The Neural Multilineage Differentiation Capacity of Human Neural Precursors from the Umbilical Cord—Ready to Bench for Clinical Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of Wharton’s Jelly Mesenchymal Stem Cells
2.2. Isolation of Umbilical Cord Blood Mesenchymal Stem Cells
2.3. Mesenchymal Stem Cells Characterization
2.4. Cell Growth Kinetics
2.5. Production of Neurospheres and Neural Precursors
2.6. Differentiation into Gabaergic-like Neurons
2.7. Differentiation into Dopaminergic-Like Neurons
2.8. Differentiation into Oligodendrocytes
2.9. Differentiation into Schwann Cells
2.10. Immunocytochemistry
2.11. Scanning Electron Microscopy
2.12. Qualitative Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
3. Results
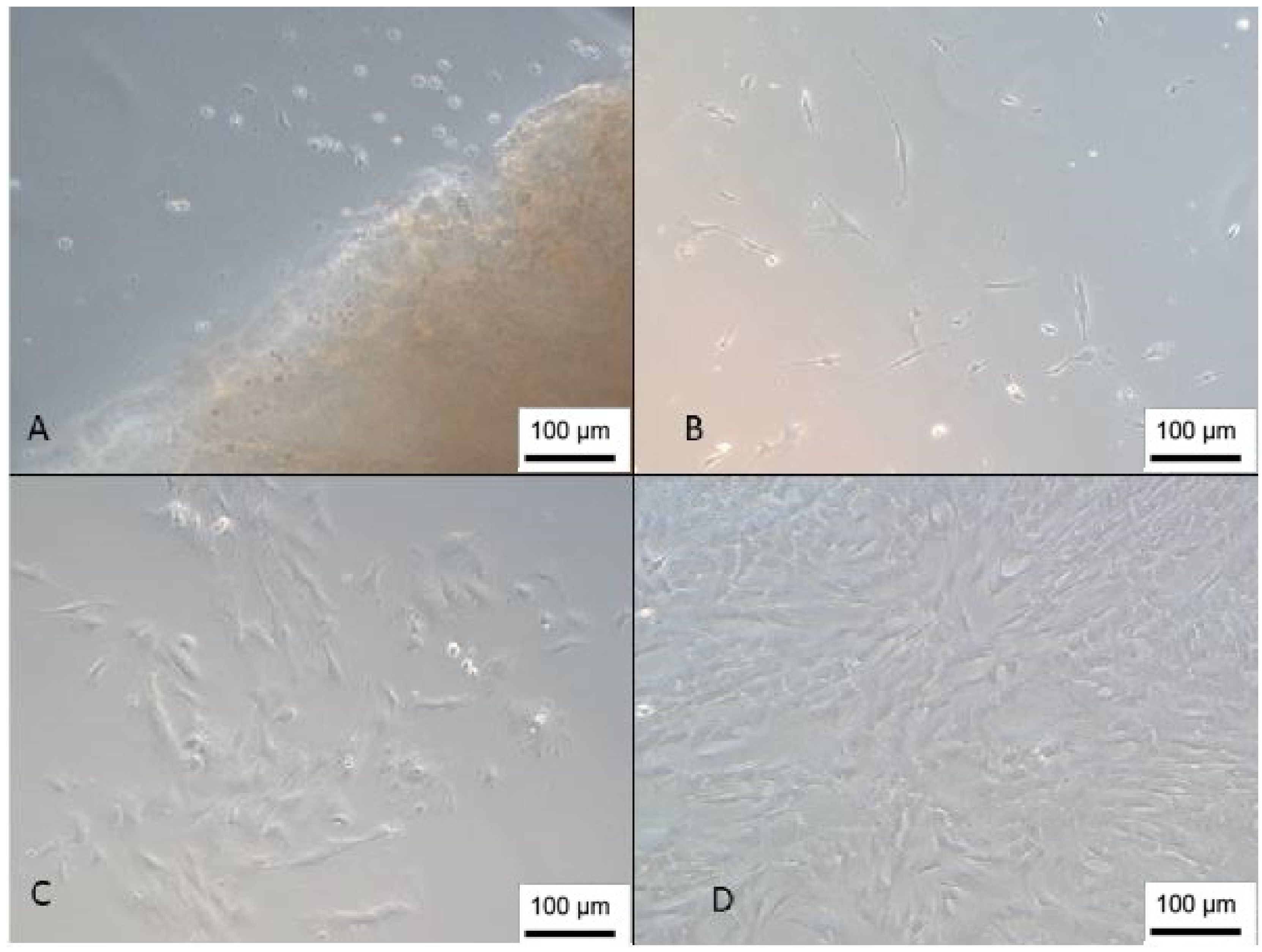
3.1. Isolation of Wharton’s Jelly Mesenchymal Stem Cells
3.2. Isolation of Umbilical Cord Blood Mesenchymal Stem Cells
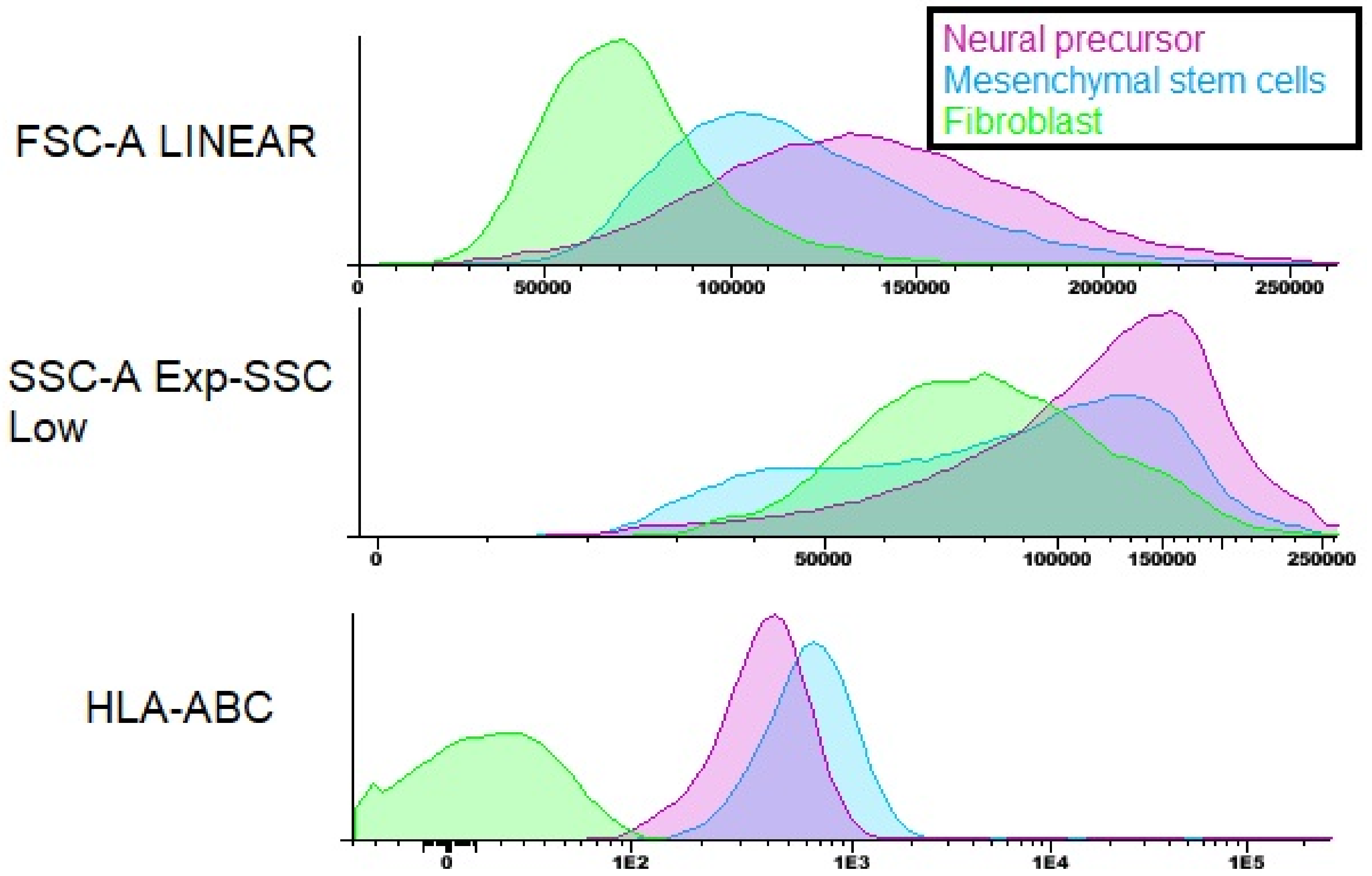
3.3. Mesenchymal Stem Cells Characterization
3.4. Cell Growth Kinetics
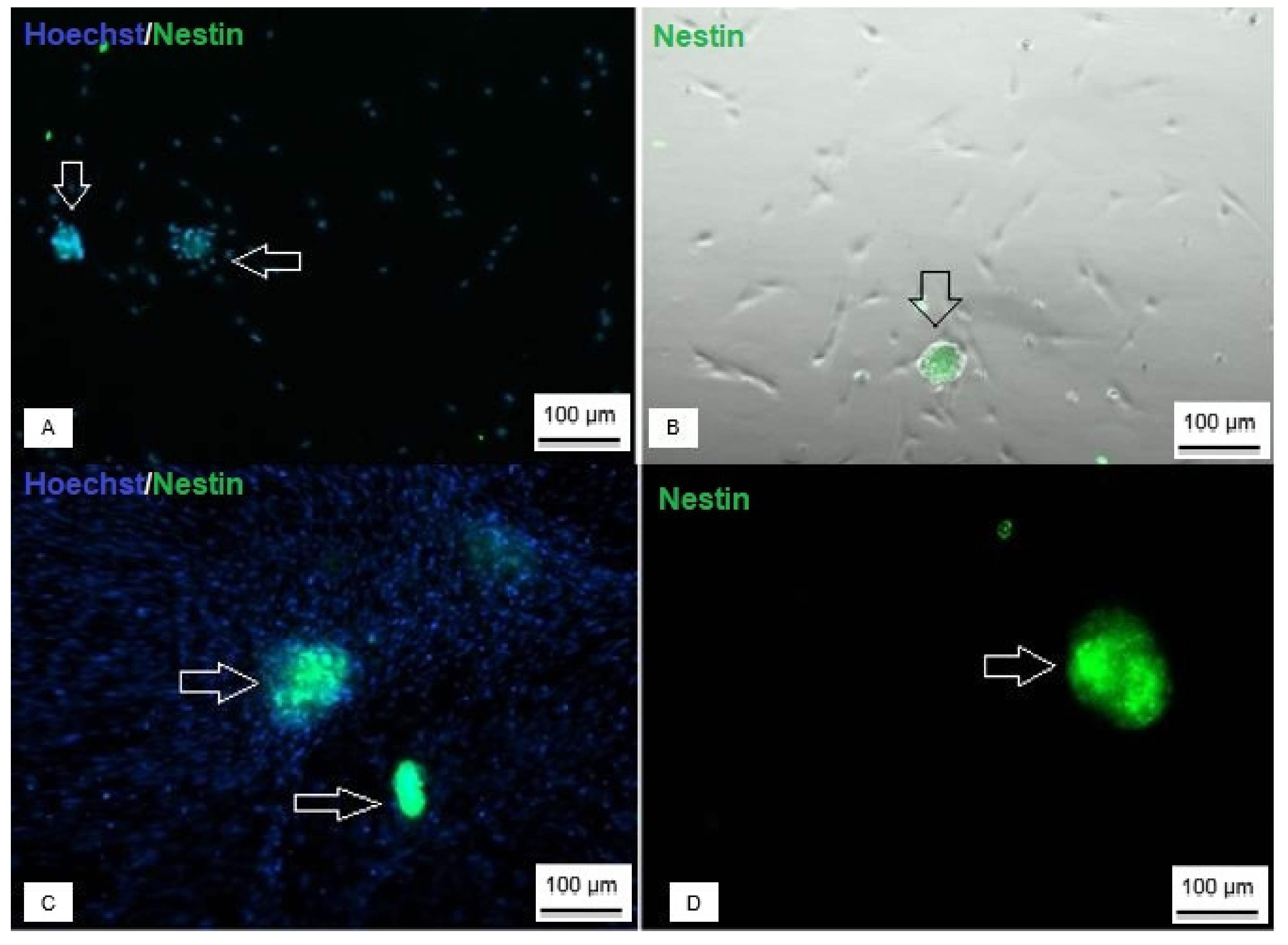
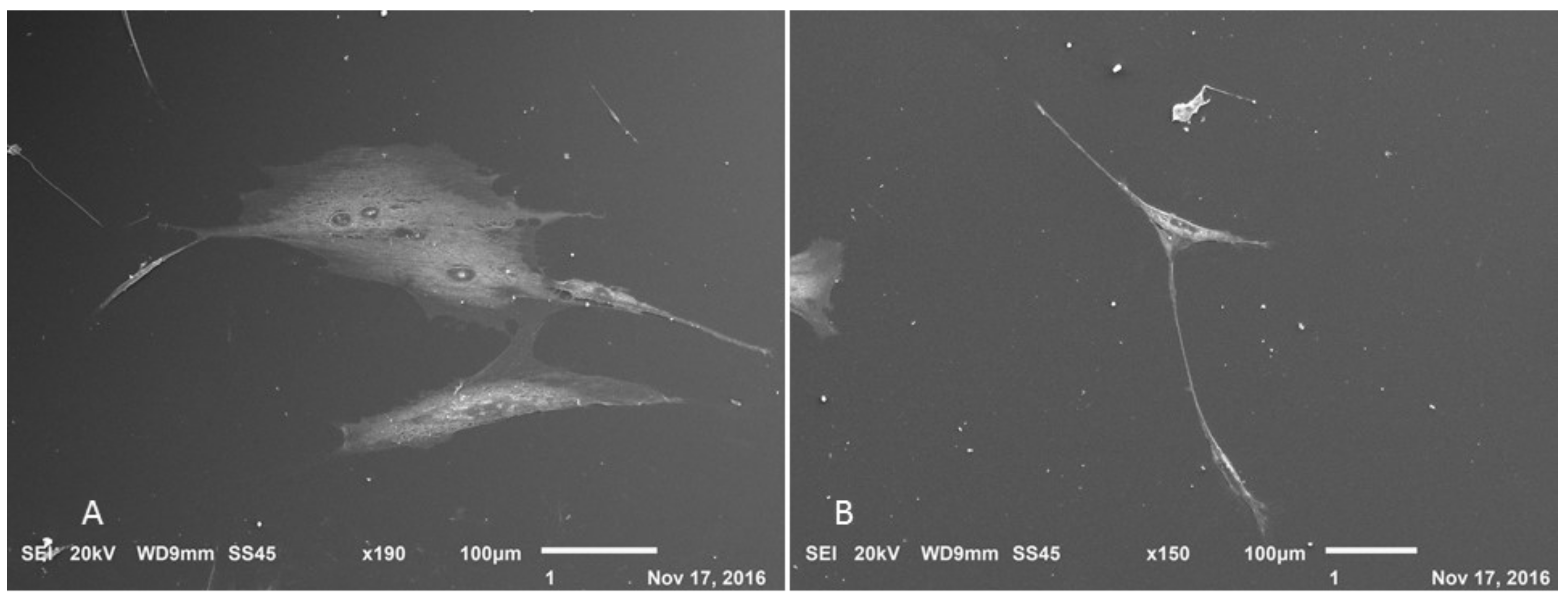
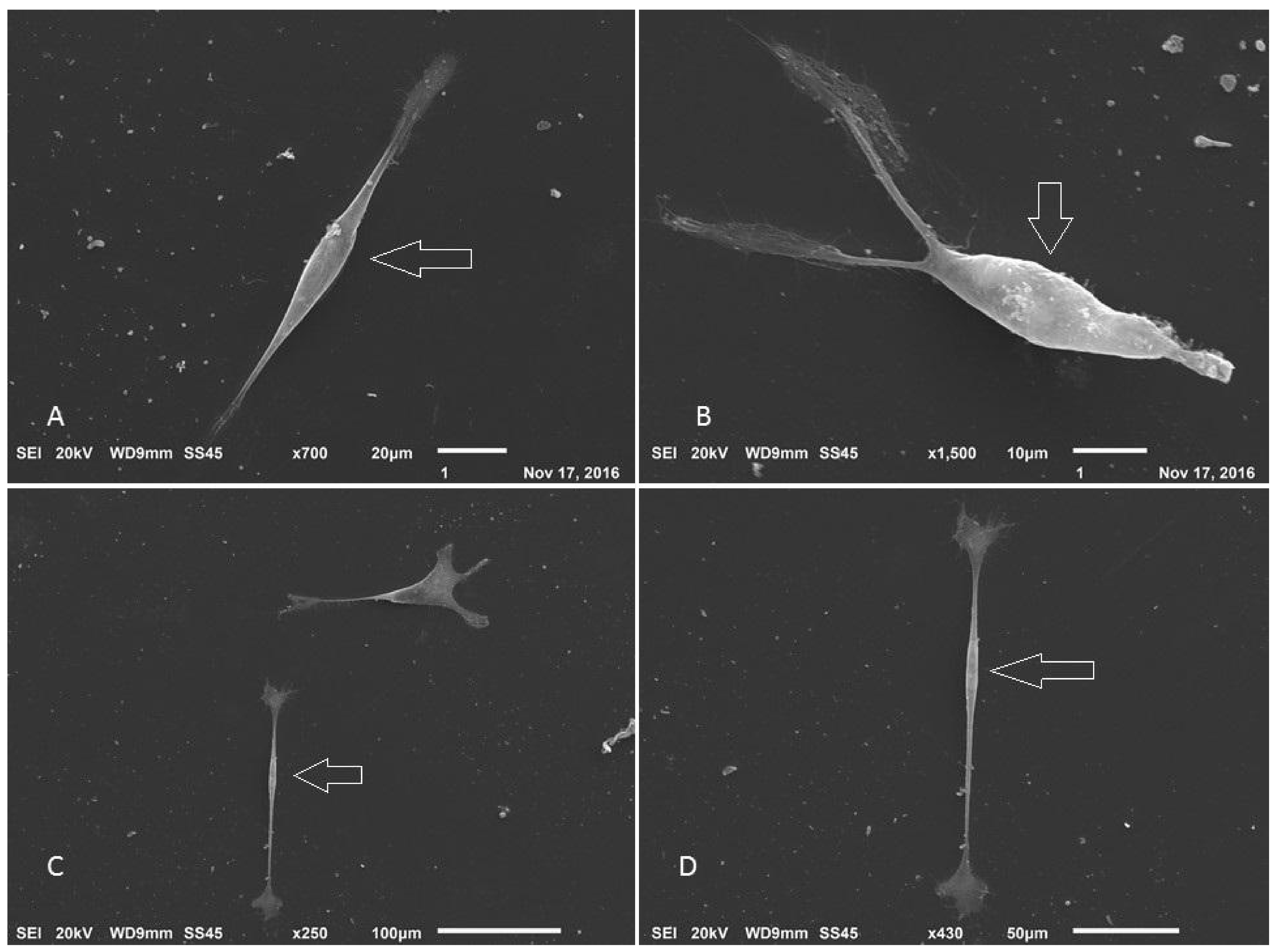
3.5. Production of Neurospheres and Neural Precursors
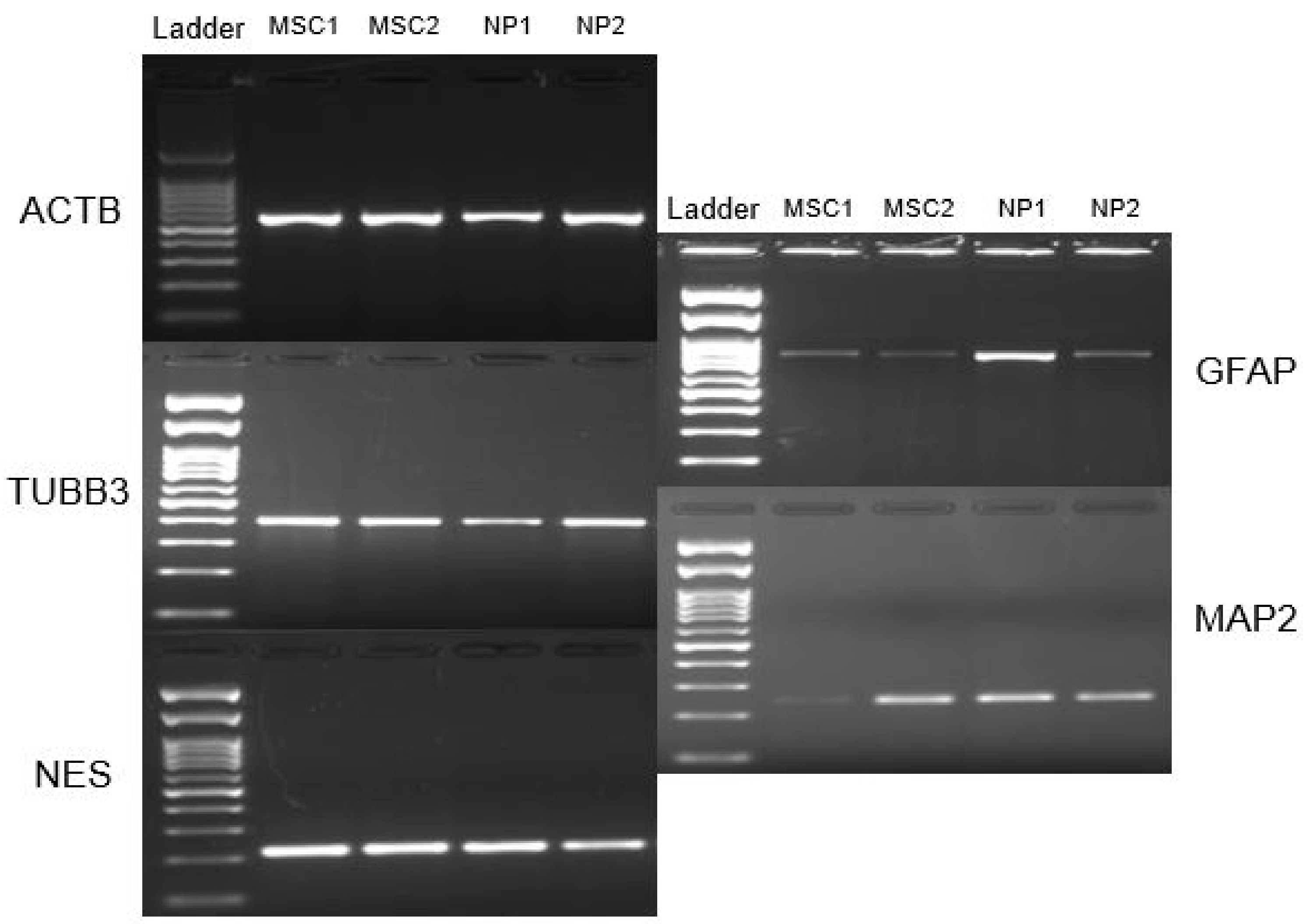
3.6. Neurosphere and Neural Precursors Characterization
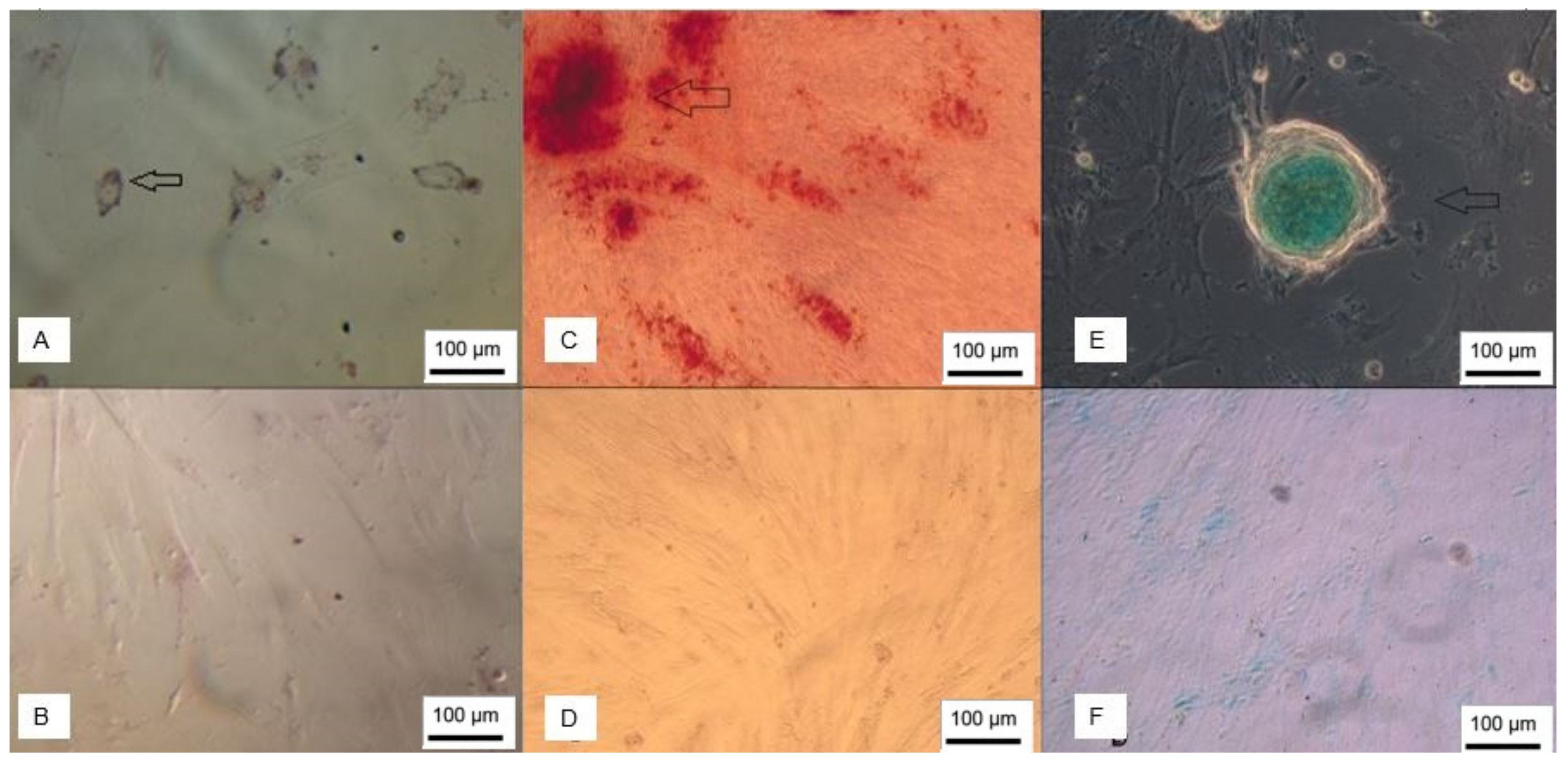
3.7. Differentiation into Cholinergic-like Neurons
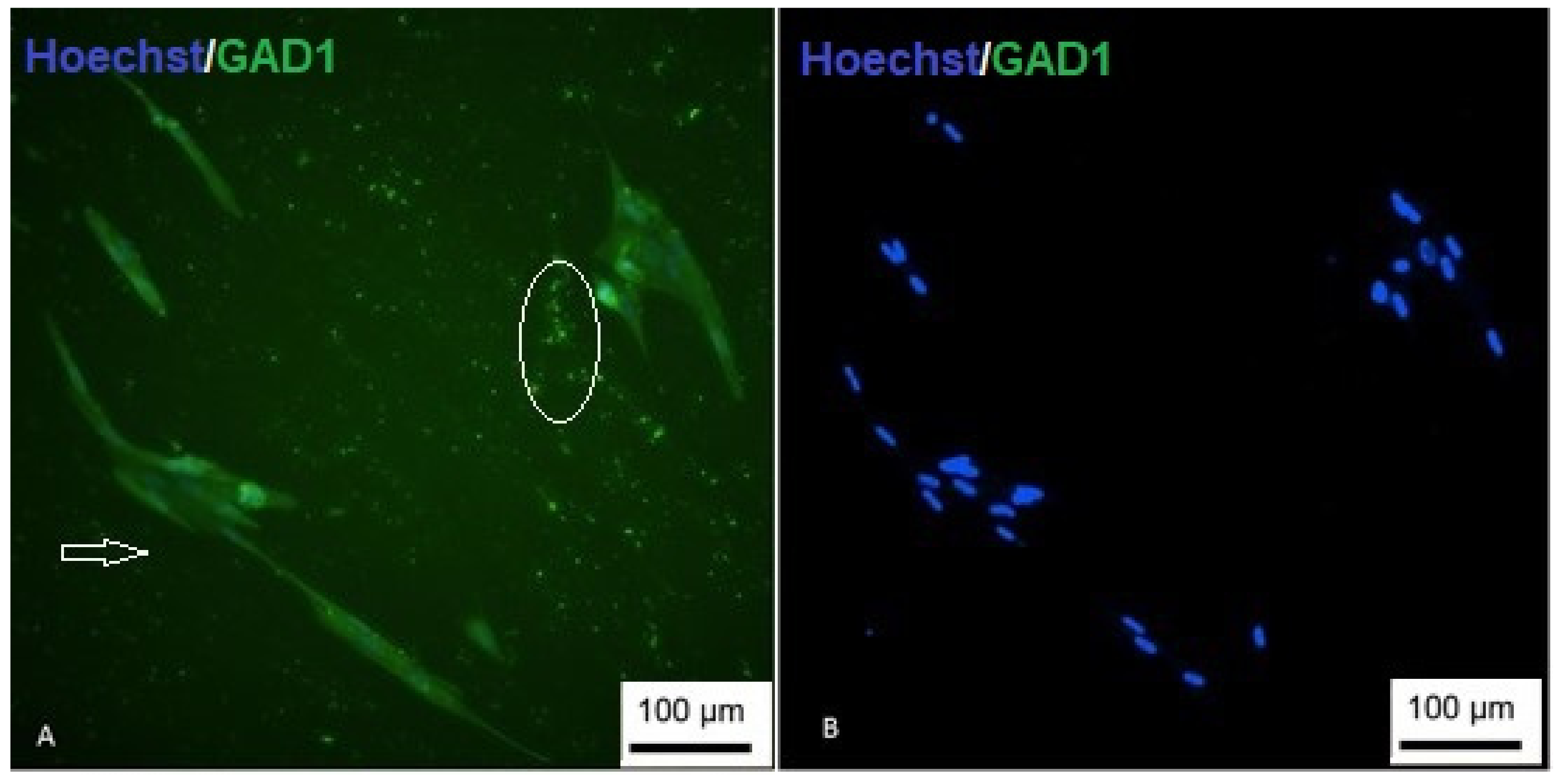
3.8. Differentiation into Gabaergic-like Neurons
3.9. Differentiation into Dopaminergic-Like Neurons
3.10. Differentiation into Oligodendrocytes
3.11. Differentiation into Schwann Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem Cells: Past, Present, and Future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef]
