Thermo-Statistical Effects of Inclusions on Vesicles: Division into Multispheres and Polyhedral Deformation
Abstract
1. Introduction
2. Total Energy of Vesicles
3. Cooperativeness of Elastic Energy of Membrane and Free Energy of Inclusions
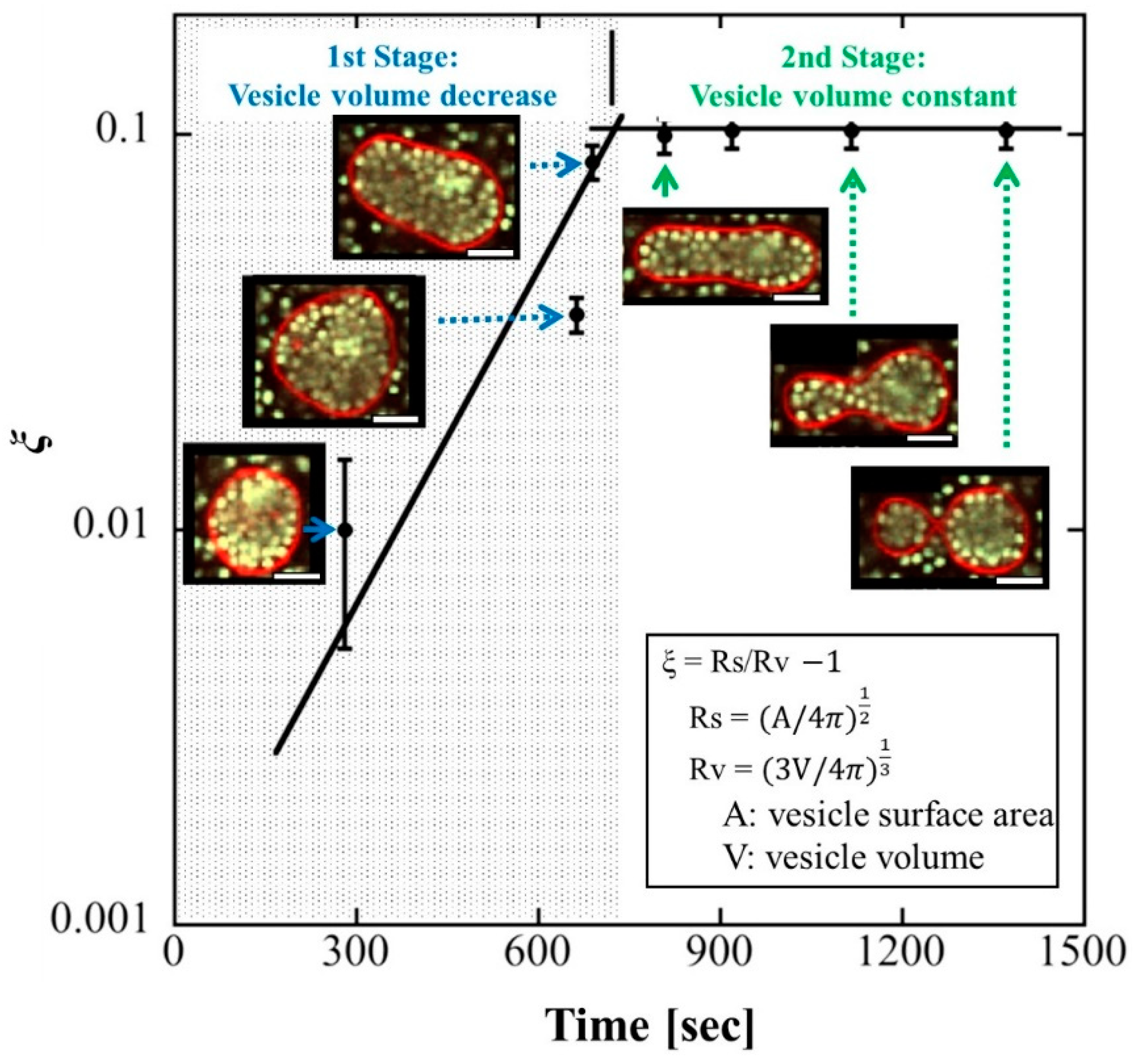
4. Division of Vesicles with Densely Encapsulated Microspheres
4.1. Change in Vesicle Volume
4.2. Division Corresponding to the Area-to-Volume Ratio
4.3. Restriction of Water Outflow by Inclusions
5. Polyhedral Deformation of Vesicles Encapsulating Microspheres at Specific Volume Fractions
5.1. Phase Separation from the Perspective of Alder Transition
5.2. Coexistence of Ordered and Disordered Phases of Microspheres in Vesicles
6. Conclusions
7. Future Directions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Ellis, R.J.; Minton, A.P. Join the crowd. Nature 2003, 425, 27–28. [Google Scholar] [CrossRef] [PubMed]
- Minton, A.P. The influence of macromolecular crowding and macromolecular confinement on biochemical reactions in physiological media. J. Biol. Chem. 2001, 276, 10577–10580. [Google Scholar] [CrossRef] [PubMed]
- Vibhute, M.A.; Schaap, M.H.; Maas, R.J.M.; Nelissen, F.H.T.; Spruijt, E.; Heus, H.A.; Hansen, M.M.K.; Huck, W.T.S. Transcription and translation in cytomimetic protocells perform most efficiently at distinct macromolecular crowding conditions. ACS Synth. Biol. 2020, 9, 2797–2807. [Google Scholar] [CrossRef]
- Garenne, D.; Noireaux, V. Analysis of cytoplasmic and membrane molecular crowding in genetically programmed synthetic cells. Biomacromolecules 2020, 21, 2808–2817. [Google Scholar] [CrossRef]
- Sheetz, M.; Yu, H. The Cell as a Machine; Cambridge University Press: Cambridge, UK, 2018; Part I, Chapter 1. [Google Scholar]
- Harry, E.J. Bacterial cell division: Regulating Z-ring formation. Microbiology 2001, 40, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Leaver, M.; Dominguez-Cuevas, P.; Coxhead, J.M.; Daniel, R.A.; Errington, J. Life without a wall or division machine in Bacillus subtilis. Nature 2009, 457, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Szostak, J.W.; Bartel, D.P.; Luisi, P.L. Synthesizing life. Nature 2001, 409, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Luisi, P.L. The Minimal Cell: The Biophysics of Cell Compartment and the Origin of Cell Functionality; Luisi, P.L., Stano, P., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 171–287. [Google Scholar]
- Luisi, P.L. The Emergence of Life: From Chemical Origins to Synthetic Biology; Cambridge University Press: Cambridge, UK, 2016; Chapters 11–12. [Google Scholar]
- Rasmussen, S.; Bedau, M.A.; Chen, L.; Deamer, D.; Krakauer, D.C.; Packard, N.H.; Stadler, P.F. Protocells: Bridging Nonliving and Living Matter; The MIT Press: Cambridge, MA, USA, 2008; Chapter 6. [Google Scholar]
