Evaluation of the Growth-Inhibitory Spectrum of Three Types of Cyanoacrylate Nanoparticles on Gram-Positive and Gram-Negative Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bacterial Strains and Culture Conditions
2.3. Synthesis of PACA Nanoparticles
2.4. Growth Inhibition Zone Assay
2.5. Analysis Cell Growth Inhibition in Liquid Medium by Measuring Turbidity
2.6. Effect of N-acetyl-L-Cysteine on Cell Growth Inhibition by ECA-NPs Exposure
2.7. Fluorescence Microscopic Observation of Membrane Damage
3. Results
3.1. Characteristics of the Synthesized PACA-MPs
3.2. Cell Growth Inhibition Activity of PACA-NPs
3.2.1. Growth Inhibition Zone Observation
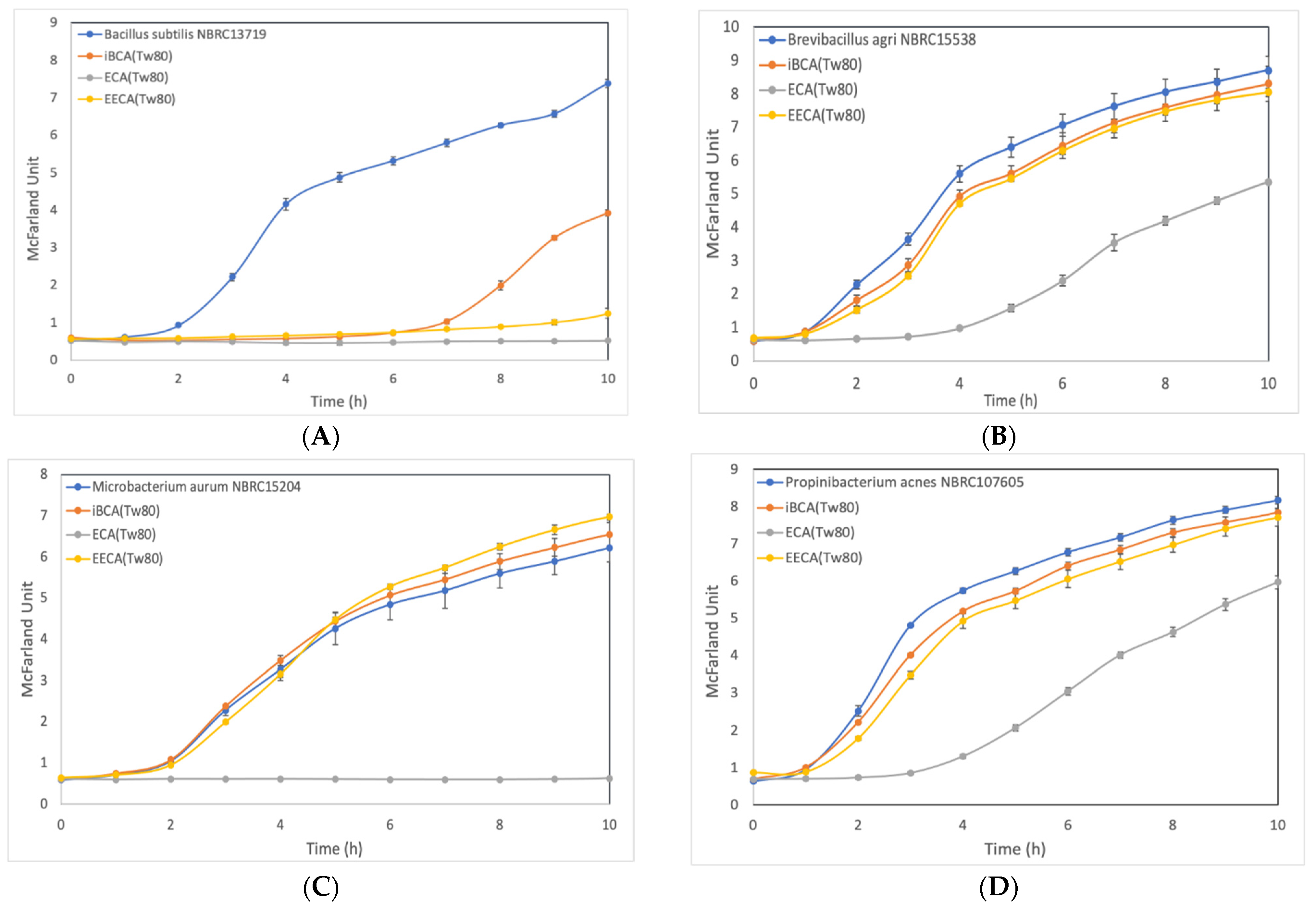
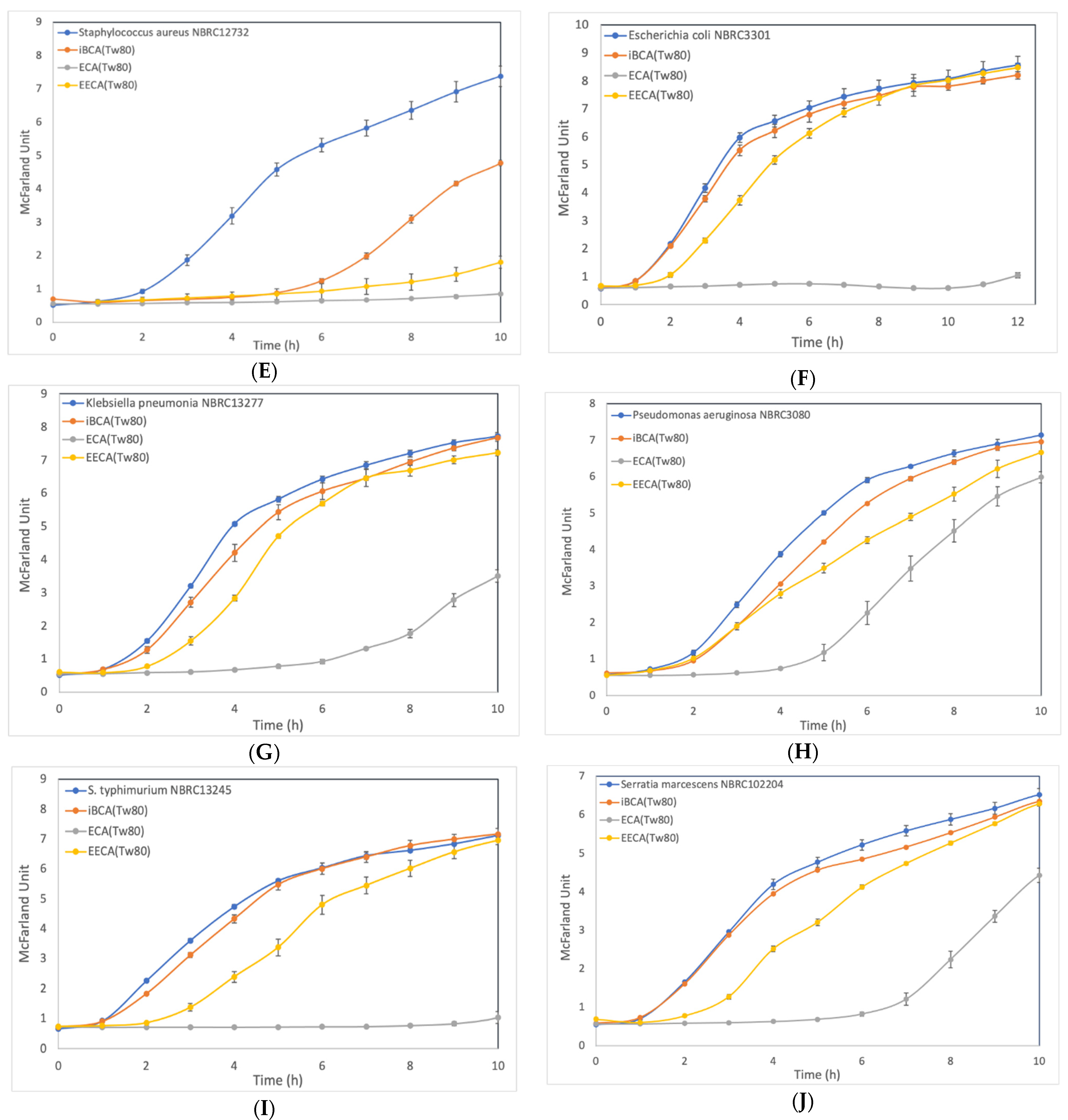
3.2.2. Effect of ECA-NPs on the Bacterial Growth Dynamics
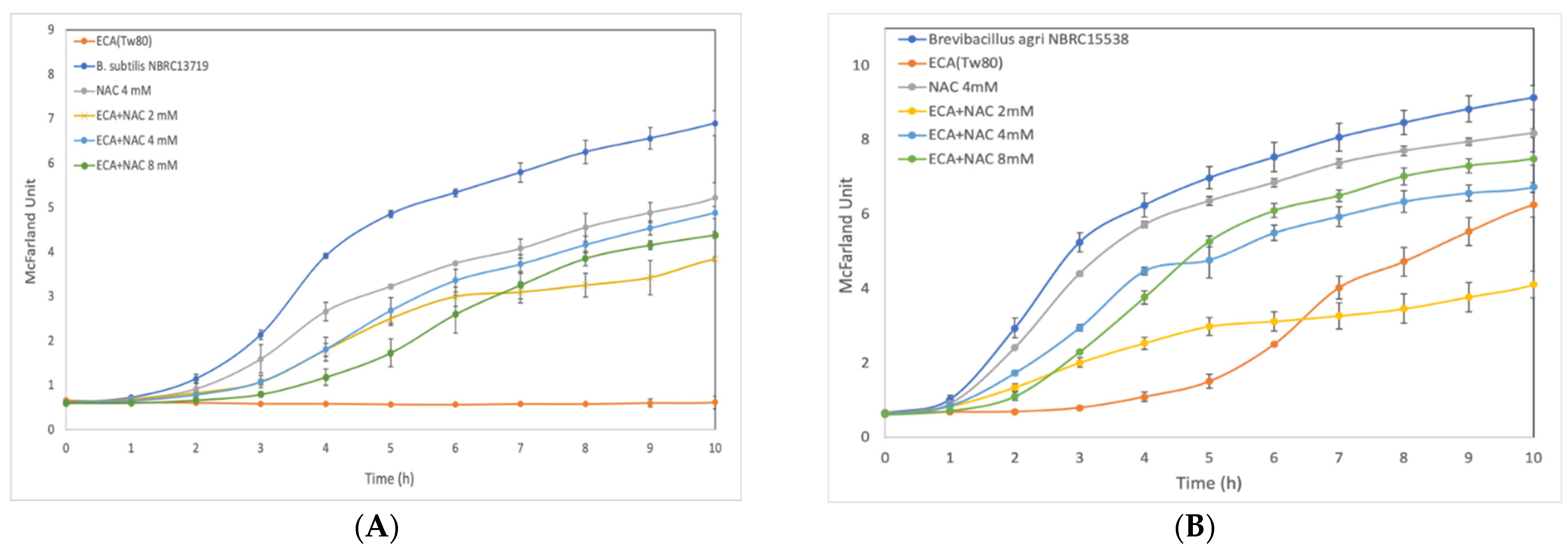
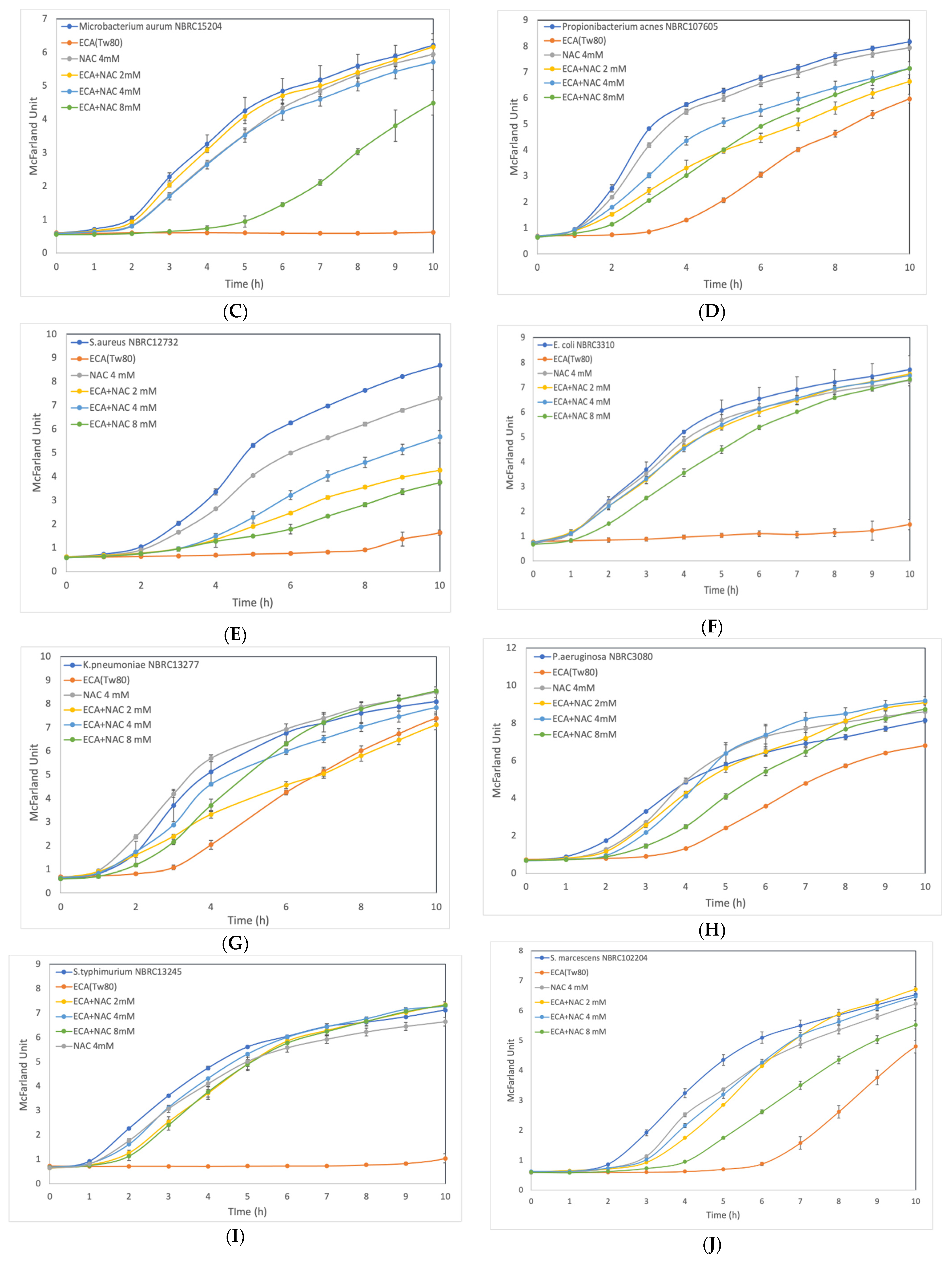
3.3. Effect of ROS Scavenger NAC on Cells after Preliminary Treatment
3.4. Fluorescent Microscopy Examination of Cytoplasmic Membranes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meredith, H.R.; Srimani, J.K.; Lee, A.J.; Lopatkin, A.J.; You, L. Collective antibiotic tolerance: Mechanisms, dynamics and intervention. Nat. Chem. Biol. 2015, 11, 182–188. [Google Scholar] [CrossRef]
- Khan, A.A.; Manzoor, K.N.; Sultan, A.; Saeed, M.; Rafique, M.; Noushad, S.; Talib, A.; Rentschler, S.; Deigner, H.P. Pulling the brakes on fast and furious multiple drug-resistant (MDR) bacteria. Int. J. Mol. Sci. 2021, 22, 859. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. In Virulence Mechanisms of Bacterial Pathogens; John Wiley and Sons: Hoboken, NJ, USA, 2016; pp. 481–511. [Google Scholar]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef] [PubMed]
