High-Efficiency Water Recovery from Urine by Vacuum Membrane Distillation for Space Applications: Water Quality Improvement and Operation Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Urine Preparation
2.3. Membrane Fabrication and Characteristics
2.4. Performance Evaluation of Deactivating Microorganisms
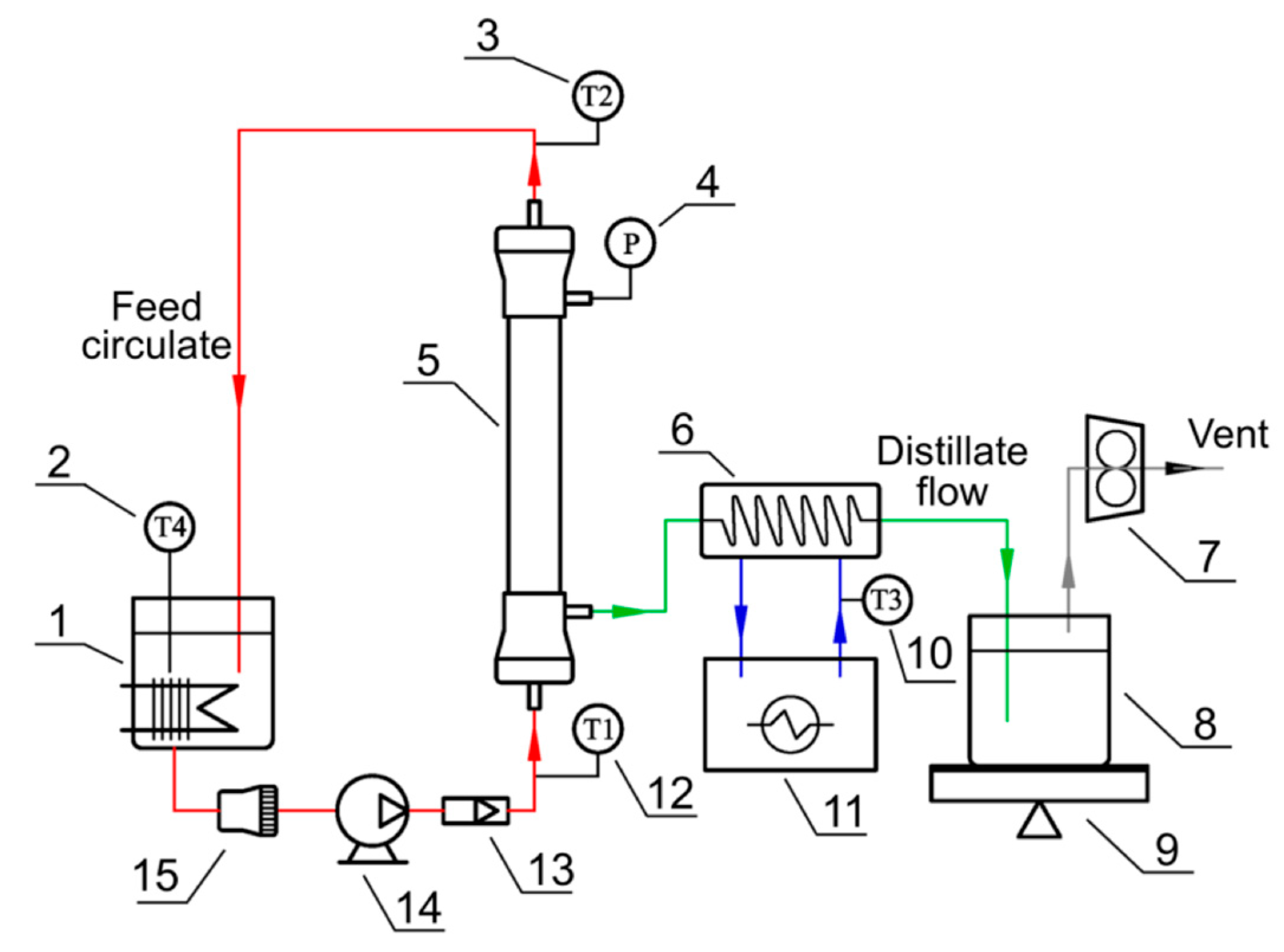
2.5. VMD Experiments and Assessment Methods
2.6. Analytical Methods
3. Results
3.1. Stabilized Urine
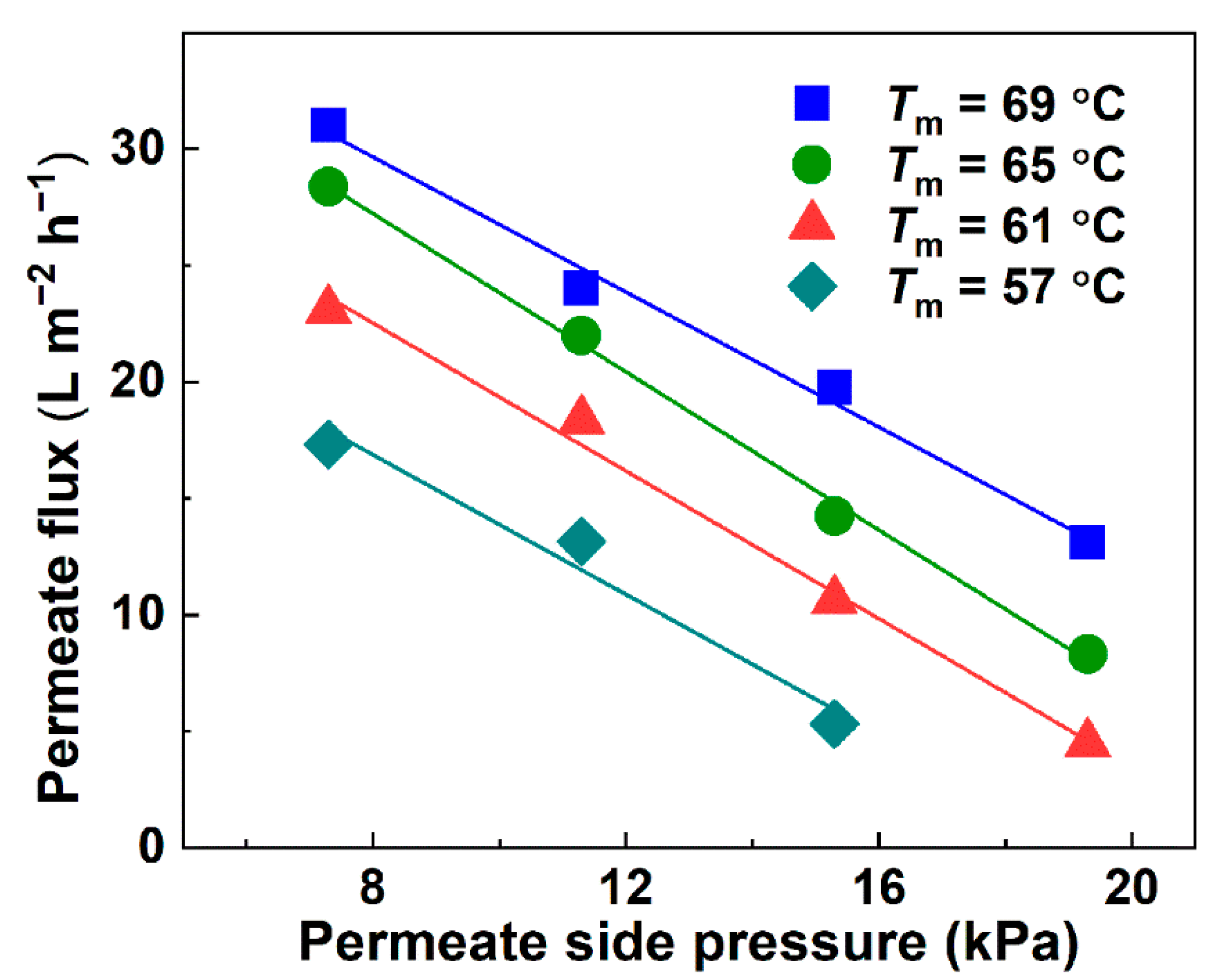
3.2. Influence of Temperature and Permeate Side Pressure on the Permeate Flux
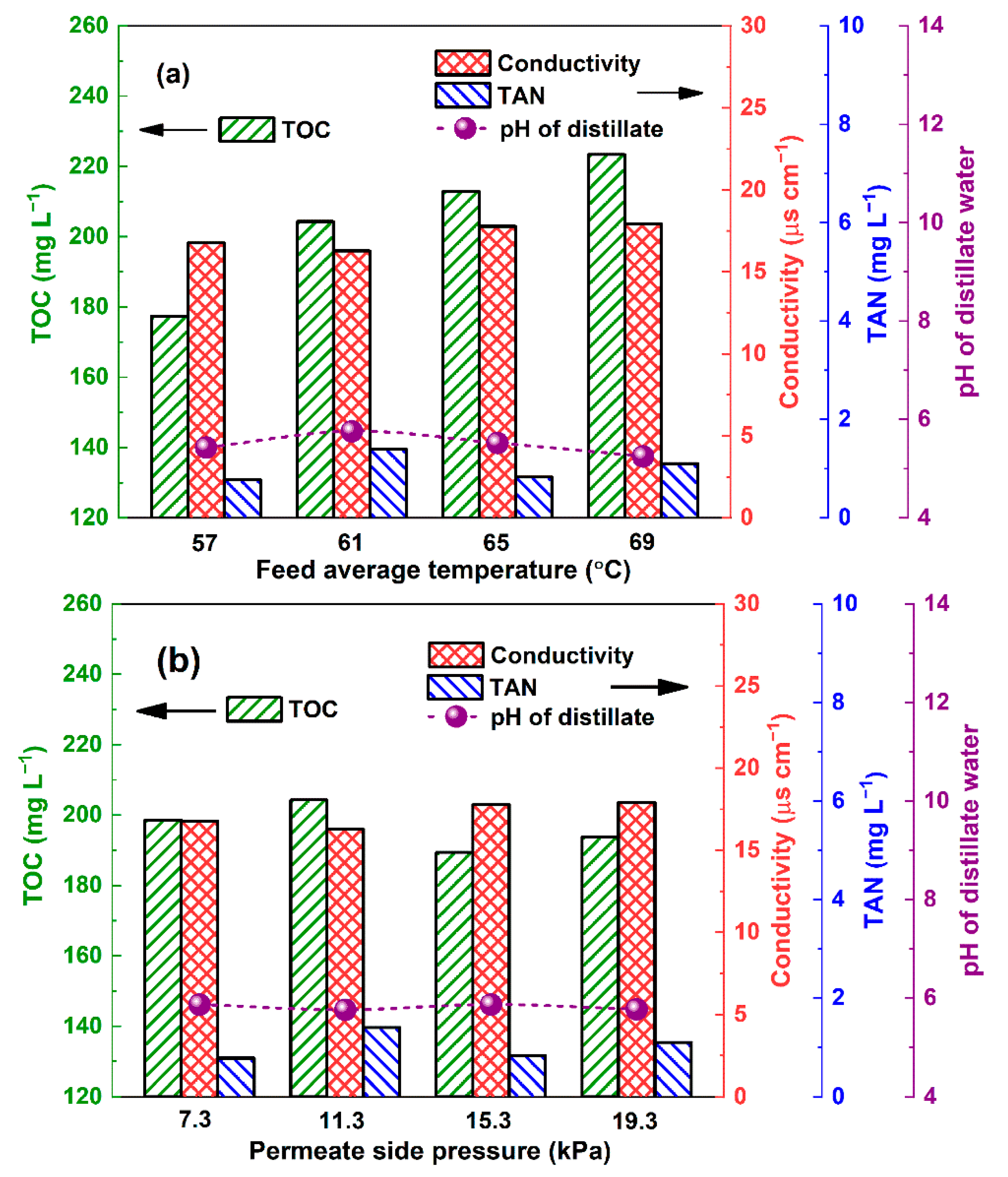
3.3. Influence of Temperature and Permeate Side Pressure on the Distillate Water Quality
3.4. Influence of Volatile Components on the Distillate Water Quality
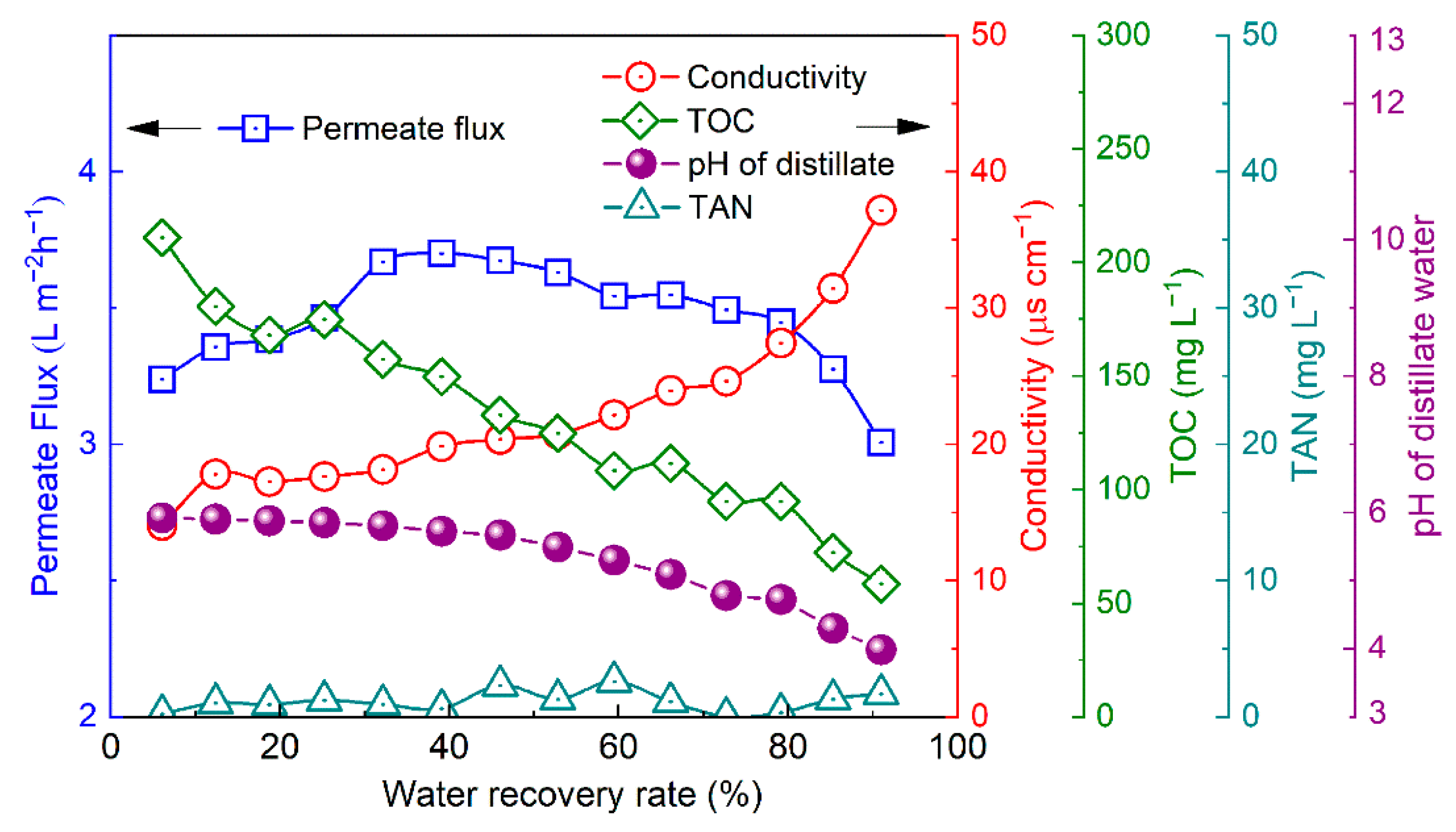
3.5. Influence of Water Recovery Rate on the Distillate Flux and Quality
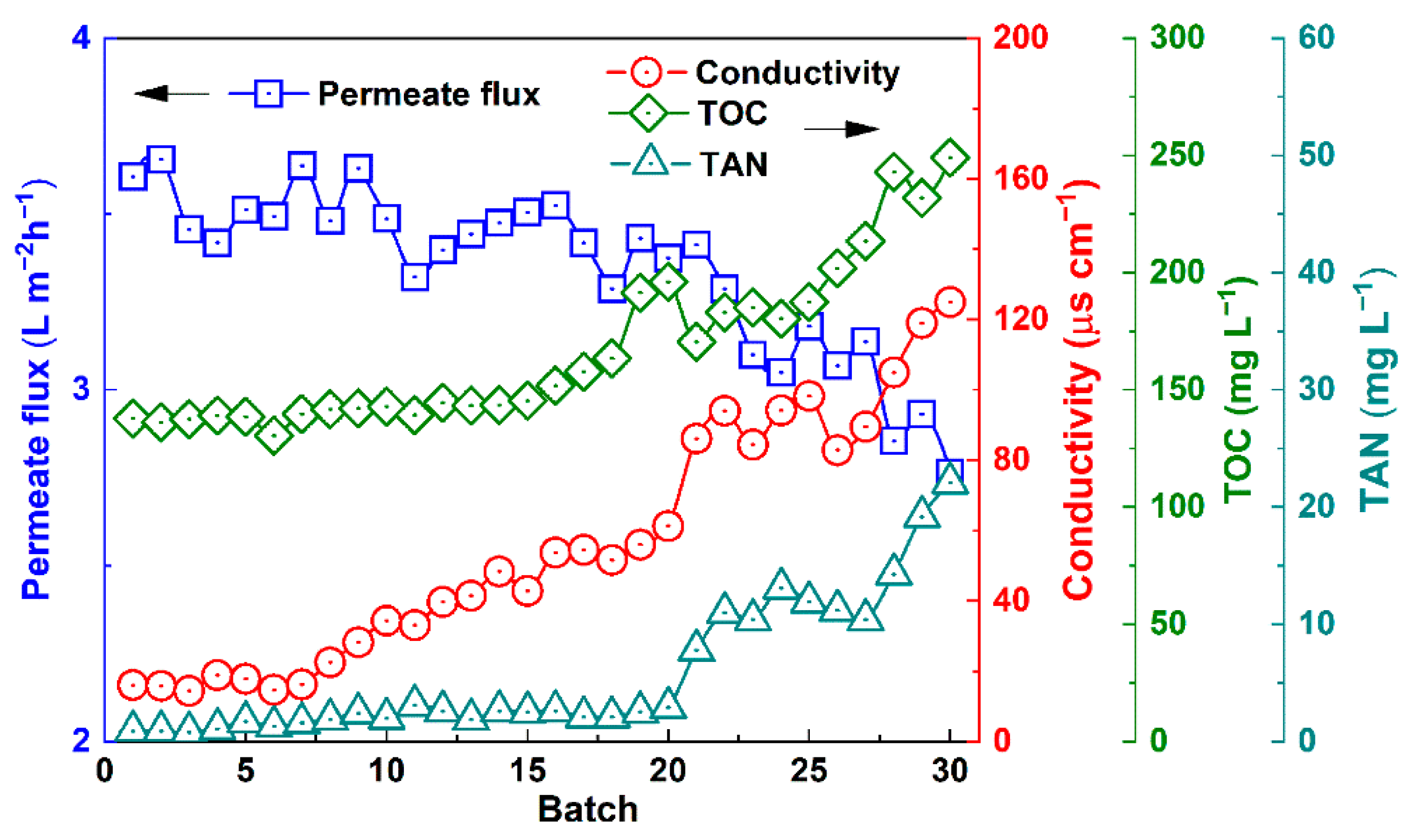
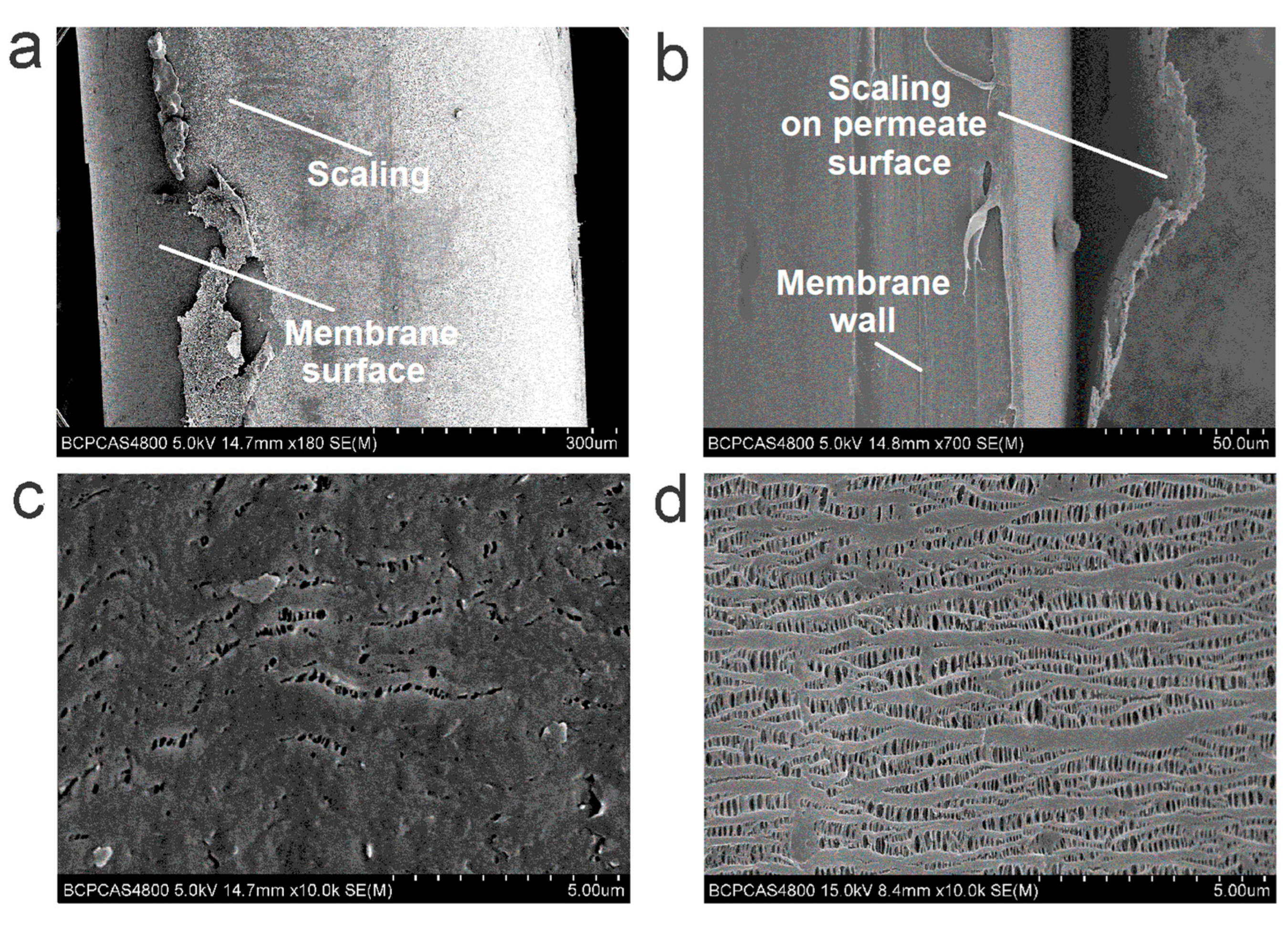
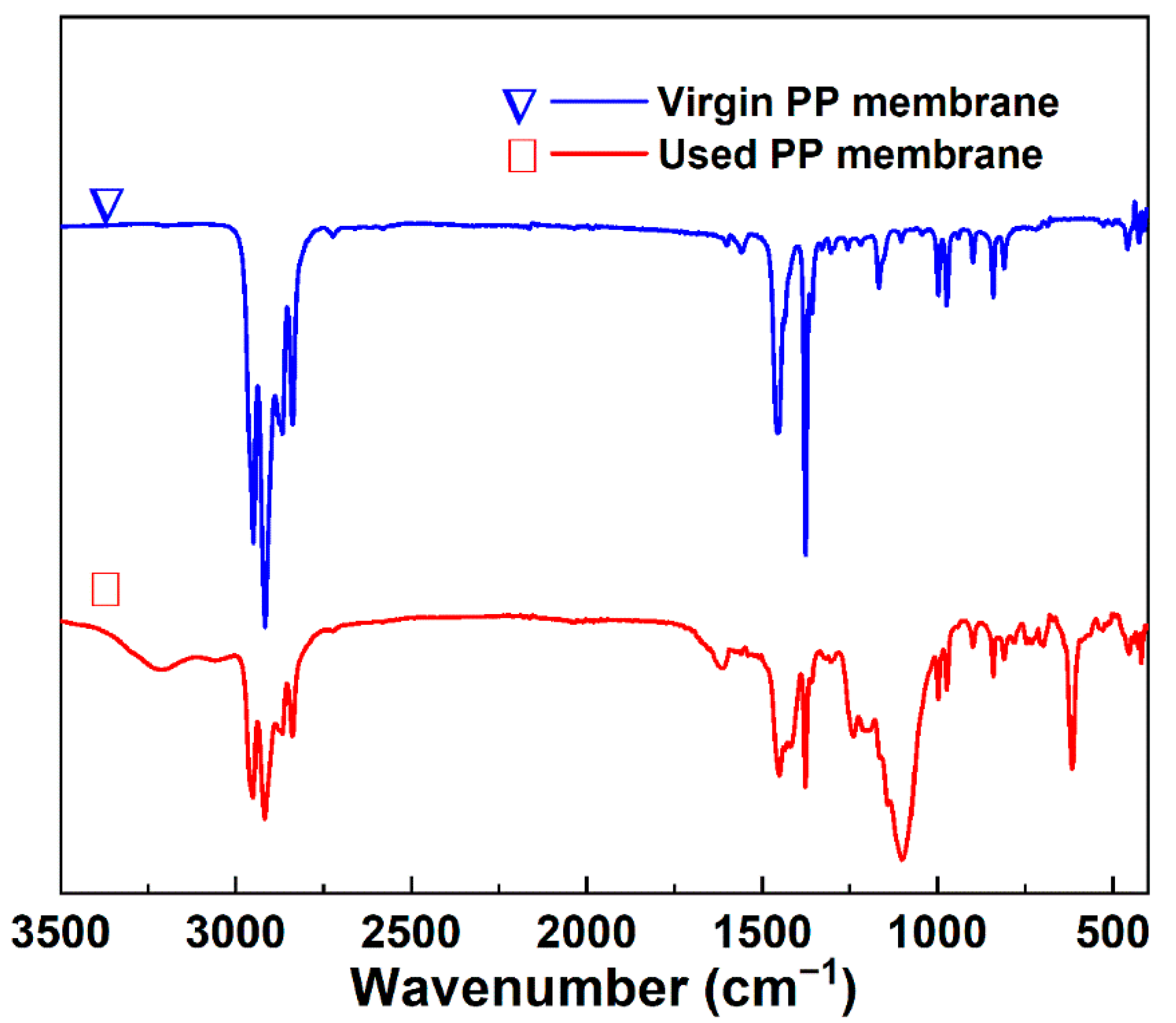
3.6. Operation Stability of Urine VMD
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pickett, M.T.; Roberson, L.B.; Calabria, J.L.; Bullard, T.J.; Turner, G.; Yeh, D.H. Regenerative water purification for space applications, Needs, challenges, and technologies towards ‘closing the loop’. Life Sci. Space Res. 2020, 24, 64–82. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Xie, B.; Liu, H. The recycle of water and nitrogen from urine in bioregenerative life support system. Acta Astronaut. 2016, 123, 86–90. [Google Scholar] [CrossRef]
- Zhang, L.; Li, T.; Ai, W.; Zhang, C.; Tang, Y.; Yu, Q.; Li, Y. Water management in a controlled ecological life support system during a 4-person-180-day integrated experiment, Configuration and performance. Sci. Total Environ. 2019, 651, 2080–2086. [Google Scholar] [CrossRef] [PubMed]