- Deamer, D.; Szostak, J.W.; Rich, A.A. The Origin of Life; Cold Spring Harbor Laboratory Press: Long Island, NY, USA, 2010; pp. 179–191. [Google Scholar]
- Nakaya, K.; Imai, M.; Komura, S.; Kawakatsu, T.; Urakami, N. Polymer-confinement-induced nematic transition of microemulsion droplets. Europhys. Lett. 2005, 71, 494–500. [Google Scholar] [CrossRef][Green Version]
- Dinsmore, A.D.; Wong, D.T.; Nelson, P.; Yodh, A.G. Hard Spheres in Vesicles: Curvature-Induced Forces and Particle-Induced Curvature. Phy. Rev. Lett. 1998, 80, 409–412. [Google Scholar] [CrossRef]
- Dimova, R.; Marques, C. The Giant Vesicle Book; CRC Press: London, UK, 2019; Chapter 5.3; pp. 79–83. [Google Scholar]
- Natsume, Y.; Pravaz, O.; Yoshida, H.; Imai, M. Shape deformation of giant vesicles encapsulating charged colloidal particles. Soft Matter 2010, 6, 5359–5366. [Google Scholar] [CrossRef]
- Natsume, Y.; Toyota, T. Giant vesicles containing microspheres with high volume fraction prepared by water-in-oil emulsion centrifugation. Chem. Lett. 2013, 42, 295–297. [Google Scholar] [CrossRef]
- Natsume, Y.; Wen, H.I.; Zhu, T.; Itoh, K.; Sheng, L.; Kurihara, K. Preparation of giant vesicles encapsulating microspheres by centrifugation of a water-in-oil emulsion. J. Vis. Exp. 2017, 119, e55282. [Google Scholar] [CrossRef] [PubMed]
- Natsume, Y.; Toyota, T. Asymmetrical polyhedral configuration of giant vesicles induced by orderly array of encapsulated colloidal particles. PLoS ONE 2016, 11, e0146683. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Natsume, Y.; Toyota, T. Appearance of crystalline pattern for colloidal particles encapsulated in giant vesicles -direct cross-sectional observation of ordered and disordered phases. Trans. Mat. Res. Soc. Japan 2016, 41, 147–149. [Google Scholar] [CrossRef][Green Version]
- Itoga, H.; Morikawa, R.; Ueta, T.; Miyakawa, T.; Natsume, Y.; Takasu, M. Effect of particles with repulsive interactions enclosed in both rigid spherical shells and flexible fluid vesicles studied by Monte Carlo simulation. Phys. Rev. E 2019, 99, 042418. [Google Scholar] [CrossRef] [PubMed]
- Terasawa, H.; Nishimura, K.; Suzuki, H.; Matsuura, T.; Yomo, T. Coupling of the fusion and budding of giant phospholipid vesicles containing macromolecules. Proc. Natl. Acad. Sci. USA 2012, 109, 5942–5947. [Google Scholar] [CrossRef]
- Briers, Y.; Walde, P.; Schuppler, M.; Loessner, M.J. How did bacterial ancestors reproduce? Lessons from L-form cells and giant lipid vesicles. Bioessays 2012, 34, 1078–1084. [Google Scholar] [CrossRef]
- Errington, J. L-form bacteria, cell walls and the origins of life. Open Biol. 2013, 3, 120143. [Google Scholar] [CrossRef]
- Romain, M.; Kawai, Y.; Errington, J. Excess membrane synthesis drives a primitive mode of cell proliferation. Cell 2013, 152, 997–1007. [Google Scholar]