- Salata, O.V. Applications of nanoparticles in biology and medicine. J. Nanobiotechnol. 2004, 2, 3. [Google Scholar] [CrossRef]
- Varier, K.M.; Gudeppu, M.; Chinnasamy, A.; Thangarajan, S.; Balasubramanian, J.; Li, Y.; Gajendran, B. Nanoparticles: Antimicrobial Applications and Its Prospects. Adv. Nanostruct. Mater. Environ. Remediat. 2019, 25, 321–355. [Google Scholar] [CrossRef]
- Sharmin, S.; Rahaman, M.M.; Sarkar, C.; Atolani, O.; Islam, M.T.; Adeyemi, O.S. Nanoparticles as antimicrobial and antiviral agents: A literature-based perspective study. Heliyon 2021, 7, e06456. [Google Scholar] [CrossRef]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Al-Sharqi, A.; Apun, K.; Vincent, M.; Kanakaraju, D.; Bilung, L.M. Enhancement of the antibacterial efficiency of silver nanoparticles against gram-positive and gram-negative bacteria using blue laser light. Int. J. Photoenergy 2019, 2019, 2528490. [Google Scholar] [CrossRef]
- Jaworski, S.; Wierzbicki, M.; Sawosz, E.; Jung, A.; Gielerak, G.; Biernat, J.; Jaremek, H.; Łojkowski, W.; Woźniak, B.; Wojnarowicz, J.; et al. Graphene oxide-based nanocomposites decorated with silver nanoparticles as an antibacterial agent. Nanoscale Res. Lett. 2018, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Perveen, S.; Ali, M.; Shah, M.R.; Khan, E.; Sharma, A.S.; Li, H.; Chen, Q. Nano-conjugates of Cefadroxil as Efficient Antibacterial Agent against Staphylococcus aureus ATCC 11632. J. Clust. Sci. 2020, 31, 811–821. [Google Scholar] [CrossRef]
- Ali, S.; Perveen, S.; Shah, M.R.; Zareef, M.; Arslan, M.; Basheer, S.; Ullah, S.; Ali, M. Bactericidal potentials of silver and gold nanoparticles stabilized with cefixime: A strategy against antibiotic-resistant bacteria. J. Nanopart. Res. 2020, 22, 201. [Google