Semi-Quantitative Evaluation of Asymmetricity of Dialysis Membrane Using Forward and Backward Ultrafiltration
Abstract
1. Introduction
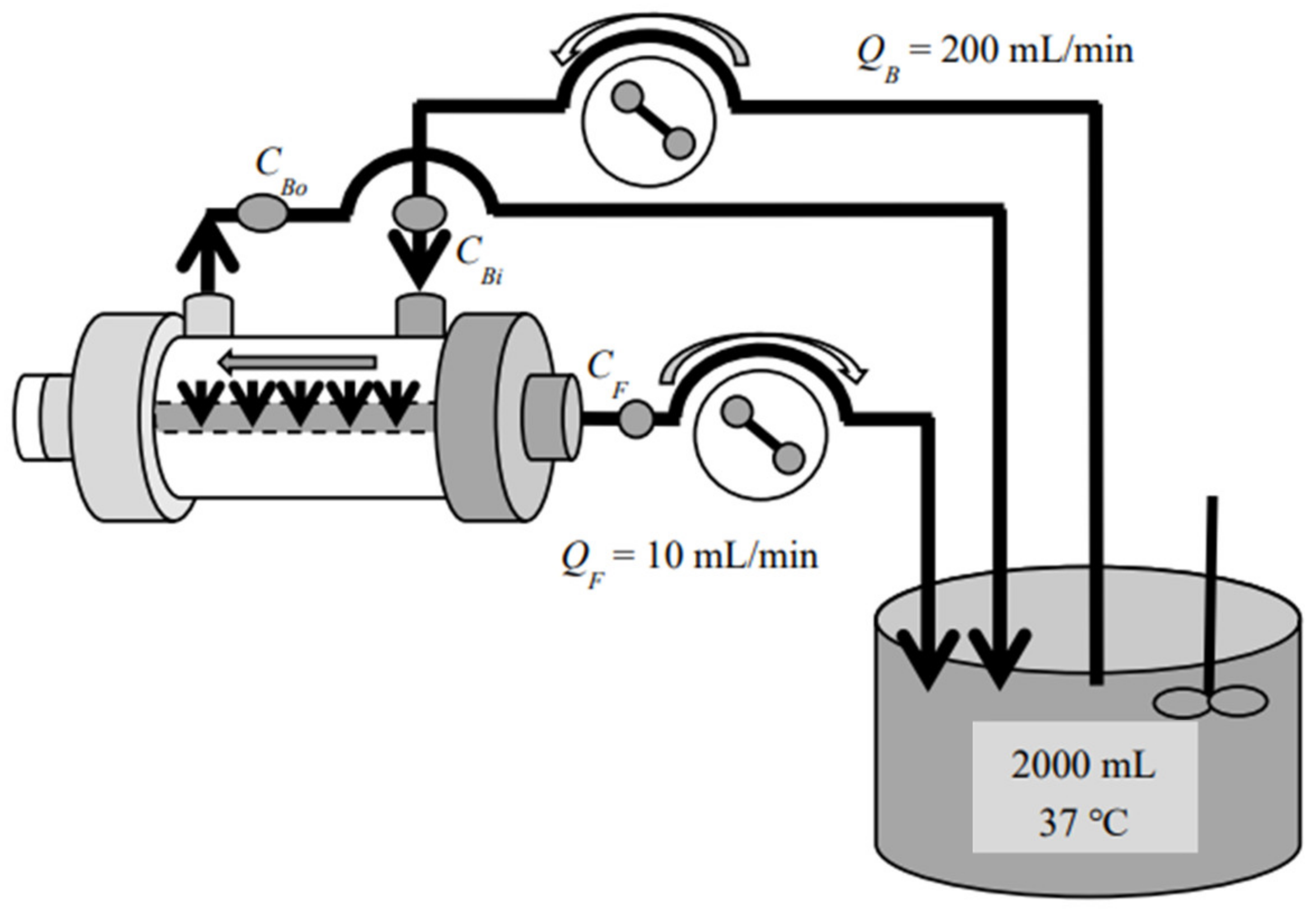
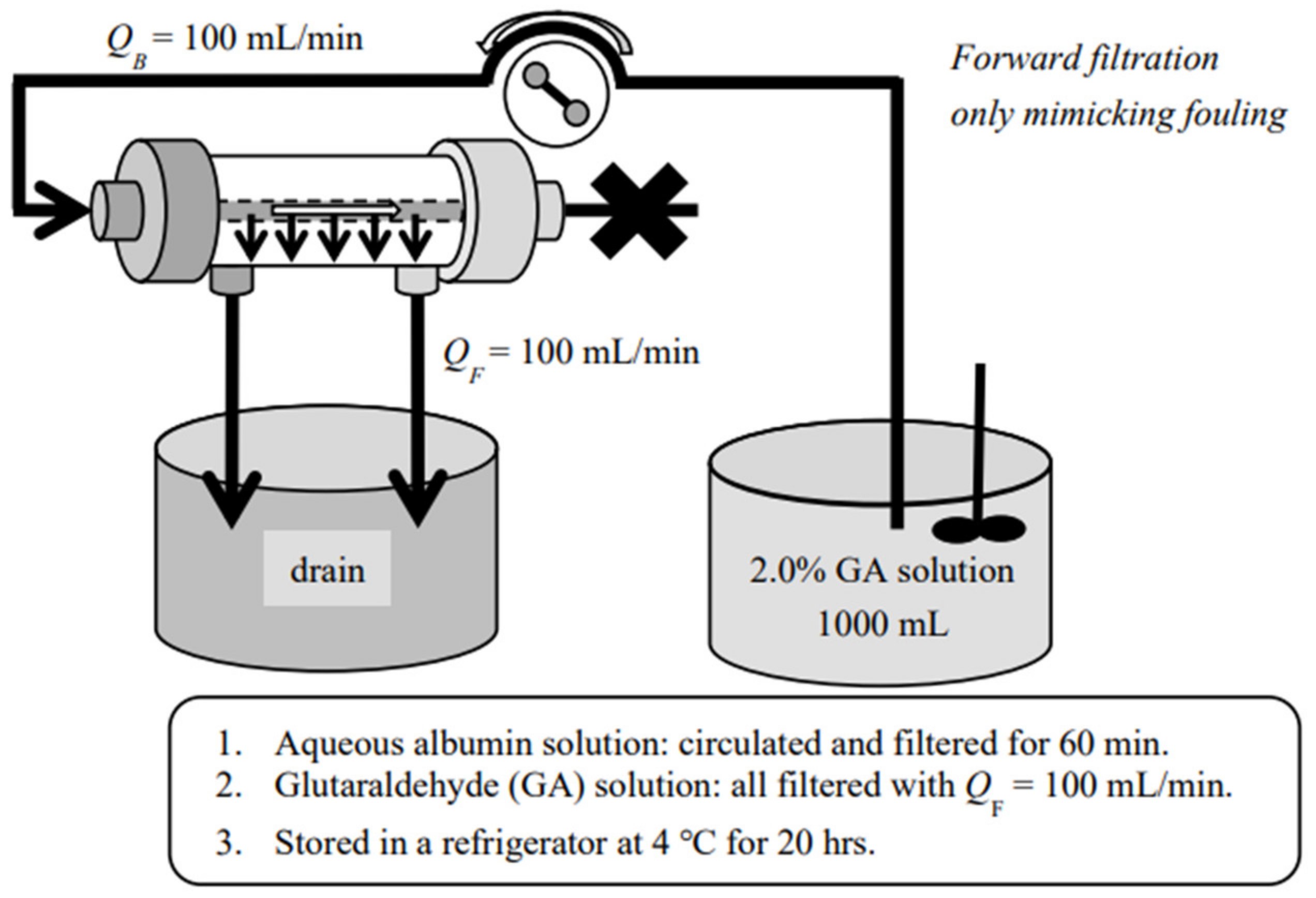
2. Materials and Method
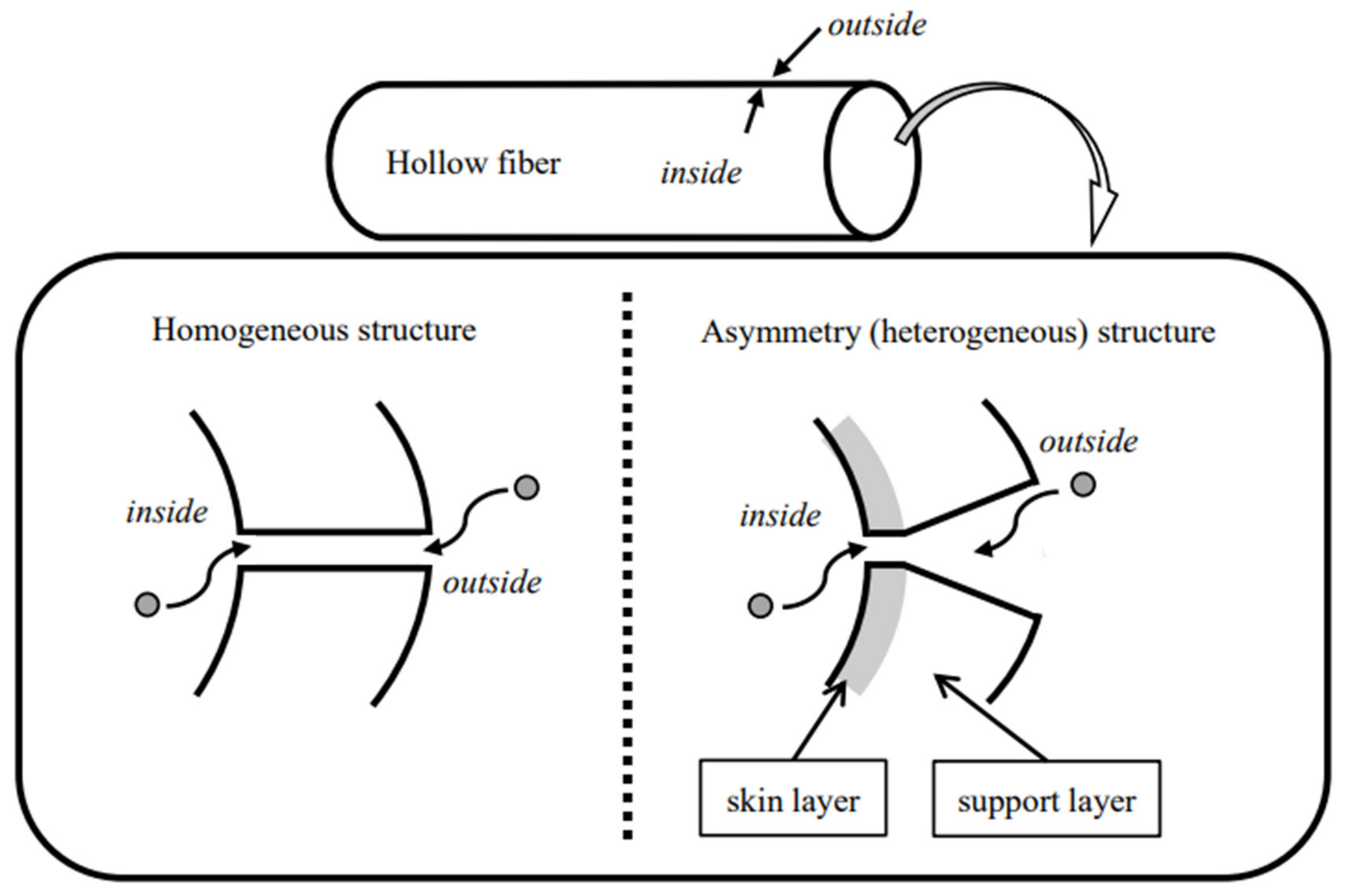
3. Theoretical
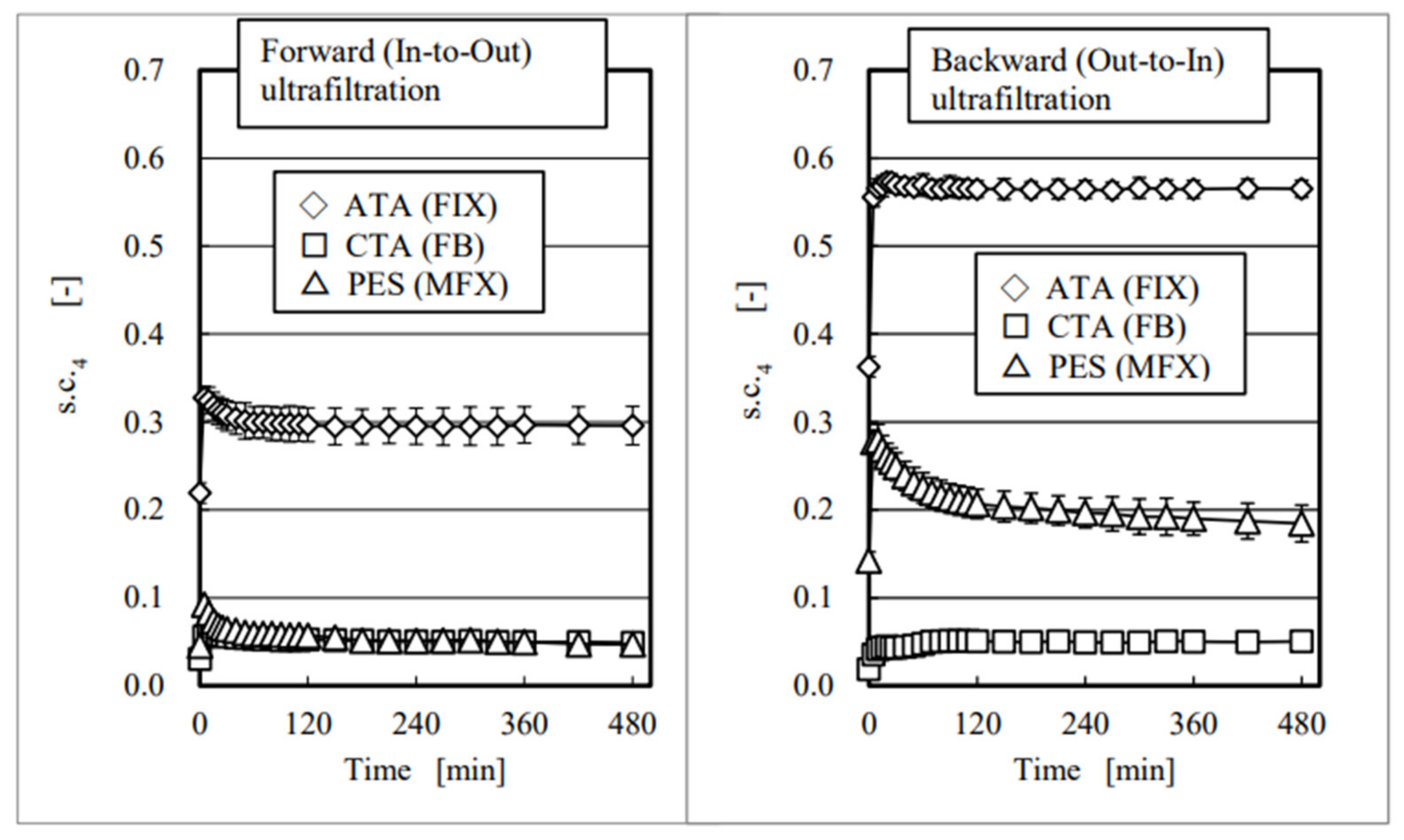
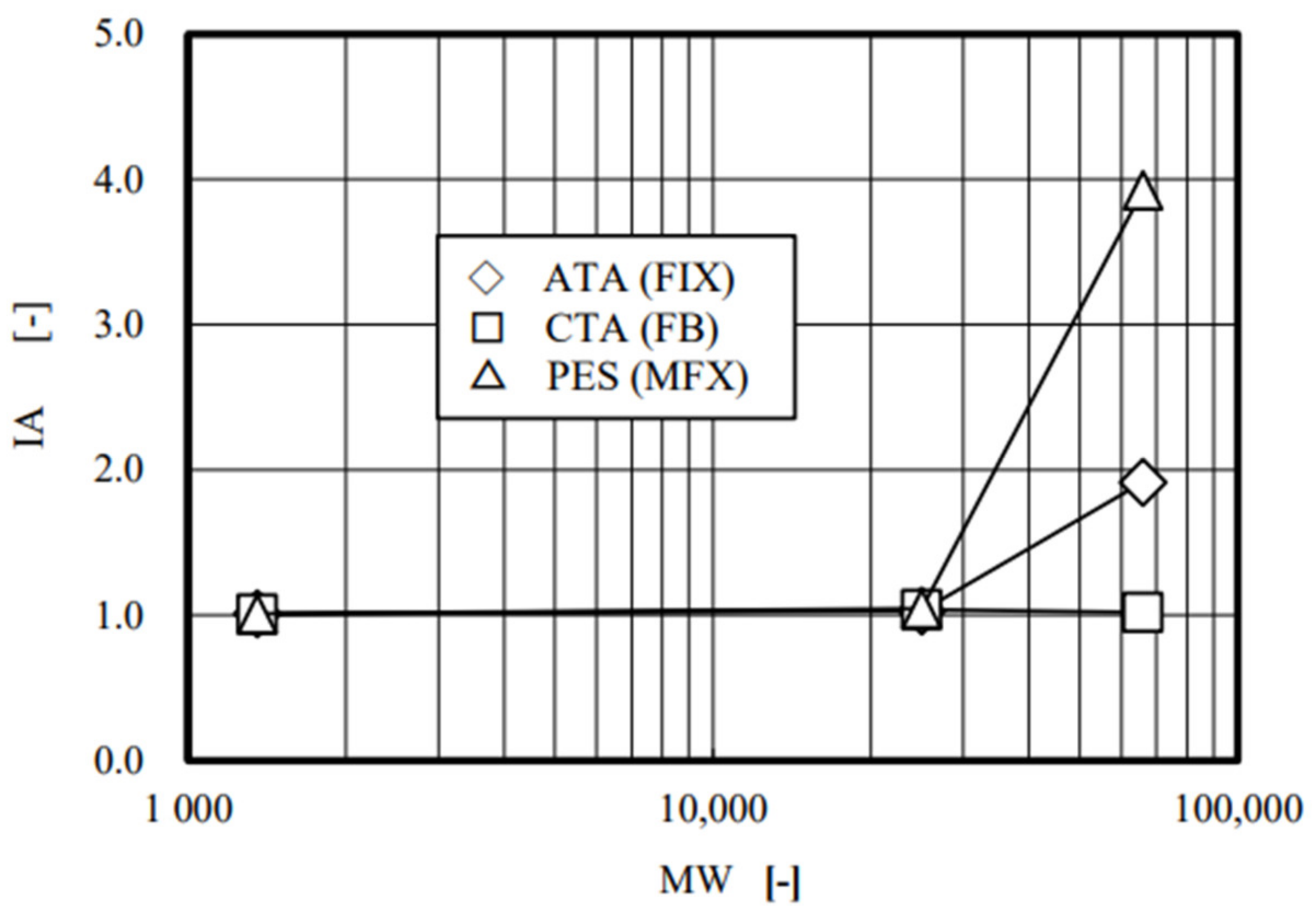
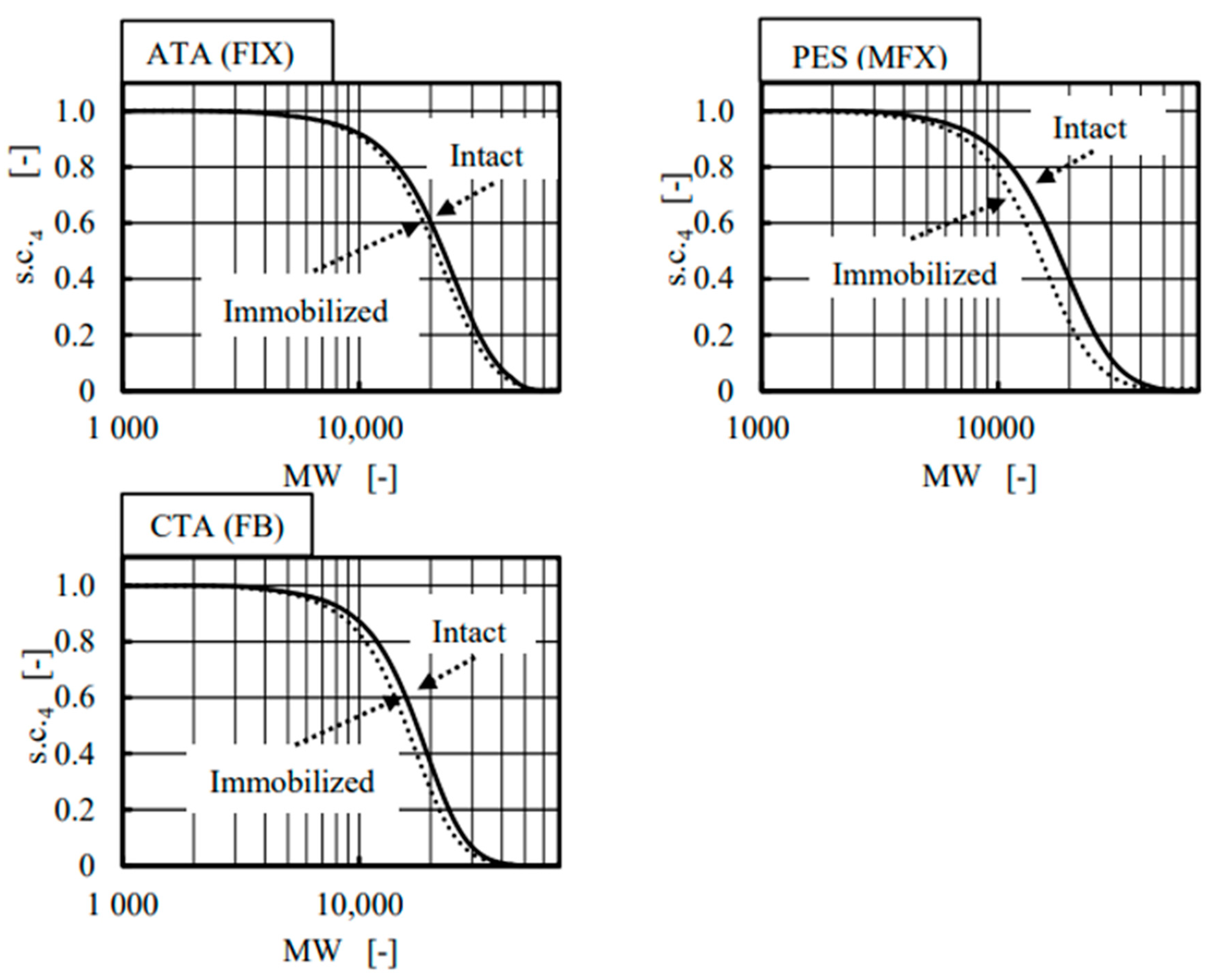
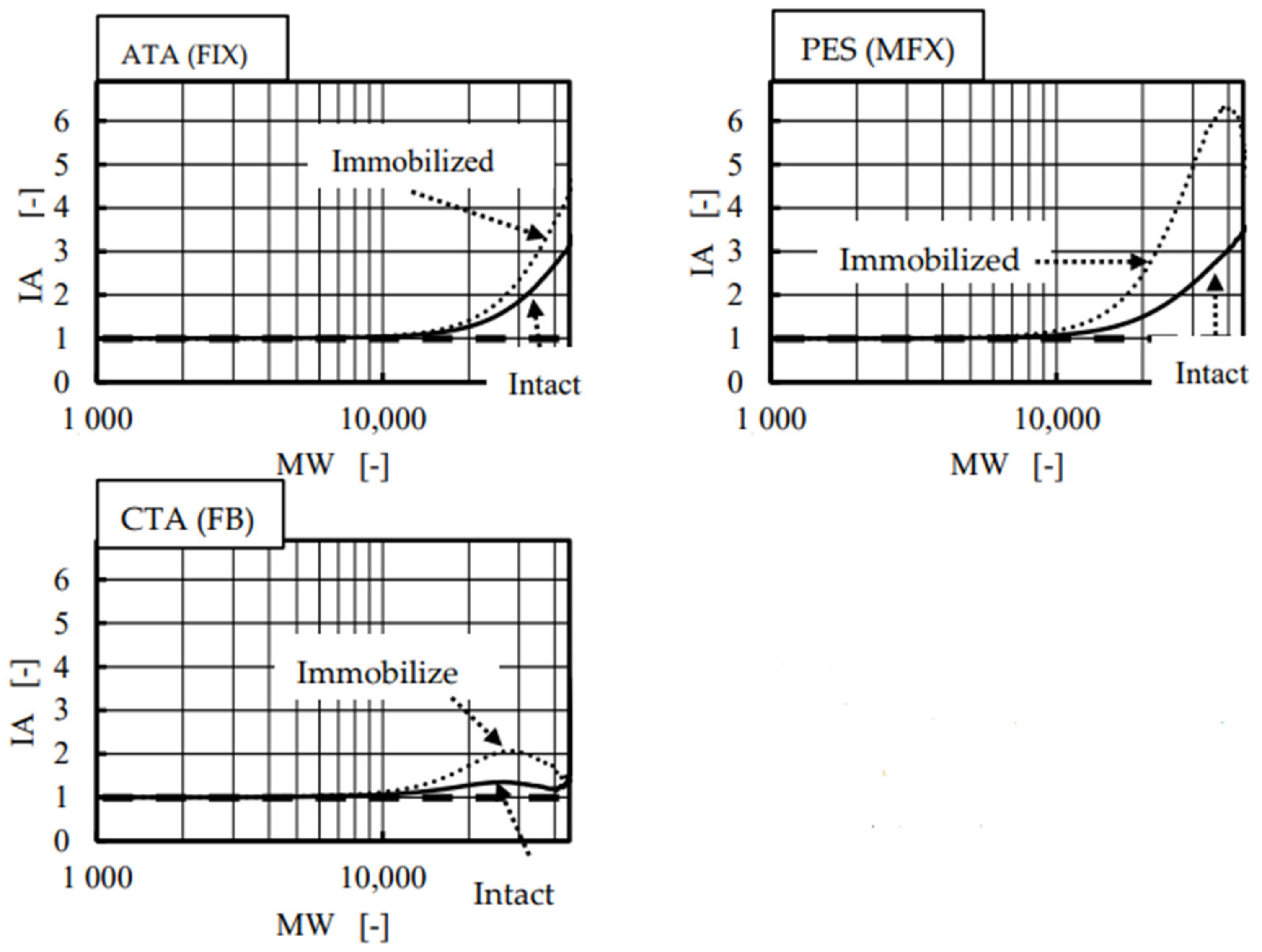
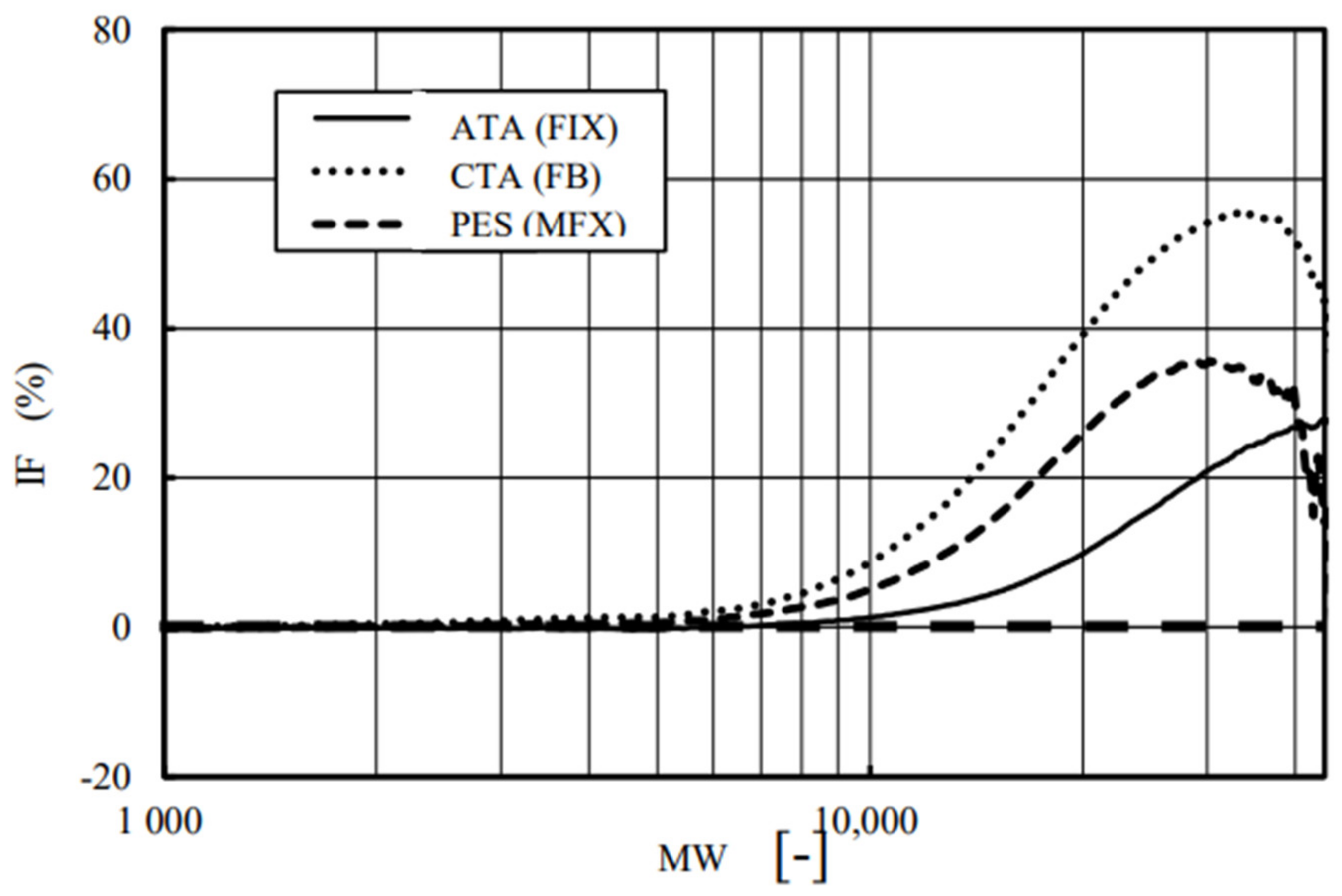
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwalbe, S.; Holzhauer, M.; Schaeffer, J.; Galanski, M.; Koch, K.M.; Floege, J. Beta 2-microglobulin associated amyloidosis: A vanishing complication of long-term hemodialysis? Kidney Int. 1997, 52, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Gejyo, F.; Yamada, T.; Odani, S.; Nakagawa, Y.; Arakawa, M.; Kunitomo, T.; Suzuki, M.; Hirasawa, Y.; Shirahama, T.; Cohen, A.S.; et al. A new form of amyloid protein associated with chronic hemodialysis was identified as beta 2-microglobulin. Biochem. Biophys. Res. Commun. 1985, 129, 701–706. [Google Scholar] [CrossRef]
- Bergström, J.; Wehle, B. No change in corrected beta 2-microglobulin concentration after cuprophane haemodialysis. Lancet 1987, 329, 628–629. [Google Scholar] [CrossRef]