- Osawa, M.; Erickson, H.P. Liposome division by a simple bacterial division machinery. Proc. Natl. Acad. Sci. USA 2013, 110, 11000–11004. [Google Scholar] [CrossRef]
- Godino, E.; López, J.N.; Zarguit, I.; Doerr, A.; Jimenez, M.; Rivas, G.; Danelon, C. Cell-free biogenesis of bacterial division proto-rings that can constrict liposomes. Commun. Biol. 2020, 3, 539. [Google Scholar] [CrossRef] [PubMed]
- Okano, T.; Inoue, K.; Koseki, K.; Suzuki, H. Deformation modes of giant unilamellar vesicles encapsulating biopolymers. ACS Synth. Biol. 2018, 7, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Tsugane, M.; Suzuki, H. Elucidating the membrane dynamics and encapsulation mechanism of large DNA molecules under molecular crowding conditions using giant unilamellar vesicles. ACS Synth. Biol. 2020, 9, 2819–2827. [Google Scholar] [CrossRef] [PubMed]
- Olivi, L.; Berger, M.; Creyghton, R.N.P.; Franceschi, N.D.; Dekker, C.; Mulder, B.M.; Claassens, N.J.; Wolde, P.R.; Oost, J. Towards a synthetic cell cycle. Nat. Commun. 2021, 12, 4531. [Google Scholar] [CrossRef]
- Hotani, H. Transformation pathways of liposomes. J. Mol. Biol. 1984, 178, 113–120. [Google Scholar] [CrossRef]
- Hotani, H.; Inada, T.; Nomura, F.; Takeda, S.; Takiguchi, K.; Itoh, T.J.; Umeda, T.; Ishijima, A. Mechanical analyses of morphological and topological transformation of liposomes. Biosystems 2003, 71, 93–100. [Google Scholar] [CrossRef]
- Berndl, K.; Kas, J.; Lipowsky, R.; Sackmann, E.; Seifert, U. Shape transformations of giant vesicles: Extreme sensitivity to bilayer asymmetry. Europhys. Lett. 1990, 13, 659–664. [Google Scholar] [CrossRef]
- Kas, J.; Sackmann, E. Shape transitions and shape stability of giant phospholipid vesicles in pure water induced by area-to-volume changes. Biophys. J. 1991, 60, 825–844. [Google Scholar] [CrossRef]
- Stoicheva, N.G.; Hui, S.W. Electrofusion of cell-size liposomes. Biochim. Biophys. Acta 1994, 1195, 31–38. [Google Scholar] [CrossRef]
- Miao, L.; Seifert, B.U.; Wortis, M.; Deobereiner, H.-G. Budding transitions of fluid-bilayer vesicles: The effect of area-difference elasticity. Phys. Rev. E 1994, 49, 5389–5407. [Google Scholar] [CrossRef]
- Seifert, U. Configurations of fluid membranes and vesicles. Adv. Phys. 1997, 46, 13–137. [Google Scholar] [CrossRef]
- Zoetekouw, B.; van Roij, R. Volume terms for charged colloids: A grand-canonical treatment. Phys. Rev. E 2006, 73, 021403. [Google Scholar] [CrossRef] [PubMed]
- Brukhno, A.V.; Akesson, T.; Jonsson, B. Phase behavior in suspensions of highly charged colloids. J. Phys. Chem. B 2009, 113, 6766–6774. [Google Scholar] [CrossRef] [PubMed]
- Lekkerkerker, H.N.W.; Tuinier, R. Colloids and the Depletion Interaction; Springer: Dordrecht, The Netherlands, 2011; Chapter 1; pp. 1–56. [Google Scholar]