- Volpin, F.; Badeti, U.; Wang, C.; Jiang, J.; Vogel, J.; Freguia, S.; Fam, D.; Cho, J.; Phuntsho, S.; Shon, H.K. Urine treatment on the International Space Station, Current practice and novel approaches. Membranes 2020, 10, 327. [Google Scholar] [CrossRef] [PubMed]
- Leonid, B.; Nikoly, S.; Gavrilov, L.; Novikov, V.; Tomashpolskiy, M.; Andreychuk, P.; Protasov, N.; Synjak, Y.; Skuratov, V. Regenerative water supply for an interplanetary space station, The experience gained on the space stations “Salut”, “Mir”, ISS and development prospects. Acta Astronaut. 2007, 61, 8–15. [Google Scholar]
- Li, H.; Yu, Q.N.; Yang, B.; Li, Z.J.; Lei, L.C. Electro-catalytic oxidation of artificial human urine by using BDD and IrO2 electrodes. J. Electroanal. Chem. 2015, 738, 14–19. [Google Scholar] [CrossRef]
- Antoniou, M.G.; Dionysios, D.D. Application of immobilized titanium dioxide photocatalysts for the degradation of creatinine and phenol, model organic contaminants found in NASA’s spacecrafts wastewater streams. Catal. Today 2007, 124, 215–223. [Google Scholar] [CrossRef]
- Leonard, J.S.; James, E.A. A urease bioreactor for water reclamation aboard manned spacecraft. Chemosphere 1995, 30, 985–994. [Google Scholar]
- Nicolau, E.; Fonseca, J.J.; Rodríguez-Martínez, J.A.; Richardson, T.; Flynn, M.; Griebenow, K.; Cabrera, C.R. Evaluation of a Urea Bioelectrochemical System for Wastewater Treatment Processes. ACS Sustain. Chem. Eng. 2014, 2, 749–754. [Google Scholar] [CrossRef]
- Cath, T.Y.; Gormly, S.; Beaudry, E.G.; Flynn, M.T.; Adams, V.D.; Childress, A.E. Membrane contactor processes for wastewater reclamation in space part I. direct osmotic concentration as pretreatment for reverse osmosis. J. Membr. Sci. 2005, 257, 85–98. [Google Scholar] [CrossRef]
- Cath, T.Y.; Adams, D.; Childress, A.E. Membrane contactor processes for wastewater reclamation in space II. Combined direct osmosis, osmotic distillation, and membrane distillation for treatment of metabolic wastewater. J. Membr. Sci. 2005, 257, 111–119. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, C.; Zhao, L.; Ma, W.C.; Liu, H.L.; Ma, J. Integrated forward osmosis-membrane distillation process for human urine treatment. Water Res. 2016, 91, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wei, Y. Recovery of monovalent mineral salts from urine in controlled ecological life support system by nanofiltration: Feasibility study. Desalination 2020, 479, 114344. [Google Scholar] [CrossRef]
- Madsen, R.F.; Thomassen, J.R.; Vial, D.; Binot, R.A. The proposal for the completely closed system in the Columbus Space Station. Desalination 1991, 83, 123–136. [Google Scholar] [CrossRef]
- Ray, H.; Perreault, F.; Boyer, T.H. Urea recovery from fresh urine by forward osmosis and membrane distillation. Environ. Sci. Water Res. Technol. 2019, 5, 1993–2003. [Google Scholar] [CrossRef]
- Garcia-Castello, E.; Cassano, A.; Criscuoli, A.; Conidi, C.; Drioli, E. Recovery and concentration of polyphenols from olive mill wastewaters by integrated membrane system. Water Res. 2010, 44, 3883–3892. [Google Scholar] [CrossRef]
- Qin, W.; Zhang, J.; Xie, Z.; Ng, D.; Ye, Y.; Gray, S.; Xie, M. Synergistic effect of combined colloidal and organic fouling in membrane distillation, Measurements and mechanisms. Environ. Sci. Water Res. Technol. 2017, 3, 119–127. [Google Scholar] [CrossRef]
- Xu, K.; Qu, D.; Zheng, M.; Guo, X.; Wang, C. Water Reduction and Nutrient Reconcentration of Hydrolyzed Urine via Direct-Contact Membrane Distillation, Ammonia Loss and Its Control. J. Environ. Eng. 2019, 145, 04018144. [Google Scholar] [CrossRef]
- Khumalo, N.; Nthunya, L.; Derese, S.; Motsa, M.; Verliefde, A.; Kuvarega, A.; Mamba, B.B.; Mhlanga, S.; Dlamini, D.S. Water recovery from hydrolysed human urine samples via direct contact membrane distillation using PVDF/PTFE membrane. Sep. Purif. Technol. 2018, 211, 610–617. [Google Scholar] [CrossRef]
- Zhao, Z.P.; Liang, X.; Xin, S.; Chen, K. Water regeneration from human urine by vacuum membrane distillation and analysis of membrane fouling characteristics. Sep. Purif. Technol. 2013, 118, 369–376. [Google Scholar] [CrossRef]