- Hakim, R.M.; Fearon, D.T.; Lazarus, J.M.; Perzanowski, C.S. Biocompatibility of dialysis membranes: Effects of chronic complement activation. Kidney Int. 1984, 26, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.R.; Hamburger, R.J.; Lysaght, M.J. Effect of membrane composition and structure on solute removal and biocompatibility in hemodialysis. Kidney Int. 1999, 56, 2005–2015. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Clark, W.R. Haemodialysis membranes. Nat. Rev. Nephrol. 2018, 14, 394–410. [Google Scholar] [CrossRef] [PubMed]
- Daugirdas, J.T.; Ing, T.S.; Roxe, D.M.; Ivanovich, P.T.; Krumlovsky, F.; Popli, S.; McLaughlin, M.M. Severe anaphylactoid reactions to cuprammonium cellulose hemodialyzers. Ann. Intern. Med. 1985, 145, 489–494. [Google Scholar] [CrossRef]
- Daugirdas, J.T.; Ing, T.S. First-use reactions during hemodialysis: A definition of subtypes. Kidney Int. Suppl. 1988, 24, S37–S43. [Google Scholar]
- Schulman, G.; Hakim, R.; Arias, R.; Silverberg, M.; Kaplan, A.P.; Arbeit, L. Bradykinin generation by dialysis membranes: Possible role in anaphylactic reaction. J. Am. Soc. Nephrol. 1993, 3, 1563–1569. [Google Scholar] [CrossRef]
- Yamashita, A.C.; Sakurai, K. Dialysis Membranes—Physicochemical Structures and Features. In Updates in Hemodialysis; Suzuki, H., Ed.; InTechOpen: London, UK, 2015; pp. 153–189. [Google Scholar] [CrossRef][Green Version]
