Intravenous Nanocarrier for Improved Efficacy of Quercetin and Curcumin against Breast Cancer Cells: Development and Comparison of Single and Dual Drug–Loaded Formulations Using Hemolysis, Cytotoxicity and Cellular Uptake Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Single and Dual Drug–Loaded Nanoemulsion
2.3. Droplet Size, Polydispersity Index and Zeta Potential Measurement
2.4. Viscosity Measurement
2.5. pH and Osmolarity
2.6. Determination of Encapsulation Efficiency
2.7. In Vitro Drug Release Study
2.8. Hemolytic Potential of Nanoemulsion and Individual Components
2.9. In Vitro Cytotoxicity Study Using MCF-7 Cells
2.10. In Vitro Cellular Uptake Study Using MCF-7 Cells
2.11. HPLC Method for Analysis of Quercetin and Curcumin
2.12. Stability Study
2.13. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characteristics of Intravenous Nanoemulsion
3.2. In Vitro Drug Release Study
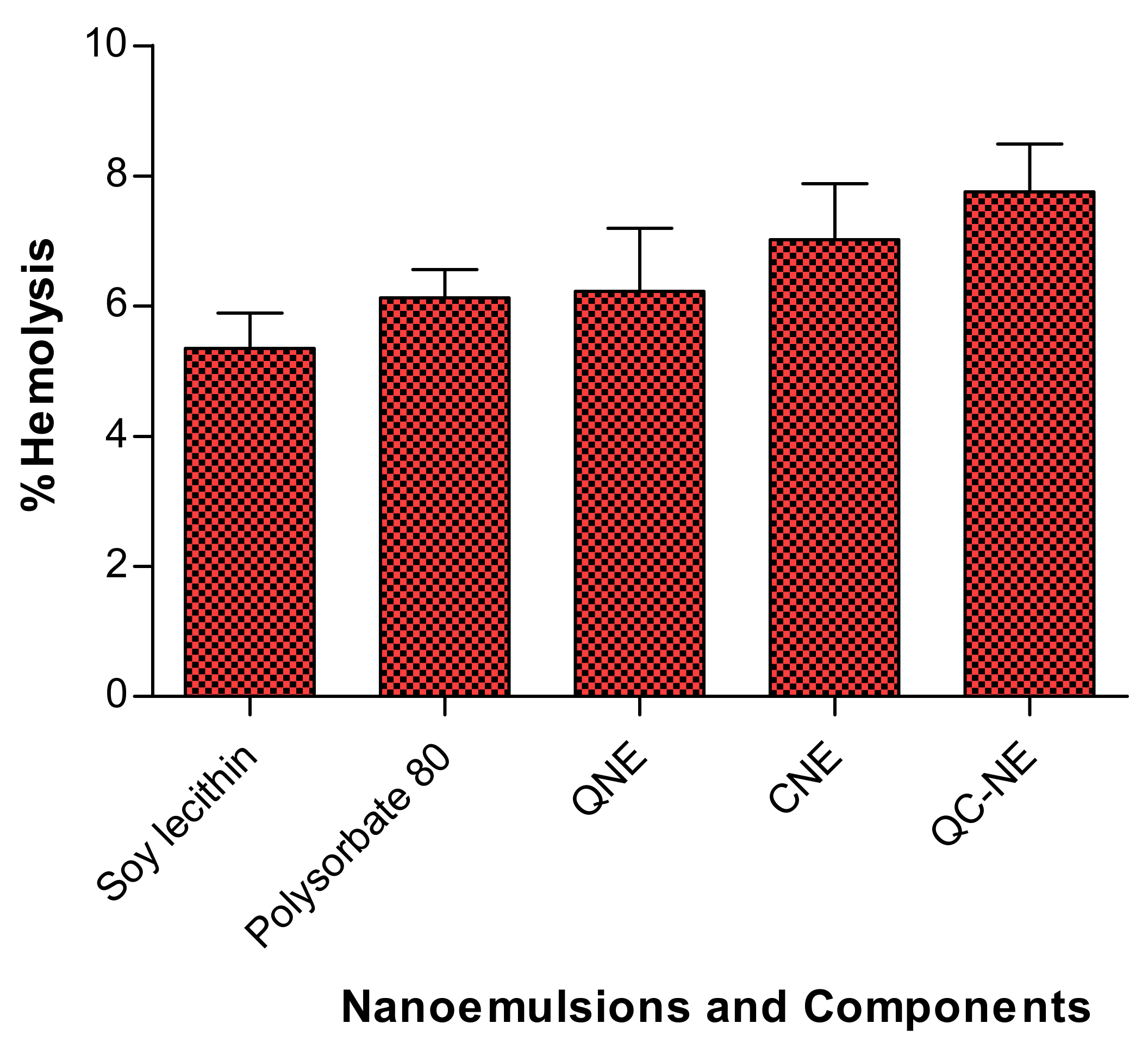
3.3. In Vitro Hemolysis of Free Drug, Nanoemulsion and Its Individual Components
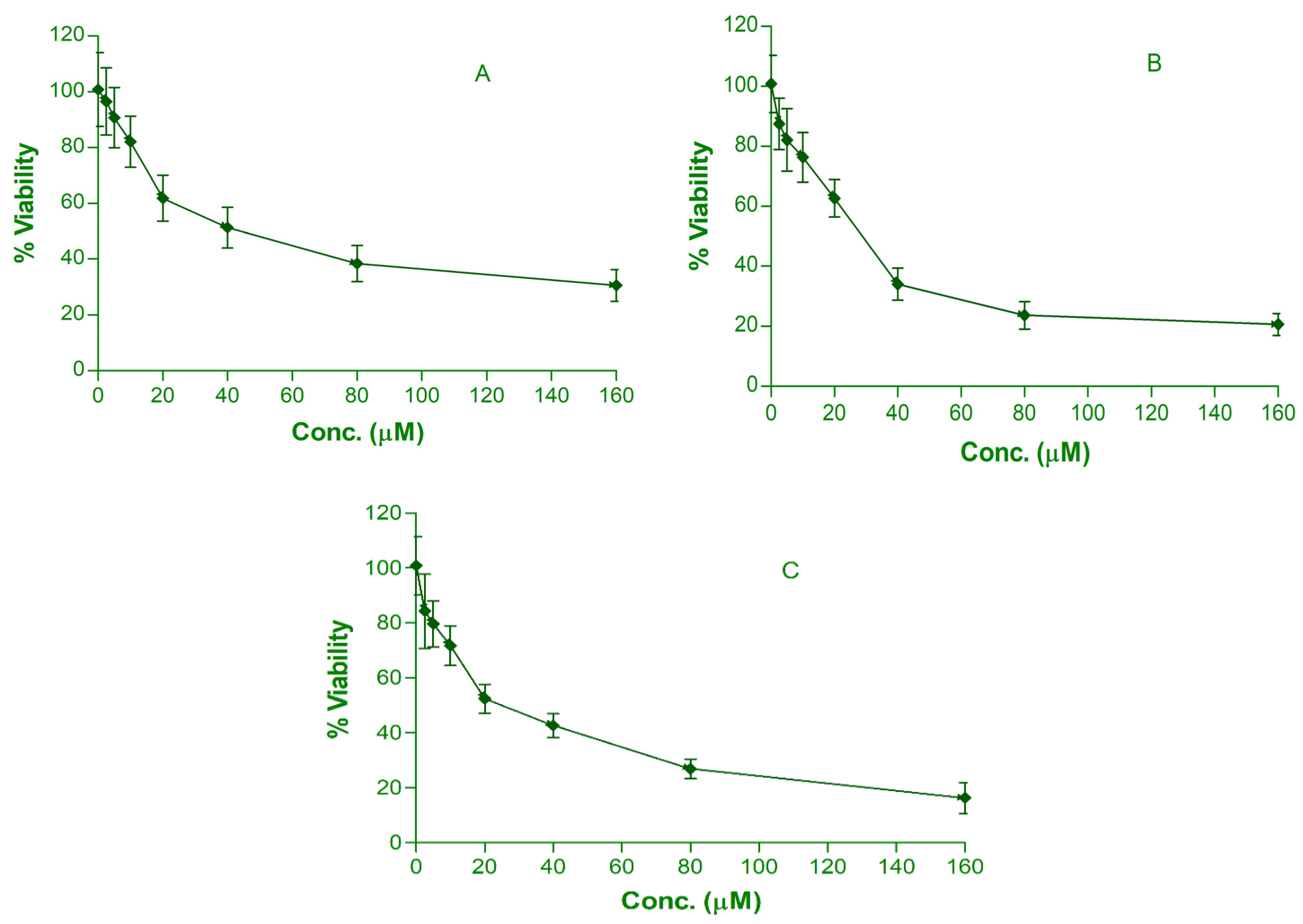
3.4. In Vitro Cytotoxic Effect of Drug from Single and Dual Drug–Loaded Nanoemulsion
3.5. Cellular Uptake of Drug from Single and Dual Drug–Loaded Nanoemulsion
3.6. Stability Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef]
- Kokuryo, T.; Yokoyama, Y.; Nagino, M. Recent advances in cancer stem cell research for cholangiocarcinoma. J. Hepatobiliary Pancreat. Sci. 2012, 19, 606–613. [Google Scholar] [CrossRef]
- Prasain, J.K.; Barnes, S. Metabolism and bioavailability of flavonoids in chemoprevention: Current analytical strategies and future prospectus. Mol. Pharm. 2007, 4, 846–864. [Google Scholar] [CrossRef] [PubMed]
- Nishino, H.; Satomi, Y.; Tokuda, H.; Masuda, M. Cancer control by phytochemicals. Curr. Pharm. Des. 2007, 13, 3394–3399. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Liu, C.; Chen, C.; Yu, X.; Chen, G.; Shi, Y.; Qin, F.; Ou, J.; Qiu, K.; Li, G. Quercetin and doxorubicin co-encapsulated biotin receptor-targeting nanoparticles for minimizing drug resistance in breast cancer. Oncotarget 2016, 7, 32184–32199. [Google Scholar] [CrossRef] [PubMed]
