Effects of Viscosity on Submerged Membrane Microfiltration Systems
Abstract
:1. Introduction
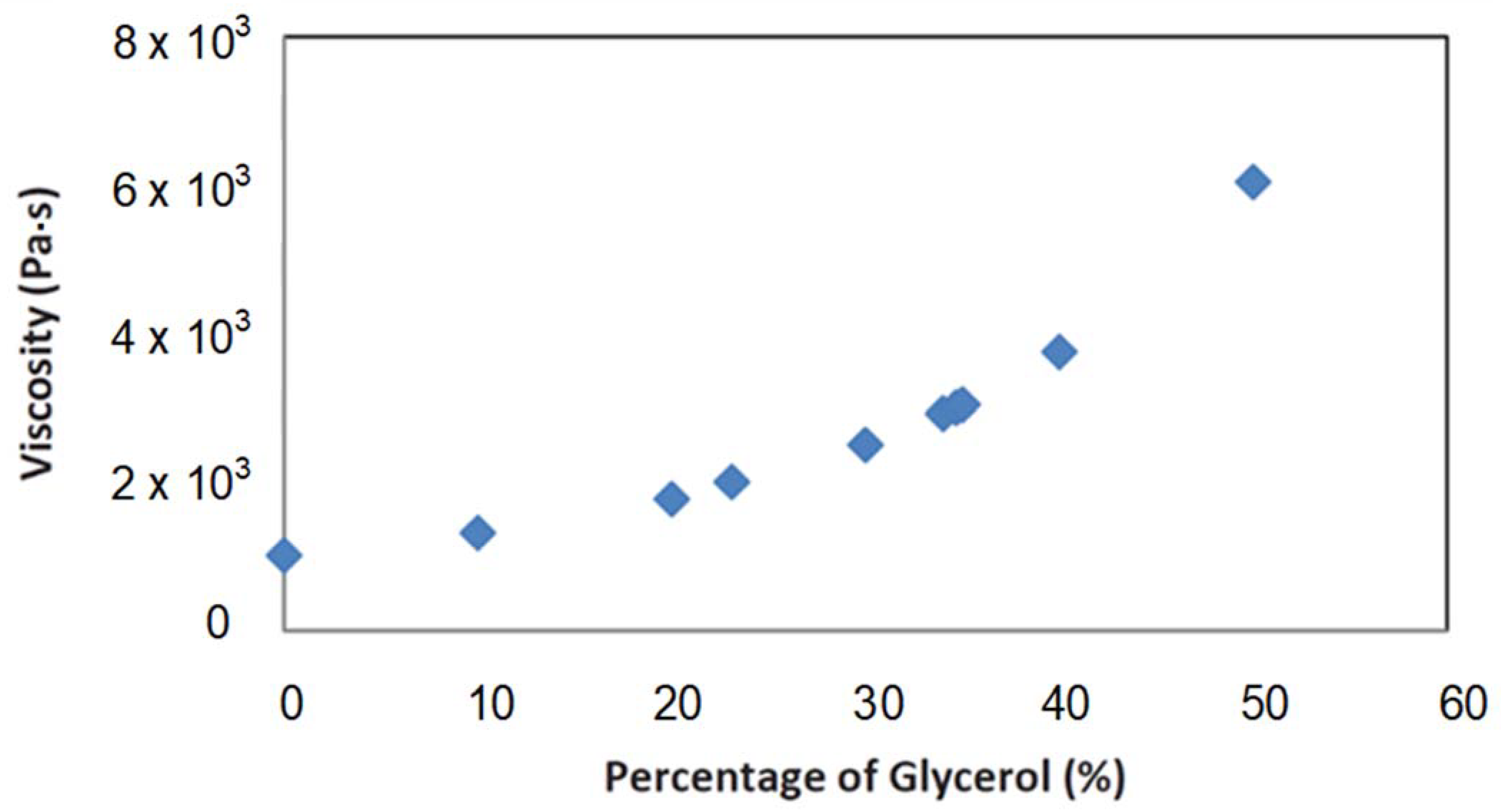
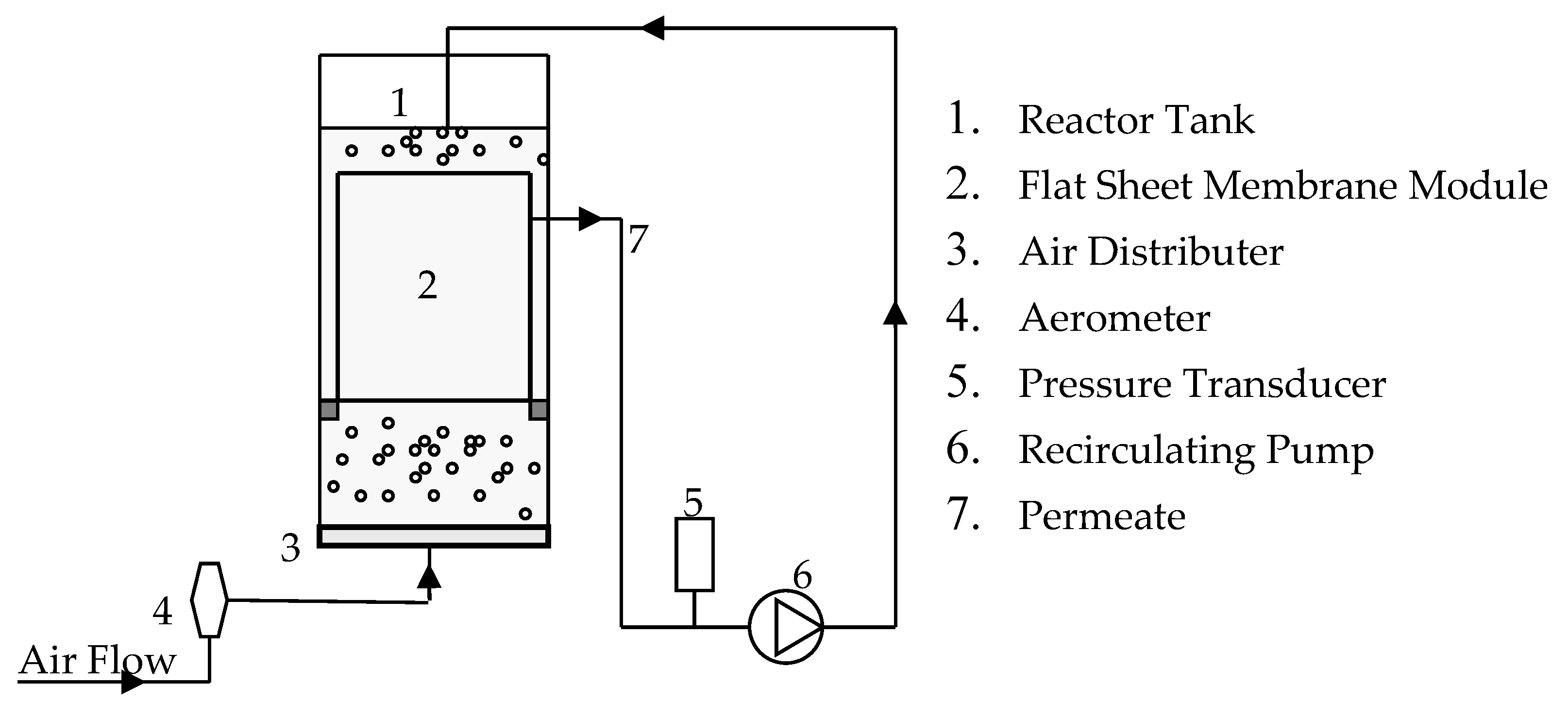
2. Materials and Methods
3. Results and Discussion
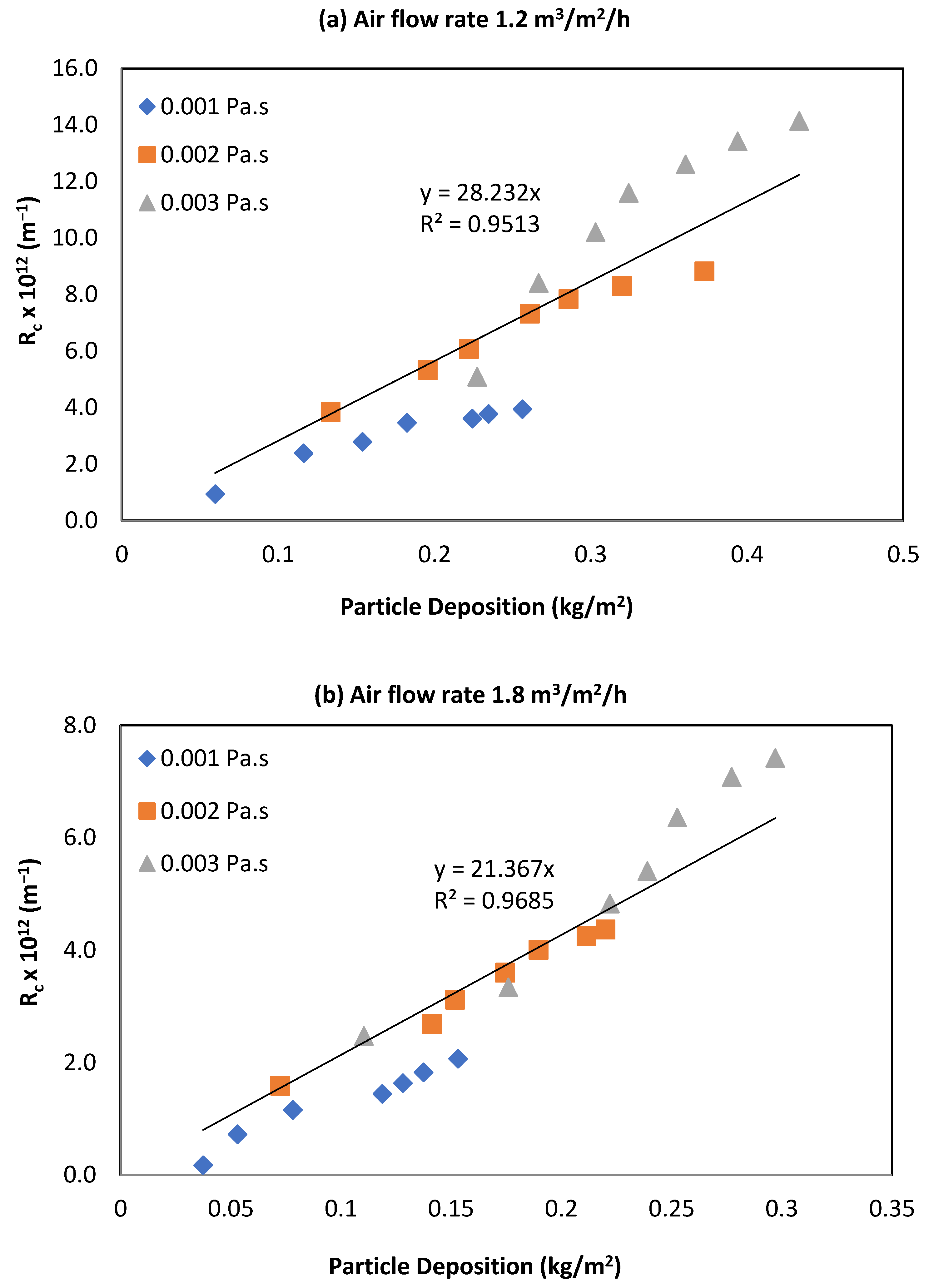
3.1. Effect of Viscosity on Rc
3.2. Effect of Viscosity on Particle Deposition (Fouling)
3.3. Relationship between Rc and Particle Deposition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EPS | Extracellular polymeric substance |
| J | Permeate flux |
| MLSS | Mixed liquor suspended solids |
| Pa·s | Pascal-second |
| Rt | Total membrane resistance |
| Rc | Cake resistance |
| Rm | Membrane resistance |
| rpm | Rotation per minutes |
| TMP | Transmembrane pressure |
| µm | Micro meter |
| µ | Viscosity |
| wc | Cake mass |
| αav | An average specific membrane resistance |
References
- Gander, M.; Jefferson, B.; Judd, S. Aerobic MBRs for domestic wastewater treatment: A review with cost considerations. Sep. Purif. Technol. 2000, 18, 119–130. [Google Scholar] [CrossRef]
- Drews, A. Membrane fouling in membrane bioreactors—Characterization, contradictions, cause and cures. J. Membr. Sci. 2010, 363, 1–28. [Google Scholar] [CrossRef]
- Meng, F.; Chae, S.-R.; Drews, A.; Kraume, M.; Shin, H.-S.; Yang, F. Recent advances in membrane bioreactors (MBRs): Membrane fouling and membrane material. Water Res. 2009, 43, 1489–1512. [Google Scholar] [CrossRef]
- Le-Clech, P.; Chen, V.; Fane, T.A. Fouling in membrane bioreactors used in wastewater treatment. J. Membr. Sci. 2006, 284, 17–53. [Google Scholar] [CrossRef]