- Bouatra, S.; Aziat, F.; Mandal, R.; An, C.G.; Wilson, M.R.; Knox, C.; Bjorndahl, T.C.; Krishnamurthy, R.; Saleem, F.; Liu, P. The human urine metabolome. PLoS ONE 2018, 8, e73076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grigoriev, A.I.; Sinyak, Y.E.; Samsonov, N.M.; Bobe, L.S.; Protasov, N.N.; Andreychuk, P.O. Regeneration of water at space stations. Acta Astronaut. 2011, 68, 1567–1573. [Google Scholar] [CrossRef]
- Saffar, A.; Carreau, P.J.; Ajji, A.; Kamal, M.R. Influence of Stretching on the Performance of Polypropylene-Based Microporous Membranes. Ind. Eng. Chem. Res. 2014, 53, 14014–14021. [Google Scholar] [CrossRef]
- Drioli, E.; Aamer, A.; Macedonio, F. Membrane distillation: Recent developments and perspectives. Desalination 2015, 356, 56–84. [Google Scholar] [CrossRef]
- Gryta, M. Long-term performance of membrane distillation process. J. Membr. Sci. 2005, 265, 153–159. [Google Scholar] [CrossRef]
- Raluy, R.G.; Schwantes, R.; Subiela, V.J.; Peate, B.; Melián, G.; Betancort, J.R. Operational experience of a solar membrane distillation demonstration plant in Pozo Izquierdo-Gran Canaria Island (Spain). Desalination 2012, 290, 1–13. [Google Scholar] [CrossRef]
- Banat, F.; Al-Asheh, S.; Qtaishat, M. Treatment of waters colored with methylene blue dye by vacuum membrane distillation. Desalination 2004, 174, 87–96. [Google Scholar] [CrossRef]
- Al-asheha, S.; Banat, F.; Qtaishat, M.; Al-khateebd, M. Concentration of sucrose solutions via vacuum membrane distillation. Desalination 2005, 195, 60–68. [Google Scholar] [CrossRef]
- Abu-Zeid, M.A.E.-R.; Zhang, Y.Q.; Dong, H.; Zhang, L.; Chen, H.L.; Hou, L. A comprehensive review of vacuum membrane distillation technique. Desalination 2015, 356, 1–14. [Google Scholar] [CrossRef]
