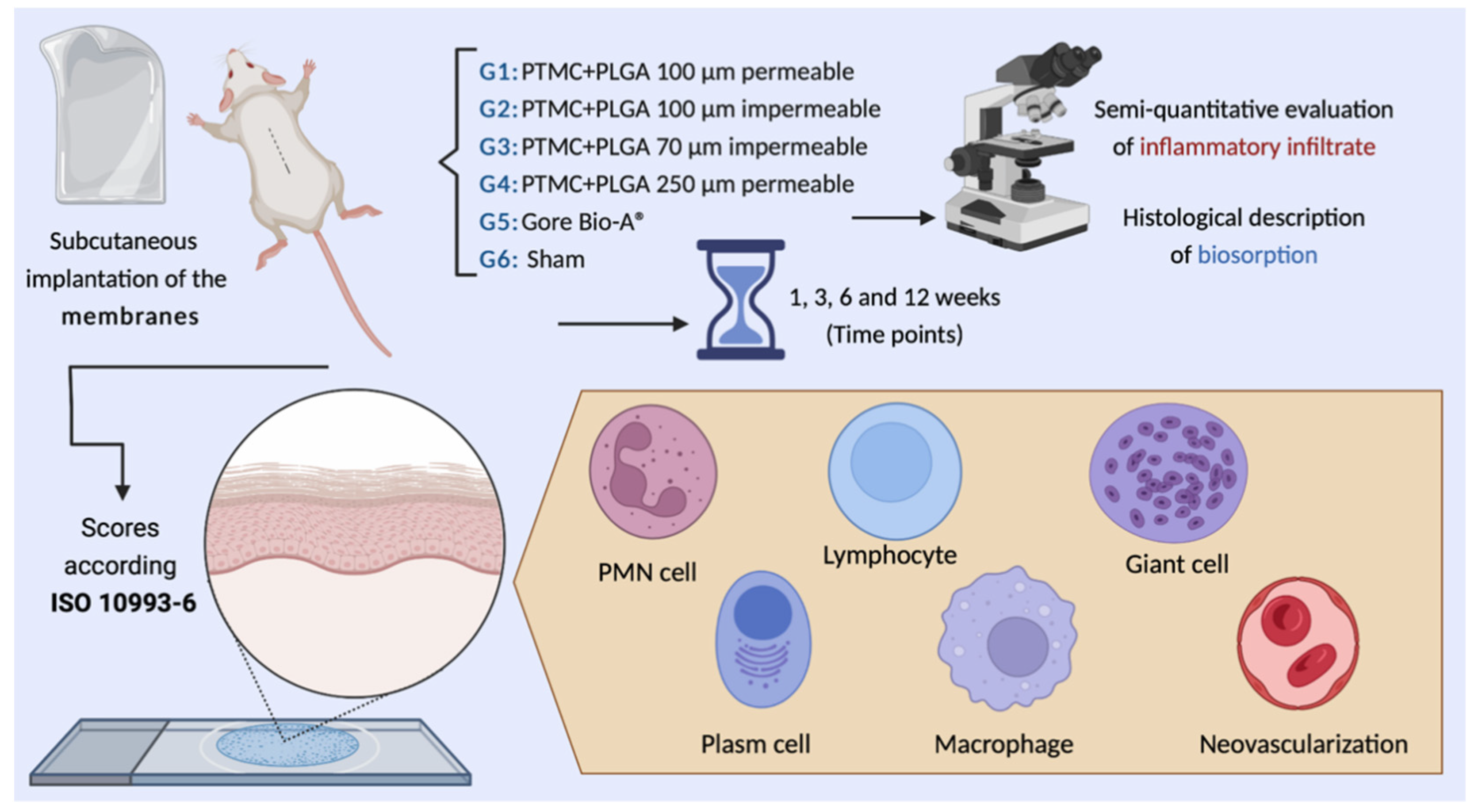
In Vivo Evaluation of Permeable and Impermeable Membranes for Guided Bone Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Materials
2.3. Physico-Chemical Characterization of the Membranes
2.4. Animal Characterization
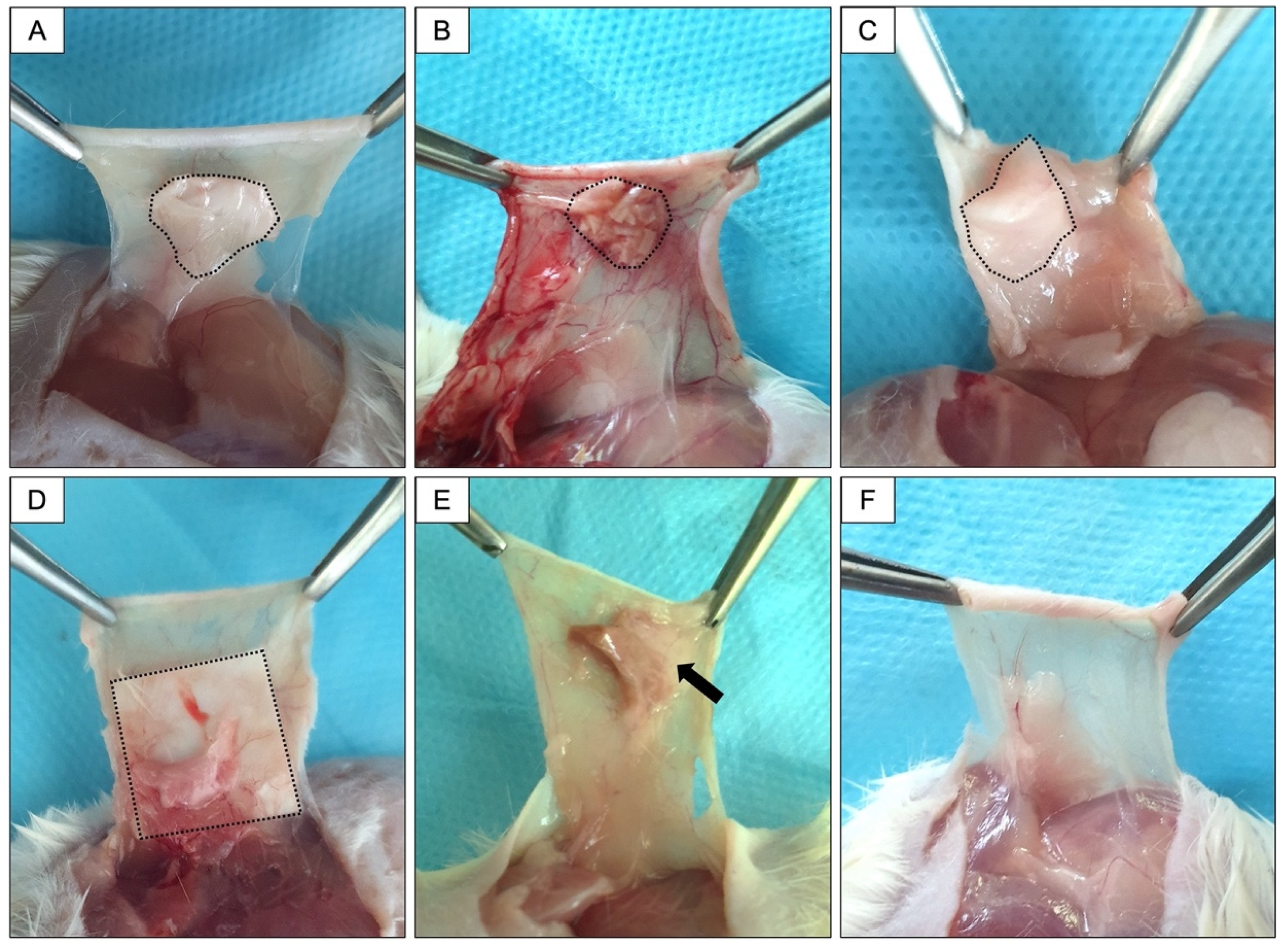
2.5. Surgical Procedures
2.6. Sample Obtention
2.7. Microscopic Evaluation
2.7.1. Histological Description
2.7.2. Semiquantitative Evaluation of Inflammatory Infiltrate
- (1)
- The quantity and distribution of inflammatory cells present at the tissue–material interface. The neutrophils, lymphocytes, plasma cells, and macrophages were classified as: absent (score zero), rare or 1–5 per high-powered (400×) field (phf) (score 1), 5–10 phf (score 2), heavy infiltrate (score 3), and packed (score 4). The multinucleated cells were classified as: absent (score zero), 1–2 per high-powered (400×) field (phf) (score 1), 3–5 phf (score 2), heavy infiltrate (score 3), and sheets (score 4).