- Wang, X. Stem Cells in Tissues, Organoids, and Cancers. Cell Mol. Life Sci. 2019, 76, 4043–4070. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, A.G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef] [PubMed]
- Main, B.J.; Maffulli, N.; Valk, J.A.; Rodriguez, H.C.; Gupta, M.; El-Amin, S.F.; Gupta, A. Umbilical Cord-Derived Wharton’s Jelly for Regenerative Medicine Applications: A Systematic Review. Pharmaceuticals 2021, 14, 1090. [Google Scholar] [CrossRef] [PubMed]
- Alatyyat, S.M.; Alasmari, H.M.; Aleid, O.A.; Abdel-Maksoud, M.S.; Elsherbiny, N. Umbilical Cord Stem Cells: Background, Processing and Applications. Tissue Cell 2020, 65, 101351. [Google Scholar] [CrossRef]
- Cui, Y.; Ma, S.; Zhang, C.; Cao, W.; Liu, M.; Li, D.; Lv, P.; Xing, Q.; Qu, R.; Yao, N.; et al. Human Umbilical Cord Mesenchymal Stem Cells Transplantation Improves Cognitive Function in Alzheimer’s Disease Mice by Decreasing Oxidative Stress and Promoting Hippocampal Neurogenesis. Behav. Brain Res. 2017, 320, 291–301. [Google Scholar] [CrossRef]
- Boroujeni, M.E.; Gardaneh, M. Umbilical Cord: An Unlimited Source of Cells Differentiable towards Dopaminergic Neurons. Neural Regen. Res. 2017, 12, 1186–1192. [Google Scholar] [CrossRef]
- Kouchakian, M.-R.; Koruji, M.; Najafi, M.; Moniri, S.F.; Asgari, A.; Shariatpanahi, M.; Moosavi, S.A.; Asgari, H.R. Human Umbilical Cord Mesenchymal Stem Cells Express Cholinergic Neuron Markers during Co-Culture with Amniotic Membrane Cells and Retinoic Acid Induction. Med. J. Islam. Repub. Iran 2021, 35, 129. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Li, M.; Zhang, X.; Du, J.; Zhao, X.; Xu, Z.; Lin, J. The Extracts of Human Fetal Brain Induce the Differentiation of Human Umbilical Cord Mesenchymal Stem Cells into Dopaminergic Neuron Containing Cells. Cell Reprogram. 2020, 22, 254–261. [Google Scholar] [CrossRef]
