Efficiencies of O-MBR and A/O-MBR for Organic Matter Removal from and Trihalomethane Formation Potential Reduction in Domestic Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Domestic Wastewater
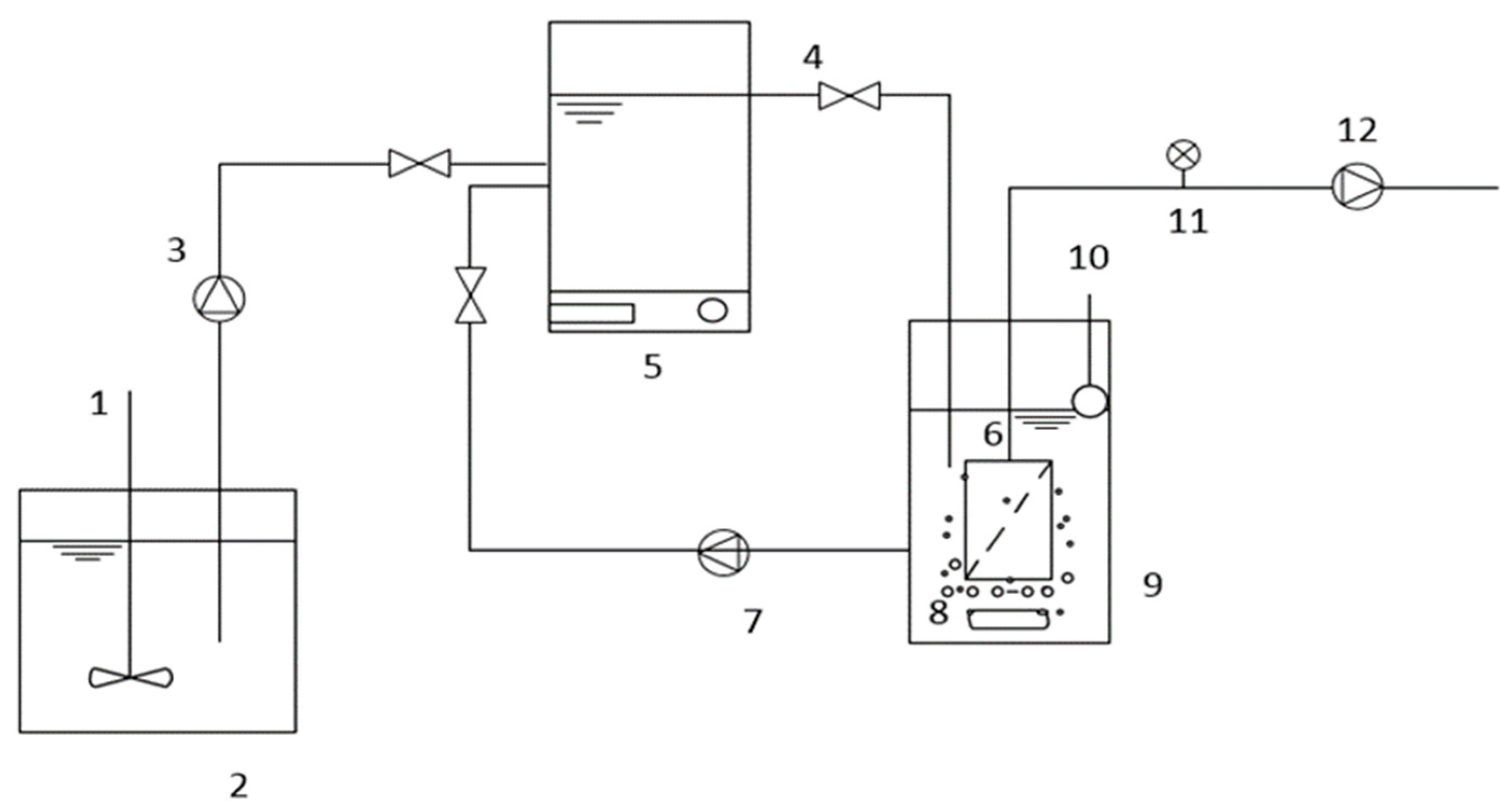
2.2. Anoxic/Oxic and Oxic Membrane Bioreactors (A/O-MBR and O-MBR)
2.3. Analytical Methods
2.4. Statistical Analysis
2.5. Trihalomethane Formation Potential (THMFP) and Analysis