- Bhatia, T.; Christ, S.; Steinkuhler, J.; Dimova, R.; Lipowsky, R. Simple sugars shape giant vesicles into multispheres with many membrane necks. Soft Matter 2020, 16, 1246–1258. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Yanagisawa, M. Generation of giant unilamellar liposomes containing biomacromolecules at physiological intracellular concentrations using hypertonic conditions. ACS Synth. Biol. 2014, 3, 870–874. [Google Scholar] [CrossRef]
- Fujiwara, K.; Yanagisawa, M.; Nomura, S.M. Reconstitution of intracellular environments in vitro and in artificial cells. Biophysics 2014, 10, 43–48. [Google Scholar] [CrossRef][Green Version]
- Jones, R.A.L. Soft Condensed Matter; Oxford University Press: Oxford, UK; New York, NY, USA, 2002; Chapter 4; pp. 49–72. [Google Scholar]
- Sekinger, J.V. Introduction to the Theory of Soft Matter; Springer International Publishing: Cham, Switzerland, 2015; Chapter 8; pp. 91–110. [Google Scholar]
- Pusey, P.N.; Megen, W. Phase behaviour of concentrated suspensions of nearly hard colloidal spheres. Nature 1986, 320, 340–342. [Google Scholar] [CrossRef]
- Ree, F.H.; Hoover, W.G. Seventh virial coefficients for hard spheres and hard disks. J. Chem. Phys. 1967, 46, 4181–4419. [Google Scholar] [CrossRef]
- Sarkar, S.; Das, S.; Dagar, S.; Joshi, M.P.; Mungi, C.V.; Sawant, A.A.; Patki, G.M.; Rajamani, S. Prebiological membranes and their role in the emergence of early cellular life. J. Membr. Biol. 2020, 253, 589–608. [Google Scholar] [CrossRef]
- Vance, J.A.; Devaraj, N.K. Membrane mimetic chemistry in artificial cells. J. Am. Chem. Soc. 2021, 143, 8223–8231. [Google Scholar] [CrossRef]
- Podolsky, K.A.; Devaraj, N.K. Synthesis of lipid membranes for artificial cells. Nat. Rev. Chem. 2021, 5, 676–694. [Google Scholar] [CrossRef]
- Blanken, D.; Foschepoth, D.; Serrão, A.C.; Danelon, C. Genetically controlled membrane synthesis in liposomes. Nat. Commun. 2020, 11, 4317. [Google Scholar] [CrossRef] [PubMed]
- Post, E.A.J.; Fletcher, S.P. Dissipative self-assembly, competition and inhibition in a self-reproducing protocell model. Chem. Sci. 2020, 11, 9434–9442. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Brea, R.J.; Niederholtmeyer, H.; Devaraj, N.K. A minimal biochemical route towards de novo formation of synthetic phospholipid membranes. Nat. Commun. 2019, 10, 300. [Google Scholar] [CrossRef]
- Walde, P.; Goto, A.; Monnard, P.A.; Wessicken, M.; Luisi, P.L. Oparin’s reactions revisited: Enzymatic synthesis of poly(adenylic acid) in micelles and self-reproducing vesicles. J. Am. Chem. Soc. 1994, 116, 7541–7547. [Google Scholar] [CrossRef]
- Hanczyc, M.M.; Fujikawa, S.M.; Szostak, J.W. Experimental models of primitive cellular compartments: Encapsulation, growth, and division. Science 2003, 302, 618–622. [Google Scholar] [CrossRef]