- De Oliveira, N.B.; Irioda, A.C.; Stricker, P.E.F.; Mogharbel, B.F.; da Rosa, N.N.; Dziedzic, D.S.M.; de Carvalho, K.A.T. Natural Membrane Differentiates Human Adipose-Derived Mesenchymal Stem Cells to Neurospheres by Mechanotransduction Related to YAP and AMOT Proteins. Membranes 2021, 11, 687. [Google Scholar] [CrossRef] [PubMed]
- Stricker, P.E.F.; de Souza Dobuchak, D.; Irioda, A.C.; Mogharbel, B.F.; Franco, C.R.C.; de Souza Almeida Leite, J.R.; de Araújo, A.R.; Borges, F.A.; Herculano, R.D.; de Oliveira Graeff, C.F.; et al. Human Mesenchymal Stem Cells Seeded on the Natural Membrane to Neurospheres for Cholinergic-like Neurons. Membranes 2021, 11, 598. [Google Scholar] [CrossRef]
- Cho, H.; Seo, Y.-K.; Jeon, S.; Yoon, H.-H.; Choi, Y.-K.; Park, J.-K. Neural Differentiation of Umbilical Cord Mesenchymal Stem Cells by Sub-Sonic Vibration. Life Sci. 2012, 90, 591–599. [Google Scholar] [CrossRef]
- Sibov, T.T.; Severino, P.; Marti, L.C.; Pavon, L.F.; Oliveira, D.M.; Tobo, P.R.; Campos, A.H.; Paes, A.T.; Amaro, E.; Gamarra, L.F.; et al. Mesenchymal Stem Cells from Umbilical Cord Blood: Parameters for Isolation, Characterization and Adipogenic Differentiation. Cytotechnology 2012, 64, 511–521. [Google Scholar] [CrossRef]
- Tondreau, T.; Meuleman, N.; Delforge, A.; Dejeneffe, M.; Leroy, R.; Massy, M.; Mortier, C.; Bron, D.; Lagneaux, L. Mesenchymal Stem Cells Derived from CD133-Positive Cells in Mobilized Peripheral Blood and Cord Blood: Proliferation, Oct4 Expression, and Plasticity. Stem Cells 2005, 23, 1105–1112. [Google Scholar] [CrossRef]
