Amphiphilic Gold Nanoparticles: A Biomimetic Tool to Gain Mechanistic Insights into Peptide-Lipid Interactions
Abstract
1. Introduction
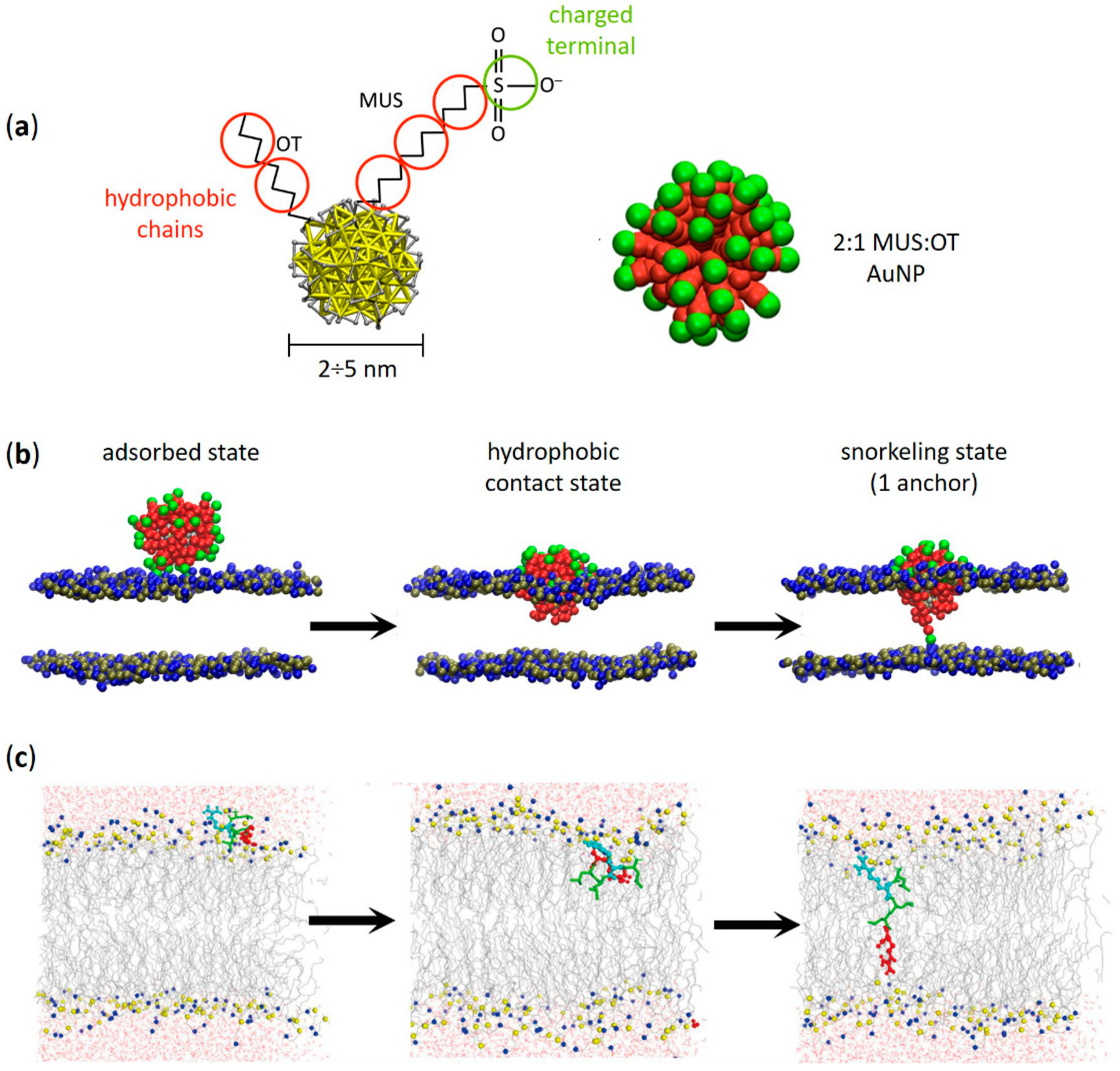
2. Amphiphilic Gold Nanoparticles and Amphiphilic Peptides: A Direct Route of Penetration into Lipid Membranes
3. Similarities in the Lipid Membrane Interactions Effects of Amphiphilic Gold Nanoparticles and Amphiphilic Peptides
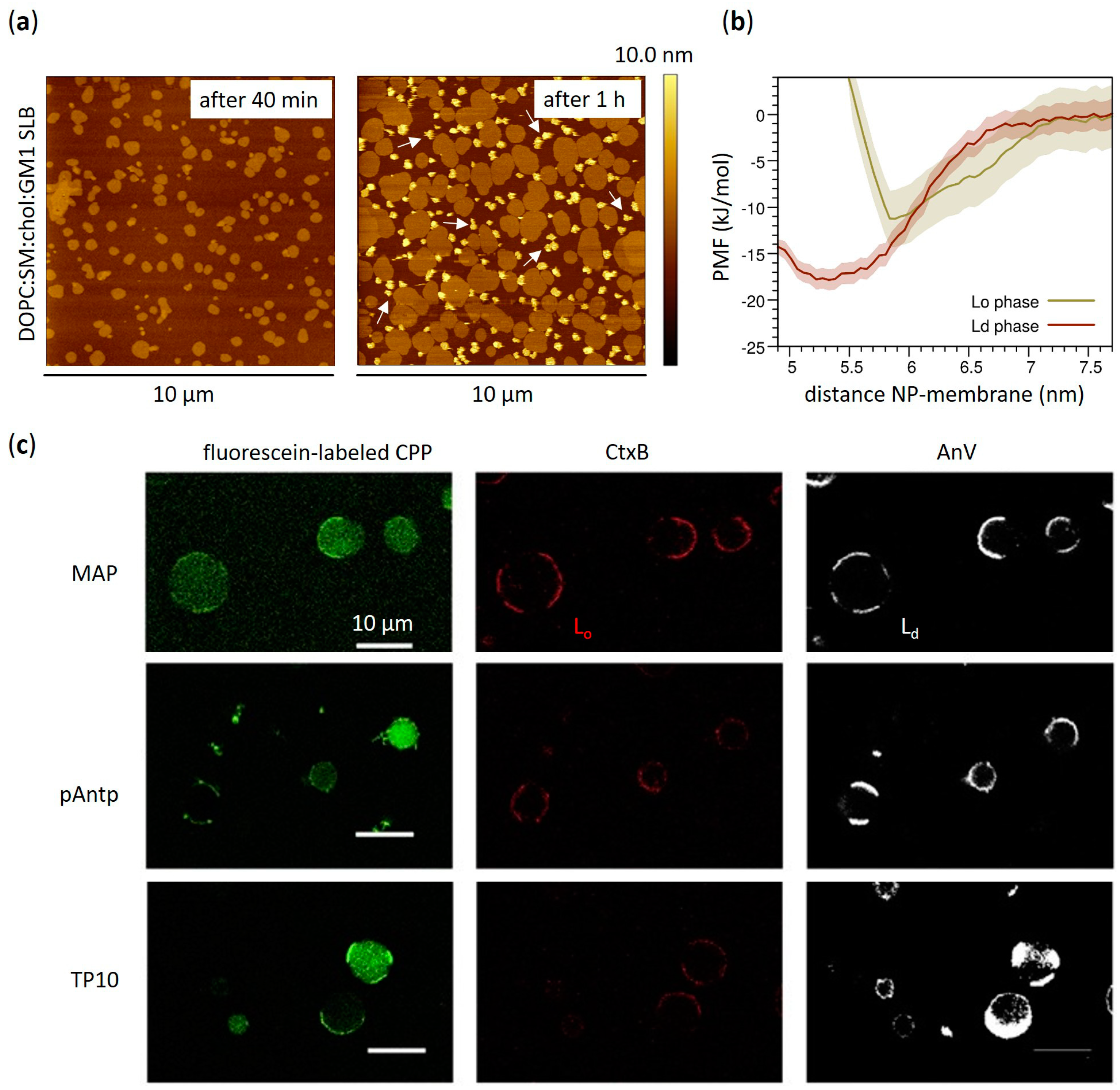
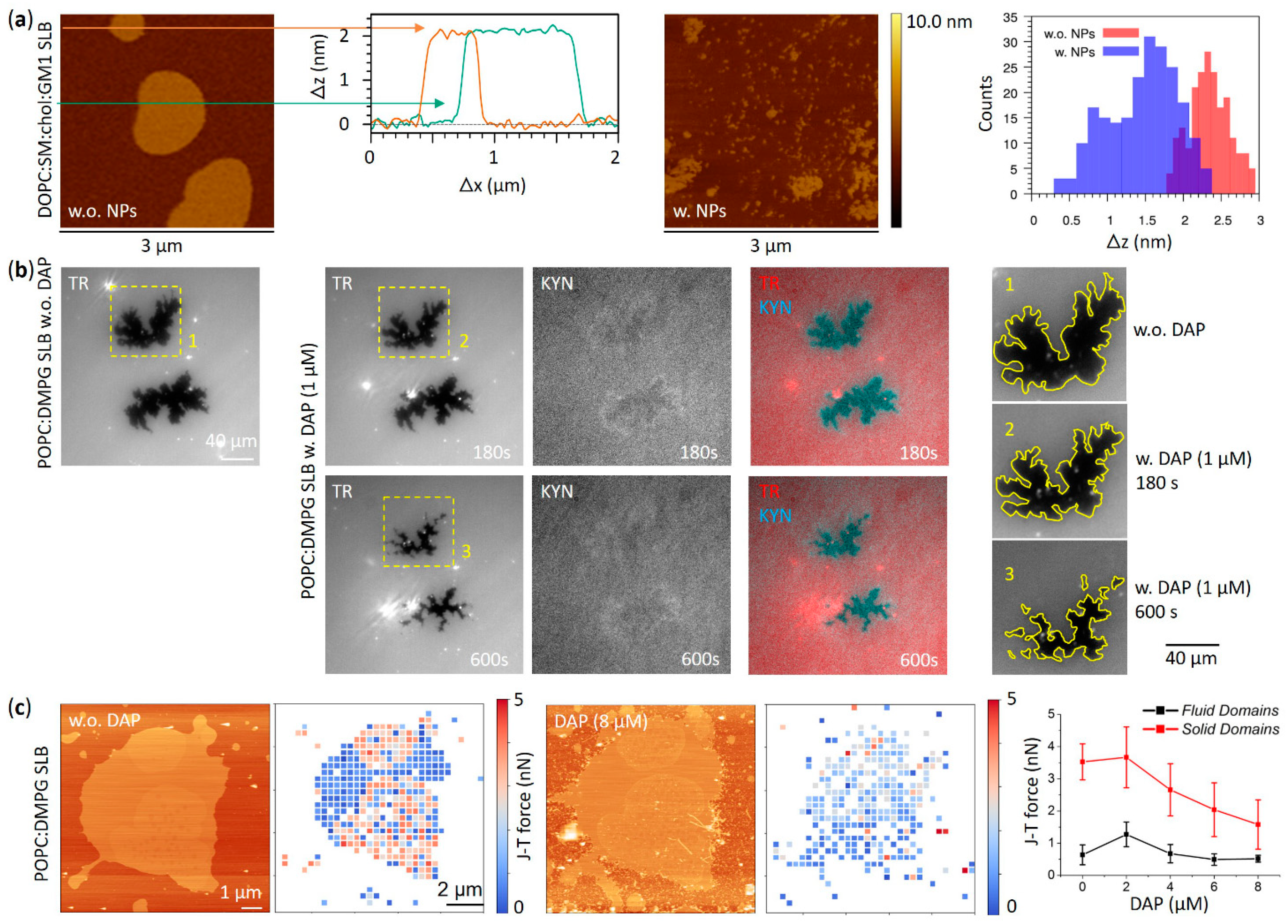
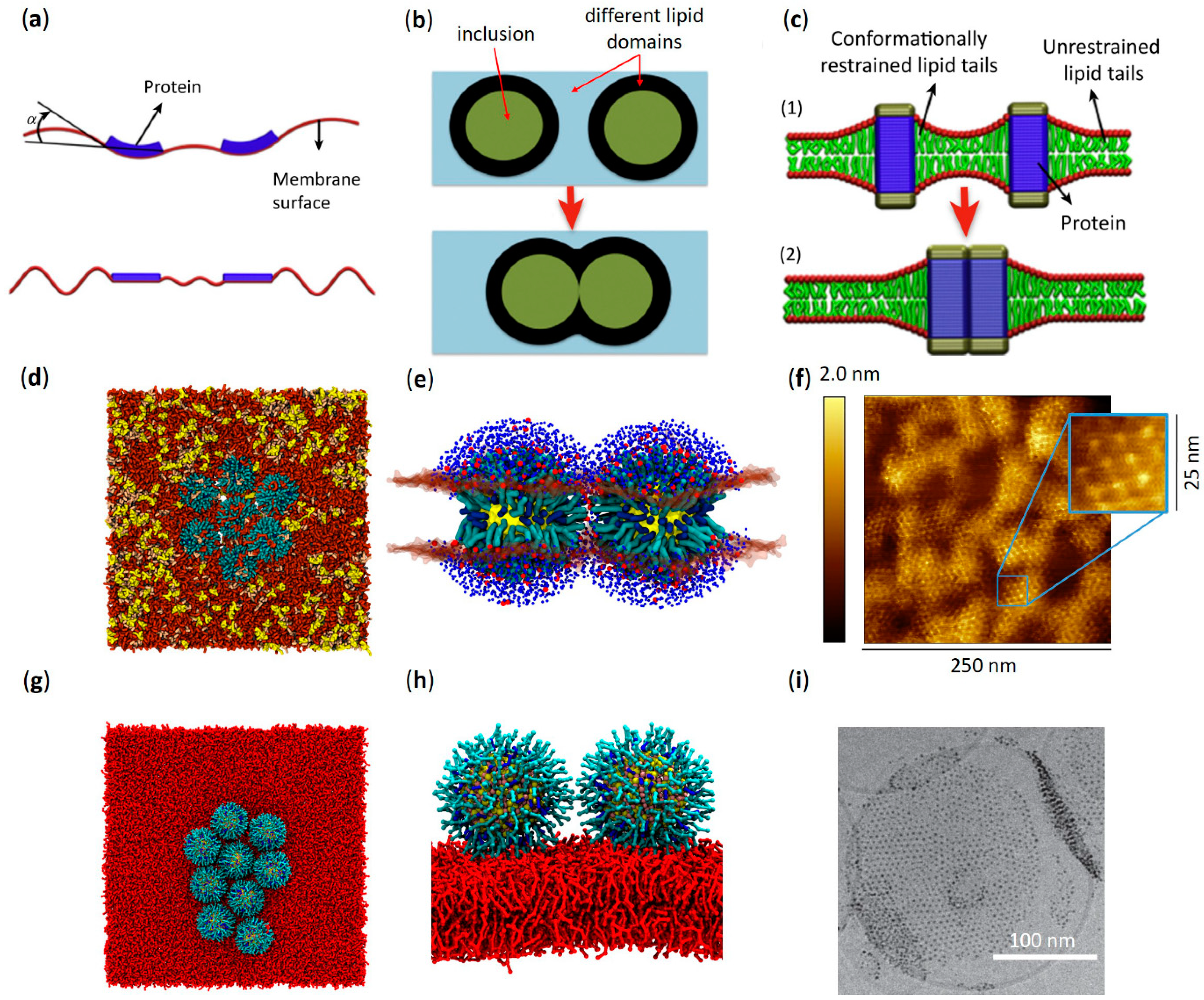
3.1. Effects on Phase Separation
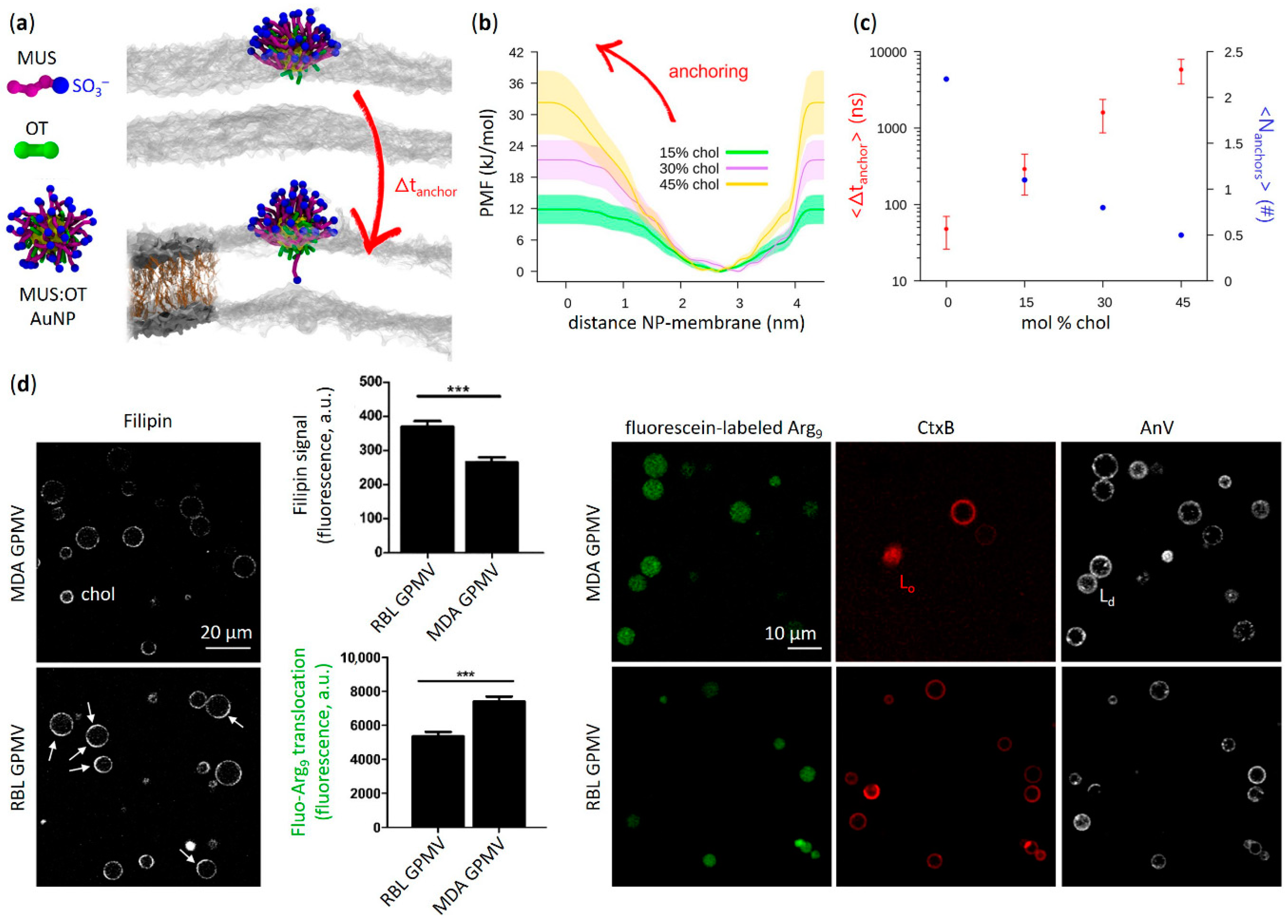
3.2. Effects of Cholesterol-Tuned Membrane Fluidity
3.3. Aggregation Effects on the Membrane Surface
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Apostolopoulos, V.; Bojarska, J.; Chai, T.-T.; Elnagdy, S.; Kaczmarek, K.; Matsoukas, J.; New, R.; Parang, K.; Lopez, O.P.; Parhiz, H.; et al. A Global Review on Short Peptides: Frontiers and Perspectives. Molecules 2021, 26, 430. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Auyeung, K. Identification of Functional Peptides from Natural and Synthetic Products on Their Anticancer Activities by Tumor Targeting. CMC 2014, 21, 2346–2356. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Wang, Y.-H.; Song, Z.; Hu, X.-Y.; Wei, J.-P.; Zhang, J.; Wang, H.-S. Recent Progress in Functional Peptides Designed for Tumor-Targeted Imaging and Therapy. J. Mater. Chem. C 2021, 9, 3749–3772. [Google Scholar] [CrossRef]
- Rong, L.; Qin, S.-Y.; Zhang, C.; Cheng, Y.-J.; Feng, J.; Wang, S.-B.; Zhang, X.-Z. Biomedical Applications of Functional Peptides in Nano-Systems. Mater. Today Chem. 2018, 9, 91–102. [Google Scholar] [CrossRef]
- Oyston, P.C.F.; Fox, M.A.; Richards, S.J.; Clark, G.C. Novel Peptide Therapeutics for Treatment of Infections. J. Med. Microbiol. 2009, 58, 977–987. [Google Scholar] [CrossRef]
- Hoffmann, A.R.; Guha, S.; Wu, E.; Ghimire, J.; Wang, Y.; He, J.; Garry, R.F.; Wimley, W.C. Broad-Spectrum Antiviral Entry Inhibition by Interfacially Active Peptides. J. Virol. 2020, 94, e01682-20. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.; Vázquez, A. Bioactive Peptides: A Review. Food Qual. Saf. 2017, 1, 29–46. [Google Scholar] [CrossRef]
- Macedo, M.W.F.S.; da Cunha, N.B.; Carneiro, J.A.; da Costa, R.A.; de Alencar, S.A.; Cardoso, M.H.; Franco, O.L.; Dias, S.C. Marine Organisms as a Rich Source of Biologically Active Peptides. Front. Mar. Sci. 2021, 8, 667764. [Google Scholar] [CrossRef]
- Kalafatovic, D.; Giralt, E. Cell-Penetrating Peptides: Design Strategies beyond Primary Structure and Amphipathicity. Molecules 2017, 22, 1929. [Google Scholar] [CrossRef]
- Luzio, J.P.; Pryor, P.R.; Bright, N.A. Lysosomes: Fusion and Function. Nat. Rev. Mol. Cell Biol. 2007, 8, 622–632. [Google Scholar] [CrossRef]
- LeCher, J.C.; Nowak, S.J.; McMurry, J.L. Breaking in and Busting out: Cell-Penetrating Peptides and the Endosomal Escape Problem. Biomol. Concepts 2017, 8, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Strandberg, E.; Killian, J.A. Snorkeling of Lysine Side Chains in Transmembrane Helices: How Easy Can It Get? FEBS Lett. 2003, 544, 69–73. [Google Scholar] [CrossRef]
- Schow, E.V.; Freites, J.A.; Myint, P.C.; Bernsel, A.; von Heijne, G.; White, S.H.; Tobias, D.J. Arginine in Membranes: The Connection Between Molecular Dynamics Simulations and Translocon-Mediated Insertion Experiments. J. Membr. Biol. 2011, 239, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Keskin, A.; Akdoğan, E.; Dunn, C.D. Evidence for Amino Acid Snorkeling from a High-Resolution, in Vivo Analysis of Fis1 Tail-Anchor Insertion at the Mitochondrial Outer Membrane. Genetics 2017, 205, 691–705. [Google Scholar] [CrossRef]
- Saha, S.; Ghosh, A.; Tiwari, N.; Kumar, A.; Kumar, A.; Goswami, C. Preferential Selection of Arginine at the Lipid-Water-Interface of TRPV1 during Vertebrate Evolution Correlates with Its Snorkeling Behaviour and Cholesterol Interaction. Sci. Rep. 2017, 7, 16808. [Google Scholar] [CrossRef]
- Jafari, M.; Mehrnejad, F.; Doustdar, F. Insight into the Interactions, Residue Snorkeling, and Membrane Disordering Potency of a Single Antimicrobial Peptide into Different Lipid Bilayers. PLoS ONE 2017, 12, e0187216. [Google Scholar] [CrossRef]
- Mishra, V.K.; Palgunachari, M.N.; Segrest, J.P.; Anantharamaiah, G.M. Interactions of Synthetic Peptide Analogs of the Class A Amphipathic Helix with Lipids. Evidence for the Snorkel Hypothesis. J. Biol Chem 1994, 269, 7185–7191. [Google Scholar] [CrossRef]
- Shinoda, W. Permeability across Lipid Membranes. Biochim. Et Biophys. Acta (BBA) Biomembr. 2016, 1858, 2254–2265. [Google Scholar] [CrossRef]
- Tejwani, R.W.; Davis, M.E.; Anderson, B.D.; Stouch, T.R. An Atomic and Molecular View of the Depth Dependence of the Free Energies of Solute Transfer from Water into Lipid Bilayers. Mol. Pharm. 2011, 8, 2204–2215. [Google Scholar] [CrossRef]
- Filipe, H.A.L.; Cardoso, R.M.S.; Loura, L.M.S.; Moreno, M.J. Interaction of Amphiphilic Molecules with Lipid Bilayers: Ki-netics of Insertion, Desorption and Translocation. In Membrane Organization and Dynamics; Chattopadhyay, A., Ed.; Springer Series in Biophysics; Springer International Publishing: Cham, Switzerland, 2017; Volume 20, pp. 49–89. ISBN 978-3-319-66600-6. [Google Scholar]
- Liu, X.; Testa, B.; Fahr, A. Lipophilicity and Its Relationship with Passive Drug Permeation. Pharm. Res. 2011, 28, 962–977. [Google Scholar] [CrossRef]