- Ruckdashel, R.; Shim, E. Effects of melt spinning parameters on polypropylene hollow fiber formation. J. Eng. Fibers Fabr. 2020, 15, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Kudelová, T.; Bartuli, E.; Strunga, A.; Hvožd’a, J.; Dohnal, M. Fully Polymeric Distillation Unit Based on Polypropylene Hollow Fibers. Polymers 2021, 13, 1031. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.J.; Wei, F.J.; Wu, B.; Zhang, K.Z.; Yao, Y.; Liang, S.M.; Qin, S.H. Effects of annealing stress field on the structure and properties of polypropylene hollow fiber membranes made by stretching. RSC Adv. 2016, 6, 4271–4279. [Google Scholar] [CrossRef]
- Liu, G.C.; Gao, C.J.; Li, X.M.; Guo, C.G.; Chen, Y.; Lv, J.L. Preparation and properties of porous polytetrafluoroethylene hollow fiber membrane through mechanical operations. J. Appl. Polym. Sci. 2015, 132, 42696. [Google Scholar] [CrossRef]
- Rácz, G.; Kerker, S.; Schmitz, O.; Schnabel, B.; Czermak, P. Experimental determination of liquid entry pressure (LEP) in vacuum membrane distillation for oily wastewaters. Membr. Water Treat. 2015, 6, 237–249. [Google Scholar] [CrossRef]
- Mohammadi, T.; Kazemi, P. Taguchi optimization approach for phenolic wastewater treatment by vacuum membrane distillation. Desalin. Water Treat. 2014, 52, 1341–1349. [Google Scholar] [CrossRef]
- Winglee, J.M.; Bossa, N.; Rosen, D.; Vardner, J.T.; Wiesner, M.R. Modeling the Concentration of Volatile and Semivolatile Contaminants in Direct Contact Membrane Distillation (DCMD) Product Water. Environ. Sci. Technol. 2017, 51, 13113–13121. [Google Scholar] [CrossRef]
- Barbe, A.M.; Hogan, P.A.; Johnson, R.A. Surface morphology changes during initial usage of hydrophobic, microporous polypropylene membranes. J. Membr. Sci. 2000, 172, 149–156. [Google Scholar] [CrossRef]
- Banat, F.A.; Simandl, J. Theoretical and experimental study in membrane distillation. Desalination 1994, 95, 39–52. [Google Scholar] [CrossRef]
- Schofield, R.W.; Fang, A.G.; Fell, C.J.D.; Macoun, R. Factors affecting flux in membrane distillation. Desalination 1990, 77, 279–294. [Google Scholar] [CrossRef]
