Negatively Charged MOF-Based Composite Anion Exchange Membrane with High Cation Selectivity and Permeability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
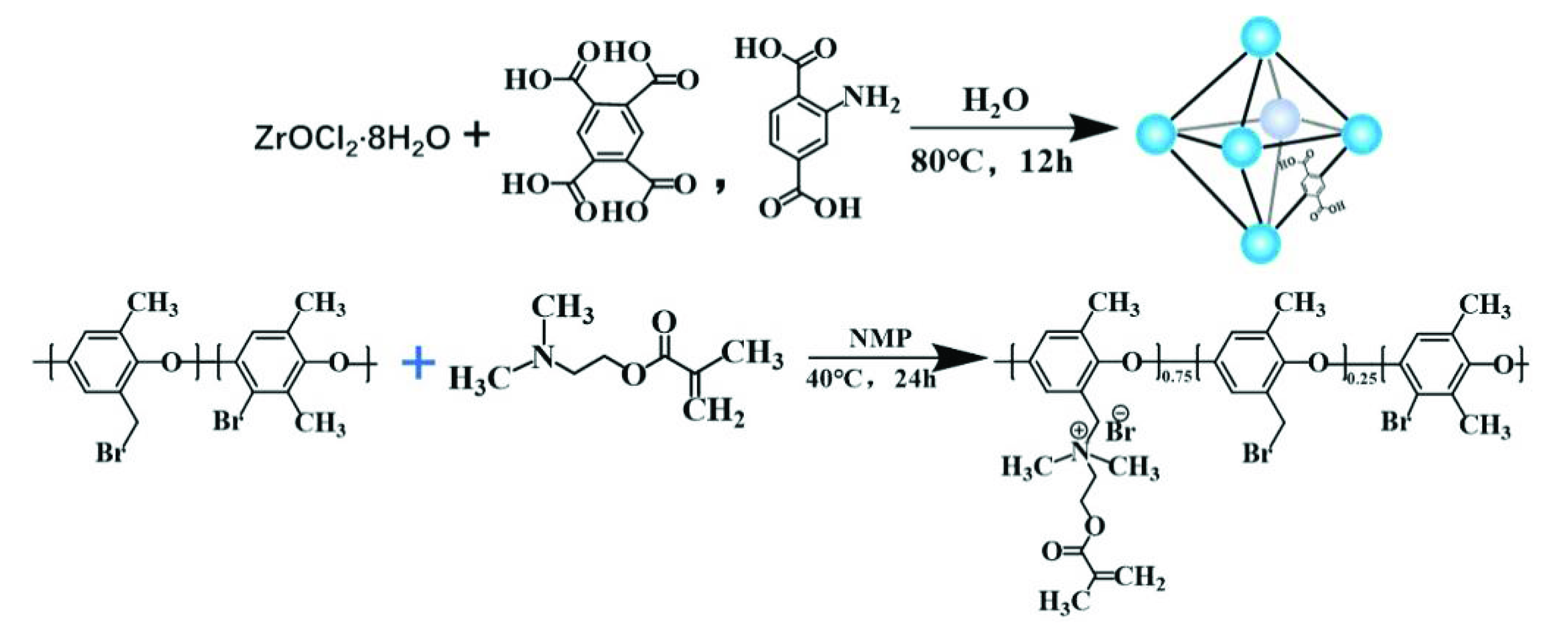
2.2. Synthesis of UiO-66-(COOH)2
2.3. Structure of UiO-66-(COOH)2/QPPO Membrane
2.3.1. Preparation of QPPO Matrix
2.3.2. Preparation of UiO-66-(COOH)2/QPPO Mixed Matrix Membrane
2.4. Characterization
2.5. Water Uptake (WU) and Linear Elongation (LER)
2.6. Ion Exchange Capacity (IEC)
2.7. Ion Separation Performance Test
3. Results and Discussion
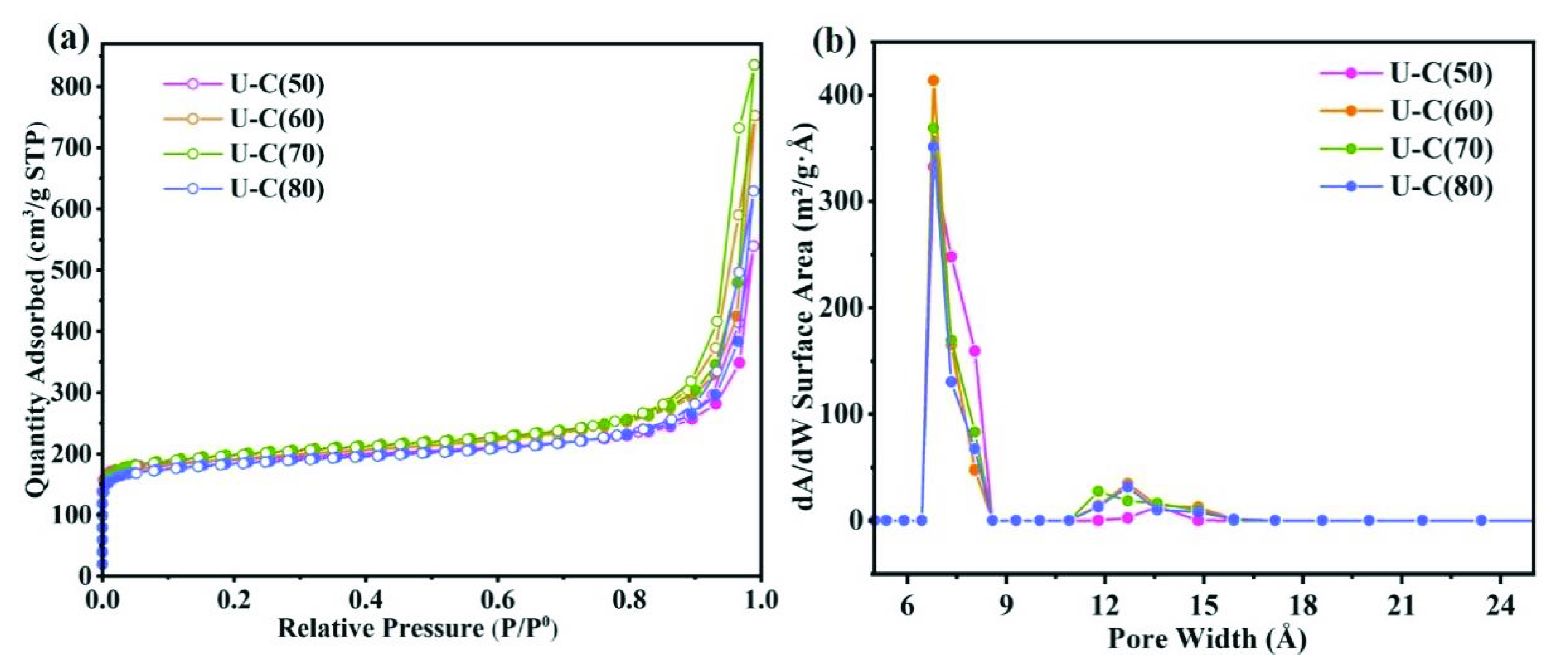
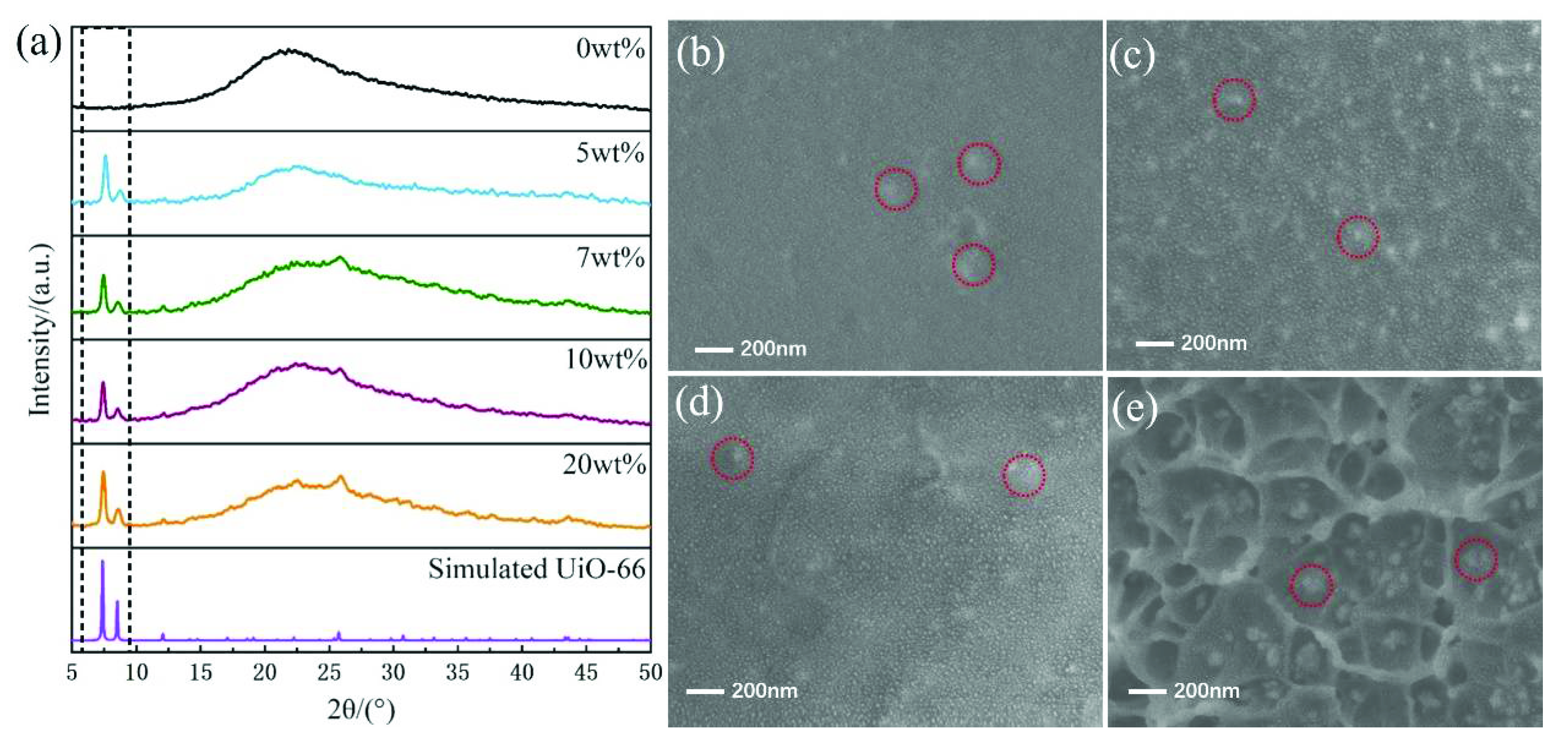
3.1. Preparation and Characterization of UiO-66-(COOH)2 Crystal
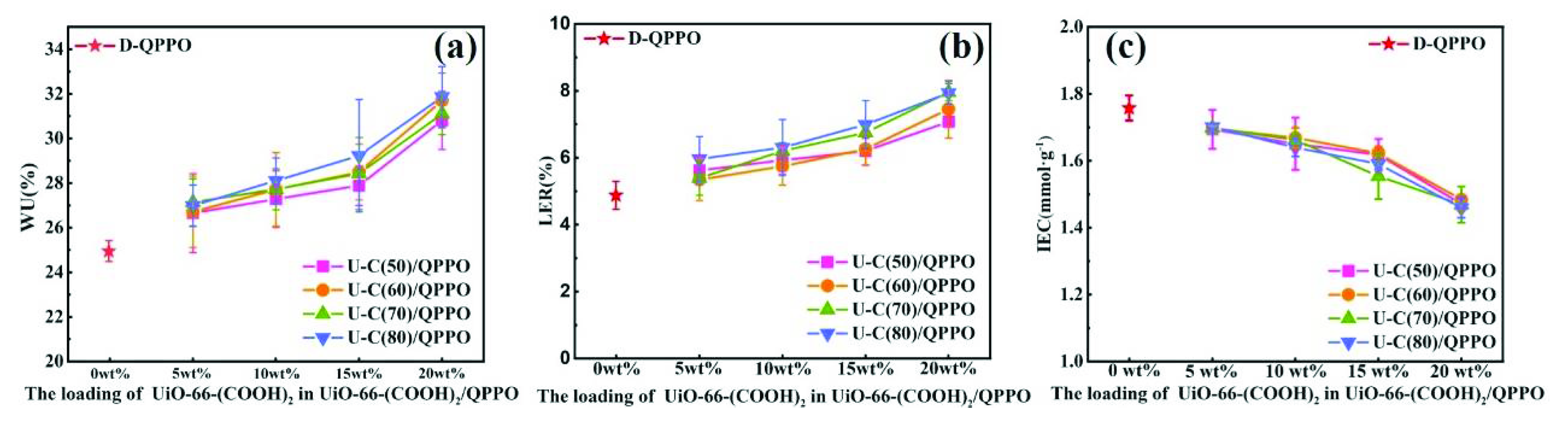
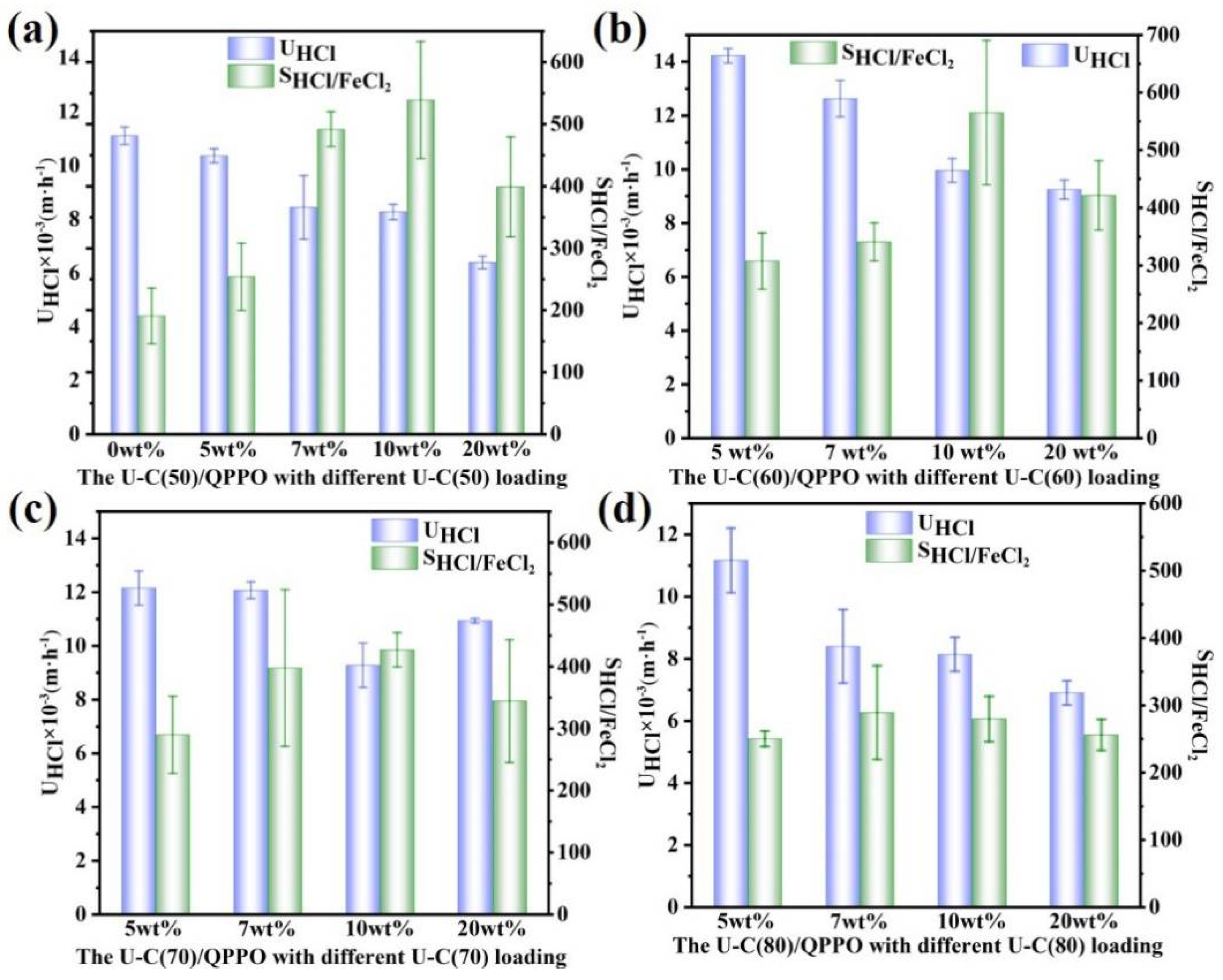
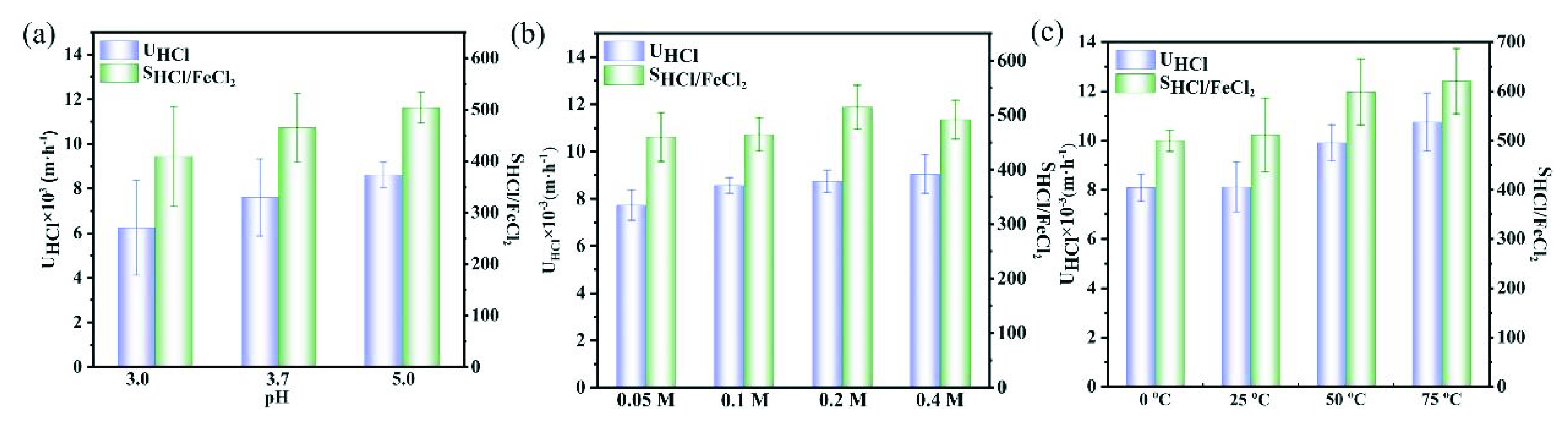
3.2. H+/Fe2+ Separation Performance of UiO-66-(COOH)2/QPPO Membrane
3.3. Structure and Characterization of U-C(6)/QPPO Membrane
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López, J.; de Oliveira, R.; Reig, M.; Vecino, X.; Gibert, O.; de Juan, A.; Cortina, J. Acid recovery from copper metallurgical process streams polluted with arsenic by diffusion dialysis. J. Environ. Chem. Eng. 2021, 9, 104692. [Google Scholar] [CrossRef]