Scholar] [CrossRef]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Muñoz, R.; Meza-Villezcas, A.; Fournier, P.G.J.; Soria-Castro, E.; Juarez-Moreno, K.; Gallego-Hernández, A.L.; Bogdanchikova, N.; Vazquez-Duhalt, R.; Huerta-Saquero, A. Enhancement of antibiotics antimicrobial activity due to the silver nanoparticles impact on the cell membrane. PLoS ONE 2019, 14, e0224904. [Google Scholar] [CrossRef] [PubMed]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver nanoparticles and their antibacterial applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; AlSalhi, M.S.; Siddiqui, M.K.J. Silver nanoparticle applications and human health. Clin. Chim. Acta 2010, 411, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32–41. [Google Scholar] [CrossRef]
- Vauthier, C.; Dubernet, C.; Fattal, E.; Pinto-Alphandary, H.; Couvreur, P. Poly(alkylcyanoacrylates) as biodegradable materials for biomedical applications. Adv. Drug Deliv. Rev. 2003, 55, 519–548. [Google Scholar] [CrossRef]
- Sulheim, E.; Baghirov, H.; Haartman, E.; Bøe, A.; Åslund, A.K.O.; Mørch, Y.; de Lange Davies, C. Cellular uptake and intracellular degradation of poly(alkyl cyanoacrylate) nanoparticles. J. Nanobiotechnol. 2016, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Korde, J.M.; Kandasubramanian, B. Biocompatible alkyl cyanoacrylates and their derivatives as bio-adhesives. Biomater. Sci. 2018, 6, 1691–1711. [Google Scholar] [CrossRef] [PubMed]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Widyaningrum, D.; Iida, D.; Tanabe, Y.; Hayashi, Y.; Kurniasih, S.D.; Ohama, T. Acutely induced cell mortality in the unicellular green alga Chlamydomonas reinhardtii (Chlorophyceae) following exposure to acrylic resin nanoparticles. J. Phycol. 2019, 55, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Al-Azab, A.J.S.; Widyaningrum, D.; Hirakawa, H.; Hayashi, Y.; Tanaka, S.; Ohama, T. A resin cyanoacrylate nanoparticle as an acute cell death inducer to broad spectrum of microalgae. Algal Res. 2021, 54, 102191. [Google Scholar] [CrossRef]