- Irioda, A.C.; Cassilha, R.; Zocche, L.; Francisco, J.C.; Cunha, R.C.; Ferreira, P.E.; Guarita-Souza, L.C.; Ferreira, R.J.; Mogharbel, B.F.; Garikipati, V.N.S.; et al. Human Adipose-Derived Mesenchymal Stem Cells Cryopreservation and Thawing Decrease A4-Integrin Expression. Stem Cells Int. 2016, 2016, e2562718. [Google Scholar] [CrossRef]
- Adib, S.; Tiraihi, T.; Darvishi, M.; Taheri, T.; Kazemi, H. Cholinergic Differentiation of Neural Stem Cells Generated from Cell Aggregates-Derived from Human Bone Marrow Stromal Cells. Tissue Eng. Regen. Med. 2015, 12, 43–52. [Google Scholar] [CrossRef]
- Ali, H.; Bayatti, N.; Lindsay, S.; Dashti, A.A.; Al-Mulla, F. Directed Differentiation of Umbilical Cord Blood Stem Cells into Cortical GABAergic Neurons. Acta Neurobiol. Exp. (Wars) 2013, 73, 250–259. [Google Scholar]
- Trzaska, K.A.; Rameshwar, P. Dopaminergic Neuronal Differentiation Protocol for Human Mesenchymal Stem Cells. Methods Mol. Biol. 2011, 698, 295–303. [Google Scholar] [CrossRef]
- Zhang, H.-T.; Fan, J.; Cai, Y.-Q.; Zhao, S.-J.; Xue, S.; Lin, J.-H.; Jiang, X.-D.; Xu, R.-X. Human Wharton’s Jelly Cells Can Be Induced to Differentiate into Growth Factor-Secreting Oligodendrocyte Progenitor-like Cells. Differentiation 2010, 79, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Kingham, P.J.; Mantovani, C.; Terenghi, G. Notch Independent Signalling Mediates Schwann Cell-like Differentiation of Adipose Derived Stem Cells. Neurosci. Lett. 2009, 467, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Salehi, H.; Amirpour, N.; Niapour, A.; Razavi, S. An Overview of Neural Differentiation