- Zhang, R.; Qin, X.; Kong, F.; Chen, P.; Pan, G. Improving Cellular Uptake of Therapeutic Entities through Interaction with Components of Cell Membrane. Drug Deliv. 2019, 26, 328–342. [Google Scholar] [CrossRef] [PubMed]
- Vorobyov, I.; Bekker, B.; Allen, T.W. Electrostatics of Deformable Lipid Membranes. Biophys. J. 2010, 98, 2904–2913. [Google Scholar] [CrossRef]
- MacCallum, J.L.; Tieleman, D.P. Hydrophobicity Scales: A Thermodynamic Looking Glass into Lipid–Protein Interactions. Trends Biochem. Sci. 2011, 36, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Rezai, T.; Bock, J.E.; Zhou, M.V.; Kalyanaraman, C.; Lokey, R.S.; Jacobson, M.P. Conformational Flexibility, Internal Hydrogen Bonding, and Passive Membrane Permeability: Successful in Silico Prediction of the Relative Permeabilities of Cyclic Peptides. J. Am. Chem. Soc. 2006, 128, 14073–14080. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, S.; Falanga, A.; Cantisani, M.; Vitiello, M.; Morelli, G.; Galdiero, M. Peptide-Lipid Interactions: Experiments and Applications. IJMS 2013, 14, 18758–18789. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, J.M. Peptide–Lipid Interactions: Insights and Perspectives. Org. Biomol. Chem. 2005, 3, 201–212. [Google Scholar] [CrossRef]
- Yang, Y.-S.S.; Atukorale, P.U.; Moynihan, K.D.; Bekdemir, A.; Rakhra, K.; Tang, L.; Stellacci, F.; Irvine, D.J. High-Throughput Quantitation of Inorganic Nanoparticle Biodistribution at the Single-Cell Level Using Mass Cytometry. Nat. Commun. 2017, 8, 14069. [Google Scholar] [CrossRef]
- Verma, A.; Stellacci, F. Effect of Surface Properties on Nanoparticle–Cell Interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef]
- Verma, A.; Uzun, O.; Hu, Y.; Hu, Y.; Han, H.-S.; Watson, N.; Chen, S.; Irvine, D.J.; Stellacci, F. Surface-Structure-Regulated Cell-Membrane Penetration by Monolayer-Protected Nanoparticles. Nat. Mater. 2008, 7, 588–595. [Google Scholar] [CrossRef]
- Jewell, C.M.; Jung, J.-M.; Atukorale, P.U.; Carney, R.P.; Stellacci, F.; Irvine, D.J. Oligonucleotide Delivery by Cell-Penetrating “Striped” Nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 12312–12315. [Google Scholar] [CrossRef]
- Carney, R.P.; Carney, T.M.; Mueller, M.; Stellacci, F. Dynamic Cellular Uptake of Mixed-Monolayer Protected Nanoparticles. Biointerphases 2012, 7, 17. [Google Scholar] [CrossRef]
- Leduc, C.; Jung, J.-M.; Carney, R.R.; Stellacci, F.; Lounis, B. Direct Investigation of Intracellular Presence of Gold Nanoparticles via Photothermal Heterodyne Imaging. ACS Nano 2011, 5, 2587–2592. [Google Scholar] [CrossRef] [PubMed]
- Atukorale, P.U.; Yang, Y.-S.; Bekdemir, A.; Carney, R.P.; Silva, P.J.; Watson, N.; Stellacci, F.; Irvine, D.J. Influence of the Glycocalyx and Plasma Membrane Composition on Amphiphilic Gold Nanoparticle Association with Erythrocytes. Nanoscale 2015, 7, 11420–11432. [Google Scholar] [CrossRef] [PubMed]
- Sabella, S.; Carney, R.P.; Brunetti, V.; Malvindi, M.A.; Al-Juffali, N.; Vecchio, G.; Janes, S.M.; Bakr, O.M.; Cingolani, R.; Stellacci, F.; et al. A General Mechanism for Intracellular Toxicity of Metal-Containing Nanoparticles. Nanoscale 2014, 6, 7052. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The Golden Age: Gold Nanoparticles for Biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef]
- Pengo, P.; Şologan, M.; Pasquato, L.; Guida, F.; Pacor, S.; Tossi, A.; Stellacci, F.; Marson, D.; Boccardo, S.; Pricl, S.; et al. Gold Nanoparticles with Patterned Surface Monolayers for Nanomedicine: Current Perspectives. Eur. Biophys. J. 2017, 46, 749–771. [Google Scholar] [CrossRef]
- Simonelli, F.; Bochicchio, D.; Ferrando, R.; Rossi, G. Monolayer-Protected Anionic Au Nanoparticles Walk into Lipid Membranes Step by Step. J. Phys. Chem. Lett. 2015, 6, 3175–3179. [Google Scholar] [CrossRef]
- Salassi, S.; Simonelli, F.; Bochicchio, D.; Ferrando, R.; Rossi, G. Au Nanoparticles in Lipid Bilayers: A Comparison between Atomistic and Coarse-Grained Models. J. Phys. Chem. C 2017, 121, 10927–10935. [Google Scholar] [CrossRef]
- Van Lehn, R.C.; Atukorale, P.U.; Carney, R.P.; Yang, Y.-S.; Stellacci, F.; Irvine, D.J.; Alexander-Katz, A. Effect of Particle Diameter and Surface Composition on the Spontaneous Fusion of Monolayer-Protected Gold Nanoparticles with Lipid Bilayers. Nano Lett. 2013, 13, 4060–4067. [Google Scholar] [CrossRef]
- Lane, J.M.D.; Grest, G.S. Spontaneous Asymmetry of Coated Spherical Nanoparticles in Solution and at Liquid-Vapor Interfaces. Phys. Rev. Lett. 2010, 104, 235501. [Google Scholar] [CrossRef]
- Henriques, S.T.; Melo, M.N.; Castanho, M.A.R.B. Cell-Penetrating Peptides and Antimicrobial Peptides: How Different Are They? Biochem. J. 2006, 399, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Van Lehn, R.C.; Ricci, M.; Silva, P.H.J.; Andreozzi, P.; Reguera, J.; Voïtchovsky, K.; Stellacci, F.; Alexander-Katz, A. Lipid Tail Protrusions Mediate the Insertion of Nanoparticles into Model Cell Membranes. Nat. Commun. 2014, 5, 4482. [Google Scholar] [CrossRef] [PubMed]
- Canepa, E.; Salassi, S.; de Marco, A.L.; Lambruschini, C.; Odino, D.; Bochicchio, D.; Canepa, F.; Canale, C.; Dante, S.; Brescia, R.; et al. Amphiphilic Gold Nanoparticles Perturb Phase Separation in Multidomain Lipid Membranes. Nanoscale 2020, 12, 19746–19759. [Google Scholar] [CrossRef] [PubMed]