- Wang, P.; Wang, P.; Guo, Y.; Rao, L.; Yan, C. Selective recovery of protonated dyes from dye wastewater by pH-responsive BCN material. Chem. Eng. J. 2021, 412, 128532. [Google Scholar] [CrossRef]
- Jallouli, W.; Keskes, S.; Guidara, W.; Rezgui, F.; Sayadi, S.; Tounsi, S. Acidic pretreatment as a chemical approach for enhanced Photorhabdus temperata bioinsecticide production from industrial wastewater. J. Environ. Manag. 2021, 278, 111476. [Google Scholar] [CrossRef] [PubMed]
- Jallouli, W.; Keskes, S.; Guidara, W.; Rezgui, F.; Sayadi, S.; Tounsi, S. Membrane distillation and dispersive solvent extraction in a closed-loop process for water, sulfuric acid and copper recycling from gold mining wastewater. Chem. Eng. J. 2022, 435, 133874. [Google Scholar]
- Yang, C.-C.; Pan, J.; Zhu, D.-Q.; Guo, Z.-Q.; Li, X.-M. Pyrometallurgical recycling of stainless-steel pickling sludge: A review. J. Iron Steel Res. Int. 2019, 26, 547–557. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Igarashi, T.; Villacorte-Tabelin, M.; Park, I.; Opiso, E.M.; Ito, M.; Hiroyoshi, N. Arsenic, selenium, boron, lead, cadmium, copper, and zinc in naturally contaminated rocks: A review of their sources, modes of enrichment, mechanisms of release, and mitigation strategies. Sci. Total Environ. 