- Demchick, P.; Koch, A.L. The permeability of the wall fabric of Escherichia coli and Bacillus subtilis. J. Bacteriol. 1996, 178, 768–773. [Google Scholar] [CrossRef]
- Shirotake, S.; Tomita, Y.; Yoshizaki, S.; Okuda, K. Preparation of cyanoacrylate nanoparticles using monosaccharides or disaccharides. Chem. Pharm. Bull. 2008, 56, 137–138. [Google Scholar] [CrossRef] [PubMed]
- Biemer, J.J. Antimicrobial susceptibility testing by the Kirby-Bauer disc diffusion method. Ann. Clin. Lab. Sci. 1973, 3, 135–140. [Google Scholar] [PubMed]
- Kourmouli, A.; Valenti, M.; van Rijn, E.; Beaumont, H.J.E.; Kalantzi, O.I.; Schmidt-Ott, A.; Biskos, G. Can disc diffusion susceptibility tests assess the antimicrobial activity of engineered nanoparticles? J. Nanopart. Res. 2018, 20, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Romero, I.L.; Malta, J.B.N.S.; Silva, C.B.; Mimica, L.M.J.; Soong, K.H.; Hida, R.Y. Antibacterial properties of cyanoacrylate tissue adhesive: Does the polymerization reaction play a role? Indian J. Ophthalmol. 2009, 57, 341–344. [Google Scholar] [CrossRef]
- Shirotake, S. A new cyanoacrylate colloidal polymer with novel antibacterial mechanism and Its application to infection control. J. Nanomed. Biother. Discov. 2014, 4, 1000122. [Google Scholar] [CrossRef]
- De Almeida Manzano, R.P.; Cayres Naufal, S.; Yudi Hida, R.; Belluzzo Guarnieri, L.O.; Nishiwaki-Dantas, M.C. Antibacterial analysis in vitro of ethyl-cyanoacrylate against ocular pathogens. Cornea 2006, 25, 350–351. [Google Scholar] [CrossRef]