- Canepa, E.; Bochicchio, D.; Gasbarri, M.; Odino, D.; Canale, C.; Ferrando, R.; Canepa, F.; Stellacci, F.; Rossi, G.; Dante, S.; et al. Cholesterol Hinders the Passive Uptake of Amphiphilic Nanoparticles into Fluid Lipid Membranes. J. Phys. Chem. Lett. 2021, 12, 8583–8590. [Google Scholar] [CrossRef] [PubMed]
- Canepa, E.; Salassi, S.; Simonelli, F.; Ferrando, R.; Rolandi, R.; Lambruschini, C.; Canepa, F.; Dante, S.; Relini, A.; Rossi, G. Non-Disruptive Uptake of Anionic and Cationic Gold Nanoparticles in Neutral Zwitterionic Membranes. Sci. Rep. 2021, 11, 1256. [Google Scholar] [CrossRef] [PubMed]
- Atukorale, P.U.; Guven, Z.P.; Bekdemir, A.; Carney, R.P.; Van Lehn, R.C.; Yun, D.S.; Jacob Silva, P.H.; Demurtas, D.; Yang, Y.-S.; Alexander-Katz, A.; et al. Structure–Property Relationships of Amphiphilic Nanoparticles That Penetrate or Fuse Lipid Membranes. Bioconjugate Chem. 2018, 29, 1131–1140. [Google Scholar] [CrossRef]
- Carney, R.P.; Astier, Y.; Carney, T.M.; Voïtchovsky, K.; Jacob Silva, P.H.; Stellacci, F. Electrical Method to Quantify Nanoparticle Interaction with Lipid Bilayers. ACS Nano 2013, 7, 932–942. [Google Scholar] [CrossRef]
- Yang, Y.-S.S.; Moynihan, K.D.; Bekdemir, A.; Dichwalkar, T.M.; Noh, M.M.; Watson, N.; Melo, M.; Ingram, J.; Suh, H.; Ploegh, H.; et al. Targeting Small Molecule Drugs to T Cells with Antibody-Directed Cell-Penetrating Gold Nanoparticles. Biomater. Sci. 2019, 7, 113–124. [Google Scholar] [CrossRef]
- Uzun, O.; Hu, Y.; Verma, A.; Chen, S.; Centrone, A.; Stellacci, F. Water-Soluble Amphiphilic Gold Nanoparticles with Structured Ligand Shells. Chem. Commun. 2008, 196–198. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, L.; Hu, G.; Bian, Y.; Li, H.; Wang, J.; Zhou, Y. Interplay of Hydrophobic and Hydrophilic Interactions in Sequence-Dependent Cell Penetration of Spontaneous Membrane-Translocating Peptides Revealed by Bias-Exchange Metadynamics Simulations. Biochim. Et Biophys. Acta (BBA) Biomembr. 2020, 1862, 183402. [Google Scholar] [CrossRef]
- Heikkilä, E.; Martinez-Seara, H.; Gurtovenko, A.A.; Vattulainen, I.; Akola, J. Atomistic Simulations of Anionic Au144(SR)60 Nanoparticles Interacting with Asymmetric Model Lipid Membranes. Biochim. Et Biophys. Acta (BBA) Biomembr. 2014, 1838, 2852–2860. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lehn, R.C.V.; Alexander-Katz, A. Pathway for Insertion of Amphiphilic Nanoparticles into Defect-Free Lipid Bilayers from Atomistic Molecular Dynamics Simulations. Soft Matter 2015, 11, 3165–3175. [Google Scholar] [CrossRef] [PubMed]
- Gkeka, P.; Sarkisov, L.; Angelikopoulos, P. Homogeneous Hydrophobic–Hydrophilic Surface Patterns Enhance Permeation of Nanoparticles through Lipid Membranes. J. Phys. Chem. Lett. 2013, 4, 1907–1912. [Google Scholar] [CrossRef] [PubMed]
- Lehn, R.C.V.; Alexander-Katz, A. Free Energy Change for Insertion of Charged, Monolayer-Protected Nanoparticles into Lipid Bilayers. Soft Matter 2013, 10, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Li, Z.; Gao, H. Surface-Structure-Regulated Penetration of Nanoparticles across a Cell Membrane. Nanoscale 2012, 4, 3768–3775. [Google Scholar] [CrossRef]
- Lehn, R.C.V.; Alexander-Katz, A. Penetration of Lipid Bilayers by Nanoparticles with Environmentally-Responsive Surfaces: Simulations and Theory. Soft Matter 2011, 7, 11392–11404. [Google Scholar] [CrossRef]
- Rossi, G.; Monticelli, L. Gold Nanoparticles in Model Biological Membranes: A Computational Perspective. Biochim. Et Biophys. Acta (BBA) Biomembr. 2016, 1858, 2380–2389. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, H.; Chen, Z.; Zheng, Y. Penetration of Lipid Membranes by Gold Nanoparticles: Insights into Cellular Uptake, Cytotoxicity, and Their Relationship. ACS Nano 2010, 4, 5421–5429. [Google Scholar] [CrossRef]
- Lin, J.; Alexander-Katz, A. Cell Membranes Open “Doors” for Cationic Nanoparticles/Biomolecules: Insights into Uptake Kinetics. ACS Nano 2013, 7, 10799–10808. [Google Scholar] [CrossRef]
- Heikkilä, E.; Martinez-Seara, H.; Gurtovenko, A.A.; Javanainen, M.; Häkkinen, H.; Vattulainen, I.; Akola, J. Cationic Au Nanoparticle Binding with Plasma Membrane-like Lipid Bilayers: Potential Mechanism for Spontaneous Permeation to Cells Revealed by Atomistic Simulations. J. Phys. Chem. C 2014, 118, 11131–11141. [Google Scholar] [CrossRef]
- Salassi, S.; Canepa, E.; Ferrando, R.; Rossi, G. Anionic Nanoparticle-Lipid Membrane Interactions: The Protonation of Anionic Ligands at the Membrane Surface Reduces Membrane Disruption. RSC Adv. 2019, 9, 13992–13997. [Google Scholar] [CrossRef] [PubMed]
- Reid, L.M.; Verma, C.S.; Essex, J.W. The Role of Molecular Simulations in Understanding the Mechanisms of Cell-Penetrating Peptides. Drug Discov. Today 2019, 24, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Fuselier, T.; Wimley, W.C. Spontaneous Membrane Translocating Peptides: The Role of Leucine-Arginine Consensus Motifs. Biophys. J. 2017, 113, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Zorko, M.; Langel, Ü. Cell-Penetrating Peptides: Mechanism and Kinetics of Cargo Delivery. Adv. Drug Deliv. Rev. 2005, 57, 529–545. [Google Scholar] [CrossRef]
- Dorairaj, S.; Allen, T.W. On the Thermodynamic Stability of a Charged Arginine Side Chain in a Transmembrane Helix. Proc. Natl. Acad. Sci. USA 2007, 104, 4943–4948. [Google Scholar] [CrossRef]
- Almeida, P.F.; Pokorny, A. Mechanisms of Antimicrobial, Cytolytic, and Cell-Penetrating Peptides: From Kinetics to Thermodynamics. Biochemistry 2009, 48, 8083–8093. [Google Scholar] [CrossRef]