3. Results and Discussion
3.1. Domestic Wastewater Characteristics
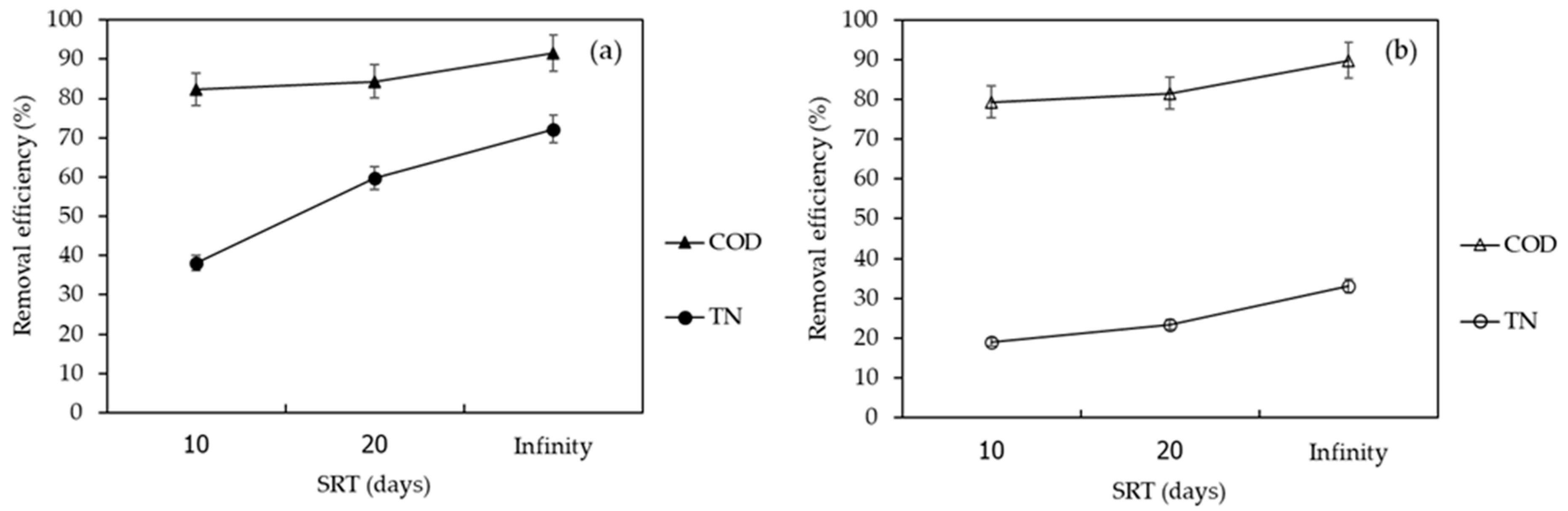
3.2. Efficiencies of the A/O-MBR and O-MBR Systems