- (2)
- The inflammatory response parameters (neovascularization, the degree of fibrosis, and fatty infiltrate.
- (3)
- The presence of necrosis.
3. Results
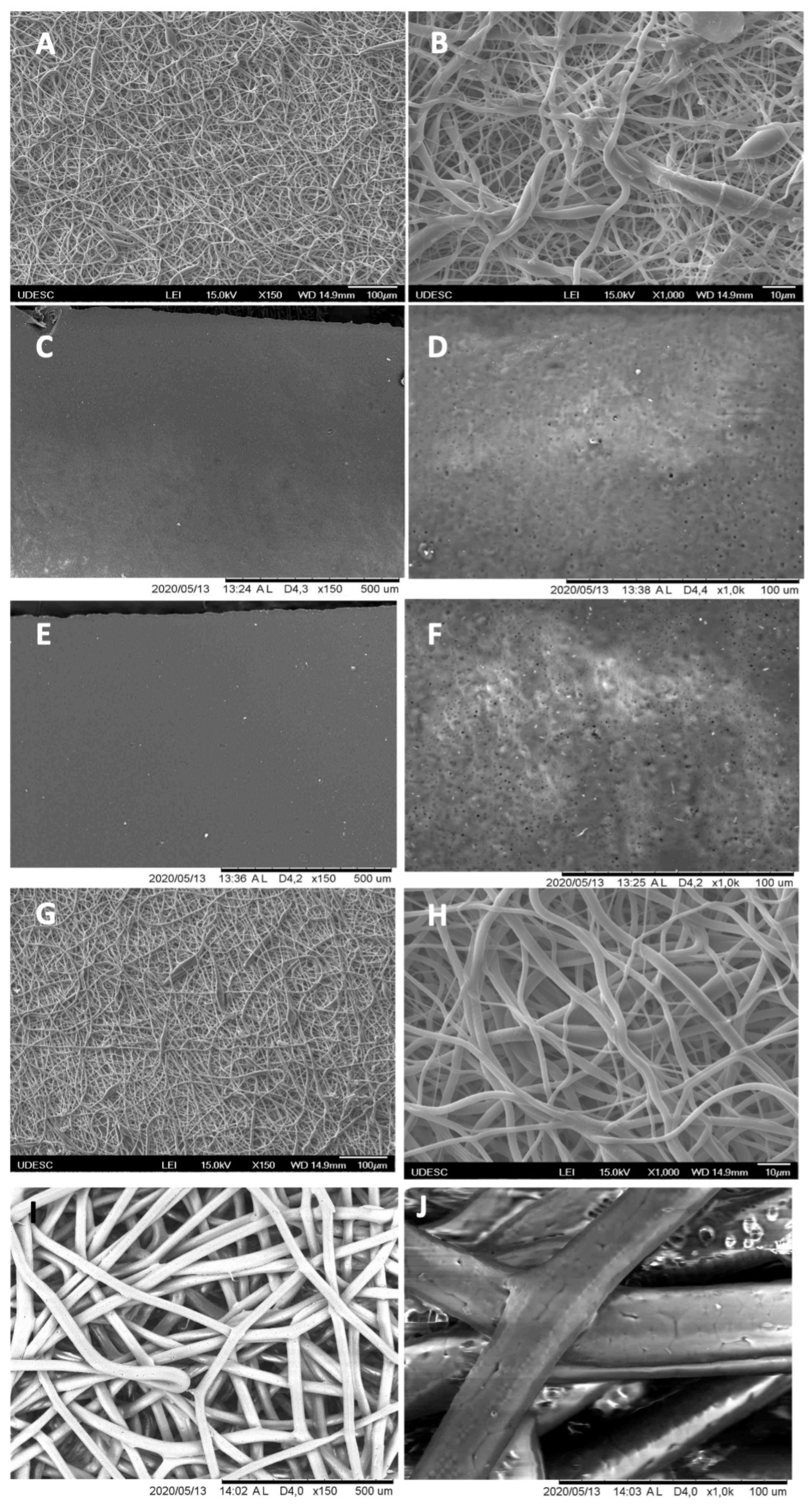
3.1. Scanning Electron Microscopy (SEM)
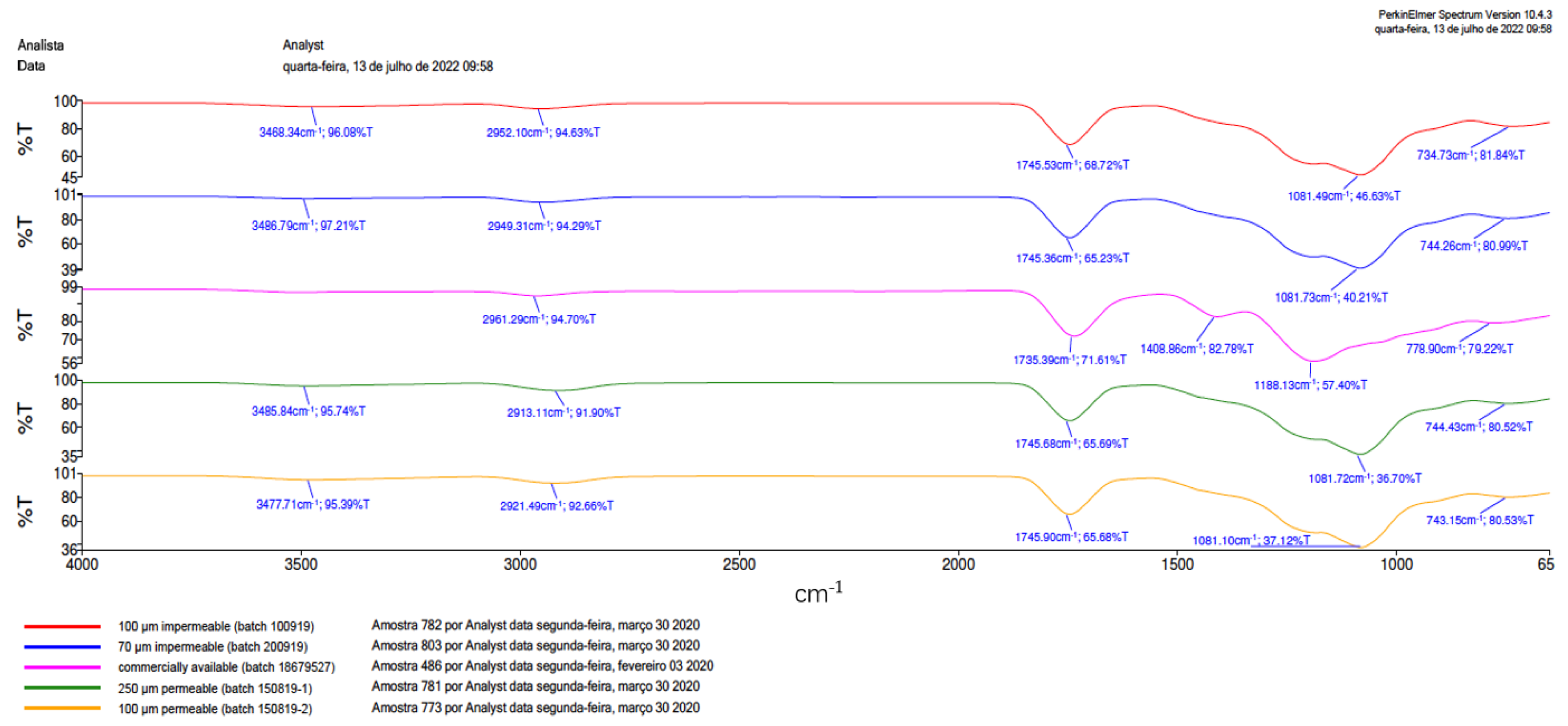
3.2. Fourier Transform Infrared Spectrometer (FTIR)
3.3. In Vivo Response to Membranes and Macroscopy Results
3.4. Descriptive Histological Analysis
3.4.1. One Week Post-Implantation
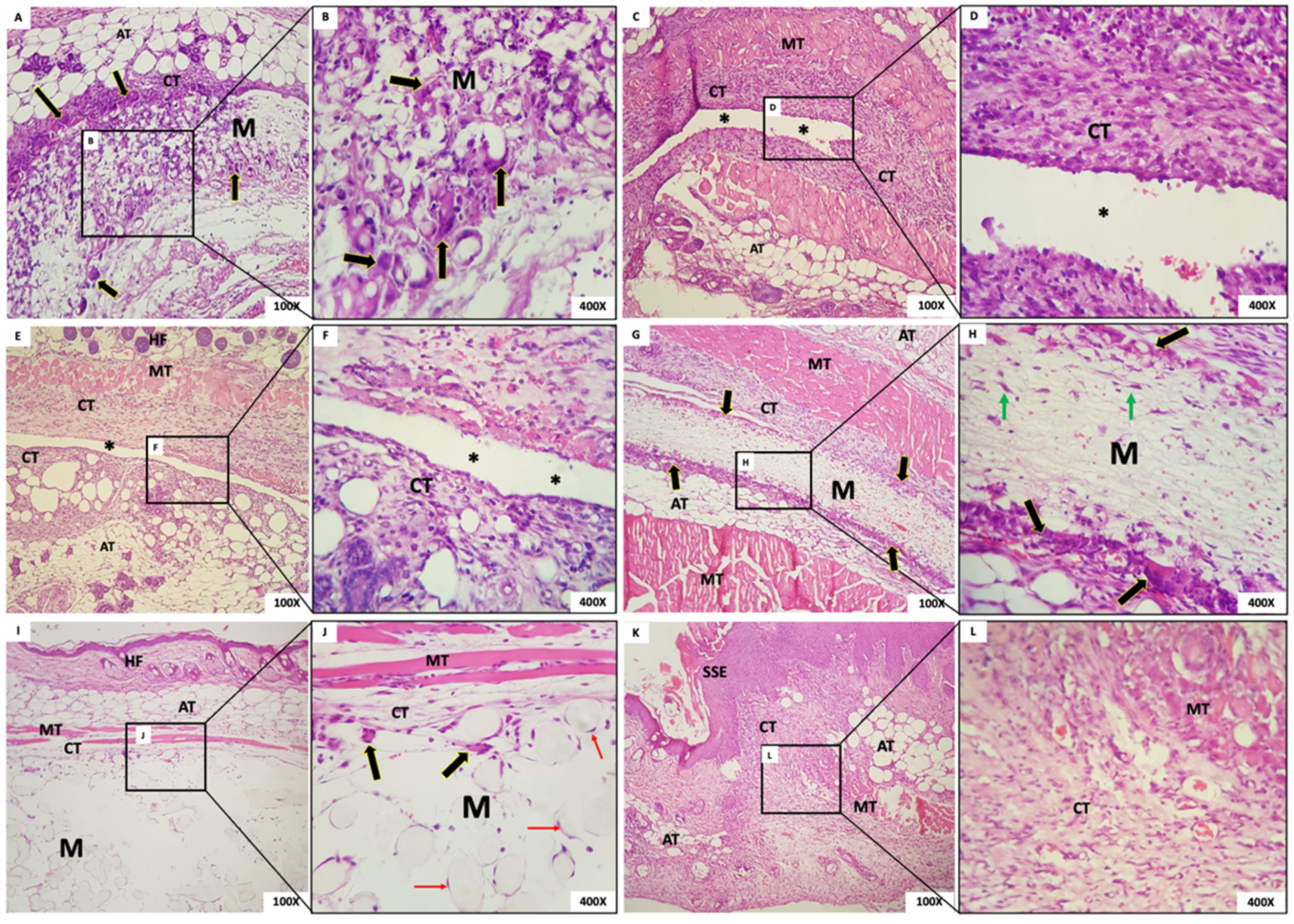
- Group 1: In G1, we observed the presence of the membrane with a fibrillar aspect and permeated with mesenchymal cells (fibroblasts), mononuclear inflammatory cells and scarce polymorphonuclear cells, and abundant multinucleated giant cells (MNGCs), both in the periphery and permeating the membrane. Some MNGCs had material in their interior. Surrounding the membrane was a narrow band of granulation reaction of predominantly mononuclear inflammatory infiltrate (Figure 6A,B).
- Group 2: In all animals in this group, it was not possible to visualize the membrane: a dense band of connective tissue containing an intense mixed inflammatory infiltrate was observed (Figure 6C,D). The membrane appeared not to have been incorporated into the underlying tissues and detached during histological processing. It was possible to observe the contour or virtual space that the membrane occupied.
- Group 3: In all animals in this group, no membrane was identified, similarly to Group 1. The membrane appeared not to have been incorporated into the underlying tissues and detached during histological processing. Additionally, in the contour, it was possible to observe connective tissue containing moderate mixed inflammatory infiltrate (Figure 6E,F).