- Rushbrook, J.L.; White, G.; Kidger, L.; Marsh, P.; Taggart, T.F.O. The antibacterial effect of 2-octyl cyanoacrylate (Dermabond®) skin adhesive. J. Infect. Prev. 2014, 15, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Lehman, R.A.W.; West, R.L.E.E.; Leonard, F. Toxicity of Alkyl 2-Cyanoacrylates: II. Bacterial Growth. Arch. Surg. 1966, 93, 447–450. [Google Scholar] [CrossRef]
- Park, D.H.; Kim, S.B.; Ahn, K.D.; Kim, E.Y.; Kim, Y.J.; Han, D.K. In vitro degradation and cytotoxicity of alkyl 2-cyanoacrylate polymers for application to tissue adhesives. J. Appl. Polym. Sci. 2003, 89, 3272–3278. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Peeters, S.H.; de Jonge, M.I. For the greater good: Programmed cell death in bacterial communities. Microbiol. Res. 2018, 207, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Wu, Y.; Wang, H.; Han, H. Synergistic antibacterial effects of curcumin modified silver nanoparticles through ROS-mediated pathways. Mater. Sci. Eng. C 2019, 99, 255–263. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef]
- Jiang, W.; Kim, B.Y.S.; Rutka, J.T.; Chan, W.C.W. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 2008, 3, 145–150. [Google Scholar] [CrossRef]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef]
- Santos, R.S.; Figueiredo, C.; Azevedo, N.F.; Braeckmans, K.; De Smedt, S.C. Nanomaterials and molecular transporters to overcome the bacterial envelope barrier: Towards advanced delivery of antibiotics. Adv. Drug Deliv. Rev. 2018, 136–137, 28–48. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Rutherford, S.T.; Silhavy, T.J.; Huang, K.C. Physical properties of the bacterial outer membrane. Nat. Rev. Microbiol. 2021, 20, 236–248. [Google Scholar] [CrossRef]