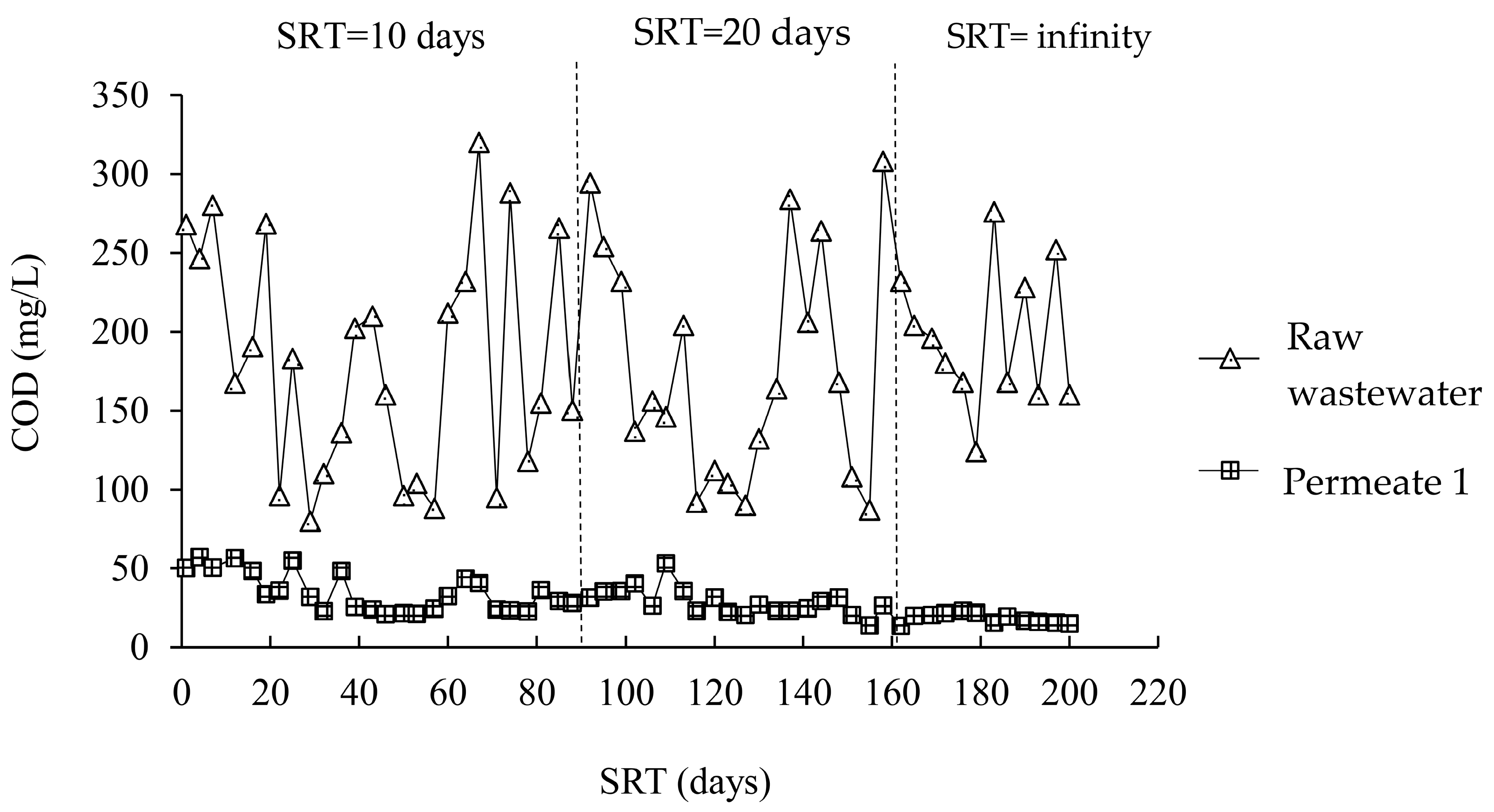
3.2.1. Chemical Oxygen Demand
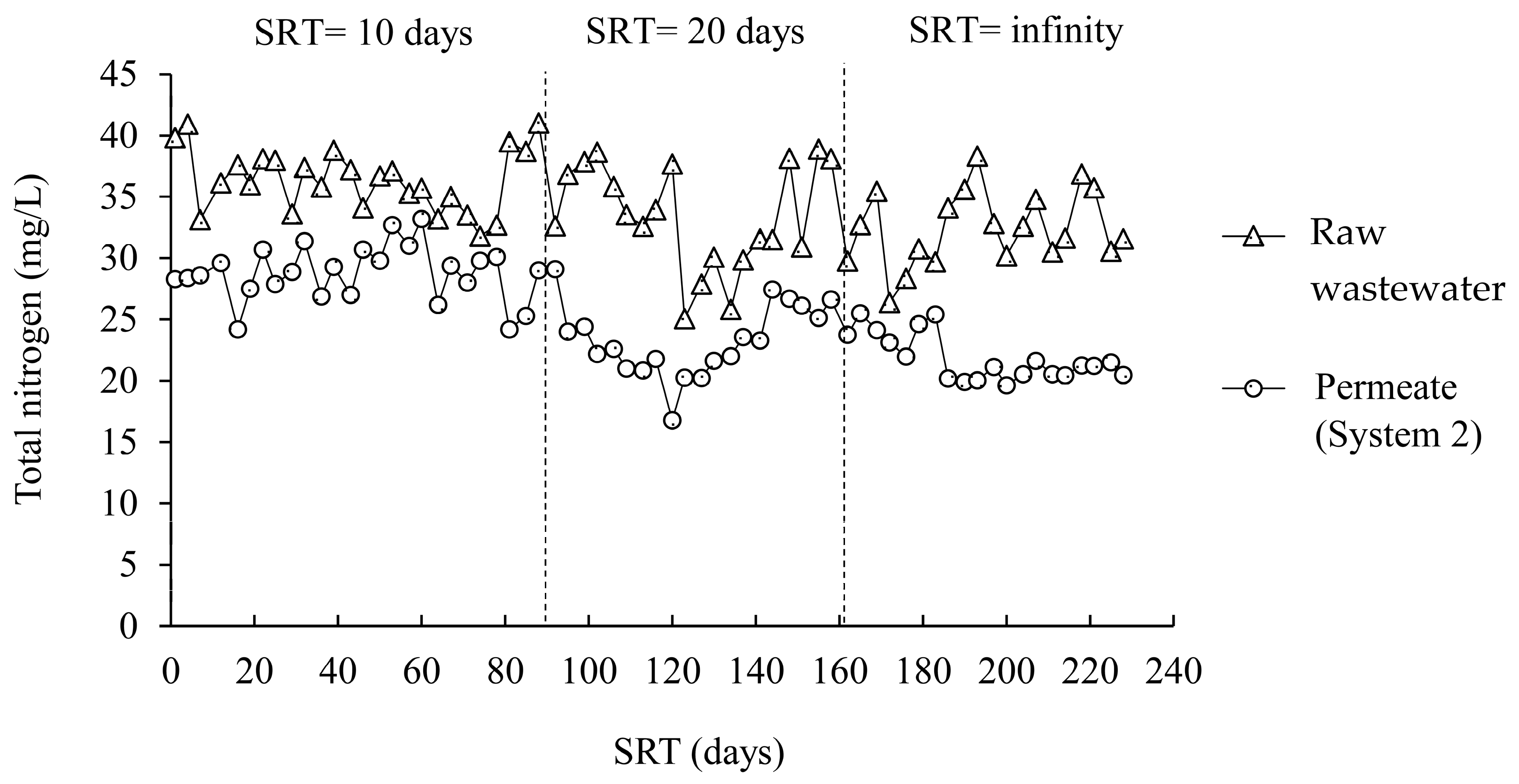
3.2.2. Effect of Nitrification/Denitrification
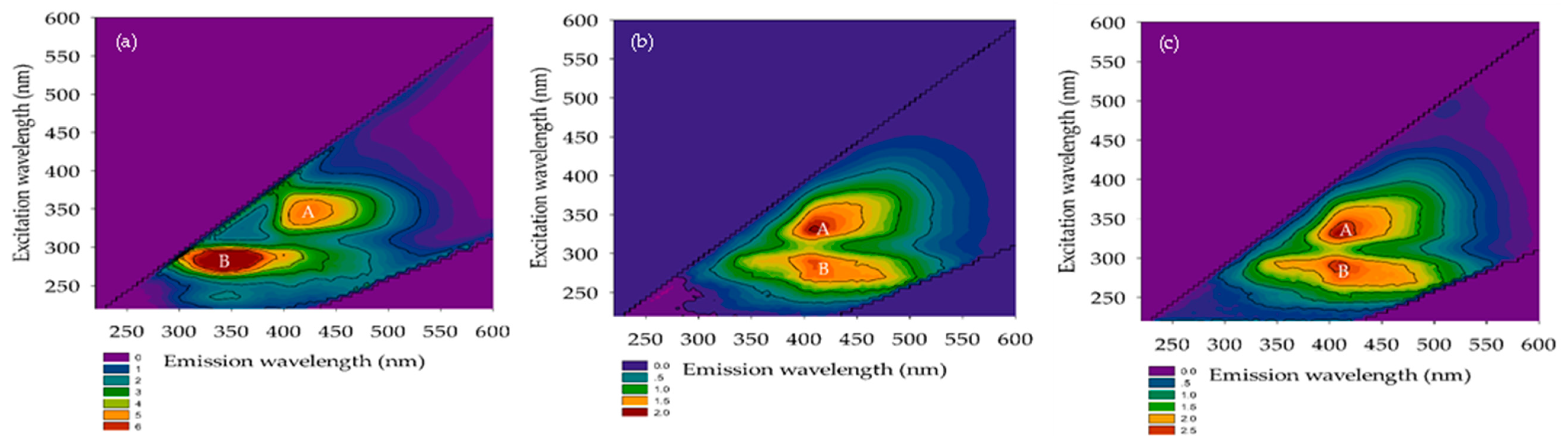