- Group 4: In this group, we observed the presence of a thick membrane (M) with a fibrillar aspect, covered by a narrow band of connective tissue with a mild to moderate granulation reaction. Multinucleated giant cells were observed in its periphery, sometimes containing material inside. Permeating the membrane, there was the presence of fusiform and starred mesenchymal cells (Figure 6G,H).
- Group 5: The presence of a thick membrane with a spherical and tubular aspect, peripherally interspersed with delicate connective tissue bundles, was observed. We noticed cell adherence on the spherical structures of the membranes and a few multinucleated giant cells. In the membrane, we observed connective tissue with a mild inflammatory infiltrate (Figure 6I,J).
- Group 6: The dermis was lined by orthokeratinized stratified squamous epithelium exhibiting areas of extensive keratinization and scar hyperplasia; underlying fibrous connective tissue was observed with a focal area of intense mixed inflammation and collagen and muscle fibrous connective tissue with focal areas of intense mixed inflammation and organized collagen and muscle fibers (Figure 6K,L).
3.4.2. Three Weeks Post-Implantation
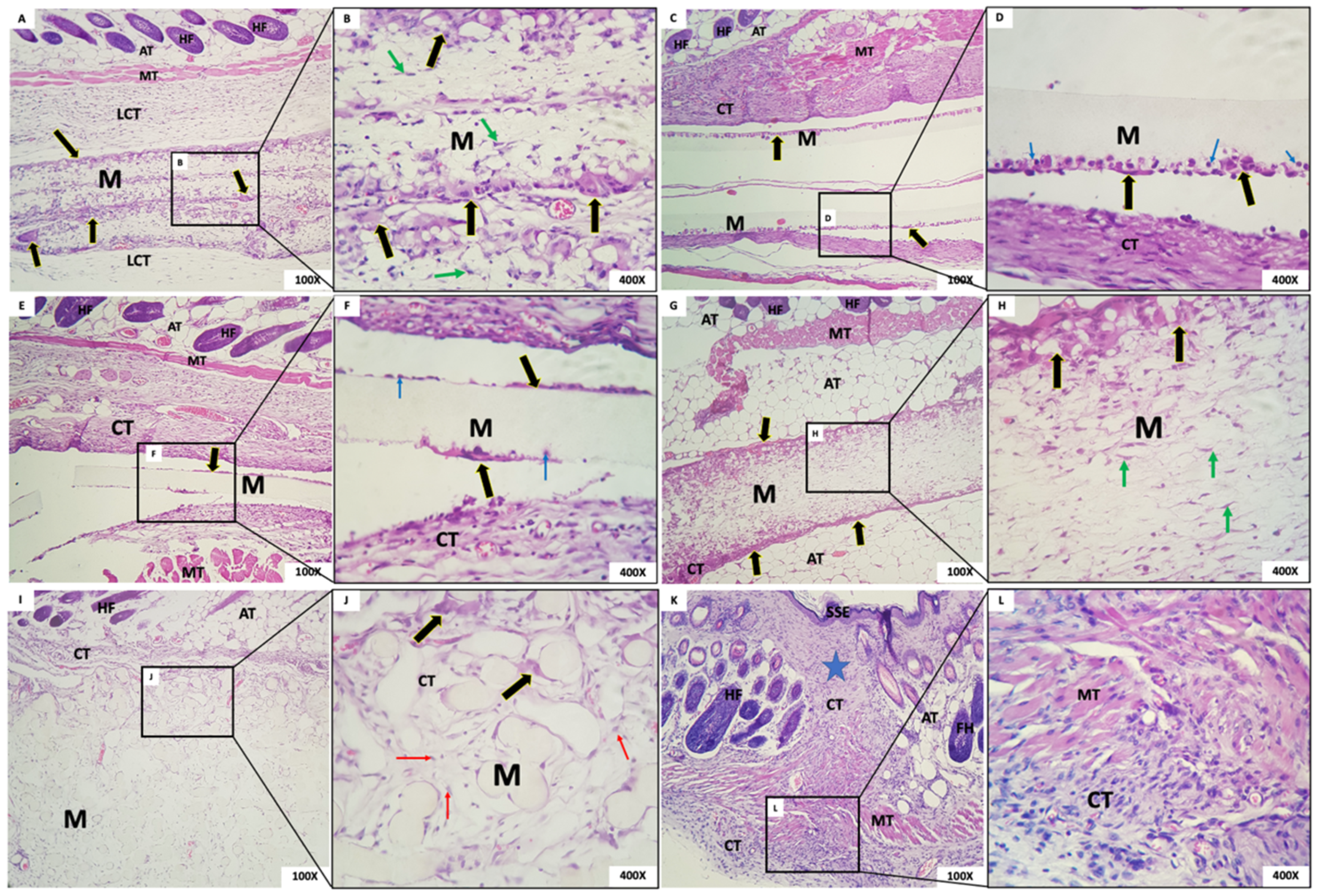
- Group 1: Membranes with a fibrillar appearance and permeating mesenchymal cells (fibroblasts), mononuclear inflammatory cells, and scarce polymorphs were observed, along with abundant multinucleated giant cells (MNGCs), both at the periphery and permeating the membrane, containing content. Some MNGCs displayed material inside. Integrated in the membrane was a delicate connective tissue with a few inflammatory cells (Figure 7A,B).
- Group 2: In G2, a rectilinear, homogeneous, and matte membrane appearing “loose” in almost the whole sample, without adherence to the connective tissue, was observed. In its periphery, multinucleated giant cells and mononuclear cells were trying to break through the membrane. A dense band of the granulation reaction in the connective tissue was subjacent (Figure 7C,D).
- Group 3: In this group, the membrane was rectilinear, homogeneous, matte, and “loose,” without adherence to the connective tissue. Multinucleated giant cells and mononuclear cells were present in its periphery, similarly to Group 2. In proximity to the membrane, there was connective tissue with a moderate mixed inflammatory infiltrate (Figure 7E,F).
- Group 4: We observed the presence of a thick fibrillar membrane permeated by sparse mesenchymal cells with a fusiform and stellate appearance. In the periphery, there was a narrow band of connective tissue with the granulation reaction, composed of a small amount of mononuclear inflammatory infiltrate with a predominance of macrophages. Multinucleated giant cells in its periphery were observed (Figure 7G,H).
- Group 5: A thick membrane with a spherical and tubular aspect interspersed with delicate connective tissue bundles was observed; cellular adherence on its surface was observed in some “spheres” of the membrane with few CGMNs. We observed connective tissue with mild inflammatory infiltrate integrated into the membrane (Figure 7I,J).
- Group 6: In the incision area, a reorganization of fibrous and muscular tissues interspersed with a moderate mononuclear inflammatory infiltrate was noted (Figure 7K,L).