2018, 645, 1522–1553. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Huang, J.; Wang, J.; Yu, J.; You, X.; Lin, X.; Van der Bruggen, B.; Zhao, S. High-performance porous anion exchange membranes for efficient acid recovery from acidic wastewater by diffusion dialysis. J. Membr. Sci. 2021, 624, 119116. [Google Scholar] [CrossRef]
- Kalaj, M.; Bentz, K.C.; Ayala, S., Jr.; Palomba, J.M.; Barcus, K.S.; Katayama, Y.; Cohen, S.M. MOF-Polymer Hybrid Materials: From Simple Composites to Tailored Architectures. Chem. Rev. 2020, 120, 8267–8302. [Google Scholar] [CrossRef]
- Rashti, A.; Lu, X.; Dobson, A.; Hassani, E.; Feyzbar-Khalkhali-Nejad, F.; He, K.; Oh, T.S. Tuning MOF-Derived Co3O4/NiCo2O4 Nanostructures for High-Performance Energy Storage. ACS Appl. Energy Mater. 2021, 4, 1537–1547. [Google Scholar] [CrossRef]
- Qian, Q.; Asinger, P.A.; Lee, M.J.; Han, G.; Rodriguez, K.M.; Lin, S.; Benedetti, F.M.; Wu, A.X.; Chi, W.S.; Smith, Z.P. MOF-Based Membranes for Gas Separations. Chem. Rev. 2020, 120, 8161–8266. [Google Scholar] [CrossRef]
