Effect of Silica Sodalite Loading on SOD/PSF Membranes during Treatment of Phenol-Containing Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Silica Sodalite (SSOD) Crystals
2.2. Fabrication and Characterization of Sodalite-Infused Polymer Membranes
2.2.1. Fabrication of SSOD/PSF Membranes
2.2.2. Characterization of SSOD Crystals and Fabricated SSOD-Infused Membranes
2.3. Preparation of Phenol-Containing Wastewater and Its Treatment Using Fabricated Membranes
3. Results
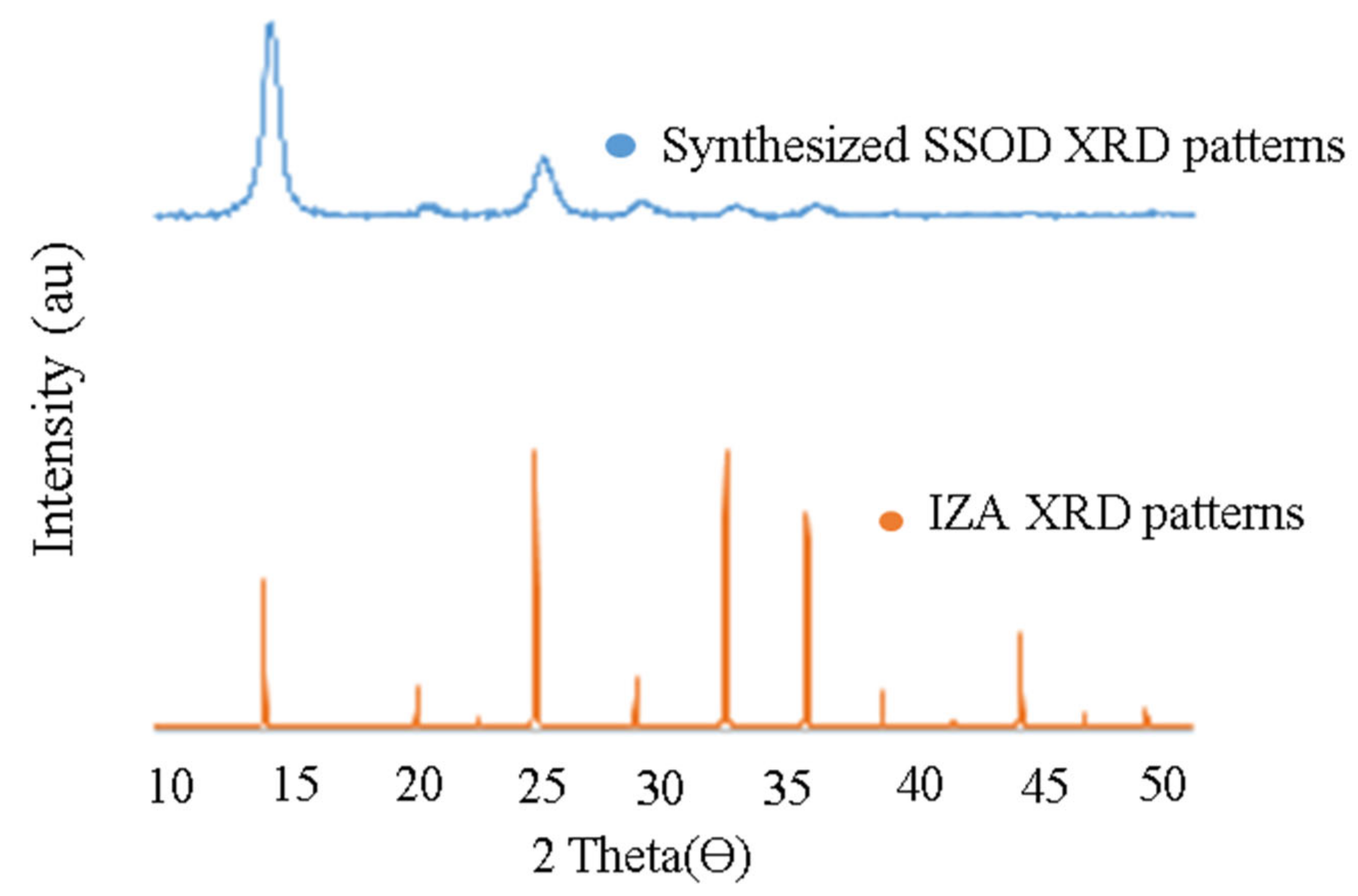
3.1. Physico-Chemical Characterization of SSOD Crystals
3.1.1. Surface Morphologies of SSOD Crystals
3.1.2. Surface Functional Groups of Sodalite Crystals via FT-IR and AFM Analysis
3.2. Characterization of Fabricated Membranes
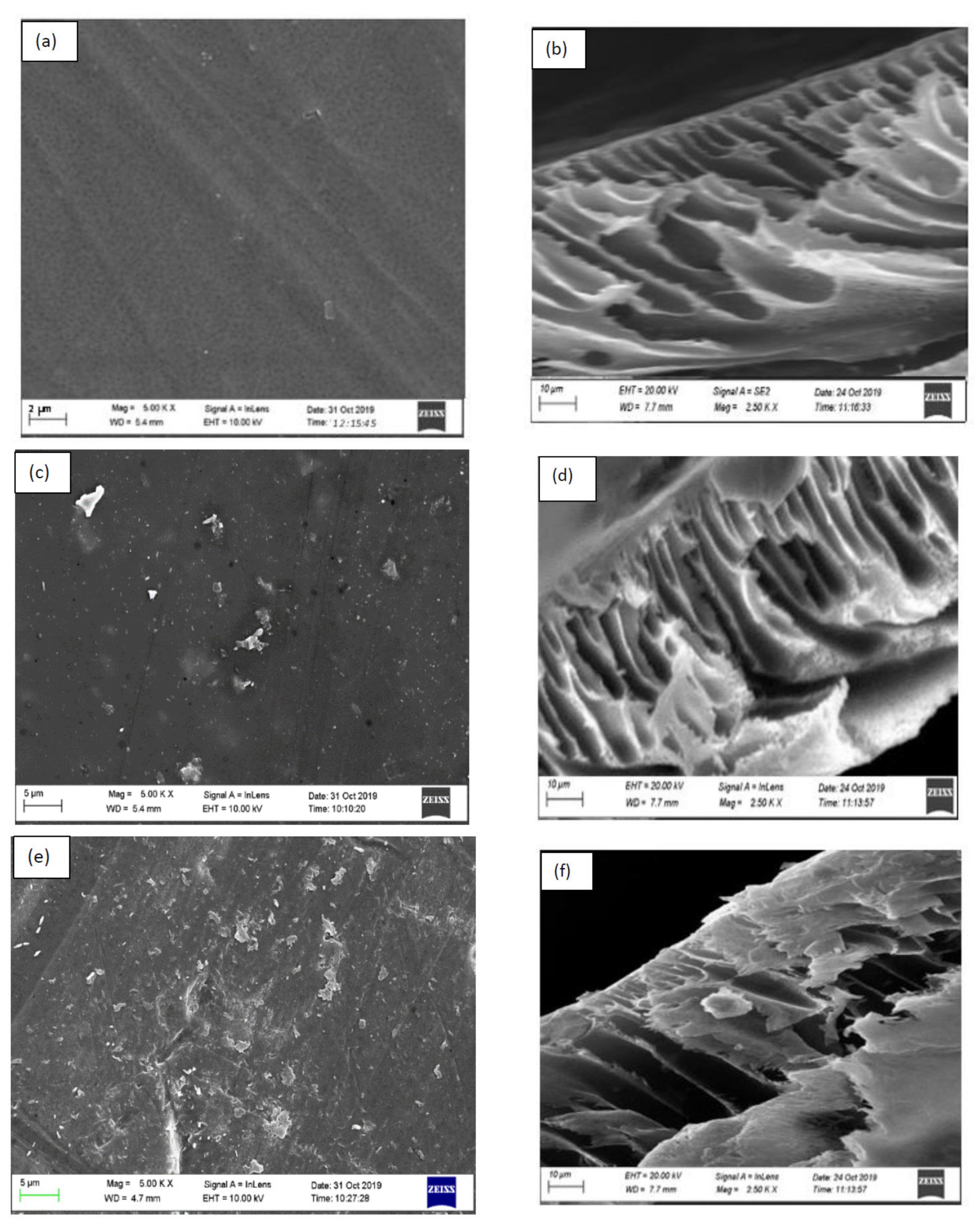
3.2.1. Surface Morphologies of Fabricated Membranes
3.2.2. Physico-Chemical Properties of Fabricated Membranes