Potential of Human Adipose Derived Stem Cells. Stem Cell Rev. Rep. 2016, 12, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Roh, E.Y.; Shin, S.; Jung, N.H.; Song, E.Y.; Chang, J.Y.; Kim, B.J.; Jeon, H.W. Comparison of Explant-Derived and Enzymatic Digestion-Derived MSCs and the Growth Factors from Wharton’s Jelly. Biomed Res. Int. 2013, 2013, 428726. [Google Scholar] [CrossRef]
- Kawasaki-Oyama, R.S.; Braile, D.M.; Caldas, H.C.; Leal, J.C.F.; Goloni-Bertollo, E.M.; Pavarino-Bertelli, É.C.; Abbud Filho, M.; Santos, I.D. Blood Mesenchymal Stem Cell Culture from the Umbilical Cord with and without Ficoll-Paque Density Gradient Method. Braz. J. Cardiovasc. Surg. 2008, 23, 29–34. [Google Scholar] [CrossRef]
- Torres, E.A.; Ramalho, R.B. O complexo HLA. Ciência Educ. 1996, 2, 70–76. [Google Scholar]
- Mishra, V.K.; Shih, H.-H.; Parveen, F.; Lenzen, D.; Ito, E.; Chan, T.-F.; Ke, L.-Y. Identifying the Therapeutic Significance of Mesenchymal Stem Cells. Cells 2020, 9, 1145. [Google Scholar] [CrossRef]
- Rodríguez-Fuentes, D.E.; Fernández-Garza, L.E.; Samia-Meza, J.A.; Barrera-Barrera, S.A.; Caplan, A.I.; Barrera-Saldaña, H.A. Mesenchymal Stem Cells Current Clinical Applications: A Systematic Review. Arch. Med. Res. 2021, 52, 93–101. [Google Scholar] [CrossRef]
- Xie, Q.; Liu, R.; Jiang, J.; Peng, J.; Yang, C.; Zhang, W.; Wang, S.; Song, J. What Is the Impact of Human Umbilical Cord Mesenchymal Stem Cell Transplantation on Clinical Treatment? Stem Cell Res. Ther. 2020, 11, 519. [Google Scholar] [CrossRef]
- Reyhani, S.; Abbaspanah, B.; Mousavi, S.H. Umbilical Cord-Derived Mesenchymal Stem Cells in Neurodegenerative Disorders: From Literature to Clinical Practice. Regen. Med. 2020, 15, 1561–1578. [Google Scholar] [CrossRef]