- Drake, T.; Ji, P.; Lin, W. Site Isolation in Metal-Organic Frameworks Enables Novel Transition Metal Catalysis. Acc. Chem. Res. 2018, 51, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, L.; Ma, S.; Hu, T. Porous MB@Cd-MOF Obtained by Post-Modification: Self-Calibrated Fluorescent Turn-on Sensor for Highly Sensitive Detection of Carbaryl. Cryst. Growth Des. 2022, 22, 2662–2669. [Google Scholar] [CrossRef]
- Cai, M.; Qin, L.; You, L.; Yao, Y.; Wu, H.; Zhang, Z.; Zhang, L.; Yin, X.; Ni, J. Functionalization of MOF-5 with mono-substituents: Effects on drug delivery behavior. RSC Adv. 2020, 10, 36862–36872. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, Y.; Wang, X.; Li, P.; Ying, W.; Chen, D.; Ma, X.; Deng, Z.; Peng, X. Simultaneous Recovery of Metal Ions and Electricity Harvesting via K-Carrageenan@ZIF-8 Membrane. ACS Appl. Mater. Interfaces 2019, 11, 34039–34045. [Google Scholar] [CrossRef]
- Liu, X.; Demir, N.K.; Wu, Z.; Li, K. Highly Water-Stable Zirconium Metal-Organic Framework UiO-66 Membranes Supported on Alumina Hollow Fibers for Desalination. J. Am. Chem. Soc. 2015, 137, 6999–7002. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Shehzad, M.A.; Wang, X.; Wu, B.; Ge, L.; Xu, T. Engineering Leaf-Like UiO-66-SO3H Membranes for Selective Transport of Cations. Nanomicro Lett. 2020, 12, 51. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, X.P.; Zong, Z.A.; Lin, R.; Zhang, X.Y.; Chen, F.S.; Zhang, L.L.; Meng, X.M.; Hou, J. Thin film nanocomposite membrane incorporated with 2D-MOF nanosheets for highly efficient reverse osmosis desalination. J. Membr. Sci. 2022, 653, 120520. [Google Scholar] [CrossRef]
- Lee, S.J.; Lim, H.W.; Park, S.H. Adsorptive seawater desalination using MOF-incorporated Cu-alginate/PVA beads: Ion removal efficiency and durability. Chemosphere 2021, 268, 128797. [Google Scholar] [CrossRef]
- Paul, M.S.; Cantwell, G.C.; Himanshu, J.; Christine, J.J.F.; Krista, S.W. Effect of water adsorption on retention of structure and surface area of metal-organic frameworks. Ind. Eng. Chem. Res. 2012, 51, 6513. [Google Scholar]
- Ahmadijokania, F.; Mohammadkhanib, R.; Ahmadipouyab, S.; Shokrgozarb, A.; Rezakazemic, M.; Molavib, H.; Aminabhavid, T.M.; Arjmanda, M. Superior chemical stability of UiO-66 metal-organic frameworks (MOFs) for selective dye adsorption. Chem. Eng. J. 2020, 399, 125346. [Google Scholar] [CrossRef]
- Molavi, H.; Hakimian, A.; Shojaei, A.; Raeiszadeh, M. Selective dye adsorption by highly water stable metal-organic framework: Long term stability analysis in aqueous media. Appl. Surf. Sci. 2018, 445, 424. [Google Scholar] [CrossRef]