- Clark, W.R.; Gao, D. Low-molecular weight proteins in end-stage renal disease: Potential toxicity and dialytic removal mechanisms. J. Am. Soc. Nephrol. 2002, 13 (Suppl. S1), S41–S47. [Google Scholar] [CrossRef]
- Tattersall, J.E.; Ward, R.A. Online haemodiafiltration: Definition, dose quantification and safety revisited. Nephrol. Dial. Transplant. 2013, 28, 542–550. [Google Scholar] [CrossRef]
- Maduell, F.; Varas, J.; Ramos, R.; Martin-Malo, A.; Pérez-Garcia, R.; Berdud, I.; Moreso, F.; Canaud, B.; Stuard, S.; Gauly, A.; et al. Hemodiafiltration reduces all-cause and cardiovascular mortality in incident hemodialysis patients: A propensity-matched cohort study. Am. J. Nephrol. 2017, 46, 288–297. [Google Scholar] [CrossRef]
- Canaud, B.; Vienken, J.; Ash, S.; Ward, R.A. Hemodiafiltration to address unmet medical needs ESKD patients. Clin. J. Am. Soc. Nephrol. 2018, 13, 1435–1443. [Google Scholar] [CrossRef]
- Masakane, I.; Kikuchi, K.; Kawanishi, H. Evidence for the clinical advantages of predilution on-line hemodiafiltration. Contrib. Nephrol. 2017, 189, 17–23. [Google Scholar] [CrossRef]
- Kikuchi, K.; Hamano, T.; Wada, A.; Nakai, S.; Masakane, I. Predilution online hemodiafiltration is associated with improved survival compared with hemodialysis. Kidney Int. 2019, 95, 929–938. [Google Scholar] [CrossRef]