- Shareghi-Oskoue, O.; Aghebati-Maleki, L.; Yousefi, M. Transplantation of Human Umbilical Cord Mesenchymal Stem Cells to Treat Premature Ovarian Failure. Stem Cell Res. Ther. 2021, 12, 454. [Google Scholar] [CrossRef]
- Romanov, Y.A.; Svintsitskaya, V.A.; Smirnov, V.N. Searching for Alternative Sources of Postnatal Human Mesenchymal Stem Cells: Candidate MSC-like Cells from Umbilical Cord. Stem Cells 2003, 21, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Wexler, S.A.; Donaldson, C.; Denning-Kendall, P.; Rice, C.; Bradley, B.; Hows, J.M. Adult Bone Marrow Is a Rich Source of Human Mesenchymal “stem” Cells but Umbilical Cord and Mobilized Adult Blood Are Not. Br. J. Haematol. 2003, 121, 368–374. [Google Scholar] [CrossRef]
- Bieback, K.; Kern, S.; Klüter, H.; Eichler, H. Critical Parameters for the Isolation of Mesenchymal Stem Cells from Umbilical Cord Blood. Stem Cells 2004, 22, 625–634. [Google Scholar] [CrossRef]
- Gang, E.J.; Hong, S.H.; Jeong, J.A.; Hwang, S.H.; Kim, S.W.; Yang, I.H.; Ahn, C.; Han, H.; Kim, H. In Vitro Mesengenic Potential of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells. Biochem. Biophys. Res. Commun. 2004, 321, 102–108. [Google Scholar] [CrossRef]
- Bahlakeh, G.; Rahbarghazi, R.; Abedelahi, A.; Sadigh-Eteghad, S.; Karimipour, M. Neurotrophic Factor-Secreting Cells Restored Endogenous Hippocampal Neurogenesis through the Wnt/β-Catenin Signaling Pathway in AD Model Mice. Stem Cell Res. Ther. 2022, 13, 343. [Google Scholar] [CrossRef]