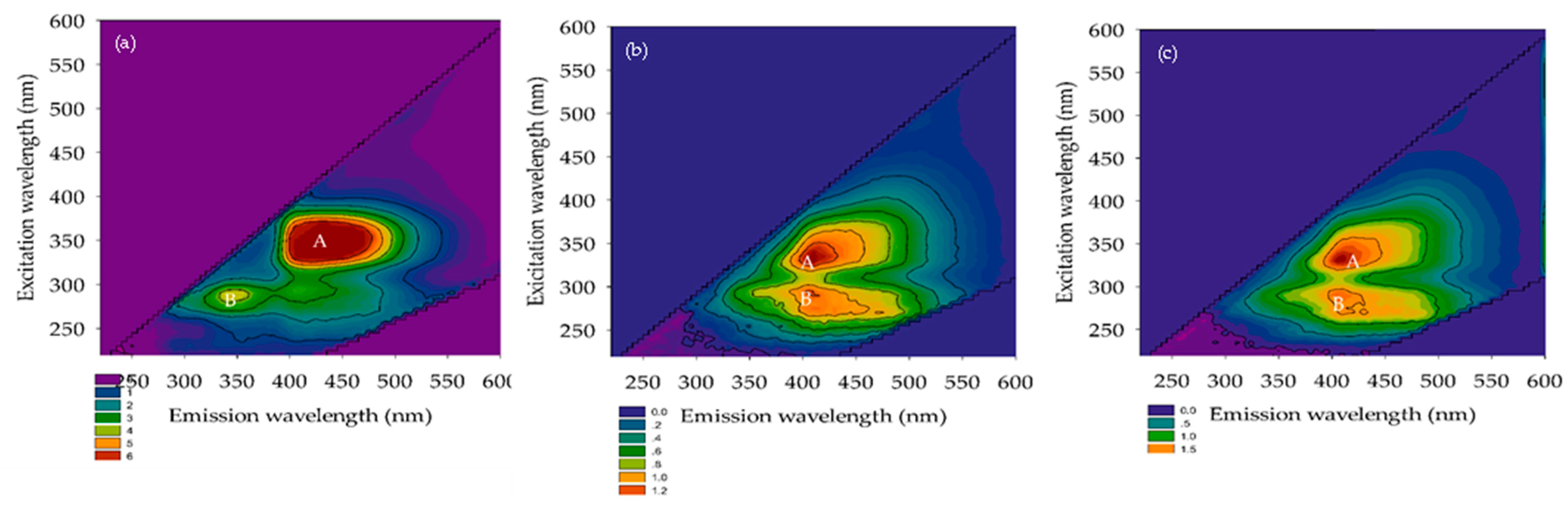
3.2.3. FEEM
3.2.4. TTHMFPs
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Domestic Wastewater. Available online: https://www.pcd.go.th/ (accessed on 2 May 2022).