- Hu, Y.; Patel, S. Thermodynamics of Cell-Penetrating HIV1 TAT Peptide Insertion into PC/PS/CHOL Model Bilayers through Transmembrane Pores: The Roles of Cholesterol and Anionic Lipids. Soft Matter 2016, 12, 6716–6727. [Google Scholar] [CrossRef]
- Herce, H.D.; Garcia, A.E. Molecular Dynamics Simulations Suggest a Mechanism for Translocation of the HIV-1 TAT Peptide across Lipid Membranes. Proc. Natl. Acad. Sci. USA 2007, 104, 20805–20810. [Google Scholar] [CrossRef]
- Cruz, J.; Mihailescu, M.; Wiedman, G.; Herman, K.; Searson, P.C.; Wimley, W.C.; Hristova, K. A Membrane-Translocating Peptide Penetrates into Bilayers without Significant Bilayer Perturbations. Biophys. J. 2013, 104, 2419–2428. [Google Scholar] [CrossRef]
- Macchi, S.; Signore, G.; Boccardi, C.; Di Rienzo, C.; Beltram, F.; Cardarelli, F. Spontaneous Membrane-Translocating Peptides: Influence of Peptide Self-Aggregation and Cargo Polarity. Sci. Rep. 2015, 5, 16914. [Google Scholar] [CrossRef]
- Hu, Y.; Patel, S. Structural and Thermodynamic Insight into Spontaneous Membrane-Translocating Peptides Across Model PC/PG Lipid Bilayers. J. Membr. Biol. 2015, 248, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Ulmschneider, J.P. Charged Antimicrobial Peptides Can Translocate across Membranes without Forming Channel-like Pores. Biophys. J. 2017, 113, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Melby, E.S.; Mensch, A.C.; Lohse, S.E.; Hu, D.; Orr, G.; Murphy, C.J.; Hamers, R.J.; Pedersen, J.A. Formation of Supported Lipid Bilayers Containing Phase-Segregated Domains and Their Interaction with Gold Nanoparticles. Environ. Sci. Nano 2016, 3, 45–55. [Google Scholar] [CrossRef]
- Sheikh, K.H.; Jarvis, S.P. Crystalline Hydration Structure at the Membrane–Fluid Interface of Model Lipid Rafts Indicates a Highly Reactive Boundary Region. J. Am. Chem. Soc. 2011, 133, 18296–18303. [Google Scholar] [CrossRef] [PubMed]
- Andersen, O.S.; Koeppe, R.E. Bilayer Thickness and Membrane Protein Function: An Energetic Perspective. Annu. Rev. Biophys. Biomol. Struct. 2007, 36, 107–130. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, R.M. Molecular Structure and Permeability at the Interface between Phase-Separated Membrane Domains. J. Phys. Chem. B 2018, 122, 6954–6965. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, S.A.; Böckmann, R.A. Coupling of Membrane Nanodomain Formation and Enhanced Electroporation near Phase Transition. Biophys. J. 2019, 116, 2131–2148. [Google Scholar] [CrossRef]
- Sheavly, J.K.; Pedersen, J.A.; Van Lehn, R.C. Curvature-Driven Adsorption of Cationic Nanoparticles to Phase Boundaries in Multicomponent Lipid Bilayers. Nanoscale 2019, 11, 2767–2778. [Google Scholar] [CrossRef]
- Balleza, D.; Mescola, A.; Marín–Medina, N.; Ragazzini, G.; Pieruccini, M.; Facci, P.; Alessandrini, A. Complex Phase Behavior of GUVs Containing Different Sphingomyelins. Biophys. J. 2019, 116, 503–517. [Google Scholar] [CrossRef]
- Säälik, P.; Niinep, A.; Pae, J.; Hansen, M.; Lubenets, D.; Langel, Ü.; Pooga, M. Penetration without Cells: Membrane Translocation of Cell-Penetrating Peptides in the Model Giant Plasma Membrane Vesicles. J. Control. Release 2011, 153, 117–125. [Google Scholar] [CrossRef]
- Trabulo, S.; Cardoso, A.L.; Mano, M.; De Lima, M.C.P. Cell-Penetrating Peptides—Mechanisms of Cellular Uptake and Generation of Delivery Systems. Pharmaceuticals 2010, 3, 961–993. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Deshayes, S.; Heitz, F.; Divita, G. Cell-Penetrating Peptides: From Molecular Mechanisms to Therapeutics. Biol. Cell 2008, 100, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Haney, E.F.; Vogel, H.J. The Expanding Scope of Antimicrobial Peptide Structures and Their Modes of Action. Trends Biotechnol. 2011, 29, 464–472. [Google Scholar] [CrossRef]
- Wimley, W.C. Describing the Mechanism of Antimicrobial Peptide Action with the Interfacial Activity Model. ACS Chem. Biol. 2010, 5, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Wimley, W.C.; Hristova, K. Antimicrobial Peptides: Successes, Challenges and Unanswered Questions. J. Membr. Biol. 2011, 239, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Bahar, A.; Ren, D. Antimicrobial Peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef] [PubMed]
- Marín-Medina, N.; Mescola, A.; Alessandrini, A. Effects of the Peptide Magainin H2 on Supported Lipid Bilayers Studied by Different Biophysical Techniques. Biochim. Et Biophys. Acta (BBA) Biomembr. 2018, 1860, 2635–2643. [Google Scholar] [CrossRef]
- Mescola, A.; Marín-Medina, N.; Ragazzini, G.; Accolla, M.; Alessandrini, A. Magainin-H2 Effects on the Permeabilization and Mechanical Properties of Giant Unilamellar Vesicles. J. Colloid Interface Sci. 2019, 553, 247–258. [Google Scholar] [CrossRef]
- Malanovic, N.; Lohner, K. Antimicrobial Peptides Targeting Gram-Positive Bacteria. Pharmaceuticals 2016, 9, 59. [Google Scholar] [CrossRef]
- Alves, I.D.; Bechara, C.; Walrant, A.; Zaltsman, Y.; Jiao, C.-Y.; Sagan, S. Relationships between Membrane Binding, Affinity and Cell Internalization Efficacy of a Cell-Penetrating Peptide: Penetratin as a Case Study. PLoS ONE 2011, 6, e24096. [Google Scholar] [CrossRef]
- Dupont, E.; Prochiantz, A.; Joliot, A. Penetratin Story: An Overview. In Cell-Penetrating Peptides; Langel, Ü., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 683, pp. 21–29. ISBN 978-1-60761-918-5. [Google Scholar]