- Pollice, A.; Brookes, A.; Jefferson, B.; Judd, S. Sub-critical flux fouling in membrane bioreactors—A review of recent literature. Desalination 2005, 174, 221–230. [Google Scholar] [CrossRef]
- Du, X.; Shi, Y.; Jegatheesan, V.; Haq, I.U. A review on the mechanism, impacts and control methods of membrane fouling in MBR system. Membranes 2020, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Dereli, R.K.; Grelot, A.; Heffernan, B.; van der Zee, F.P.; van Lier, J.B. Implications of changes in solids retention time on long term evolution of sludge filterability in anaerobic membrane bioreactors treating high strength industrial wastewater. Water Res. 2014, 59, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, X.C.; Yu, Z.; Ngo, H.H.; Sun, Q.; Zhang, Q. New insight into fouling behavior and foulants accumulation property of cake sludge in a full-scale membrane bioreactor. J. Membr. Sci. 2016, 510, 10–17. [Google Scholar] [CrossRef]
- Rosenberger, S.; Krüger, U.; Witzig, R.; Manz, W.; Szewzyk, U.; Kraume, M. Performance of a bioreactor with submerged membranes for aerobic treatment of municipal waste water. Water Res. 2002, 36, 413–420. [Google Scholar] [CrossRef]
- Itonaga, T.; Kimura, K.; Watanabe, Y. Influence of suspension viscosity and colloidal particles on permeability of membrane used in membrane bioreactor (MBR). Water Sci. Technol. 2004, 50, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Hasar, H.; Kinaci, C.; Ünlü, A.; Toǧrul, H.; Ipek, U. Rheological properties of activated sludge in a sMBR. Biochem. Eng. J. 2004, 20, 1–6. [Google Scholar] [CrossRef]