- Ahmadijokani, F.; Ahmadipouya, S.; Molavi, H.; Rezakazemi, M.; Aminabhavi, T.M.; Arjmand, M. Impact of scale, activation solvents, and aged conditions on gas adsorption properties of UiO-66. J. Environ. Manag. 2020, 274, 111155. [Google Scholar] [CrossRef] [PubMed]
- Ahmadijokani, F.; Tajahmadi, S.; Bahi, A.; Molavi, H.; Rezakazemi, M.; Ko, F.; Aminabhavi, T.M.; Arjmand, M. Ethylenediamine-functionalized Zr-based MOF for efficient removal of heavy metal ions from water. Chemosphere 2021, 264, 128466. [Google Scholar] [CrossRef] [PubMed]
- Ahmadijokani, F.; Molavi, H.; Rezakazemi, M.; Tajahmadi, S.; Bahi, A.; Ko, F.; Aminabhavi, T.M.; Li, J.-R.; Arjmand, M. UiO-66 metal-organic frameworks in water treatment: A critical review. Prog. Mater. Sci. 2022, 125, 100904. [Google Scholar] [CrossRef]
- Ahmadipouya, S.; Mousavi, S.A.; Shokrgozar, A.; Mousavi, D.V. Improving dye removal and antifouling performance of polysulfone nanofiltration membranes by incorporation of UiO-66 metal-organic framework. J. Environ. Chem. Eng. 2022, 10, 107535. [Google Scholar] [CrossRef]
- Lin, R.; Hernandez, B.V.; Ge, L.; Zhu, Z. Metal organic framework based mixed matrix membranes: An overview on filler/polymer interface. J. Mater. Chem. A 2018, 6, 293–312. [Google Scholar] [CrossRef]