3.2.3. Surface Roughness of Fabricated Membranes
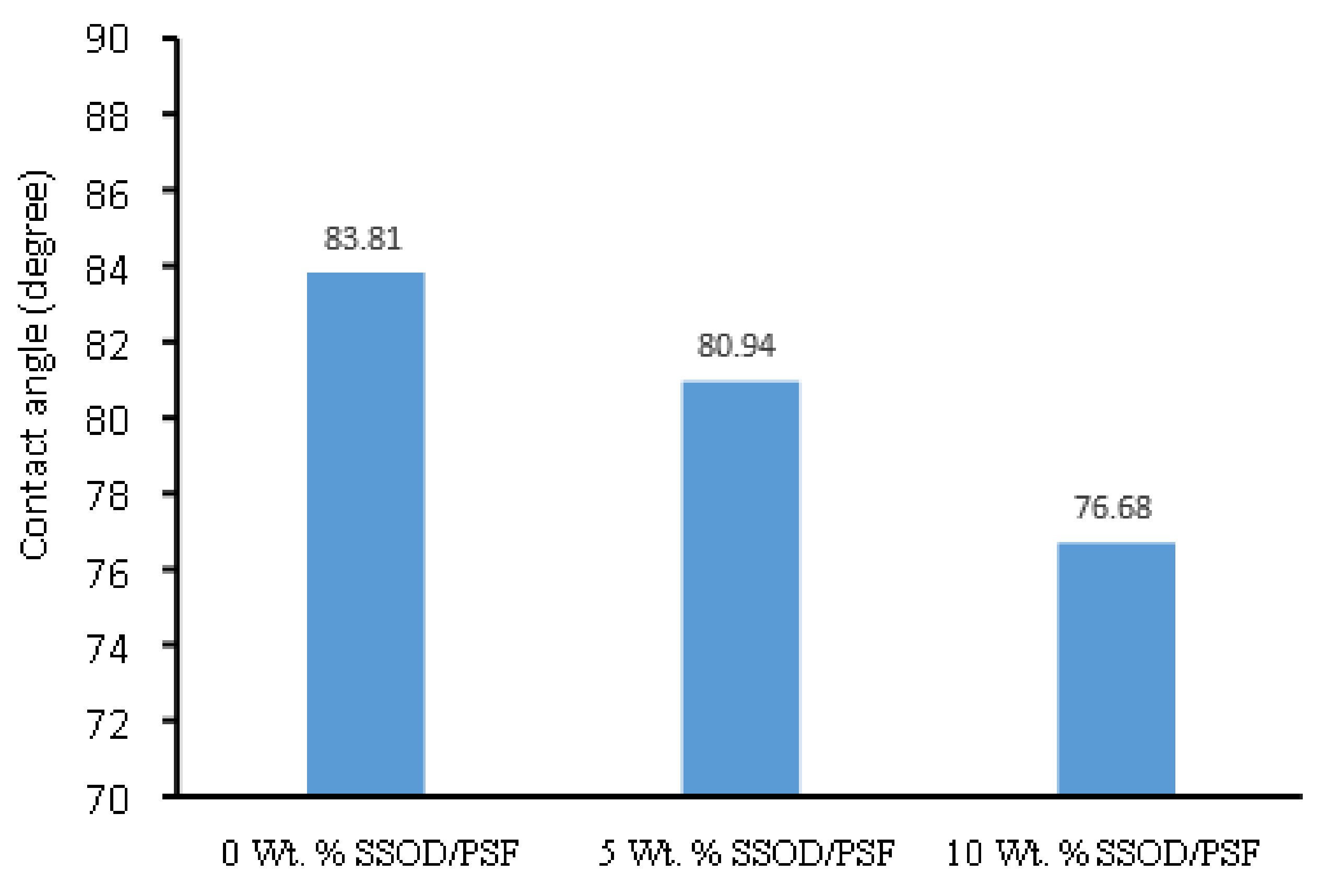
3.2.4. Hydrophilicity Measurement of Fabricated Membranes
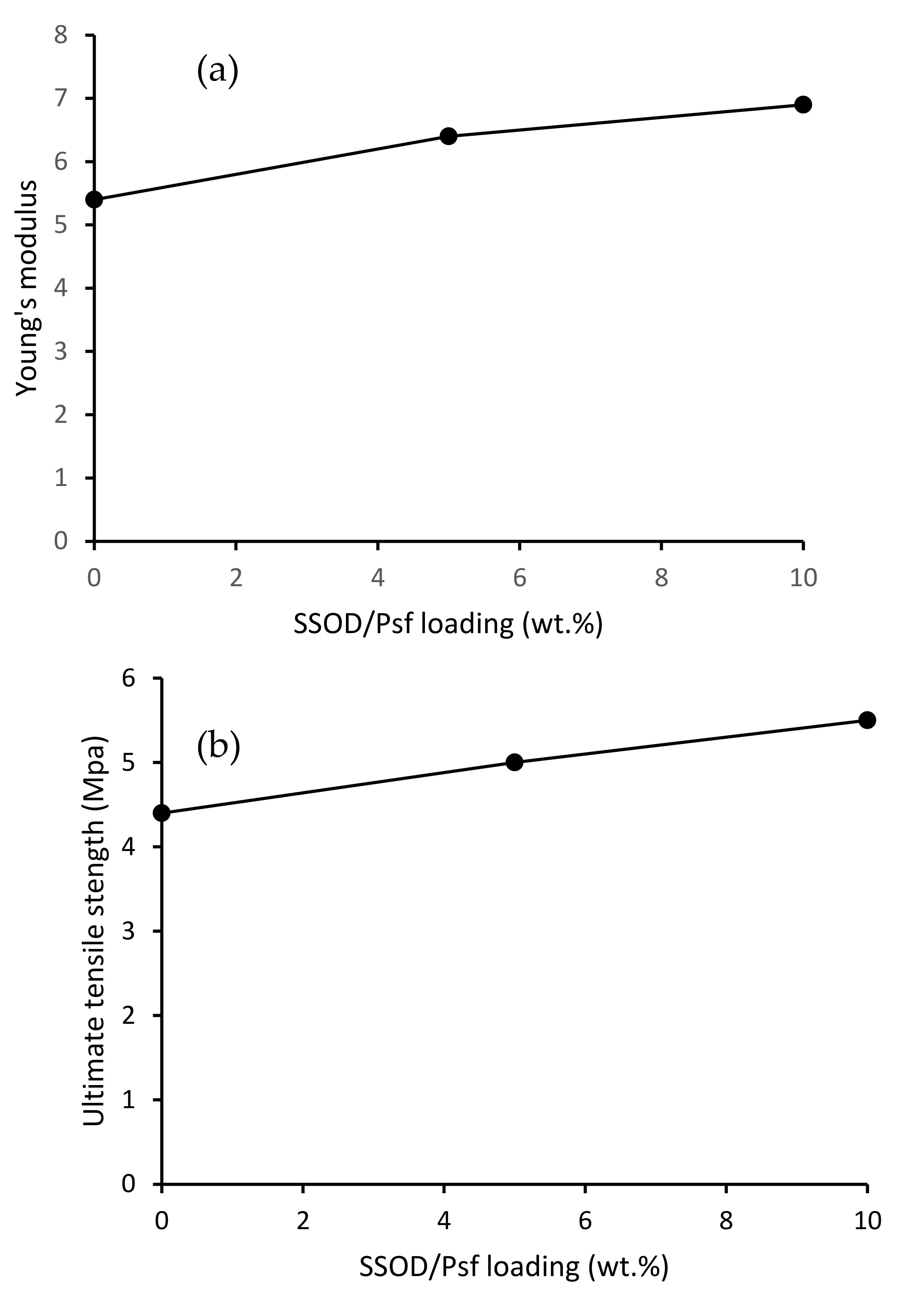
3.2.5. Mechanical Properties of Fabricated Membranes
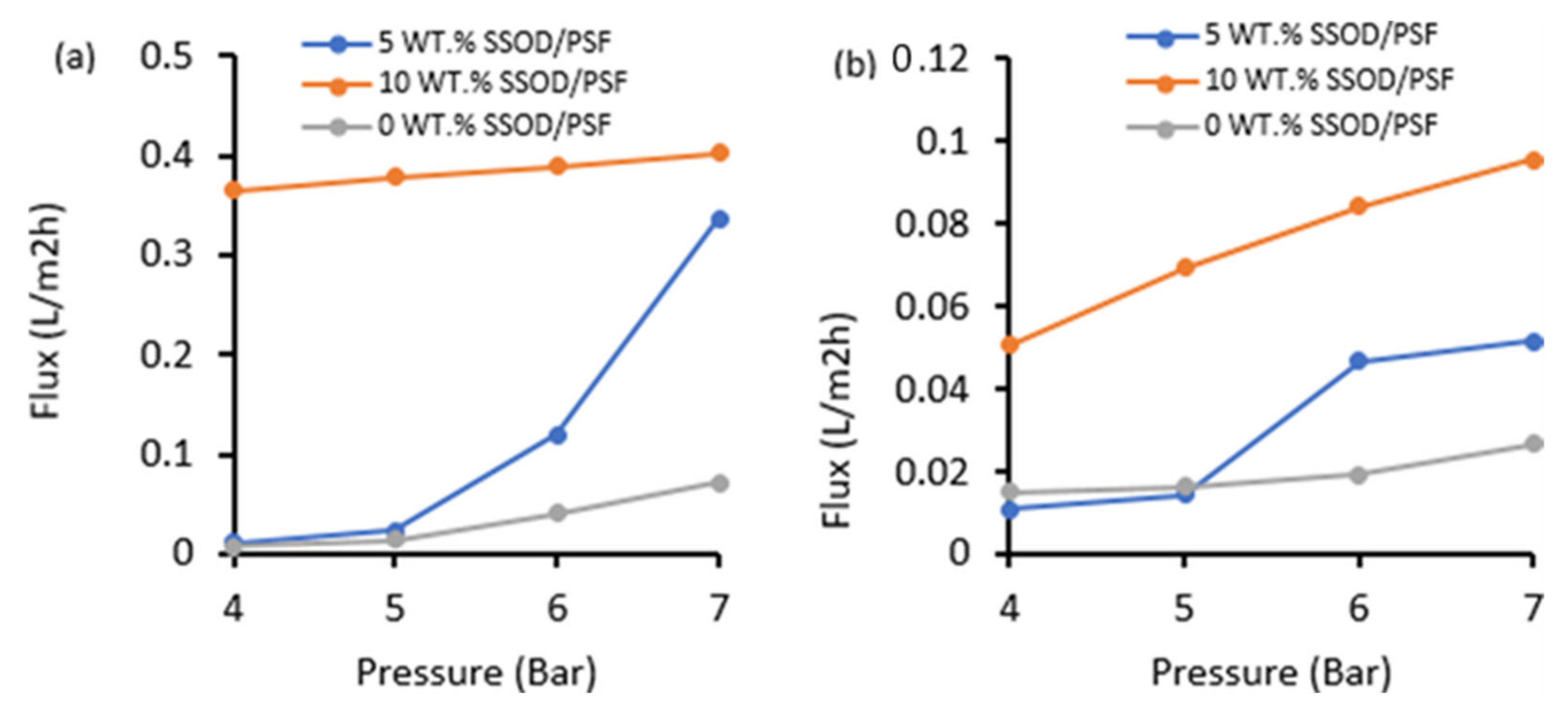
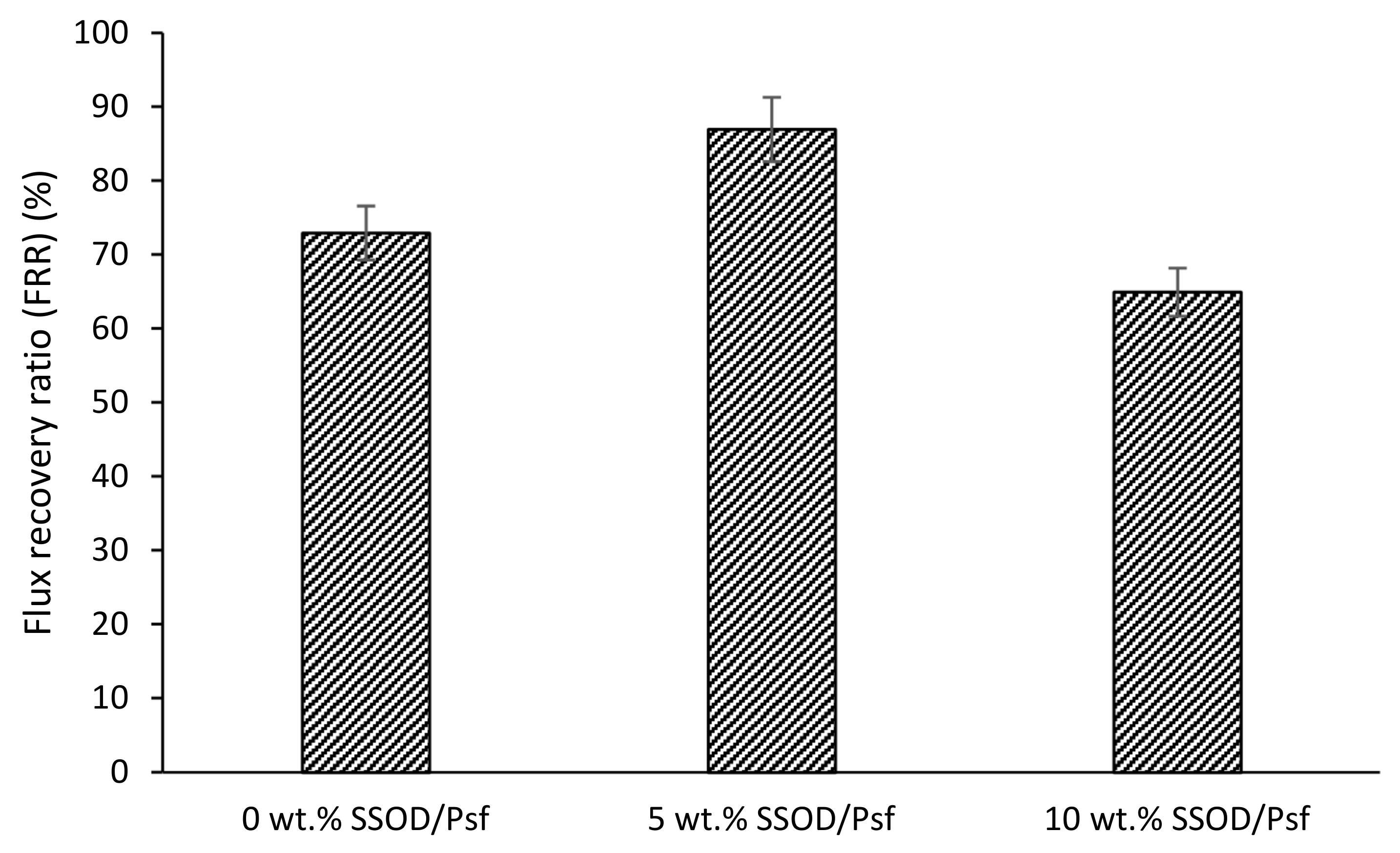
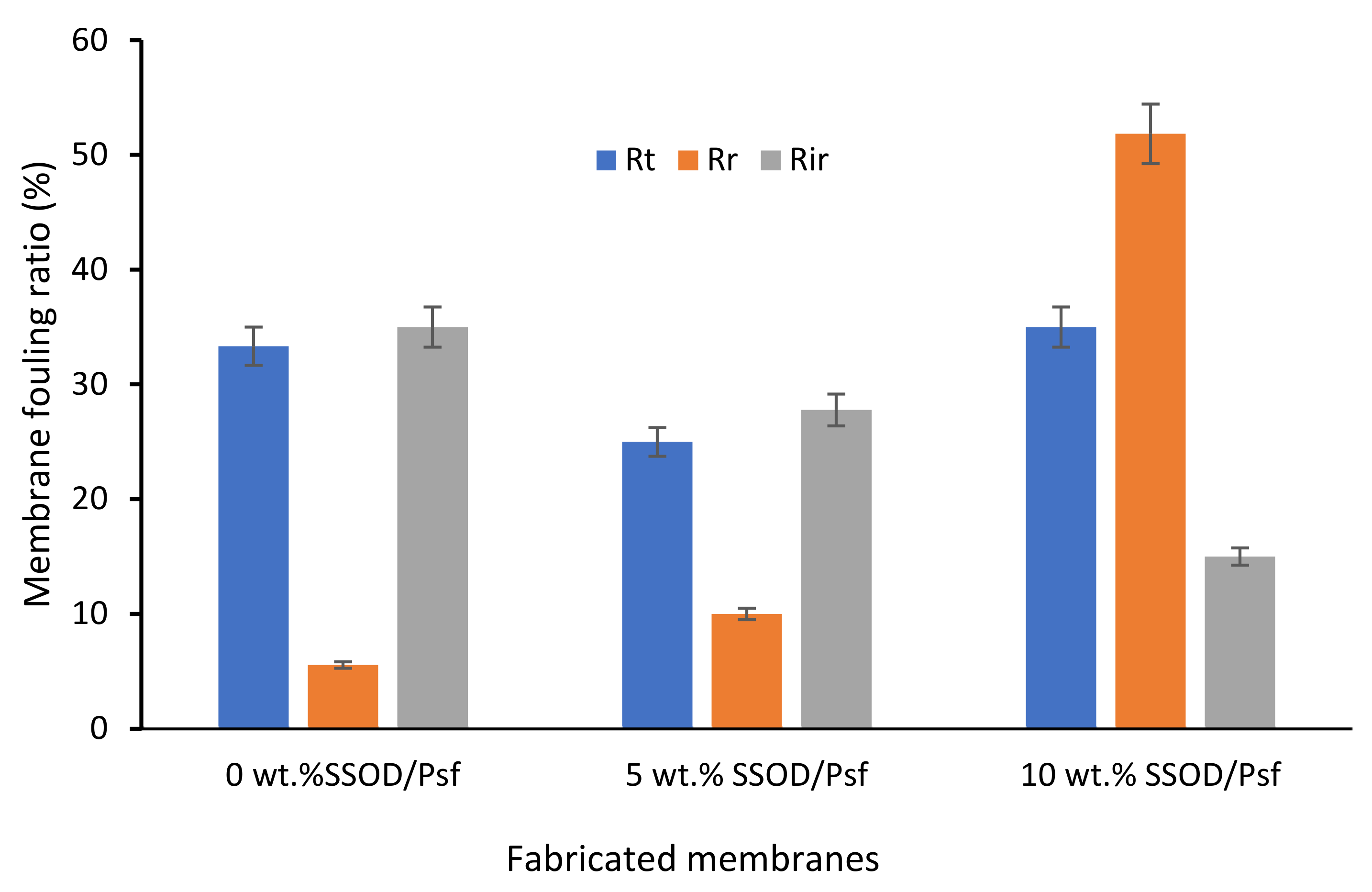
3.3. Evaluation of the Membranes’ Performance during Phenol-Containing Wastewater Treatment
3.4. Conclusions
- SSOD nanoparticles are successfully synthesized via topotactic conversion as confirmed by the XRD and SEM results, which is similar to what has been reported in the literature.
- SEM images showed that incorporation of the SSOD nanoparticles changed the structure of the membrane.
- The increased concentration of SSOD in the polymer matrix decreased the porosity of the membranes as depicted in SEM images.
- The pure water flux for all the membranes is noticeably higher than phenol-containing water flux, this is due to competitive sorption between phenol and water molecules.
- Increasing the concentration of nanoparticles in a polymer of mixed matrix membrane in this study enhanced the hydrophilicity of the membrane, mechanical strength, and permeation flux. The 10 wt.% SSOD/Psf membrane displayed highest flux.