- MartíNez-DíEz, L.; Vázquez-González, M. Temperature and concentration polarization in membrane distillation of aqueous salt solutions. J. Membr. Sci. 1999, 156, 265–273. [Google Scholar] [CrossRef]
- Mengual, J.I.; Khayet, M.; Godino, M.P. Heat and mass transfer in vacuum membrane distillation. Int. J. Heat Mass Transf. 2004, 47, 865–875. [Google Scholar] [CrossRef]
- Jiraratananon, R.; Fane, A.G. Heat transport and membrane distillation coefficients in direct contact membrane distillation. J. Membr. Sci. 2003, 212, 177–193. [Google Scholar]
- Tijing, L.D.; Woo, Y.C.; Choi, J.S.; Lee, S.; Kim, S.H.; Shon, H.K. Fouling and its control in membrane distillation—a review. J. Membr. Sci. 2015, 475, 215–244. [Google Scholar] [CrossRef]
- Lin, Y.L. Effects of organic, biological and colloidal fouling on the removal of pharmaceuticals and personal care products by nanofiltration and reverse osmosis membranes. J. Membr. Sci. 2017, 542, 342–351. [Google Scholar] [CrossRef]
- Guillen-Burrieza, E.; Thomas, R.; Mansoor, B.; Johnson, D.; Hilal, N.; Arafat, H. Effect of dry-out on the fouling of PVDF and PTFE membranes under conditions simulating intermittent seawater membrane distillation (SWMD). J. Membr. Sci. 2013, 438, 126–139. [Google Scholar] [CrossRef]
- Rezaei, M.; Warsinger, D.M.; Lienhard, V.J.H.; Duke, M.C.; Matsuura, T.; Samhaber, W.M. Wetting phenomena in membrane distillation, Mechanisms, reversal, and prevention. Water Res. 2018, 139, 329–352. [Google Scholar] [CrossRef]
- Christie, K.S.; Yin, Y.M.; Lin, S.H.; Tong, T.Z. Distinct Behaviors between Gypsum and Silica Scaling in Membrane Distillation. Environ. Sci. Technol. 2020, 54, 568–576. [Google Scholar] [CrossRef]
- Ren, W. FTIR spectroscopic and NMR studies of hard elastic polypropylene. Colloid Polym. Sci. 1992, 270, 747–752. [Google Scholar] [CrossRef]
- Madhurambal, G.; Mariappan, M.; Mojumdar, S.C. Thermal, UV and FTIR spectral studies of urea–thiourea zinc chloride single crystal. J. Therm. Anal. Calorim. 2010, 100, 763–768. [Google Scholar] [CrossRef]