- Penkova, A.V.; Kuzminova, A.I.; Dmitrenko, M.E.; Surkova, V.A.; Liamin, V.P.; Markelov, D.A.; Komolkin, A.V.; Poloneeva, D.Y.; Selyutin, A.A.; Mazur, A.S.; et al. Novel pervaporation mixed matrix membranes based on polyphenylene isophtalamide modified by metal–organic framework UiO-66(NH2)-EDTA for highly efficient methanol isolation. Sep. Purif. Technol. 2021, 263, 118370. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Wang, J.; Gascon, J.; Li, J.; Van der Bruggen, B. Metal-organic frameworks-based membranes for liquid separation. Chem. Soc. Rev. 2017, 46, 7124–7144. [Google Scholar] [CrossRef]
- Pakamorė, I.; Rousseau, J.; Rousseau, C.; Monflier, E.; Szilágyi, P. An ambient-temperature aqueous synthesis of zirconium-based metal–organic frameworks. Green Chem. 2018, 20, 5292–5298. [Google Scholar] [CrossRef]
- Choi, K.M.; Na, K.; Somorjai, G.A.; Yaghi, O.M. Chemical Environment Control and Enhanced Catalytic Performance of Platinum Nanoparticles Embedded in Nanocrystalline Metal-Organic Frameworks. J. Am. Chem. Soc. 2015, 137, 7810–7816. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Kango, H.; Yamashita, H. Catalytic Transfer Hydrogenation of Biomass-Derived Levulinic Acid and Its Esters to γ-Valerolactone over Sulfonic Acid-Functionalized UiO-66. ACS Sustain. Chem. Eng. 2016, 5, 1141–1152. [Google Scholar] [CrossRef]