- Membranes without SSOD particles displayed the highest phenol rejection of 99.55%. However, 10 wt.% SSOD/Psf showed the highest mechanical strength
- Nonetheless, the permeation flux was increased with increasing nanoparticles loading, implying that nanoparticles cause stronger attraction of water molecules.
- Findings also showed that the SSOD-loaded membranes displayed an improved phenol fouling resistance during a filtration process.
- For an improved performance by enhancing the surface area-to-volume ratio of the membrane, a hollow fiber configuration could be considered [53].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Drinking-Water: Fact Sheet No. 391; WHO: Geneva, Switzerland, 2015; Available online: http://www.who.int/mediacentre/factsheets/fs391/en/ (accessed on 5 May 2020).
- WHO; UNICEF. Progress in Drinking Water and Sanitation 2012 Update, Joint Monitoring Programme for Water Supply and Sanitation; WHO: Geneva, Switzerland, 2018; p. 58. [Google Scholar]
- Shirazi, M.; Kargari, A.; Shirazi, M. Direct contact membrane distillation for seawater desalination. Desalination Water Treat. 2012, 49, 368–375. [Google Scholar] [CrossRef]
- Unesco. Water for a Sustainable World | United Nations Educational, Scientific and Cultural Organization. 2019. Available online: http://www.unesco.org/new/en/natural-sciences/environment/water/wwap/wwdr/015-water-for-a-sustainable-world (accessed on 26 October 2019).
- Choi, H.; Spengler, J. Source attribution of personal exposure to airborne polycyclic aromatic hydrocarbon mixture using concurrent personal, indoor, and outdoor measurements. Environ. Int. 2014, 63, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.J.; Vijaya, J.J.; Kayalvizhi, K.; Sekaran, G. Adsorption of phenol from aqueous solutions using mesoporous carbon prepared by two-stage process. Chem. Eng. J. 2007, 132, 279–287. [Google Scholar] [CrossRef]
- Venkateswaran, P.; Palanivelu, K. Recovery of phenol from aqueous solution by supported liquid membrane using vegetable oils as liquid membrane. J. Hazard. Mater. B 2006, 131, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, O.; Amin, N.K.; El-Ashtoukhy, E.S.Z. Electrochemical removal of phenol from oil refinery wastewater. J. Hazard. Mater. 2009, 163, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, X.; Jin, Q.; Liu, Y.; Wang, Y. Removal of phenol from aqueous solution by adsorption onto OTMAC-modified attapulgite. J. Environ. Manag. 2007, 84, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Said, K.A.M.; Ismail, A.F.; Zulhairun, A.K.; Abdullah, M.S.; Usman, J.; Ariff Azali, M.; Tepe, O.; Dursun, A.Y. Combined effects of external mass transfer and biodegradation rates on removal of phenol by immobilized Ralstonia eutropha in a packed bed reactor. J. Hazard. Mater. 2008, 51, 9–16. [Google Scholar]
- Palma, M.S.A.; Paiva, J.L.; Zilli, M.; Converti, A. Batch phenol removal from methyl isobutyl ketone by liquid-liquid extraction with chemical reaction. Chem. Eng. Process. 2007, 46, 764–768. [Google Scholar] [CrossRef]
- Busca, G.; Berardinelli, S.; Resini, C.; Arrighi, L. Technologies for the removal of phenol from fluid streams: A short review of recent developments. J. Hazard. Mater. 2008, 160, 265–288. [Google Scholar] [CrossRef]
- Mohamad Said, K.A.; Ismail, A.F.; Karim, Z.A.; Abdullah, M.S.; Usman, J.; Raji, Y.O. Innovation in membrane fabrication: Magnetic induced photocatalytic membrane. J. Taiwan Inst. Chem. Eng. 2020, 113, 372–395. [Google Scholar] [CrossRef]
- Hofman, J.A.M.H.; Beerendonk, E.F.; Folmer, H.C.; Kruithoff, J.C. Removal of pesticides and other micropollutants with cellulose acetate, polyamide and ultra-low pressure reverse osmosis membranes. Desalination 1997, 113, 209–214. [Google Scholar] [CrossRef]
- Košutic, K.; Kunst, B. Removal of organics from aqueous solutions by commercial RO and NF membranes of characterized porosities. Desalination 2002, 142, 47–56. [Google Scholar] [CrossRef]
- Lipski, C.; Cote, P. The use of pervaporation for the removal of organic contaminants from water. Environ. Prog. 1990, 9, 254–261. [Google Scholar] [CrossRef]
- Tomaszewska, M.; Mozia, S. Removal of organic matter from water by PAC/UF system. Water Res. 2002, 36, 4137–4143. [Google Scholar] [CrossRef]
- Li, Q.; Marinas, B.J.; Snoeyink, V.L.; Campos, C. Three-component competitive adsorption model for flow-through PAC systems. 1. Model development and verification with a PAC/membrane system. Environ. Sci. Technol. 2003, 37, 2997–3004. [Google Scholar] [CrossRef]