- Leenheer, J.A.; Croué, J.P. Peer Reviewed: Characterizing Aquatic Dissolved Organic Matter. Environ. Sci. Technol. 2003, 37, 18A–26A. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Loper, J.C.; Lang, D.R.; Schoeny, R.S.; Richmond, B.B.; Gallagher, P.M.; Smith, C.C. Residue organic mixtures from drinking water show in vitro mutagenic and transforming activity. J. Toxicol. Environ. Health 1978, 4, 919–938. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, L.; Zeng, Q.; Zhang, S.H.; Xie, H.; Liu, A.L.; Lu, W.Q. Comparison of DNA damage in human-derived he-patoma line (HepG2) exposed to the fifteen drinking water disinfection byproducts using the single cell gel electrophoresis assay. Mutat. Res. 2011, 741, 89–94. [Google Scholar] [CrossRef]
- Richardson, S.D.; Plewa, M.J.; Wagner, E.D.; Schoeny, R.; DeMarini, D. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research. Mutat. Res. Mutat. Res. 2007, 636, 178–242. [Google Scholar] [CrossRef]
- Gao, D.W.; Zhang, T.; Tang, C.Y.Y.; Wu, W.M.; Wong, C.Y.; Lee, Y.H.; Yeh, D.H.; Criddle, C.S. Membrane fouling in an anaerobic membrane bioreactor: Differences in relative abundance of bacterial species in the membrane foulant layer and in suspension. J. Membr. Sci. 2010, 364, 331–338. [Google Scholar] [CrossRef]
- Skouteris, G.; Hermosilla, D.; López, P.; Negro, C.; Blanco, Á. Anaerobic membrane bioreactors for wastewater treatment: A review. Chem. Eng. J. 2012, 198–199, 138–148. [Google Scholar] [CrossRef]
- Stuckey, D.C. Recent developments in anaerobic membrane reactors. Bioresour. Technol. 2012, 122, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Dereli, R.K.; Ersahin, M.E.; Ozgun, H.; Ozturk, I.; Jeison, D.; van der Zee, F.; van Lier, J.B. Potentials of anaerobic membrane bioreactors to overcome treatment limitations induced by industrial wastewaters. Bioresour. Technol. 2012, 122, 160–170. [Google Scholar] [CrossRef]
- Adoonsook, D.; Chia-Yuan, C.; Wongrueng, A.; Pumas, C. A simple way to improve a conventional A/O-MBR for high simultaneous carbon and nutrient removal from synthetic municipal wastewater. PLoS ONE 2019, 14, e0214976. [Google Scholar] [CrossRef]
- Adoonsook, D.; Chia-Yuan, C.; Wongrueng, A.; Pumas, C. Microbial community composition in different carbon source types of biofilm A/O-MBR systems with complete sludge retention. Environ. Technol. 2021, 42, 2950–2967. [Google Scholar]
- Liu, J.; Zhang, H.; Zhang, P.; Wu, Y.; Gou, X.; Song, Y.; Tian, Z.; Zeng, G. Two-stage anoxic/oxic combined membrane bioreactor system for landfill leachate treatment: Pollutant removal performances and microbial community. Bioresour. Technol. 2017, 243, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Guo, W.; Lee, W. Formation of disinfection byproducts upon chlorine dioxide preoxidation followed by chlorination or chloramination of natural organic matter. Chemosphere 2013, 91, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Plewa, M.J.; Wagner, E.D. Risks of Disinfection Byproducts in Drinking Water: Comparative Mammalian Cell Cytotoxicity and Genotoxicity. In Encyclopedia of Environmental Health, 2nd ed.; Nriagu, J., Ed.; Elsevier: Oxford, UK, 2019; pp. 559–566. [Google Scholar]