- Le-Clech, P.; Jefferson, B.; Judd, S. Impact of aeration, solids concentration and membrane characteristics on the hydraulic performance of a membrane bioreactor. J. Membr. Sci. 2003, 218, 117–129. [Google Scholar] [CrossRef]
- Delrue, F.; Stricker, A.E.; Mietton-Peuchot, M.; Racault, Y. Relationships between mixed liquor properties, operating conditions and fouling on two full-scale MBR plants. Desalination 2011, 272, 9–19. [Google Scholar] [CrossRef]
- Komesli, O.T.; Gökçay, C.F. Investigation of sludge viscosity and its effects on the performance of a vacuum rotation membrane bioreactor. Environ. Technol. 2014, 35, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Chang, S.; Fane, A. The use of gas bubbling to enhance membrane processes. J. Membr. Sci. 2003, 221, 1–35. [Google Scholar] [CrossRef]
- Kumar, S.; Kusakabe, K.; Raghunathan, K.; Fan, L.S. Mechanism of heat transfer in bubbly liquid and liquid-solid systems: Single bubble injection. AICHE J. 1992, 38, 733–741. [Google Scholar] [CrossRef]
- Wicaksana, F.; Fane, A.G.; Chen, V. Fibre movement induced by bubbling using submerged hollow fibre membranes. J. Membr. Sci. 2006, 271, 186–195. [Google Scholar] [CrossRef]
- Zhi-Hua, W.; Xiao-Yu, L.; Hong-Qi, Z.; Yong, W.; Yun-Fei, X.; Bao-Liang, P.; Yang, L. Modeling of kinetic charteristics of alkaline-surfactant-polymer-strengthened foams decay under ultrasonic standing wave. Pet. Sci. 2022, in press. [Google Scholar] [CrossRef]