- Lamazière, A.; Chassaing, G.; Trugnan, G.; Ayala-Sanmartin, J. Tubular Structures in Heterogeneous Membranes Induced by the Cell Penetrating Peptide Penetratin. Commun. Integr. Biol. 2009, 2, 223–224. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lamazière, A.; Wolf, C.; Lambert, O.; Chassaing, G.; Trugnan, G.; Ayala-Sanmartin, J. The Homeodomain Derived Peptide Penetratin Induces Curvature of Fluid Membrane Domains. PLoS ONE 2008, 3, e1938. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.; Lamazière, A.; Filleau, A.; Corvis, Y.; Espeau, P.; Ayala-Sanmartin, J. Membrane Re-Arrangements and Rippled Phase Stabilisation by the Cell Penetrating Peptide Penetratin. Biochim. Et Biophys. Acta (BBA) Biomembr. 2016, 1858, 2584–2591. [Google Scholar] [CrossRef] [PubMed]
- Allolio, C.; Magarkar, A.; Jurkiewicz, P.; Baxová, K.; Javanainen, M.; Mason, P.E.; Šachl, R.; Cebecauer, M.; Hof, M.; Horinek, D.; et al. Arginine-Rich Cell-Penetrating Peptides Induce Membrane Multilamellarity and Subsequently Enter via Formation of a Fusion Pore. Proc. Natl. Acad. Sci. USA 2018, 115, 11923–11928. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.M.S.; Trabulo, S.; Cardoso, A.L.; Lorents, A.; Morais, C.M.; Gomes, P.; Nunes, C.; Lúcio, M.; Reis, S.; Padari, K.; et al. S4(13)-PV Cell-Penetrating Peptide Induces Physical and Morphological Changes in Membrane-Mimetic Lipid Systems and Cell Membranes: Implications for Cell Internalization. Biochim. Et Biophys. Acta (BBA) Biomembr. 2012, 1818, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Hassan-Zadeh, E.; Hussain, F.; Huang, J. Gramicidin Peptides Alter Global Lipid Compositions and Bilayer Thicknesses of Coexisting Liquid-Ordered and Liquid-Disordered Membrane Domains. Langmuir 2017, 33, 3324–3332. [Google Scholar] [CrossRef] [PubMed]
- Mescola, A.; Ragazzini, G.; Alessandrini, A. Daptomycin Strongly Affects the Phase Behavior of Model Lipid Bilayers. J. Phys. Chem. B 2020, 124, 8562–8571. [Google Scholar] [CrossRef]
- Su, J.; Marrink, S.J.; Melo, M.N. Localization Preference of Antimicrobial Peptides on Liquid-Disordered Membrane Domains. Front. Cell Dev. Biol. 2020, 8, 350. [Google Scholar] [CrossRef]
- Lee, M.-T.; Hung, W.-C.; Hsieh, M.-H.; Chen, H.; Chang, Y.-Y.; Huang, H.W. Molecular State of the Membrane-Active Antibiotic Daptomycin. Biophys. J. 2017, 113, 82–90. [Google Scholar] [CrossRef]
- Balleza, D.; Mescola, A.; Alessandrini, A. Model Lipid Systems and Their Use to Evaluate the Phase State of Biomembranes, Their Mechanical Properties and the Effect of Non-Conventional Antibiotics: The Case of Daptomycin. Eur. Biophys. J. 2020, 49, 401–408. [Google Scholar] [CrossRef]
- Huang, H.W. DAPTOMYCIN, Its Membrane-Active Mechanism vs. That of Other Antimicrobial Peptides. Biochim. Et Biophys. Acta (BBA) Biomembr. 2020, 1862, 183395. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.A.; Millán, J. The Role of Lipid Rafts in Signalling and Membrane Trafficking in T Lymphocytes. J. Cell Sci. 2001, 114, 3957–3965. [Google Scholar] [CrossRef] [PubMed]
- Ikonen, E. Roles of Lipid Rafts in Membrane Transport. Curr. Opin. Cell Biol. 2001, 13, 470–477. [Google Scholar] [CrossRef]
- Krause, M.R.; Regen, S.L. The Structural Role of Cholesterol in Cell Membranes: From Condensed Bilayers to Lipid Rafts. Acc. Chem. Res. 2014, 47, 3512–3521. [Google Scholar] [CrossRef]
- Liscum, L.; Dahl, N. Intracellular Cholesterol Transport. J. Lipid Res. 1992, 33, 1239–1254. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Pasenkiewicz-Gierula, M.; Widomska, J.; Mainali, L.; Raguz, M. High Cholesterol/Low Cholesterol: Effects in Biological Membranes: A Review. Cell Biochem. Biophys. 2017, 75, 369–385. [Google Scholar] [CrossRef]
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane Lipids: Where They Are and How They Behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Eid, J.; Razmazma, H.; Jraij, A.; Ebrahimi, A.; Monticelli, L. On Calculating the Bending Modulus of Lipid Bilayer Membranes from Buckling Simulations. J. Phys. Chem. B 2020, 124, 6299–6311. [Google Scholar] [CrossRef]
- Lorents, A.; Säälik, P.; Langel, Ü.; Pooga, M. Arginine-Rich Cell-Penetrating Peptides Require Nucleolin and Cholesterol-Poor Subdomains for Translocation across Membranes. Bioconjugate Chem. 2018, 29, 1168–1177. [Google Scholar] [CrossRef]
- Brock, R. The Uptake of Arginine-Rich Cell-Penetrating Peptides: Putting the Puzzle Together. Bioconjugate Chem. 2014, 25, 863–868. [Google Scholar] [CrossRef]
- Crosio, M.A.; Via, M.A.; Cámara, C.I.; Mangiarotti, A.; Del Pópolo, M.G.; Wilke, N. Interaction of a Polyarginine Peptide with Membranes of Different Mechanical Properties. Biomolecules 2019, 9, 625. [Google Scholar] [CrossRef] [PubMed]
- Sharmin, S.; Islam, M.Z.; Karal, M.A.S.; Alam Shibly, S.U.; Dohra, H.; Yamazaki, M. Effects of Lipid Composition on the Entry of Cell-Penetrating Peptide Oligoarginine into Single Vesicles. Biochemistry 2016, 55, 4154–4165. [Google Scholar] [CrossRef] [PubMed]