- Maetzel, D.; Denzel, S.; Mack, B.; Canis, M.; Went, P.; Benk, M.; Kieu, C.; Papior, P.; Baeuerle, P.A.; Munz, M.; et al. Nuclear signalling by tumour-associated antigen EpCAM. Nat. Cell Biol. 2009, 11, 162–171. [Google Scholar] [CrossRef]
- Ediriweera, M.K.; Tennekoon, K.H.; Adhikari, A.; Samarakoon, S.R.; Thabrew, I.; de Silva, E.P. New halogenated constituents from Mangifera zeylanica Hook. f. and their potential anti-cancer effects in breast and ovarian cancer cells. J. Ethnopharmacol. 2016, 189, 165–174. [Google Scholar] [CrossRef]
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.R.; Thabrew, I.; de Silva, E.P. Protective effects of six selected dietary compounds against leptin-induced proliferation of oestrogen receptor positive (MCF-7) breast cancer cells. Medicines 2017, 4, 56. [Google Scholar] [CrossRef]
- Noolu, B.; Gogulothu, R.; Bhat, M.; Qadri, S.S.Y.H.; Reddy, V.S.; Reddy, G.B.; Ismail, A. In-vivo inhibition of proteasome activity and tumour growth by Murraya koenigii leaf extract in breast cancer xenografts and by its active flavonoids in breast cancer cells. Anticancer Agents Med. Chem. 2016, 16, 1605–1614. [Google Scholar] [CrossRef]
- Ramachandran, C.; Rodriguez, S.; Ramachandran, R.; Nair, P.R.; Fonseca, H.; Khatib, Z.; Escalon, E.; Melnick, S.J. Expression profiles of apoptotic genes induced by curcumin in human breast cancer and mammary epithelial cell lines. Anticancer Res. 2005, 25, 3293–3302. [Google Scholar]
- Karunagaran, D.; Rashmi, R.; Kumar, T.R. Induction of apoptosis by curcumin and its implications for cancer therapy. Curr. Cancer Drug Targets 2005, 5, 117–129. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Evangelopoulos, A.; Schizas, N.; Kazazis, C. Potential anticancer properties and mechanisms of action of curcumin. Anticancer Res. 2015, 35, 645–651. [Google Scholar]
- Gera, M.; Sharma, N.; Ghosh, M.; Huynh, D.L.; Lee, S.J.; Min, T.; Kwon, T.; Jeong, D. Nanoformulations of curcumin: An emerging paradigm for improved remedial application. Oncotarget 2017, 8, 66680–66698. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, N.; Ahmad, R.; Naqvi, A.A.; Alam, M.A.; Rub, R.A.; Ahmad, F.J. Enhancement of quercetin oral bioavailability by self-nanoemulsifying drug delivery system and their quantification through ultra-high performance liquid chromatography and mass spectrometry in cerebral ischemia. Drug Res. 2017, 67, 564–575. [Google Scholar] [CrossRef]