- Ozawa, R.; Natsume, Y. Spontaneous growth and deformation of giant vesicles by adding heterogeneous amphiphilic molecules. In Proceedings of the Physical Society of Japan 2022 Autumn Meeting, Tokyo, Japan, 12–15 September 2022. [Google Scholar]
- Babu, D.; Scanes, J.H.; Plamont, R.; Ryabchun, A.; Lancia, F.; Kudernac, T.; Fletcher, S.P. Acceleration of lipid reproduction by emergence of microscopic motion. Nat. Commun. 2021, 12, 2959. [Google Scholar] [CrossRef]
- Taylor, J.W.; Eghtesadi, S.A.; Points, L.J.; Liu, T.; Cronin, L. Autonomous model protocell division driven by molecular replication. Nat. Commun. 2017, 8, 237. [Google Scholar] [CrossRef]
- Morrows, M.; Colomer, I.; Fletcher, S.P. A chemically fuelled self-replicator. Nat. Commun. 2019, 10, 1011. [Google Scholar] [CrossRef]
- Hollo, G.; Miele, Y.; Rosi, F.; Lagzi, I. Shape changes and budding of giant vesicles induced by an internal chemical trigger: An interplay between osmosis and pH change. Phys. Chem. Chem. Phys. 2021, 23, 4262–4270. [Google Scholar] [CrossRef]
- Vutukuri, H.R.; Hoore, M.; Velasco, C.A.; Buren, L.; Dutto, A.; Auth, T.; Fedosov, D.A.; Gompper, G.; Vermant, J. Active particles induce large shape deformation in giant lipid vesicles. Nature 2020, 586, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Peterson, M.S.E.; Baskaran, A.; Hagan, M.F. Vesicle shape transformations driven by confined active filaments. Nat. Commun. 2021, 12, 7247. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Papoian, G.A. Membrane-MEDIYAN: Simulating deformable vesicles containing complex cytoskeletal networks. J. Phys. Chem. B 2021, 125, 10710–10719. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Nishiyama, M.; Kazayama, Y.; Toyota, T.; Harada, Y.; Takiguchi, K. Reversible morphological control of tubulin encapsulating giant liposome by hydrostatic pressure. Langmuir 2016, 32, 3794–3802. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natsume, Y. Thermo-Statistical Effects of Inclusions on Vesicles: Division into Multispheres and Polyhedral Deformation. Membranes 2022, 12, 608. https://doi.org/10.3390/membranes12060608
Natsume Y. Thermo-Statistical Effects of Inclusions on Vesicles: Division into Multispheres and Polyhedral Deformation. Membranes. 2022; 12(6):608. https://doi.org/10.3390/membranes12060608
Chicago/Turabian StyleNatsume, Yuno. 2022. "Thermo-Statistical Effects of Inclusions on Vesicles: Division into Multispheres and Polyhedral Deformation" Membranes 12, no. 6: 608. https://doi.org/10.3390/membranes12060608
APA StyleNatsume, Y. (2022). Thermo-Statistical Effects of Inclusions on Vesicles: Division into Multispheres and Polyhedral Deformation. Membranes, 12(6), 608. https://doi.org/10.3390/membranes12060608