- Beveridge, T.J. Structures of gram-negative cell walls and their derived membrane vesicles. J. Bacteriol. 1999, 181, 4725–4733. [Google Scholar] [CrossRef] [PubMed]
- Schäffer, C.; Messner, P. The structure of secondary cell wall polymers: How Gram-positive bacteria stick their cell walls together. Microbiology 2005, 151, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Reid, A.N.; Szymanski, C.M. Chapter 20—Biosynthesis and assembly of capsular polysaccharides. In Microbial Glycobiology; Holst, O., Brennan, P.J., von Itzstein, M., Moran, A.P.B.T.-M.G., Eds.; Academic Press: San Diego, CA, USA, 2010; pp. 351–373. ISBN 978-0-12-374546-0. [Google Scholar]
- Baez, A.; Shiloach, J. Effect of elevated oxygen concentration on bacteria, yeasts, and cells propagated for production of biological compounds. Microb. Cell Fact. 2014, 13, 181. [Google Scholar] [CrossRef]
- Dayem, A.A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.M.; Choi, H.Y.; Cho, S.G. The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef]
| Size (nm) | Zeta Potential (mV) | |
|---|---|---|
| EECA-NPs | 248 ± 2 | −65.9 ± 3.53 |
| ECA-NPs | 82.5 ± 0.42 | −6.72 ± 2.78 |
| iBCA-NPs | 22.9 ± 0.46 | −1.91 ± 4.11 |
| Micro Organism | Species | PACA-NPs | C a | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ECA-NPs (mg/L) | EECA-NPs (mg/L) | iBCA-NPs (mg/L) | |||||||||
| 10 | 100 | 1000 | 10 | 100 | 1000 | 10 | 100 | 1000 | |||
| Gram-positive bacteria | Bacillus subtilis | 7 | 9 | 11.5 | n.a. | 6.5 | 12.5 | 7 | 7.5 | 10 | n.a. |
| Brevibacillus agri | 7.5 | 8.5 | 11 | n.a. | n.a. | 7.5 | n.a. | n.a. | n.a. | n.a. | |
| Microbacterium aurum | 9 | 10 | 16 | 7 | 9 | 15 | 8 | 10 | 12 | n.a. | |
| Propionibacterium acnes | n.a. | n.a. | 7.5 | n.a. | n.a. | n.a. | n.a. | n.a. | 6.5 | n.a. | |