- Dian, L.; Yu, E.; Chen, X.; Wen, X.; Zhang, Z.; Qin, L.; Wang, Q.; Li, G.; Wu, C. Enhancing oral bioavailability of quercetin using novel soluplus polymeric micelles. Nanoscale Res. Lett. 2014, 9, 684. [Google Scholar] [CrossRef] [Green Version]
- Turner, P.V.; Brabb, T.; Pekow, C.; Vasbinder, M.A. Administration of substances to laboratory animals: Routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 600–613. [Google Scholar]
- Constantinides, P.P.; Tustian, A.; Kessler, D.R. Tocol emulsions for drug solubilization and parenteral delivery. Adv. Drug Deliv. Rev. 2004, 56, 1243–1255. [Google Scholar] [CrossRef]
- Constantinides, P.P.; Chaubal, M.V.; Shorr, R. Advances in lipid nanodispersions for parenteral drug delivery and targeting. Adv. Drug Deliv. Rev. 2008, 60, 757–767. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Clogston, J.D.; Neun, B.W.; Hall, J.B.; Patri, A.K.; McNeil, S.E. Method for analysis of nanoparticle hemolytic properties In-Vitro. Nano Lett. 2008, 8, 2180–2187. [Google Scholar] [CrossRef]
- Manaargadoo-Catin, M.; Ali-Cherif, A.; Pougnas, J.L.; Perrin, C. Hemolysis by surfactants—A review. Adv. Colloid Interface Sci. 2016, 228, 1–16. [Google Scholar] [CrossRef]
- Partearroyo, M.A.; Ostolaza, H.; Goni, F.M.; Barbera-Guillem, E. Surfactant-induced cell toxicity and cell lysis. Biochem. Pharmacol. 1990, 40, 1323–1328. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; McNeil, S.E. Understanding the correlation between in-vitro and in-vivo immunotoxicity tests for nanomedicines. J. Control. Release 2013, 172, 456–466. [Google Scholar] [CrossRef] [Green Version]
- Liptrott, N.J.; Giardiello, M.; McDonald, T.O.; Rannard, S.P.; Owen, A. Assessment of interactions of efavirenz solid drug nanoparticles with human immunological and haematological systems. J. Nanobiotechnol. 2018, 16, 22. [Google Scholar] [CrossRef] [Green Version]
- Silva, H.D.; Cerqueira, M.A.; Souza, B.W.S.; Ribeiro, C.; Avides, M.C.; Quintas, M.A.C.; Coimbra, J.S.R.; Cameiro-da-Cunha, M.G.; Vicente, A.A. Nanoemulsions of β-carotene using a high-energy emulsification-evaporation technique. J. Food Eng. 2011, 102, 130–135. [Google Scholar] [CrossRef] [Green Version]