- Pappenheimer, J.R.; Renkin, E.M.; Borrero, L.M. Filtration, diffusion and molecular sieving through peripheral capillary membranes—A contribution to the pore theory of capillary permeability. Amer. J. Physiol. 1951, 167, 13–46. [Google Scholar] [CrossRef]
- Verniory, A.; Dubois, R.; Decoodt, P.; Gassee, J.P.; Lambert, P.P. Measurement of the permeability of biological membranes—Application to the glomerular wall. J. Gen. Physiol. 1973, 62, 489–507. [Google Scholar] [CrossRef]
- Sakai, K.; Takesawa, S.; Mimura, R.; Ohashi, H. Determination of pore radius of hollow fiber dialysis membranes using tritium-labeled water. J. Chem. Eng. Jpn. 1988, 21, 207–210. [Google Scholar] [CrossRef]
- Tomisawa, N.; Yamashita, A.C. Amount of adsorbed albumin loss by dialysis membranes with protein adsorption. J. Artif. Organs 2009, 12, 194–199. [Google Scholar] [CrossRef]
- Gomez, M.; Bañon-Maneus, E.; Arias-Guillén, M.; Fontseré, N.; Broseta, J.J.; Ojeda, R.; Maduell, F. Distinct solute removal patterns by similar surface high-flux membranes in haemodiafiltration: The adsorption point of View. Blood Purif. 2022, 51, 38–46. [Google Scholar] [CrossRef]
- Kiguchi, T.; Tomisawa, N.; Yamashita, A.C. Replication of Fouling In Vitro in Hollow Fiber Dialyzers by Albumin Immobilization. J. Artif. Organs 2022, 1–7. [Google Scholar] [CrossRef]