- Pae, J.; Säälik, P.; Liivamägi, L.; Lubenets, D.; Arukuusk, P.; Langel, Ü.; Pooga, M. Translocation of Cell-Penetrating Peptides across the Plasma Membrane Is Controlled by Cholesterol and Microenvironment Created by Membranous Proteins. J. Control. Release 2014, 192, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Caesar, C.E.B.; Esbjörner, E.K.; Lincoln, P.; Nordén, B. Membrane Interactions of Cell-Penetrating Peptides Probed by Tryptophan Fluorescence and Dichroism Techniques: Correlations of Structure to Cellular Uptake. Biochemistry 2006, 45, 7682–7692. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.Z.; Sharmin, S.; Levadnyy, V.; Alam Shibly, S.U.; Yamazaki, M. Effects of Mechanical Properties of Lipid Bilayers on the Entry of Cell-Penetrating Peptides into Single Vesicles. Langmuir 2017, 33, 2433–2443. [Google Scholar] [CrossRef] [PubMed]
- Futaki, S.; Nakase, I. Cell-Surface Interactions on Arginine-Rich Cell-Penetrating Peptides Allow for Multiplex Modes of Internalization. Acc. Chem. Res. 2017, 50, 2449–2456. [Google Scholar] [CrossRef]
- Stanzl, E.G.; Trantow, B.M.; Vargas, J.R.; Wender, P.A. Fifteen Years of Cell-Penetrating, Guanidinium-Rich Molecular Transporters: Basic Science, Research Tools, and Clinical Applications. Acc. Chem. Res. 2013, 46, 2944–2954. [Google Scholar] [CrossRef]
- Liu, B.R.; Chiou, S.-H.; Huang, Y.-W.; Lee, H.-J. Bio-Membrane Internalization Mechanisms of Arginine-Rich Cell-Penetrating Peptides in Various Species. Membranes 2022, 12, 88. [Google Scholar] [CrossRef]
- Murayama, T.; Masuda, T.; Afonin, S.; Kawano, K.; Takatani-Nakase, T.; Ida, H.; Takahashi, Y.; Fukuma, T.; Ulrich, A.S.; Futaki, S. Loosening of Lipid Packing Promotes Oligoarginine Entry into Cells. Angew. Chem. Int. Ed. 2017, 56, 7644–7647. [Google Scholar] [CrossRef]
- Watkins, C.L.; Schmaljohann, D.; Futaki, S.; Jones, A.T. Low Concentration Thresholds of Plasma Membranes for Rapid Energy-Independent Translocation of a Cell-Penetrating Peptide. Biochem. J. 2009, 420, 179–191. [Google Scholar] [CrossRef]
- Neundorf, I. Antimicrobial and Cell-Penetrating Peptides: How to Understand Two Distinct Functions Despite Similar Physicochemical Properties. In Antimicrobial Peptides; Matsuzaki, K., Ed.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2019; Volume 1117, pp. 93–109. ISBN 9789811335877. [Google Scholar]
- MacCallum, J.L.; Bennett, W.F.D.; Tieleman, D.P. Distribution of Amino Acids in a Lipid Bilayer from Computer Simulations. Biophys. J. 2008, 94, 3393–3404. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Koh, J.-J.; Liu, S.; Lakshminarayanan, R.; Verma, C.S.; Beuerman, R.W. Membrane Active Antimicrobial Peptides: Translating Mechanistic Insights to Design. Front. Neurosci. 2017, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Edwards-Gayle, C.J.C.; Barrett, G.; Roy, S.; Castelletto, V.; Seitsonen, J.; Ruokolainen, J.; Hamley, I.W. Selective Antibacterial Activity and Lipid Membrane Interactions of Arginine-Rich Amphiphilic Peptides. ACS Appl. Bio Mater. 2020, 3, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.V.; de Oliveira, C.F.R.; dos Santos, E.L.; dos Santos, H.F.; Júnior, E.C.; Marchetto, R.; da Cruz, L.A.; Ferreira, A.M.T.; Gomes, V.M.; Taveira, G.B.; et al. Differential Interactions of the Antimicrobial Peptide, RQ18, with Phospholipids and Cholesterol Modulate Its Selectivity for Microorganism Membranes. Biochim. Et Biophys. Acta (BBA) Gen. Subj. 2021, 1865, 129937. [Google Scholar] [CrossRef]
- Li, J.; Lu, X.; Ma, W.; Chen, Z.; Sun, S.; Wang, Q.; Yuan, B.; Yang, K. Cholesterols Work as a Molecular Regulator of the Antimicrobial Peptide-Membrane Interactions. Front. Mol. Biosci. 2021, 8, 638988. [Google Scholar] [CrossRef]
- Sood, R.; Kinnunen, P.K.J. Cholesterol, Lanosterol, and Ergosterol Attenuate the Membrane Association of LL-37(W27F) and Temporin L. Biochim. Et Biophys. Acta (BBA) Biomembr. 2008, 1778, 1460–1466. [Google Scholar] [CrossRef]
- Henderson, J.M.; Iyengar, N.S.; Lam, K.L.H.; Maldonado, E.; Suwatthee, T.; Roy, I.; Waring, A.J.; Lee, K.Y.C. Beyond Electrostatics: Antimicrobial Peptide Selectivity and the Influence of Cholesterol-Mediated Fluidity and Lipid Chain Length on Protegrin-1 Activity. Biochim. Et Biophys. Acta (BBA) Biomembr. 2019, 1861, 182977. [Google Scholar] [CrossRef]
- Ishitsuka, Y.; Pham, D.S.; Waring, A.J.; Lehrer, R.I.; Lee, K.Y.C. Insertion Selectivity of Antimicrobial Peptide Protegrin-1 into Lipid Monolayers: Effect of Head Group Electrostatics and Tail Group Packing. Biochim. Et Biophys. Acta (BBA) Biomembr. 2006, 1758, 1450–1460. [Google Scholar] [CrossRef]
- Losada-Pérez, P.; Khorshid, M.; Hermans, C.; Robijns, T.; Peeters, M.; Jiménez-Monroy, K.L.; Truong, L.T.N.; Wagner, P. Melittin Disruption of Raft and Non-Raft-Forming Biomimetic Membranes: A Study by Quartz Crystal Microbalance with Dissipation Monitoring. Colloids Surf. B Biointerfaces 2014, 123, 938–944. [Google Scholar] [CrossRef]
- Prenner, E.J.; Lewis, R.N.A.H.; Jelokhani-Niaraki, M.; Hodges, R.S.; McElhaney, R.N. Cholesterol Attenuates the Interaction of the Antimicrobial Peptide Gramicidin S with Phospholipid Bilayer Membranes. Biochim. Et Biophys. Acta (BBA) Biomembr. 2001, 1510, 83–92. [Google Scholar] [CrossRef]
- Sani, M.-A.; Whitwell, T.C.; Separovic, F. Lipid Composition Regulates the Conformation and Insertion of the Antimicrobial Peptide Maculatin 1.1. Biochim. Et Biophys. Acta (BBA) Biomembr. 2012, 1818, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Mason, A.J.; Marquette, A.; Bechinger, B. Zwitterionic Phospholipids and Sterols Modulate Antimicrobial Peptide-Induced Membrane Destabilization. Biophys. J. 2007, 93, 4289–4299. [Google Scholar] [CrossRef] [PubMed]