- Hijroudi, F.; Rahbarghazi, R.; Sadigh-Eteghad, S.; Bahlakeh, G.; Hassanpour, M.; Shimia, M.; Karimipour, M. Neural Stem Cells Secretome Increased Neurogenesis and Behavioral Performance and the Activation of Wnt/β-Catenin Signaling Pathway in Mouse Model of Alzheimer’s Disease. Neuromolecular Med. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Pruszak, J.; Ludwig, W.; Blak, A.; Alavian, K.; Isacson, O. CD15, CD24, and CD29 Define a Surface Biomarker Code for Neural Lineage Differentiation of Stem Cells. Stem Cells 2009, 27, 2928–2940. [Google Scholar] [CrossRef] [Green Version]
















| Tube | Content |
|---|---|
| 1 | Unmarked cells |
| 2 | Isotypic control |
| 3 | CD34 FITC/CD271 PE/7-AAD PERCP/CD45 PECY-7 |
| 4 | CD34 FITC/CD29 PE/7-AAD PERCP/CD45 PECY-7 |
| 5 | CD73 FITC/CD105 PE/7-AAD PERCP/CD45 PECY-7/CD90 APC |
| Antibodies | Manufactured |
|---|---|
| Anti-CHAT | Merck Millipore®, Burlington, MA, USA |
| Anti-β Tubulin III | Sigma-Aldrich®, St. Louis, MO, USA |
| Anti-O4 | R&D System®, Minneapolis, MN, USA |
| Anti-GAD1/GAD67 | R&D System®, Minneapolis, MN, USA |
| Anti-tyrosine hydroxylase (TH) | Sigma-Aldrich®, St. Louis, MO, USA |
| Anti-NGFR p75 | Sigma-Aldrich®, St. Louis, MO, USA |
| Anti-S100β | Sigma-Aldrich®, St. Louis, MO, USA |
| Anti-Nestin | Sigma-Aldrich®, St. Louis, MO, USA |
| Gene | Sequence (5′-3′) | Annealing Temperature | Amplifier Size |
|---|---|---|---|
| MAP2/F | GCTAAATCGTAAGTGAGGGCTG | 60 °C | 241 bp |
| MAP2/R | TGGCTCTCTGGCTCTCTAGC | 60 °C | 241 bp |
| TUBB3/F | GGAGATCGTGCACATCCAGG | 62 °C | 385 bp |
| TUBB3/R | CAGGCAGTCGCAGTTTTCAC | 62 °C | 385 bp |
| NES/F | AACAGCGACGGAGGTCTCTA | 58 °C | 220 bp |
| NES/R | TTCTCTTGTCCCGCAGACTT | 58 °C | 220 bp |
| GFAP/F | CTCACCAAATTCCACCCGCA | 60 °C | 769 bp |
| GFAP/R | ACCGCACACAGTACCTGAAG | 60 °C | 769 bp |