3.4.3. Six Weeks Post-Implantation
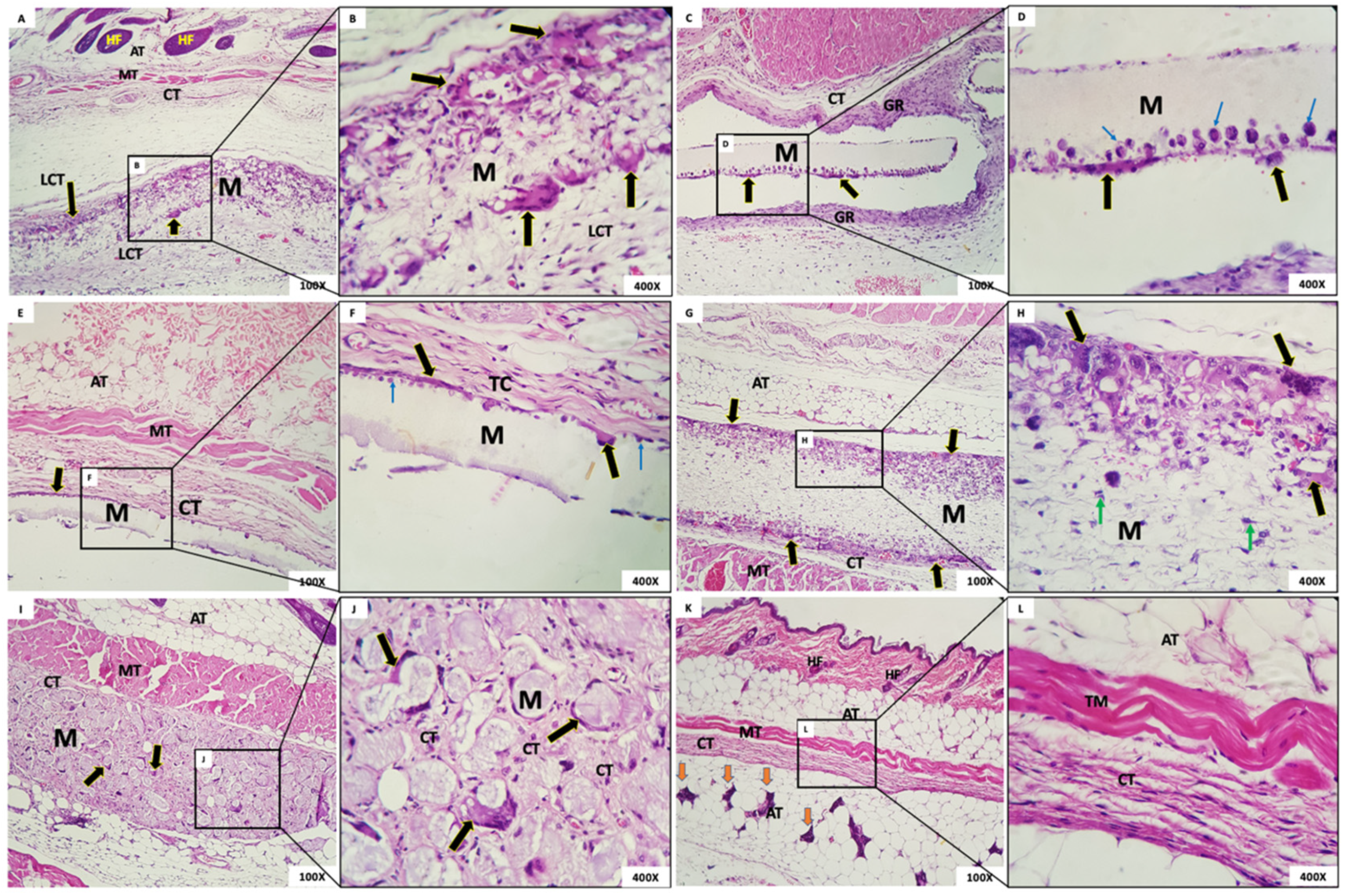
- Group 1: In this group, it was observed that, six weeks after implantation, the membrane was interspersed with loosely arranged connective tissue and multinucleated giant cells with contents inside. The membrane was present in all animals (Figure 8A,B). The membrane, despite being permeated by a cell population and connective tissue, maintained its scaffold.
- Group 2: The presence of a rectilinear and homogeneous membrane with a microscopic matte appearance and without adherence to the adjacent connective tissue was observed. In the connective tissue surrounding the membrane, a granulation reaction with intense mononuclear infiltration also was observed, in addition to the presence of multinucleated giant cells in the periphery of the membrane (Figure 8C,D).
- Group 3: The membrane of G3 had a matte, rectilinear, and homogeneous aspect. Microscopically, it looked partially loose, without adherence to the adjacent connective tissue, limited by mild multinucleated giant cells and mononuclear cells. In the adjacent membrane, connective tissue with inflammatory cells was present (Figure 8E,F).
- Group 4: A thick membrane with a fibrillar aspect containing in its periphery a narrow range of the granulation reaction and multinucleated giant cells with content inside was observed. It is possible that the membrane was permeated by mesenchymal cells with a fusiform and stellate aspect (Figure 8G,H).
- Group 5: In this group, we observed a membrane composed of multiple structures with a clear basophilic spheroidal appearance bounded by multinucleated giant cells and macrophages. Fibrocellular connective tissue permeated the spheres (Figure 8I,J).
- Group 6: In this group, organized connective tissue with sparse inflammatory cells was observed. Adipose, muscular, and glandular tissues were present in the region (Figure 8K,L).
3.4.4. Twelve Weeks Post-Implantation
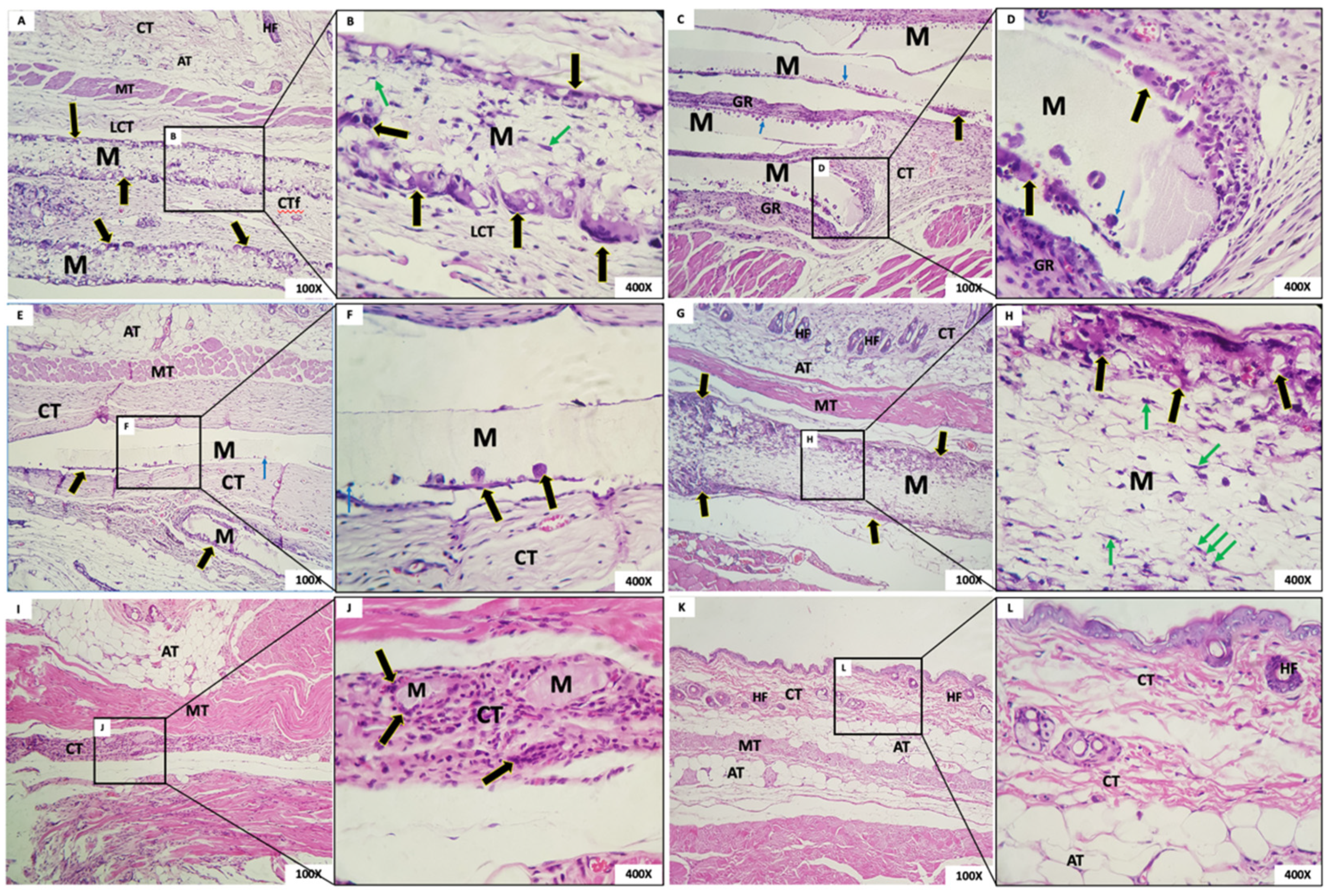
- Group 1: In this group, the membrane was surrounded by loosely arranged connective tissue with scant inflammatory cells and giant cells in the periphery. At the highest enlargement of the membrane, it was observed that multinucleated giant cells in the membrane presented contents inside, and mesenchymal cells were present permeating the membrane (Figure 9A,B). As in the case at six weeks, the membrane, although permeated by a cell population and connective tissue, had maintained its framework.