- Yeow, E.K.L.; Clayton, A.H.A. Enumeration of Oligomerization States of Membrane Proteins in Living Cells by Homo-FRET Spectroscopy and Microscopy: Theory and Application. Biophys. J. 2007, 92, 3098–3104. [Google Scholar] [CrossRef]
- Gorbenko, G.; Trusova, V. Protein Aggregation in a Membrane Environment. In Advances in Protein Chemistry and Structural Biology; Donev, R., Ed.; Academic Press: Cambridge, MA, USA, 2011; Volume 84, pp. 113–142. [Google Scholar]
- Bouvier, M. Oligomerization of G-Protein-Coupled Transmitter Receptors. Nat. Rev. Neurosci. 2001, 2, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, A.; Schlessinger, J. Signal Transduction by Receptors with Tyrosine Kinase Activity. Cell 1990, 61, 203–212. [Google Scholar] [CrossRef]
- Parton, R.G.; McMahon, K.-A.; Wu, Y. Caveolae: Formation, Dynamics, and Function. Curr. Opin. Cell Biol. 2020, 65, 8–16. [Google Scholar] [CrossRef]
- Blood, P.D.; Voth, G.A. Direct Observation of Bin/Amphiphysin/Rvs (BAR) Domain-Induced Membrane Curvature by Means of Molecular Dynamics Simulations. Proc. Natl. Acad. Sci. USA 2006, 103, 15068–15072. [Google Scholar] [CrossRef]
- Kozlov, M.M.; Campelo, F.; Liska, N.; Chernomordik, L.V.; Marrink, S.J.; McMahon, H.T. Mechanisms Shaping Cell Membranes. Curr. Opin. Cell Biol. 2014, 29, 53–60. [Google Scholar] [CrossRef]
- Peter, B.J.; Kent, H.M.; Mills, I.G.; Vallis, Y.; Butler, P.J.G.; Evans, P.R.; McMahon, H.T. BAR Domains as Sensors of Membrane Curvature: The Amphiphysin BAR Structure. Science 2004, 303, 495–499. [Google Scholar] [CrossRef]
- Luckey, M. Membrane Structural Biology: With Biochemical and Biophysical Foundations; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef] [PubMed]
- Mani, R.; Cady, S.D.; Tang, M.; Waring, A.J.; Lehrer, R.I.; Hong, M. Membrane-Dependent Oligomeric Structure and Pore Formation of a β-Hairpin Antimicrobial Peptide in Lipid Bilayers from Solid-State NMR. Proc. Natl. Acad. Sci. USA 2006, 103, 16242–16247. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-H.; Wang, C.-F.; Chang, T.-W.; Wang, Y.-J.; Liao, Y.-D. Oligomerization and Insertion of Antimicrobial Peptide TP4 on Bacterial Membrane and Membrane-Mimicking Surfactant Sarkosyl. PLoS ONE 2019, 14, e0216946. [Google Scholar] [CrossRef]
- Chiti, F.; Dobson, C.M. Protein Misfolding, Amyloid Formation, and Human Disease: A Summary of Progress Over the Last Decade. Annu Rev. Biochem. 2017, 86, 27–68. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.; Chan, W.C.W. Effect of Gold Nanoparticle Aggregation on Cell Uptake and Toxicity. ACS Nano 2011, 5, 5478–5489. [Google Scholar] [CrossRef]
- Yang, Z.; Malinick, A.S.; Yang, T.; Cheng, W.; Cheng, Q. Gold Nanoparticle-Coupled Liposomes for Enhanced Plasmonic Biosensing. Sens. Actuators Rep. 2020, 2, 100023. [Google Scholar] [CrossRef]
- Johannes, L.; Pezeshkian, W.; Ipsen, J.H.; Shillcock, J.C. Clustering on Membranes: Fluctuations and More. Trends Cell Biol. 2018, 28, 405–415. [Google Scholar] [CrossRef]
- Goswami, D.; Gowrishankar, K.; Bilgrami, S.; Ghosh, S.; Raghupathy, R.; Chadda, R.; Vishwakarma, R.; Rao, M.; Mayor, S. Nanoclusters of GPI-Anchored Proteins Are Formed by Cortical Actin-Driven Activity. Cell 2008, 135, 1085–1097. [Google Scholar] [CrossRef]
- Jaqaman, K.; Kuwata, H.; Touret, N.; Collins, R.; Trimble, W.S.; Danuser, G.; Grinstein, S. Cytoskeletal Control of CD36 Diffusion Promotes Its Receptor and Signaling Function. Cell 2011, 146, 593–606. [Google Scholar] [CrossRef]
- Raghupathy, R.; Anilkumar, A.A.; Polley, A.; Singh, P.P.; Yadav, M.; Johnson, C.; Suryawanshi, S.; Saikam, V.; Sawant, S.D.; Panda, A.; et al. Transbilayer Lipid Interactions Mediate Nanoclustering of Lipid-Anchored Proteins. Cell 2015, 161, 581–594. [Google Scholar] [CrossRef]
- Bassereau, P.; Sens, P. Physics of Biological Membranes; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Helfrich, W. Elastic Properties of Lipid Bilayers: Theory and Possible Experiments. Z. Für Nat. C 1973, 28, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Neu, J.; Oster, G. Curvature-Mediated Interactions Between Membrane Proteins. Biophys. J. 1998, 75, 2274–2291. [Google Scholar] [CrossRef]
- Auth, T.; Gompper, G. Budding and Vesiculation Induced by Conical Membrane Inclusions. Phys. Rev. E 2009, 80, 031901. [Google Scholar] [CrossRef] [PubMed]
- Deserno, M.; Kremer, K.; Paulsen, H.; Peter, C.; Schmid, F. Computational Studies of Biomembrane Systems: Theoretical Considerations, Simulation Models, and Applications. In From Single Molecules to Nanoscopically Structured Materials; Basché, T., Müllen, K., Schmidt, M., Eds.; Advances in Polymer Science; Springer International Publishing: Cham, Switzerland, 2014; pp. 237–283. ISBN 978-3-319-05828-3. [Google Scholar]
- Goulian, M.; Bruinsma, R.; Pincus, P. Long-Range Forces in Heterogeneous Fluid Membranes. EPL 1993, 22, 145–150. [Google Scholar] [CrossRef]
- Dommersnes, P.G.; Fournier, J.B.; Galatola, P. Long-Range Elastic Forces between Membrane Inclusions in Spherical Vesicles. Europhys. Lett. 1998, 42, 233–238. [Google Scholar] [CrossRef][Green Version]
- Safouane, M.; Berland, L.; Callan-Jones, A.; Sorre, B.; Römer, W.; Johannes, L.; Toombes, G.E.S.; Bassereau, P. Lipid Cosorting Mediated by Shiga Toxin Induced Tubulation. Traffic 2010, 11, 1519–1529. [Google Scholar] [CrossRef]
- Schweitzer, Y.; Kozlov, M.M. Membrane-Mediated Interaction between Strongly Anisotropic Protein Scaffolds. PLoS Comput. Biol. 2015, 11, e1004054. [Google Scholar] [CrossRef]