- Sadare, O.O.; Yoro, K.O.; Moothi, K.; Daramola, M.O. Lignocellulosic Biomass-Derived Nanocellulose Crystals as Fillers in Membranes for Water and Wastewater Treatment: A Review. Membranes 2022, 12, 320. [Google Scholar] [CrossRef]
- Ntshangase, N.C.; Sadare, O.O.; Daramola, M.O. Effect of Silica Sodalite Functionalization and PVA Coating on Performance of Sodalite Infused PSF Membrane during Treatment of Acid Mine Drainage. Membranes 2021, 11, 315. [Google Scholar] [CrossRef]
- Ngobeni, R.; Sadare, O.; Daramola, M.O. Synthesis and Evaluation of HSOD/PSF and SSOD/PSF Membranes for Removal of Phenol from Industrial Wastewater. Polymers 2021, 13, 1253. [Google Scholar] [CrossRef]
- Daramola, M.; Silinda, B.; Masondo, S.; Oluwasina, O. Polyethersulphone-sodalite (PES-SOD) mixed-matrix membranes: Prospects for acid mine drainage (AMD) treatment. J. S. Afr. Inst. Min. Metall. 2015, 115, 1221–1228. [Google Scholar] [CrossRef]
- Mukherjee, R.; De, S. Novel carbon-nanoparticle polysulfone hollow fiber mixed matrix ultrafiltration membrane: Adsorptive removal of benzene, phenol and toluene from aqueous solution. Sep. Purif. Technol. 2016, 157, 229–240. [Google Scholar] [CrossRef]
- Moteki, T.; Chaikittisilp, W.; Sakamoto, Y.; Shimojima, A.; Okubo, T. Role of Acidic Pretreatment of Layered Silicate RUB-15 in Its Topotactic Conversion into Pure Silica Sodalite. Chem. Mater. 2011, 23, 3564–3570. [Google Scholar] [CrossRef]
- Gohil, J.M.; Ray, P. Polyvinyl alcohol as the barrier layer in thin film composite nanofiltration membranes: Preparation, characterization, and performance evaluation. J. Colloid Interface Sci. 2009, 338, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Tansel, B. Extracellular Polymeric Substances and Membrane Fouling: Microtopographical Characterization of Fouling Phenomena by Atomic Force Microscopy. Proc. Water Environ. Fed. 2008, 1, 432–437. [Google Scholar] [CrossRef]
- Emadzadeh, D.; Matsuura, T.; Ghanbari, M.; Ismail, A. Hybrid forward osmosis/ultrafiltration membrane bag for water purification. Desalination 2019, 468, 114071. [Google Scholar] [CrossRef]
- Dwivedi, M.; Harishchandra, R.; Koshkina, O.; Maskos, M.; Galla, H. Size Influences the Effect of Hydrophobic Nanoparticles on Lung Surfactant Model Systems. Biophys. J. 2014, 106, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Sadare, O.O.; Daramola, M.O. Blended Polysulfone/Polyethersulfone (PSF/PES) Membrane with Enhanced Antifouling Property for Separation of Succinate from Organic Acids from Fermentation Broth. ACS Sustain. Chem. Eng. 2021, 9, 13068–13083. [Google Scholar] [CrossRef]
- Daramola, M.O.; Adeogun, A.G. Empirical modelling of chemically enhanced backwash during ultrafiltration process. Membr. Water Treat. 2011, 2, 225–237. [Google Scholar] [CrossRef]
- Daramola, M.; Sadare, O.; Oluwasina, O.; Iyuke, S. Synthesis and Application of Functionalized Carbon Nanotube Infused Polymer Membrane (fCNT/PSF/PVA) for Treatment of Phenol-Containing Wastewater. J. Membr. Sci. Res. 2019, 5, 310–316. [Google Scholar]
- Shareef, U.; Othman, M.H.D.; Ismail, A.F.; Jilani, A. Facile removal of bisphenol A from water through novel Ag-doped TiO2 photocatalytic hollow fiber ceramic membrane. J. Aust. Ceram. Soc. 2020, 56, 29–39. [Google Scholar] [CrossRef]
- Dustgeer, M.R.; Asma, S.T.; Jilani, A.; Raza, K.; Hussain, S.Z.; Shakoorf, M.B.; Iqbal, J.; Abdel-wahab, M.S.; Darwesh, R. Synthesis and characterization of a novel single-phase sputtered Cu2O thin films: Structural, antibacterial activity and photocatalytic degradation of methylene blue. Inorg. Chem. Commun. 2021, 128, 108606. [Google Scholar] [CrossRef]
- Ma, X.; Wang, C.; Guo, H.; Wang, Z.; Sun, N.; Huo, P.; Gu, J.; Liu, Y. Novel dopamine-modified cellulose acetate ultrafiltration membranes with improved separation and antifouling performances. J. Mater. Sci. 2022, 57, 6474–6486. [Google Scholar] [CrossRef]
- Pakade, V.; Nchoe, O.; Hlungwane, L.; Tavengwa, N. Sequestration of hexavalent chromium from aqueous solutions by activated carbon derived from Macadamia nutshells. Water Sci. Technol. 2016, 75, 196–206. [Google Scholar] [CrossRef]
- Özsin, G.; Kılıç, M.; Apaydın-Varol, E.; Pütün, A. Chemically activated carbon production from agricultural waste of chickpea and its application for heavy metal adsorption: Equilibrium, kinetic, and thermodynamic studies. Appl. Water Sci. 2019, 9, 56. [Google Scholar] [CrossRef]
- Iza-Structure.org. XPD Plot. 2019. Available online: http://www.iza-structure.org/IZA-SC/pow_plot.php (accessed on 11 September 2019).