- Boschetti-de-Fierro, A.; Voigt, M.; Storr, M.; Krause, B. MCO membranes: Enhanced selectivity in high-flux class. Sci. Rep. 2015, 5, 18448. [Google Scholar] [CrossRef]
- Japanese Society for Artificial Organs: A Guideline for Performance Evaluation of Hemodialyzers; Japanese Society for Artificial Organs: Tokyo, Japan, 1982. (In Japanese)
- Kawanishi, H.; Mineshima, M.; Hirakata, H.; Akizawa, T. A method for performance evaluation of blood purification devices. Jap. J. Dial. Ther. 2012, 45, 435–445. [Google Scholar] [CrossRef]
- Henderson, L.W.; Colton, C.K.; Ford, C.A. Kinetics of hemodiafiltration. II. Clinical characterization of a new blood cleansing modality. J. Lab. Clin. Med. 1975, 85, 372–391. [Google Scholar]
- Colton, C.K.; Henderson, L.W.; Ford, C.A.; Lysaght, M.J. Kinetics of hemodiafiltration. I. In Vitro transport characteristics of a hollow-fiber blood ultrafilter. J. Lab. Clin. Med. 1975, 85, 355–371. [Google Scholar]
- Yamashita, A.C. New dialysis membrane for removal of middle molecule uremic toxins. Amer. J. Kid. Dis. 2001, 38, S217–S219. [Google Scholar] [CrossRef]
- Yamashita, A.C.; Sakiyama, R.; Hamada, H.; Tojo, K. Two novel definitive equations of the sieving coefficient. Kidney and Dialysis Jin-To-Toseki 1998, 45 (Suppl. S1), 36–38. (In Japanese) [Google Scholar]