- Bouchemal, K.; Briancon, S.; Perrier, E.; Fessi, H. Nanoemulsion formulation using spontaneous emulsification: Solvent, oil and surfactant optimization. Int. J. Pharm. 2004, 280, 241–251. [Google Scholar] [CrossRef]
- Sze, A.; Erickson, D.; Ren, L.; Li, D. Zeta-potential measurement using the Smoluchowski equation and the slope of the current–time relationship in electroosmotic flow. J. Colloid Interface Sci. 2003, 261, 402–441. [Google Scholar] [CrossRef]
- Florence, A.T.; Whitehill, D. Stability and stabilization of water-in-oil-in-water multiple emulsions. Macro Microemulsions 1985, 23, 359–380. [Google Scholar]
- Matsumoto, S.; Kita, Y.; Yonezawa, D. An attempt at preparing water-in-oil-in-water multiple-phase emulsions. J. Colloid Interface Sci. 1976, 57, 353–361. [Google Scholar] [CrossRef]
- Seguy, L.; Groo, A.; Goux, D.; Hennequin, D.; Malzert-Freon, A. Design of non-haemolytic nanoemulsions for intravenous administration of hydrophobic APIs. Pharmaceutics 2020, 12, 1141. [Google Scholar] [CrossRef]
- Harun, S.N.; Nordin, S.A.; Gani, S.S.A.; Shamsuddin, A.F.; Basri, M.; Basri, H.B. Development of nanoemulsion for efficient brain parenteral delivery of cefuroxime: Designs, characterizations, and pharmacokinetics. Int. J. Nanomed. 2018, 13, 2571–2584. [Google Scholar] [CrossRef] [Green Version]
- Karami, Z.; Khoshkam, M.; Hamidi, M. Optimization of olive oil-based nanoemulsion preparation for intravenous drug delivery. Drug Res. 2019, 69, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Li, S.D.; Huang, L. Pharmacokinetics and biodistribution of nanoparticles. Mol. Pharm. 2008, 5, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Schuh, R.S.; Bruxel, F.; Teixeira, H.F. Physicochemical properties of lecithin-based nanoemulsions obtained by spontaneous emulsification or high-pressure homogenization. Quim. Nova 2014, 37, 1193–1198. [Google Scholar]
- Joshi, M.; Patravale, V. Formulation and evaluation of nanostructured lipid carrier (NLC)-based gel of valdecoxib. Drug Dev. Ind. Pharm. 2006, 32, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Liu, R. Water-Insoluble Drug Formulation, 2nd ed; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]




| Nanocarriers | Droplet Size (nm) | Polydispersity Index | Zeta Potential (−mV) | Viscosity (cps) | pH | Osmolarity (mOsm) | % EE | % DL |