- Schäfer, R.; Merten, C.; Eigenberger, G. Bubble size distributions in a bubble column reactor under industrial conditions. Exp. Therm. Fluid Sci. 2002, 26, 595–604. [Google Scholar] [CrossRef]
- Li, Q.; Cui, Z.; Pepper, D. Effect of bubble size and frequency on the permeate flux of gas sparged ultrafiltration with tubular membranes. Chem. Eng. J. 1997, 67, 71–75. [Google Scholar] [CrossRef]
- Pradhan, M. Study on Fouling Control in Submerged Membrane Microfiltration. Ph.D. Thesis, University of Technology Sydney, Sydney, Australia, November 2012. [Google Scholar]
- Sheely, M.L. Glycerol viscosity tables. Ind. Eng. Chem. 1932, 24, 1060–1064. [Google Scholar] [CrossRef]
- Belfort, G.; Davis, R.H.; Zydney, A.L. The behavior of suspensions and macromolecular solutions in crossflow microfiltration. J. Membr. Sci. 1994, 96, 1–58. [Google Scholar] [CrossRef]
- Pradhan, M.; Aryal, R.; Vigneswaran, S.; Kandasamy, J. Application of air flow for mitigation of particle deposition in submerged membrane microfiltration. Desalin. Water Treat. 2011, 32, 201–207. [Google Scholar] [CrossRef]
- Pradhan, M.; Vigneswaran, S.; Kandasamy, J. Assessment of fouling behaviour in submerged microfiltration system coupled with flocculation. J. Ind. Eng. Chem. 2015, 21, 254–260. [Google Scholar] [CrossRef]
- Pradhan, M.; Vigneswaran, S.; Kandasamy, J.; Aim, R.B. Combined effect of air and mechanical scouring of membranes for fouling reduction in submerged membrane reactor. Desalination 2012, 288, 58–65. [Google Scholar] [CrossRef]