- Group 2: The membrane appeared rectilinear, intact, homogeneous, and, with a matte aspect, “loose”, without adherence to the connective tissue. In its periphery was observed the presence of multinucleated giant cells and mononuclear cells inside the membrane. A band of the granulation reaction was found underneath the membrane (Figure 9C,D).
- Group 3: The membrane was rectilinear, intact, homogeneous, matte, and “loose”, with no adherence to the connective tissue. In its periphery, the presence of multinucleated giant cells and a few mononuclear cells were observed. In proximity to the membrane, connective tissue with a few inflammatory cells was noted (Figure 9E,F).
- Group 4: In this group, the membrane had a fibrillar aspect and, contained in the periphery, a sparse mononuclear inflammatory infiltrate, and a large number of multinucleated giant cells were observed. It was possible to observe, at the highest magnification, the membrane permeated by mesenchymal cells with a fusiform and striated aspect and, in the periphery, multinucleated giant cells with content inside (Figure 9G,H).
- Group 5: The presence of a few membrane fragments interspersed with a band of connective tissue exhibiting granulation reaction was observed. From the details at the higher magnification, it was possible to observe membrane residues interspersed with the granulation reaction and multinucleated giant cells (Figure 9I,J).
- Group 6: In this group, well-organized tissue with scarce inflammatory cells and a normal healing appearance was observed for the 12-week period (Figure 9K,L).
3.5. Semiquantitative Histological Analysis of Local Biological Effect of Implanted Membranes: ISO 10993-6: 2016/Part 6/Annex E
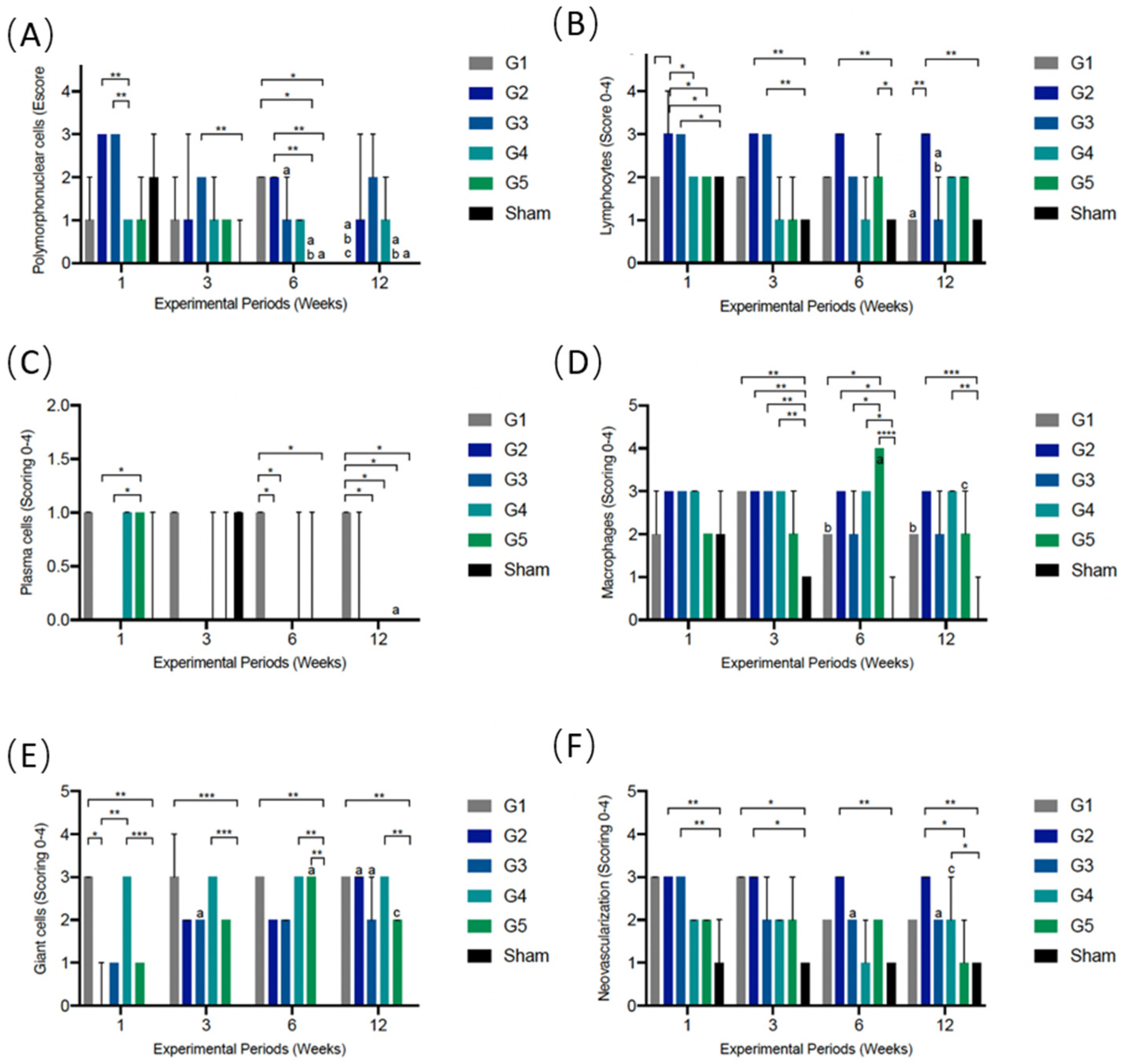
3.5.1. PMN Cells
3.5.2. Lymphocytes
3.5.3. Plasma Cells
3.5.4. Macrophages
3.5.5. Giant Cells
3.5.6. Neovascularization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nyman, S.; Lindhe, J.; Karring, T.; Rylander, H. New attachment following surgical treatment of human periodontal disease. J. Clin. Periodontol. 1982, 9, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Gottlow, J.; Nyman, S.; Karring, T.; Lindhe, J. New attachment formation as the result of controlled tissue regeneration. J. Clin. Periodontol. 1984, 11, 494–503. [Google Scholar] [CrossRef]
- Dahlin, C.; Linde, A.; Gottlow, J.; Nyman, S. Healing of bone defects by guided tissue regeneration. Plast. Reconstr. Surg. 1988, 81, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Bassett, C.A.; Campbell, J.B.; Girado, J.M.; Rossi, J.P.; Seymour, R.J. Application of monomolecular filter tubes in bridging gaps in peripheral nerves and for prevention of neuroma formation: A preliminary report. J. Neurosurg. 1956, 13, 635–637. [Google Scholar] [CrossRef] [PubMed]
- Hammerle, C.H.; Schmid, J.; Lang, N.P.; Olah, A.J. Temporal dynamics of healing in rabbit cranial defects using guided bone regeneration. J. Oral Maxillofac. Surg. 1995, 53, 167–174. [Google Scholar] [CrossRef]
- Linde, A.; Thoren, C.; Dahlin, C.; Sandberg, E. Creation of new bone by an osteopromotive membrane technique: An experimental study in rats. J. Oral Maxillofac. Surg. 1993, 51, 892–897. [Google Scholar] [CrossRef]
- Karring, T.; Nyman, S.; Gottlow, J.; Laurell, L. Development of the biological concept of guided tissue regeneration—Animal and human studies. Periodontology 1993, 1, 26–35. [Google Scholar] [CrossRef]
- Becmeur, F.; Geiss, S.; Laustriat, S.; Bientz, J.; Marcellin, L.; Sauvage, P. History of Teflon. Eur. Urol. 1990, 17, 299–300. [Google Scholar] [CrossRef]
- Hoornaert, A.; d’Arros, C.; Heymann, M.F.; Layrolle, P. Biocompatibility, resorption and biofunctionality of a new synthetic biodegradable membrane for guided bone regeneration. Biomed. Mater. 2016, 11, 045012. [Google Scholar] [CrossRef]
- Neto, A.M.D.; Sartoretto, S.C.; Duarte, I.M.; Resende, R.F.B.; Neves Novellino Alves, A.T.; Mourao, C.; Calasans-Maia, J.; Montemezzi, P.; Tristao, G.C.; Calasans-Maia, M.D. In Vivo Comparative Evaluation of Biocompatibility and Biodegradation of Bovine and Porcine Collagen Membranes. Membranes 2020, 10, 423. [Google Scholar] [CrossRef]