|---|---|---|---|---|---|---|---|---|
| QNE | 26.4 ± 1.34 | 0.126 ± 0.002 | 8.6 ± 1.62 | 1.64 | 7.1 ± 0.2 | 279 ± 8.67 | 90.28 | 0.73 |
| CNE | 27.8 ± 1.87 | 0.129 ± 0.001 | 8.4 ± 1.38 | 1.76 | 7.2 ± 0.1 | 279 ± 7.89 | 87.54 | 0.81 |
| QC-NE | 25.9 ± 1.59 | 0.127 ± 0.003 | 8.9 ± 1.57 | 1.69 | 7.0 ± 0.3 | 280 ± 9.36 | Q: 88.83 | Q: 0.71 |
| C: 85.37 | C: 0.83 |
| Test Sample | % Hemolysis | In Vitro Cytotoxicity | In Vitro Cellular Uptake (ng/μg) | ||
|---|---|---|---|---|---|
| Max. Viability (%) | Min. Viability (%) | IC50 (µM) | |||
| Quercetin | - | - | - | - | 158.3 ± 13.5 |
| Curcumin | - | - | - | - | 167.5 ± 15.8 |
| Soy lecithin | 5.35 ± 0.54 | - | - | - | - |
| Polysorbate 80 | 6.13 ± 0.43 | - | - | - | - |
| QNE | 6.23 ± 0.97 | 96.54 ± 12.05 | 30.67 ± 5.62 | 40.2 ± 2.34 | 612.3 ± 41.4 |
| CNE | 7.02 ± 0.86 | 87.44 ± 10.15 | 20.48 ± 3.43 | 28.12 ± 2.07 | 648.6 ± 39.6 |
| QC-NE | 7.76 ± 0.73 | 84.33 ± 8.26 | 16.27 ± 2.87 | 21.23 ± 2.16 | Q:627.5 ± 53.8 |
| C:632.4 ± 47.6 | |||||
| Nanoemulsions | Time (Month) | Droplet Size (nm) at 4 °C and 25 °C | Polydispersity Index at 4 °C and 25 °C | Zeta Potential (−mV) at 4 °C and 25 °C | Osmolarity (mOsm) at 4 °C and 25 °C | pH at 4 °C and 25 °C |
|---|---|---|---|---|---|---|
| QNE | 0 | 26.4 ± 1.34 | 0.126 ± 0.002 | 8.6 ± 1.62 | 279 ± 8.67 | 7.1 ± 0.2 |
| 1 | 26.5 ± 1.28 | 0.126 ± 0.004 | 8.3 ± 1.34 | 279 ± 7.05 | 7.0 ± 0.1 | |
| 26.5 ± 1.33 | 0.126 ± 0.002 | 8.3 ± 1.27 | 279 ± 8.35 | 6.8 ± 0.2 | ||
| 3 | 26.5 ± 1.31 | 0.125 ± 0.001 | 8.2 ± 1.42 | 279 ± 6.83 | 6.9 ± 0.1 | |
| 26.6 ± 1.28 | 0.125 ± 0.003 | 8.1 ± 1.36 | 280 ± 5.76 | 6.5 ± 0.1 | ||
| 6 | 26.6 ± 1.24 | 0.125 ± 0.003 | 8.2 ± 1.48 | 280 ± 5.97 | 6.9 ± 0.3 | |
| 26.6 ± 1.18 | 0.124 ± 0.002 | 8.0 ± 1.29 | 280 ± 6.44 | 6.3 ± 0.3 | ||
| CNE | 0 | 27.8 ± 1.87 | 0.129 ± 0.001 | 8.4 ± 1.38 | 279 ± 7.89 | 7.2 ± 0.2 |
| 1 | 28.0 ± 1.65 | 0.129 ± 0.002 | 8.4 ± 1.13 | 280 ± 6.54 | 7.2 ± 0.1 | |
| 28.2 ± 1.38 | 0.129 ± 0.003 | 8.4 ± 1.24 | 279 ± 7.38 | 7.2 ± 0.2 | ||
| 3 | 28.3 ± 1.41 | 0.132 ± 0.004 | 8.6 ± 1.32 | 280 ± 5.76 | 7.0 ± 0.1 | |
| 28.5 ± 1.39 | 0.130 ± 0.003 | 8.7 ± 1.41 | 279 ± 6.38 | 6.7 ± 0.3 | ||
| 6 | 28.5 ± 1.33 | 0.131 ± 0.002 | 8.6 ± 1.42 | 281 ± 7.04 | 7.0 ± 0.2 | |
