(VI). Membranes. 2022; 12(9):835. https://doi.org/10.3390/membranes12090835
Chicago/Turabian StyleBat-Amgalan, Munkhpurev, Naoto Miyamoto, Naoki Kano, Ganchimeg Yunden, and Hee-Joon Kim. 2022. "Preparation and Characterization of Low-Cost Ceramic Membrane Coated with Chitosan: Application to the Ultrafine Filtration of Cr(VI)" Membranes 12, no. 9: 835. https://doi.org/10.3390/membranes12090835
APA StyleBat-Amgalan, M., Miyamoto, N., Kano, N., Yunden, G., & Kim, H.-J. (2022). Preparation and Characterization of Low-Cost Ceramic Membrane Coated with Chitosan: Application to the Ultrafine Filtration of Cr(VI). Membranes, 12(9), 835. https://doi.org/10.3390/membranes12090835