- Simons, K.; Gerl, M.J. Revitalizing Membrane Rafts: New Tools and Insights. Nat. Rev. Mol. Cell Biol. 2010, 11, 688–699. [Google Scholar] [CrossRef]
- Rossy, J.; Ma, Y.; Gaus, K. The Organisation of the Cell Membrane: Do Proteins Rule Lipids? Curr. Opin. Chem. Biol. 2014, 20, 54–59. [Google Scholar] [CrossRef]
- Johannes, L.; Wunder, C.; Shafaq-Zadah, M. Glycolipids and Lectins in Endocytic Uptake Processes. J. Mol. Biol. 2016, 428, 4792–4818. [Google Scholar] [CrossRef]
- Anderson, R.G.W.; Jacobson, K. Cell Biology: A Role for Lipid Shells in Targeting Proteins to Caveolae, Rafts, and Other Lipid Domains. Science 2002, 296, 1821–1825. [Google Scholar] [CrossRef] [PubMed]
- Marčelja, S. Lipid-Mediated Protein Interaction in Membranes. Biochim. Et Biophys. Acta (BBA) Biomembr. 1976, 455, 1–7. [Google Scholar] [CrossRef]
- Sintes, T.; Baumgärtner, A. Protein Attraction in Membranes Induced by Lipid Fluctuations. Biophys. J. 1997, 73, 2251–2259. [Google Scholar] [CrossRef]
- Haselwandter, C.A.; Wingreen, N.S. The Role of Membrane-Mediated Interactions in the Assembly and Architecture of Chemoreceptor Lattices. PLoS Comput. Biol. 2014, 10, e1003932. [Google Scholar] [CrossRef]
- Morozova, D.; Guigas, G.; Weiss, M. Dynamic Structure Formation of Peripheral Membrane Proteins. PLoS Comput. Biol. 2011, 7, e1002067. [Google Scholar] [CrossRef]
- Lavagna, E.; Bochicchio, D.; Marco, A.L.D.; Güven, Z.P.; Stellacci, F.; Rossi, G. Ion-Bridges and Lipids Drive Aggregation of Same-Charge Nanoparticles on Lipid Membranes. Nanoscale 2022, 14, 6912–6921. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.; Král, P. Nanoparticles Self-Assembly within Lipid Bilayers. ACS Omega 2018, 3, 10631–10637. [Google Scholar] [CrossRef]
- Rasch, M.R.; Rossinyol, E.; Hueso, J.L.; Goodfellow, B.W.; Arbiol, J.; Korgel, B.A. Hydrophobic Gold Nanoparticle Self-Assembly with Phosphatidylcholine Lipid: Membrane-Loaded and Janus Vesicles. Nano Lett. 2010, 10, 3733–3739. [Google Scholar] [CrossRef]
- Angelikopoulos, P.; Sarkisov, L.; Cournia, Z.; Gkeka, P. Self-Assembly of Anionic, Ligand-Coated Nanoparticles in Lipid Membranes. Nanoscale 2017, 9, 1040–1048. [Google Scholar] [CrossRef]
- Lavagna, E.; Barnoud, J.; Rossi, G.; Monticelli, L. Size-Dependent Aggregation of Hydrophobic Nanoparticles in Lipid Membranes. Nanoscale 2020, 12, 9452–9461. [Google Scholar] [CrossRef]
- Petretto, E.; Ong, Q.K.; Olgiati, F.; Ting, M.; Campomanes, P.; Stellacci, F.; Vanni, S. Ion-Mediated Charge-Charge Interactions Drive Aggregation of Surface-Functionalized Gold Nanoparticles. 2021. Available online: https://doi.org/10.26434/chemrxiv-2021-kq8f0 (accessed on 22 November 2021).
- Lavagna, E.; Güven, Z.P.; Bochicchio, D.; Olgiati, F.; Stellacci, F.; Rossi, G. Amphiphilic Nanoparticles Generate Curvature in Lipid Membranes and Shape Liposome–Liposome Interfaces. Nanoscale 2021, 13, 16879–16884. [Google Scholar] [CrossRef] [PubMed]
- Gessner, I.; Neundorf, I. Nanoparticles Modified with Cell-Penetrating Peptides: Conjugation Mechanisms, Physicochemical Properties, and Application in Cancer Diagnosis and Therapy. IJMS 2020, 21, 2536. [Google Scholar] [CrossRef] [PubMed]
- Chiarpotti, M.V.; Longo, G.S.; Del Pópolo, M.G. Nanoparticles Modified with Cell Penetrating Peptides: Assessing Adsorption on Membranes Containing Acidic Lipids. Colloids Surf. B Biointerfaces 2021, 197, 111373. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Kumar, D.; Khang, G.; Lim, D.-K. Recent Advances in Gold Nanoparticle-Based Bioengineering Applications. J. Mater. Chem. B 2015, 3, 8433–8444. [Google Scholar] [CrossRef]
- Li, D.; He, Q.; Li, J. Smart Core/Shell Nanocomposites: Intelligent Polymers Modified Gold Nanoparticles. Adv. Colloid Interface Sci. 2009, 149, 28–38. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, X. Recent Advances in Amphiphilic Polymers as the Stabilizers of Colloidal Gold Nanoparticles. Macromol. Mater. Eng. 2018, 303, 1800105. [Google Scholar] [CrossRef]
- Tahir, M.A.; Guven, Z.P.; Arriaga, L.R.; Tinao, B.; Yang, Y.-S.S.; Bekdemir, A.; Martin, J.T.; Bhanji, A.N.; Irvine, D.; Stellacci, F.; et al. Calcium-Triggered Fusion of Lipid Membranes Is Enabled by Amphiphilic Nanoparticles. Proc. Natl. Acad. Sci. USA 2020, 117, 18470–18476. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canepa, E.; Relini, A.; Bochicchio, D.; Lavagna, E.; Mescola, A. Amphiphilic Gold Nanoparticles: A Biomimetic Tool to Gain Mechanistic Insights into Peptide-Lipid Interactions. Membranes 2022, 12, 673. https://doi.org/10.3390/membranes12070673
Canepa E, Relini A, Bochicchio D, Lavagna E, Mescola A. Amphiphilic Gold Nanoparticles: A Biomimetic Tool to Gain Mechanistic Insights into Peptide-Lipid Interactions. Membranes. 2022; 12(7):673. https://doi.org/10.3390/membranes12070673
Chicago/Turabian StyleCanepa, Ester, Annalisa Relini, Davide Bochicchio, Enrico Lavagna, and Andrea Mescola. 2022. "Amphiphilic Gold Nanoparticles: A Biomimetic Tool to Gain Mechanistic Insights into Peptide-Lipid Interactions" Membranes 12, no. 7: 673. https://doi.org/10.3390/membranes12070673
APA StyleCanepa, E., Relini, A., Bochicchio, D., Lavagna, E., & Mescola, A. (2022). Amphiphilic Gold Nanoparticles: A Biomimetic Tool to Gain Mechanistic Insights into Peptide-Lipid Interactions. Membranes, 12(7), 673. https://doi.org/10.3390/membranes12070673