| ACTB/F | CTGGGACGACATGGAGAAAA | 56 °C | 564 bp |
| ACTB/R | AAGGAAGGCTGGAAGAGTGC | 56 °C | 564 bp |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza Dobuchak, D.; Stricker, P.E.F.; de Oliveira, N.B.; Mogharbel, B.F.; da Rosa, N.N.; Dziedzic, D.S.M.; Irioda, A.C.; Athayde Teixeira de Carvalho, K. The Neural Multilineage Differentiation Capacity of Human Neural Precursors from the Umbilical Cord—Ready to Bench for Clinical Trials. Membranes 2022, 12, 873. https://doi.org/10.3390/membranes12090873
de Souza Dobuchak D, Stricker PEF, de Oliveira NB, Mogharbel BF, da Rosa NN, Dziedzic DSM, Irioda AC, Athayde Teixeira de Carvalho K. The Neural Multilineage Differentiation Capacity of Human Neural Precursors from the Umbilical Cord—Ready to Bench for Clinical Trials. Membranes. 2022; 12(9):873. https://doi.org/10.3390/membranes12090873
Chicago/Turabian Stylede Souza Dobuchak, Daiany, Priscila Elias Ferreira Stricker, Nathalia Barth de Oliveira, Bassam Felipe Mogharbel, Nádia Nascimento da Rosa, Dilcele Silva Moreira Dziedzic, Ana Carolina Irioda, and Katherine Athayde Teixeira de Carvalho. 2022. "The Neural Multilineage Differentiation Capacity of Human Neural Precursors from the Umbilical Cord—Ready to Bench for Clinical Trials" Membranes 12, no. 9: 873. https://doi.org/10.3390/membranes12090873
APA Stylede Souza Dobuchak, D., Stricker, P. E. F., de Oliveira, N. B., Mogharbel, B. F., da Rosa, N. N., Dziedzic, D. S. M., Irioda, A. C., & Athayde Teixeira de Carvalho, K. (2022). The Neural Multilineage Differentiation Capacity of Human Neural Precursors from the Umbilical Cord—Ready to Bench for Clinical Trials. Membranes, 12(9), 873. https://doi.org/10.3390/membranes12090873