- Koike, M.; Asakura, Y.; Sugihara, M.; Kuroda, Y.; Tsuzura, H.; Wada, H.; Shimojima, A.; Kuroda, K. Topotactic conversion of layered silicate RUB-15 to silica sodalite through interlayer condensation in N-methylformamide. Dalton Trans. 2017, 46, 10232–10239. [Google Scholar] [CrossRef]
- Mehmood, Y.; Khan, I.U.; Shahzad, Y.; Khalid, S.H.; Asghar, S.; Irfan, M.; Asif, M.; Khalid, I.; Yousaf, A.M.; Hussain, T. Facile synthesis of mesoporous silica nanoparticles using modified sol-gel method: Optimization and in vitro cytotoxicity studies. Pak. J. Pharm. Sci. 2019, 32, 1805–1812. [Google Scholar]
- Singh, K.; Devi, S.; Bajaj, H.; Ingole, P.; Choudhari, J.; Bhrambhatt, H. Optical Resolution of Racemic Mixtures of Amino Acids through Nanofiltration Membrane Process. Sep. Sci. Technol. 2014, 49, 2630–2641. [Google Scholar] [CrossRef]
- Alruwailia, S.F.; Alsohaimia, I.H.; El-Sayeda, M.Y.; Hassana, H.M.A.; Aldawsarib, A.M.; Alshahranic, A.A.; Alra, T.S. Antifouling efficiency and high-flux ultrafiltration membrane comprising sulfonated poly (ether sulfone) and TNTs-g-PSPA nanofiller. J. Taiwan Inst. Chem. Eng. 2021, 129, 350–360. [Google Scholar] [CrossRef]
- Unuigbe, C.; Fayemiwo, O.; Daramola, M. Performance evaluation of iron nanoparticles infused polyethersulphone (Fe-NPs/PES) membrane during treatment of BTEX-contaminated wastewater. Water Environ. J. 2019, 34, 74–86. [Google Scholar] [CrossRef]
- Mukhkerjee, M.; De, S. Reduction of microbial contamination from drinking water using an iron oxide nanoparticle-impregnated ultrafiltration mixed matrix membrane: Preparation, characterization and antimicrobial properties. Environ. Sci. Water Res. Technol. 2015, 1, 204–217. [Google Scholar] [CrossRef]
- Singh, P.S.; Joshi, S.V.; Trivedi, J.J.; Devmurari, C.V.; Rao, A.P.; Ghosh, P.K. Probing the structural variations of thin film composite RO membranes obtained by coating polyamide over polysulfone membranes of different pore dimensions. J. Membr. Sci. 2006, 278, 19–25. [Google Scholar] [CrossRef]
- Bowen, W.R.; Hilal, N.; Lovitt, R.W.; Wright, C.J. A new technique for membrane characterisation: Direct measurement of the force of adhesion of a single particle using an atomic force microscope. J. Membr. Sci. 1998, 139, 269–274. [Google Scholar] [CrossRef]
- Caglayan, M. Atomic Force Microscopy as a Characterization Tool for Contact Lenses: Indentation Tests and Grain Analysis. Int. J. Polym. Mater. Polym. Biomater. 2014, 63, 680–684. [Google Scholar] [CrossRef]
- Van der Bruggen, B. Chemical modification of polyethersulfone nanofiltration membranes: A review. J. Appl. Polym. Sci. 2009, 114, 630–642. [Google Scholar] [CrossRef]
- Chunjin, W.; Li, A.; Li, L.; Zhang, L.; Wang, H.; Qi, X.; Zhang, Q. Treatment of oily water by a poly(vinyl alcohol) ultrafiltration membrane. Desalination 2008, 225, 312–321. [Google Scholar]
- Maphutha, S.; Moothi, K.; Meyyappan, M.; Iyuke, S. A carbon nanotube-infused polysulfone membrane with polyvinyl alcohol layer for treating oil-containing waste water. Sci. Rep. 2013, 3, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Rameetse, M.S.; Aberefa, O.; Daramola, M.O. Effect of Loading and Functionalization of Carbon Nanotube on the Performance of Blended Polysulfone/Polyethersulfone Membrane during Treatment of Wastewater Containing Phenol and Benzene. Membranes 2020, 10, 54. [Google Scholar] [CrossRef]
- Shen, X.; Xie, T.; Wang, J.; Wang, F. Improved fouling resistance of poly(vinylidene fluoride) membrane modified with poly(acryloyl morpholine)-based amphiphilic copolymer. Colloid Polym. Sci. 2017, 295, 1211–1221. [Google Scholar] [CrossRef]
- Shen, X.; Yin, X.; Zhao, Y.; Chen, L. Improved protein fouling resistance of PVDF membrane grafted with the polyampholyte layers. Colloid Polym. Sci. 2015, 293, 1205–1213. [Google Scholar] [CrossRef]
- Imtiaz, A.; Othman, M.H.D.; Jilani, A.; Khan, I.U.; Kamaludin, R.; Iqbal, J.; Al-Sehemi, A.G. Challenges, Opportunities and Future Directions of Membrane Technology for Natural Gas Purification: A Critical Review. Membranes 2022, 12, 646. [Google Scholar] [CrossRef]





| Membrane | EWC (%) | Porosity (%) |
|---|---|---|
| 0 wt.% SSOD/Psf | 55.01 | 40.45 ± 1.9 |
| 5 wt.% SSOD/Psf | 64.05 | 55.40 ± 2.3 |
| 10 wt.% SSOD/Psf | 73.24 | 61.09 ± 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadare, O.O.; Ngobeni, R.; Daramola, M.O. Effect of Silica Sodalite Loading on SOD/PSF Membranes during Treatment of Phenol-Containing Wastewater. Membranes 2022, 12, 800. https://doi.org/10.3390/membranes12080800
Sadare OO, Ngobeni R, Daramola MO. Effect of Silica Sodalite Loading on SOD/PSF Membranes during Treatment of Phenol-Containing Wastewater. Membranes. 2022; 12(8):800. https://doi.org/10.3390/membranes12080800
Chicago/Turabian StyleSadare, Olawumi O., Rivoningo Ngobeni, and Michael O. Daramola. 2022. "Effect of Silica Sodalite Loading on SOD/PSF Membranes during Treatment of Phenol-Containing Wastewater" Membranes 12, no. 8: 800. https://doi.org/10.3390/membranes12080800
APA StyleSadare, O. O., Ngobeni, R., & Daramola, M. O. (2022). Effect of Silica Sodalite Loading on SOD/PSF Membranes during Treatment of Phenol-Containing Wastewater. Membranes, 12(8), 800. https://doi.org/10.3390/membranes12080800