| Staphylococcus aureus | n.a. | 10 | 14 | n.a. | 7 | 10 | n.a. | 7 | 10 | n.a. | |
| Gram-negative bacteria | Escherichia coli | 6.5 | 9 | 10 | n.a. | 6.5 | 7.5 | n.a. | n.a. | n.a. | n.a. |
| Klebsiella pneumoniae | n.a. | 9 | 12 | n.a. | n.a. | 7 | n.a. | n.a. | n.a. | n.a. | |
| Pseudomonas aeruginosa | n.a. | 8 | 11 | n.a. | n.a. | 6.5 | n.a. | n.a. | n.a. | n.a. | |
| Serratia marcescens | 7 | 8.5 | 11 | n.a. | n.a. | 6.5 | n.a. | n.a. | n.a. | n.a. | |
| Salmonella typhimurium | 7 | 10 | 10 | n.a. | 8.5 | 9 | n.a. | n.a. | n.a. | n.a. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarian, F.D.; Ando, K.; Tsurumi, S.; Miyashita, R.; Ute, K.; Ohama, T. Evaluation of the Growth-Inhibitory Spectrum of Three Types of Cyanoacrylate Nanoparticles on Gram-Positive and Gram-Negative Bacteria. Membranes 2022, 12, 782. https://doi.org/10.3390/membranes12080782
Sarian FD, Ando K, Tsurumi S, Miyashita R, Ute K, Ohama T. Evaluation of the Growth-Inhibitory Spectrum of Three Types of Cyanoacrylate Nanoparticles on Gram-Positive and Gram-Negative Bacteria. Membranes. 2022; 12(8):782. https://doi.org/10.3390/membranes12080782
Chicago/Turabian StyleSarian, Fean Davisunjaya, Kazuki Ando, Shota Tsurumi, Ryohei Miyashita, Koichi Ute, and Takeshi Ohama. 2022. "Evaluation of the Growth-Inhibitory Spectrum of Three Types of Cyanoacrylate Nanoparticles on Gram-Positive and Gram-Negative Bacteria" Membranes 12, no. 8: 782. https://doi.org/10.3390/membranes12080782
APA StyleSarian, F. D., Ando, K., Tsurumi, S., Miyashita, R., Ute, K., & Ohama, T. (2022). Evaluation of the Growth-Inhibitory Spectrum of Three Types of Cyanoacrylate Nanoparticles on Gram-Positive and Gram-Negative Bacteria. Membranes, 12(8), 782. https://doi.org/10.3390/membranes12080782