| Flux (L/m2/h) | Feed Viscosity: 0.001 Pa·s | Feed Viscosity: 0.002 Pa·s | Feed Viscosity: 0.003 Pa·s | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Airflow Increment (m3/m2/h) | Airflow Increment (m3/m2/h) | Airflow Increment (m3/m2/h) | |||||||
| 0.6–1.2 | 0.6–1.8 | 1.2–1.8 | 0.6–1.2 | 0.6–1.8 | 1.2–1.8 | 0.6–1.2 | 0.6–1.8 | 1.2–1.8 | |
| 10 | 33 | 51 | 31 | 28 | 59 | 45 | 42 | 66 | 45 |
| 15 | 25 | 60 | 46 | 20 | 62 | 51 | 29 | 65 | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pradhan, M.; Johir, M.A.H.; Kandasamy, J.; Ratnaweera, H.; Vigneswaran, S. Effects of Viscosity on Submerged Membrane Microfiltration Systems. Membranes 2022, 12, 780. https://doi.org/10.3390/membranes12080780
Pradhan M, Johir MAH, Kandasamy J, Ratnaweera H, Vigneswaran S. Effects of Viscosity on Submerged Membrane Microfiltration Systems. Membranes. 2022; 12(8):780. https://doi.org/10.3390/membranes12080780
Chicago/Turabian StylePradhan, Muna, Md Abu Hasan Johir, Jaya Kandasamy, Harsha Ratnaweera, and Saravanamuthu Vigneswaran. 2022. "Effects of Viscosity on Submerged Membrane Microfiltration Systems" Membranes 12, no. 8: 780. https://doi.org/10.3390/membranes12080780
APA StylePradhan, M., Johir, M. A. H., Kandasamy, J., Ratnaweera, H., & Vigneswaran, S. (2022). Effects of Viscosity on Submerged Membrane Microfiltration Systems. Membranes, 12(8), 780. https://doi.org/10.3390/membranes12080780