- Zainudin, F.; Abu Hasan, H.; Abdullah, S. An overview of the technology used to remove trihalomethane (THM), trihalomethane precursors, and trihalomethane formation potential (THMFP) from water and wastewater. J. Ind. Eng. Chem. 2017, 57, 1–14. [Google Scholar] [CrossRef]
- Rakruam, P.; Wattanachira, S. Reduction of DOM fractions and their trihalomethane formation potential in surface river water by in-line coagulation with ceramic membrane filtration. J. Environ. Sci. 2014, 26, 529–536. [Google Scholar] [CrossRef]
- APHA; AWWA; WPCF. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Water Works Association: Washington, DC, USA, 2005. [Google Scholar]
- Amatya, I.; Kansakar, B.; Tare, V.; Fiksdal, L. Role of pH on biological Nitrification Process. J. Inst. Eng. 2011, 8, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Ovez, B. Batch biological denitrification using Arundo donax, Glycyrrhiza glabra, and Gracilaria verrucosa as carbon source. Process Biochem. 2006, 41, 1289–1295. [Google Scholar] [CrossRef]
- Ding, Y.; Guo, Z.; Ma, B.; Wang, F.; You, H.; Mei, J.; Hou, X.; Liang, Z.; Li, Z.; Jin, C. The Influence of Different Operation Conditions on the Treatment of Mariculture Wastewater by the Combined System of Anoxic Filter and Membrane Bioreactor. Membranes 2021, 11, 729. [Google Scholar] [CrossRef]
- Fu, Z.; Yang, F.; An, Y.; Xue, Y. Simultaneous nitrification and denitrification coupled with phosphorus removal in an modified anoxic/oxic-membrane bioreactor (A/O-MBR). Biochem. Eng. J. 2009, 43, 191–196. [Google Scholar] [CrossRef]
- Painter, H.A.; Loveless, J.E. Effect of temperature and pH value on the growth-rate constants of nitrifying bacteria in the activated-sludge process. Water Res. 1983, 17, 237–248. [Google Scholar] [CrossRef]
- Metcalf and Eddy. Wastewater Engineering Treatment: Treatment, Disposal and Reuse, 3rd ed.; McGraw-Hill: Singapore, 1991; ISBN 0-07-041690-7. [Google Scholar]
- Do, K.U.; Chu, X.Q. Chapter 5-Performances of membrane bioreactor technology for treating domestic wastewater operated at different sludge retention time. In Development in Wastewater Treatment Research and Processes; Shah, M., Rodriguez-Couto, S., Biswas, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 107–122. [Google Scholar]
- Phanwilai, S.; Noophan, P.; Li, C.W.; Choo, K.H. Effect of COD:N ratio on biological nitrogen removal using full-scale step-feed in municipal wastewater treatment plants. Sustain. Environ. Res. 2020, 30, 1–9. [Google Scholar]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence excitation−emission matrix regional integration to quantify spectra for dissolved organic matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.L.; Liu, T.T.; Cui, F.; Ni, J.R. Fluorescence evolution of leachates during treatment processes from two contrasting landfills. Environ. Technol. 2008, 29, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Sriboonnak, S.; Induvesa, P.; Wattanachira, S.; Rakruam, P.; Siyasukh, A.; Pumas, C.; Wongrueng, A.; Khan, E. Trihalomethanes in Water Supply System and Water Distribution Networks. Int. J. Environ. Res. Public Health 2021, 18, 9066. [Google Scholar] [CrossRef] [PubMed]