- Yamashita, A.C.; Ono, T.; Tomisawa, N. Verification of physicochemical structures of dialysis membrane using reversal dialysis technique. Hemodial. Int. 2017, 20 (Suppl. S2), S3–S9. [Google Scholar] [CrossRef]
- Yamashita, A.C.; Masaki, H.; Kobayashi, E.; Sukegawa, T. Evaluation of solute penetration across the polysulfone membrane with vitamin E coating. Hemodial. Int. 2015, 19 (Suppl. S2), S20–S25. [Google Scholar] [CrossRef]
- Yamashita, A.C.; Sakurai, K. Clinical effect of pre-dilution hemodiafiltration based on the permeation of the hemodiafilter, “Chronic kidney disease–Recent advances in clinical and basic research”. Contrib. Nephrol. 2015, 185, 1–7. [Google Scholar] [CrossRef]
- Sunohara, T.; Masuda, T. Fundamental characteristics of the newly developed ATATM membrane dialyzer. Contrib. Nephrol. 2017, 189, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Maduell, F.; Ojeda, R.; Arias-Guillén, M.; Fontseré, N.; Vera, M.; Rodas, L.; Gómez, M.; Huablocho, K.P.; Esquivel, F.; Mori, P.D.; et al. A new generation of cellulose triacetate suitable for online haemodiafiltration. Nefrología 2018, 38, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.R.; Hadidi, M.; Motevalian, S.P.; Sunohara, T.; Zydney, A.L. Effects of plasma proteins on the transport and