- Hurzeler, M.B.; Quinones, C.R.; Schupbach, P. Guided bone regeneration around dental implants in the atrophic alveolar ridge using a bioresorbable barrier. An experimental study in the monkey. Clin. Oral Implant. Res. 1997, 8, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, E.; Dahlin, C.; Linde, A. Bone regeneration by the osteopromotion technique using bioabsorbable membranes: An experimental study in rats. J. Oral Maxillofac. Surg. 1993, 51, 1106–1114. [Google Scholar] [CrossRef]
- Zellin, G.; Gritli-Linde, A.; Linde, A. Healing of mandibular defects with different biodegradable and non-biodegradable membranes: An experimental study in rats. Biomaterials 1995, 16, 601–609. [Google Scholar] [CrossRef]
- Brunel, G.; Benque, E.; Elharar, F.; Sansac, C.; Duffort, J.F.; Barthet, P.; Baysse, E.; Miller, N. Guided bone regeneration for immediate non-submerged implant placement using bioabsorbable materials in Beagle dogs. Clin. Oral Implant. Res. 1998, 9, 303–312. [Google Scholar] [CrossRef]
- Hammerle, C.H.; Jung, R.E. Bone augmentation by means of barrier membranes. Periodontology 2003, 33, 36–53. [Google Scholar] [CrossRef] [Green Version]
- Warrer, K.; Karring, T.; Nyman, S.; Gogolewski, S. Guided tissue regeneration using biodegradable membranes of polylactic acid or polyurethane. J. Clin. Periodontol. 1992, 19, 633–640. [Google Scholar] [CrossRef]
- da Costa Pereira, L.; de Almeida Barros Mourao, C.F.; Neves Novellino Alves, A.T.; Figueiredo de Brito Resende, R.; Pinheiro Guedes de Uzeda, M.J.; Granjeiro, J.M.; Seabra Louro, R.; Diuana Calasans-Maia, M. In Vitro Physico-Chemical Characterization and Standardized In Vivo Evaluation of Biocompatibility of a New Synthetic Membrane for Guided Bone Regeneration. Materials 2019, 12, 1186. [Google Scholar] [CrossRef] [Green Version]
- Guney, A.; Malda, J.; Dhert, W.J.A.; Grijpma, D.W. Triblock copolymers based on epsilon-caprolactone and trimethylene carbonate for the 3D printing of tissue engineering scaffolds. Int. J. Artif. Organs 2017, 40, 176–184. [Google Scholar] [CrossRef]
- Kang, H.; Jiang, X.; Liu, Z.; Liu, F.; Yan, G.; Li, F. Biodegradable 3D Printed Scaffolds of Modified Poly (Trimethylene Carbonate) Composite Materials with Poly (L-Lactic Acid) and Hydroxyapatite for Bone Regeneration. Nanomaterials 2021, 11, 3215. [Google Scholar] [CrossRef]
- Zhang, Z.; Kuijer, R.; Bulstra, S.K.; Grijpma, D.W.; Feijen, J. The in vivo and in vitro degradation behavior of poly(trimethylene carbonate). Biomaterials 2006, 27, 1741–1748. [Google Scholar] [CrossRef]
- Levin, L.; Halperin-Sternfeld, M. Tooth preservation or implant placement: A systematic review of long-term tooth and implant survival rates. J. Am. Dent. Assoc. 2013, 144, 1119–1133. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.; van Leeuwen, A.; Yuan, H.; Bos, R.R.; Grijpma, D.W.; Kuijer, R. Evaluation of novel resorbable membranes for bone augmentation in a rat model. Clin. Oral Implant. Res. 2016, 27, e8–e14. [Google Scholar] [CrossRef] [PubMed]
- Martin-Thome, H.; Bourdin, D.; Strube, N.; Saffarzadeh, A.; Morlock, J.F.; Campard, G.; Evanno, C.; Hoornaert, A.; Layrolle, P. Clinical Safety of a New Synthetic Resorbable Dental Membrane: A Case Series Study. J. Oral Implant. 2018, 44, 138–145. [Google Scholar] [CrossRef] [PubMed]
- NC3Rs Reporting Guidelines Working Group. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. J. Physiol. 2010, 588, 2519–2521. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef]
- Smith, A.J.; Clutton, R.E.; Lilley, E.; Hansen, K.E.A.; Brattelid, T. PREPARE: Guidelines for planning animal research and testing. Lab. Anim. 2018, 52, 135–141. [Google Scholar] [CrossRef] [Green Version]
- ISO 10993-6; Biological Evaluation of Medical Devices; Part 6: Tests for Local Effects after Implantation. International Organization for Standardization: Geneva, Switzerland, 2016.