- Yimyam, K.; Wongrueng, A.; Rakruam, P.; Nitayavardhana, S.; Phetrak, A.; Theepharaksapan, S.; Wattanachira, S. Reduction of DBP precursors and their THMFPs in leachate contaminated groundwater by PAC adsorption. Eng. J. 2017, 21, 11–23. [Google Scholar] [CrossRef]





| Parameters | Range | Value (n = 5) |
|---|---|---|
| pH | 7.14–7.98 | 7.51 ± 0.1 |
| SS (mg/L) | 50–920 | 268.0 ± 172.2 |
| Temperature (°C) | 20.4–33.8 | 28.2 ± 3.1 |
| Alkalinity (mg/L as CaCO3) | 86–176 | 128.5 ± 20.2 |
| COD (mg/L) | 80–308 | 191.1 ± 69.1 |
| BOD (mg/L) | 98.3–133.8 | 116.7 ± 12.4 |
| TKN (mg/L) Ammonia (mg/L) | 24.6–39.4 18.4–32.8 | 33.5 ± 3.4 24.0 ± 3.2 |
| Nitrite (mg/L) | 0.0–1.8 | 0.4 ± 0.4 |
| Nitrate (mg/L) | 0.0–1.4 | 0.3 ± 0.3 |
| Phosphorus (mg/L) | 1.21–1.78 | 1.5 ± 0.2 |
| SRT (days) | COD (mg/L) | ||
|---|---|---|---|
| Raw Wastewater | Permeate from A/O-MBR (System 1) | Permeate from O-MBR (System 2) | |
| 10 | 193.5 ± 85.3 | 28.9 ± 8.9 | 29.3 ± 10.7 |
| 20 | 174.1 ± 80.6 | 28.9 ± 5.1 | 30.5 ± 3.7 |
| Infinity | 218.6 ± 61.1 | 17.4 ± 4.1 | 21.2 ± 4.2 |
| p-value | 0.301 | 0.000 | 0.000 |
| SRT (days) | Total Nitrogen (mg/L) | ||
|---|---|---|---|
| Raw Wastewater | Permeate from A/O-MBR (System 1) | Permeate from O-MBR (System 2) | |
| 10 | 35.5 ± 2.9 | 22.0 ± 2.0 | 29.0 ± 2.6 |
| 20 | 31.6 ± 4.6 | 12.7 ± 0.9 | 23.9 ± 2.5 |
| Infinity | 32.4 ± 3.1 | 8.9 ± 0.6 | 21.4 ± 1.6 |
| p-value | 0.030 | 0.000 | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sriboonnak, S.; Yanun, A.; Induvesa, P.; Pumas, C.; Duangjan, K.; Rakruam, P.; Nitayavardhana, S.; Sittisom, P.; Wongrueng, A. Efficiencies of O-MBR and A/O-MBR for Organic Matter Removal from and Trihalomethane Formation Potential Reduction in Domestic Wastewater. Membranes 2022, 12, 761. https://doi.org/10.3390/membranes12080761
Sriboonnak S, Yanun A, Induvesa P, Pumas C, Duangjan K, Rakruam P, Nitayavardhana S, Sittisom P, Wongrueng A. Efficiencies of O-MBR and A/O-MBR for Organic Matter Removal from and Trihalomethane Formation Potential Reduction in Domestic Wastewater. Membranes. 2022; 12(8):761. https://doi.org/10.3390/membranes12080761
Chicago/Turabian StyleSriboonnak, Sornsiri, Aegkapan Yanun, Phacharapol Induvesa, Chayakorn Pumas, Kritsana Duangjan, Pharkphum Rakruam, Saoharit Nitayavardhana, Prattakorn Sittisom, and Aunnop Wongrueng. 2022. "Efficiencies of O-MBR and A/O-MBR for Organic Matter Removal from and Trihalomethane Formation Potential Reduction in Domestic Wastewater" Membranes 12, no. 8: 761. https://doi.org/10.3390/membranes12080761
APA StyleSriboonnak, S., Yanun, A., Induvesa, P., Pumas, C., Duangjan, K., Rakruam, P., Nitayavardhana, S., Sittisom, P., & Wongrueng, A. (2022). Efficiencies of O-MBR and A/O-MBR for Organic Matter Removal from and Trihalomethane Formation Potential Reduction in Domestic Wastewater. Membranes, 12(8), 761. https://doi.org/10.3390/membranes12080761