surface characteristics of polysulfone/polyethersulfone and asymmetric cellulose triacetate high flux dialyzers. Artif. Organs 2018, 42, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Fujimori, A.; Naito, H.; Miyazaki, T. Adsorption of complement, cytokines, and proteins by different dialysis membrane materials: Evaluation by confocal laser scanning fluorescence microscopy. Artif. Organs 1998, 22, 1014–1017. [Google Scholar] [CrossRef]
- Grandi, F.; Bolasco, P.; Palladino, G.; Sereni, L.; Caiazzo, M.; Atti, M.; Ghezzi, P.M. Adsorption in Extracorporeal Blood Purification: How to Enhance Solutes Removal beyond Diffusion and Convection; InTechOpen: London, UK, 2013; pp. 381–408. [Google Scholar] [CrossRef]


| Commercial Name | Membrane | Physicochemical Structure of the Membrane | Abbreviations of the Commercial Name |
|---|---|---|---|
| FIX-210Seco | CTA (ATA®) | Asymmetry | FIX |
| FB-210UHβeco | CTA | Homogeneous | FB |
| MFX-21Seco | PES | Asymmetry | MFX |
| Solutes | Molecular Weight (-) | Produced | Purpose | Initial Concentration (mg/mL) |
|---|---|---|---|---|
| vitamin B12 | 1355 | FUJIFILM Wako Pure Chemical Co., Osaka, Japan. | Test solute | 0.025 |
| α-chymotripsin 1 | 25,000 | Sigma-Aldrich, St. Louis, MO, USA. | 0.305 | |
| albumin 1 | 66,000 | FUJIFILM Wako Pure Chemical Co., Osaka, Japan. | Test solute & Foulant 2 | 24.0 |