- Bernhard, A.M.; Czekaj, I.; Elsener, M.; Wokaun, A.; KröCher, O. Evaporation of urea at atmospheric pressure. J. Phys. Chem. A 2011, 115, 2581–2589. [Google Scholar] [CrossRef]
- Al-Hosney, H.A.; Grassian, V.H. Water, sulfur dioxide and nitric acid adsorption on calcium carbonate: A transmission and ATR-FTIR study. Phys. Chem. Chem. Phys. 2005, 7, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Guillen-Burrieza, E.; Ruiz-Aguirre, A.; Zaragoza, G.; Arafat, H.A. Membrane fouling and cleaning in long term plant-scale membrane distillation operations. J. Membr. Sci. 2014, 468, 360–372. [Google Scholar] [CrossRef]
- Long, D.N.; Cath, T. A scaling mitigation approach during direct contact membrane distillation. Sep. Purif. Technol. 2011, 80, 315–322. [Google Scholar]
- Zhang, J.; Li, J.-D.; Duke, M.; Hoang, M.; Xie, Z.; Groth, A.; Tun, C.; Gray, S. Modelling of vacuum membrane distillation. J. Memb. Sci. 2013, 434, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane distillation: A comprehensive review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.-D.; Duke, M.; Xie, Z.; Gray, S. Performance of asymmetric hollow fibre membranes in membrane distillation under various configurations and vacuum enhancement. J. Memb. Sci. 2010, 362, 517–528. [Google Scholar] [CrossRef] [Green Version]
- Ashoor, B.B.; Mansour, S.; Giwa, A.; Dufour, V.; Hasan, S.W. Principles and applications of direct contact membrane distillation (DCMD): A comprehensive review. Desalination 2016, 398, 222–246. [Google Scholar] [CrossRef]
- El-Bourawi, M.S.; Khayet, M.; Ma, R.; Ding, Z.; Li, Z.; Zhang, X. Application of vacuum membrane distillation for ammonia removal. J. Memb. Sci. 2007, 301, 200–209. [Google Scholar] [CrossRef]
- Binns, E.H. The dissociation constant of phenol in water between 25 °C and 60 °C. Trans. Faraday Soc. 1959, 55, 1900–1903. [Google Scholar] [CrossRef]


| Index b | Ersatz Urine | Stabilized Ersatz Urine |