| 28.9 ± 1.54 | 0.130 ± 0.003 | 8.4 ± 1.29 | 280 ± 6.46 | 6.4 ± 0.2 | ||
| QC-NE | 0 | 25.9 ± 1.59 | 0.127 ± 0.003 | 8.9 ± 1.57 | 280 ± 9.36 | 7.0 ± 0.2 |
| 25.9 ± 1.59 | 1 | 25.9 ± 1.46 | 0.127 ± 0.002 | 8.9 ± 1.82 | 280 ± 5.89 | 7.2 ± 0.3 |
| 25.7 ± 1.37 | 0.128 ± 0.003 | 8.7 ± 1.46 | 280 ± 7.28 | 6.9 ± 0.2 | ||
| 3 | 26.3 ± 1.53 | 0.128 ± 0.004 | 8.7 ± 1.37 | 279 ± 8.74 | 7.2 ± 0.1 | |
| 25.1 ± 1.48 | 0.129 ± 0.001 | 8.4 ± 1.29 | 279 ± 7.46 | 6.6 ± 0.2 | ||
| 6 | 26.5 ± 1.52 | 0.128 ± 0.002 | 8.5 ± 1.64 | 279 ± 7.03 | 7.3 ± 0.2 | |
| 24.8 ± 1.27 | 0.130 ± 0.003 | 8.4 ± 1.72 | 278 ± 8.16 | 6.3 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.A.; Mittal, V.; Wahab, S.; Alsayari, A.; Bin Muhsinah, A.; Almaghaslah, D. Intravenous Nanocarrier for Improved Efficacy of Quercetin and Curcumin against Breast Cancer Cells: Development and Comparison of Single and Dual Drug–Loaded Formulations Using Hemolysis, Cytotoxicity and Cellular Uptake Studies. Membranes 2022, 12, 713. https://doi.org/10.3390/membranes12070713
Rahman MA, Mittal V, Wahab S, Alsayari A, Bin Muhsinah A, Almaghaslah D. Intravenous Nanocarrier for Improved Efficacy of Quercetin and Curcumin against Breast Cancer Cells: Development and Comparison of Single and Dual Drug–Loaded Formulations Using Hemolysis, Cytotoxicity and Cellular Uptake Studies. Membranes. 2022; 12(7):713. https://doi.org/10.3390/membranes12070713
Chicago/Turabian StyleRahman, Mohammad Akhlaquer, Vineet Mittal, Shadma Wahab, Abdulrhman Alsayari, Abdullatif Bin Muhsinah, and Dalia Almaghaslah. 2022. "Intravenous Nanocarrier for Improved Efficacy of Quercetin and Curcumin against Breast Cancer Cells: Development and Comparison of Single and Dual Drug–Loaded Formulations Using Hemolysis, Cytotoxicity and Cellular Uptake Studies" Membranes 12, no. 7: 713. https://doi.org/10.3390/membranes12070713
APA StyleRahman, M. A., Mittal, V., Wahab, S., Alsayari, A., Bin Muhsinah, A., & Almaghaslah, D. (2022). Intravenous Nanocarrier for Improved Efficacy of Quercetin and Curcumin against Breast Cancer Cells: Development and Comparison of Single and Dual Drug–Loaded Formulations Using Hemolysis, Cytotoxicity and Cellular Uptake Studies. Membranes, 12(7), 713. https://doi.org/10.3390/membranes12070713