| dextran 3 | ~1500 | Sigma-Aldrich, St. Louis, MO, USA | Test solute | 0.50 4 |
| ~25,000 | ||||
| ~40,000 | ||||
| ~60,000 | ||||
| ~200,000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamashita, A.C.; Kakee, T.; Ono, T.; Motegi, J.; Yamaguchi, S.; Sunohara, T. Semi-Quantitative Evaluation of Asymmetricity of Dialysis Membrane Using Forward and Backward Ultrafiltration. Membranes 2022, 12, 624. https://doi.org/10.3390/membranes12060624
Yamashita AC, Kakee T, Ono T, Motegi J, Yamaguchi S, Sunohara T. Semi-Quantitative Evaluation of Asymmetricity of Dialysis Membrane Using Forward and Backward Ultrafiltration. Membranes. 2022; 12(6):624. https://doi.org/10.3390/membranes12060624
Chicago/Turabian StyleYamashita, Akihiro C., Toshiki Kakee, Takahisa Ono, Jun Motegi, Satoru Yamaguchi, and Takashi Sunohara. 2022. "Semi-Quantitative Evaluation of Asymmetricity of Dialysis Membrane Using Forward and Backward Ultrafiltration" Membranes 12, no. 6: 624. https://doi.org/10.3390/membranes12060624
APA StyleYamashita, A. C., Kakee, T., Ono, T., Motegi, J., Yamaguchi, S., & Sunohara, T. (2022). Semi-Quantitative Evaluation of Asymmetricity of Dialysis Membrane Using Forward and Backward Ultrafiltration. Membranes, 12(6), 624. https://doi.org/10.3390/membranes12060624