|---|---|---|
| pH | 6.0–7.0 | 1.5–2.0 |
| Conductivity | 21 ± 1.0 | 32 ± 1.0 |
| Plate count (CFU/mL) | 2.2 × 105 | — |
| TOC (mg/L) | 4271.5 ± 53.31 | 4247 ± 19.20 |
| TAN (mg/L) | 340 ± 12.73 | 337 ± 15.35 |
| Urea (mg/L) | 14,376.6 ± 576.36 | 14,321.79 ± 321.31 |
| TP (mg/L) | 341.4 ± 15.34 | 268.75 ± 35.55 |
| Phenol (mg/L) | 164.32 ± 11.15 | 159.44 ± 10.93 |
| Na+ (mg/L) | 3227.91 ± 108.22 | 3194.15 ± 106.36 |
| K+ (mg/L) | 1569.91 ± 27.89 | 1580.65 ± 118.56 |
| Mg2+ (mg/L) | 125.03 ± 3.72 | 121.99 ± 7.25 |
| Ca2+ (mg/L) | 14.39 ± 0.26 | 4.145 ± 0.80 |
| Cr (mg/L) | — | 202.5 ± 6.73 |
| SO42− (mg/L) | 673.255 ± 14.14 | 5130.94 ± 112.27 |
| Cl− (mg/L) | 6325.69 ± 164.47 | 6344.12 ± 315.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Liu, J.; Li, D.; Liu, Z.; Zhang, J.; Ding, P.; Liu, G.; Feng, Y. High-Efficiency Water Recovery from Urine by Vacuum Membrane Distillation for Space Applications: Water Quality Improvement and Operation Stability. Membranes 2022, 12, 629. https://doi.org/10.3390/membranes12060629
Wang F, Liu J, Li D, Liu Z, Zhang J, Ding P, Liu G, Feng Y. High-Efficiency Water Recovery from Urine by Vacuum Membrane Distillation for Space Applications: Water Quality Improvement and Operation Stability. Membranes. 2022; 12(6):629. https://doi.org/10.3390/membranes12060629
Chicago/Turabian StyleWang, Fei, Junfeng Liu, Da Li, Zheng Liu, Jie Zhang, Ping Ding, Guochang Liu, and Yujie Feng. 2022. "High-Efficiency Water Recovery from Urine by Vacuum Membrane Distillation for Space Applications: Water Quality Improvement and Operation Stability" Membranes 12, no. 6: 629. https://doi.org/10.3390/membranes12060629
APA StyleWang, F., Liu, J., Li, D., Liu, Z., Zhang, J., Ding, P., Liu, G., & Feng, Y. (2022). High-Efficiency Water Recovery from Urine by Vacuum Membrane Distillation for Space Applications: Water Quality Improvement and Operation Stability. Membranes, 12(6), 629. https://doi.org/10.3390/membranes12060629