- Aghaloo, T.L.; Misch, C.; Lin, G.H.; Iacono, V.J.; Wang, H.L. Bone Augmentation of the Edentulous Maxilla for Implant Placement: A Systematic Review. Int. J. Oral Maxillofac. Implant. 2016, 31, s19–s30. [Google Scholar] [CrossRef] [Green Version]
- Bizelli, V.F.; Ramos, E.U.; Veras, A.S.C.; Teixeira, G.R.; Faverani, L.P.; Bassi, A.P.F. Calvaria Critical Size Defects Regeneration Using Collagen Membranes to Assess the Osteopromotive Principle: An Animal Study. Membranes 2022, 12, 461. [Google Scholar] [CrossRef]
- Abe, G.L.; Sasaki, J.I.; Katata, C.; Kohno, T.; Tsuboi, R.; Kitagawa, H.; Imazato, S. Fabrication of novel poly(lactic acid/caprolactone) bilayer membrane for GBR application. Dent. Mater. 2020, 36, 626–634. [Google Scholar] [CrossRef]
- Sculean, A.; Nikolidakis, D.; Schwarz, F. Regeneration of periodontal tissues: Combinations of barrier membranes and grafting materials—Biological foundation and preclinical evidence: A systematic review. J. Clin. Periodontol. 2008, 35, 106–116. [Google Scholar] [CrossRef]
- Sartoretto, S.C.; Calasans-Maia, M.D.; Alves, A.; Resende, R.F.B.; da Costa Fernandes, C.J.; de Magalhaes Padilha, P.; Rossi, A.M.; Teti, A.; Granjeiro, J.M.; Zambuzzi, W.F. The role of apoptosis associated speck-like protein containing a caspase-1 recruitment domain (ASC) in response to bone substitutes. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110965. [Google Scholar] [CrossRef] [PubMed]
- Moerbeck-Filho, P.; Sartoretto, S.C.; Uzeda, M.J.; Barreto, M.; Medrado, A.; Alves, A.; Calasans-Maia, M.D. Evaluation of the In Vivo Biocompatibility of Amorphous Calcium Phosphate-Containing Metals. J. Funct. Biomater. 2020, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Geremias, T.C.; Sartoretto, S.C.; Batistella, M.A.; Souza, A.A.U.; Alves, A.; Uzeda, M.J.P.; Calasans-Maia, J.; Montemezzi, P.; Mourao, C.; Calasans-Maia, M. In Vivo Biological Evaluation of Biodegradable Nanofibrous Membranes Incorporated with Antibiofilm Compounds. Polymers 2021, 13, 2457. [Google Scholar] [CrossRef]
- Naung, N.Y.; Shehata, E.; Van Sickels, J.E. Resorbable Versus Nonresorbable Membranes: When and Why? Dent. Clin. 2019, 63, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.S.; Choi, J.W.; Kim, J.H.; Chung, H.Y.; Jin, S.; Shim, J.H.; Yun, W.S.; Jeong, C.M.; Huh, J.B. Comparative Efficacies of Collagen-Based 3D Printed PCL/PLGA/beta-TCP Composite Block Bone Grafts and Biphasic Calcium Phosphate Bone Substitute for Bone Regeneration. Materials 2017, 10, 421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, X.L.; Sui, G.; Zhao, M.L.; Chen, G.Q.; Yang, X.P. Poly(L-lactic acid)/hydroxyapatite hybrid nanofibrous scaffolds prepared by electrospinning. J. Biomater. Sci. Polym. Ed. 2007, 18, 117–130. [Google Scholar] [CrossRef]
- Chen, G.; Xia, Y.; Lu, X.; Zhou, X.; Zhang, F.; Gu, N. Effects of surface functionalization of PLGA membranes for guided bone regeneration on proliferation and behavior of osteoblasts. J. Biomed. Mater. Res. A 2013, 101, 44–53. [Google Scholar] [CrossRef]
- Liao, H.; Walboomers, X.F.; Habraken, W.J.; Zhang, Z.; Li, Y.; Grijpma, D.W.; Mikos, A.G.; Wolke, J.G.; Jansen, J.A. Injectable calcium phosphate cement with PLGA, gelatin and PTMC microspheres in a rabbit femoral defect. Acta Biomater. 2011, 7, 1752–1759. [Google Scholar] [CrossRef]
- Hu, B.; Du, H.J.; Yan, G.P.; Zhuo, R.X.; Wu, Y.; Fan, C.L. Magnetic polycarbonate microspheres for tumor-targeted delivery of tumor necrosis factor. Drug Deliv. 2014, 21, 204–212. [Google Scholar] [CrossRef] [Green Version]
- de Santana, R.B.; de Mattos, C.M.; Francischone, C.E.; Van Dyke, T. Superficial topography and porosity of an absorbable barrier membrane impacts soft tissue response in guided bone regeneration. J. Periodontol. 2010, 81, 926–933. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sartoretto, S.C.; Gens, N.d.F.; de Brito Resende, R.F.; Alves, A.T.N.N.; Cecato, R.C.; Uzeda, M.J.; Granjeiro, J.M.; Calasans-Maia, M.D.; Calasans-Maia, J.A. In Vivo Evaluation of Permeable and Impermeable Membranes for Guided Bone Regeneration. Membranes 2022, 12, 711. https://doi.org/10.3390/membranes12070711
Sartoretto SC, Gens NdF, de Brito Resende RF, Alves ATNN, Cecato RC, Uzeda MJ, Granjeiro JM, Calasans-Maia MD, Calasans-Maia JA. In Vivo Evaluation of Permeable and Impermeable Membranes for Guided Bone Regeneration. Membranes. 2022; 12(7):711. https://doi.org/10.3390/membranes12070711
Chicago/Turabian StyleSartoretto, Suelen Cristina, Natalia de Freitas Gens, Rodrigo Figueiredo de Brito Resende, Adriana Terezinha Neves Novellino Alves, Rafael Cury Cecato, Marcelo José Uzeda, Jose Mauro Granjeiro, Monica Diuana Calasans-Maia, and Jose Albuquerque Calasans-Maia. 2022. "In Vivo Evaluation of Permeable and Impermeable Membranes for Guided Bone Regeneration" Membranes 12, no. 7: 711. https://doi.org/10.3390/membranes12070711
APA StyleSartoretto, S. C., Gens, N. d. F., de Brito Resende, R. F., Alves, A. T. N. N., Cecato, R. C., Uzeda, M. J., Granjeiro, J. M., Calasans-Maia, M. D., & Calasans-Maia, J. A. (2022). In Vivo Evaluation of Permeable and Impermeable Membranes for Guided Bone Regeneration. Membranes, 12(7), 711. https://doi.org/10.3390/membranes12070711