- Foo, M.L.; Horike, S.; Fukushima, T.; Hijikata, Y.; Kubota, Y.; Takata, M.; Kitagawa, S. Ligand-based solid solution approach to stabilisation of sulphonic acid groups in porous coordination polymer Zr6O4(OH)4(BDC)6(UiO-66). Dalton Trans. 2012, 41, 13791–13794. [Google Scholar]
- Morris, W.; Doonan, C.J.; Yaghi, O.M. Postsynthetic modification of a metal-organic framework for stabilization of a hemiaminal and ammonia uptake. Inorg. Chem. 2011, 50, 6853–6855. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Nicoul, M.; Shymanovich, U.; Brinks, F.; Afshari, M.; Tarasevitch, A.; Von Der Linde, D.; Sokolowski-Tinten, K. Acoustic response of a laser-excited polycrystalline Au-film studied by ultrafast Debye-Scherrer diffraction at a table-top short-pulse X-ray source. AIP Adv. 2019, 10, 35015. [Google Scholar] [CrossRef]
- Yang, C.; Hou, L.X.; Yao, Z.K.; Zhao, J.J.; Hou, L.A.; Zhang, L. High proton selectivity membrane based on the keto-linked cationic covalent organic framework for acid recovery. J. Membr. Sci. 2021, 640, 119800. [Google Scholar] [CrossRef]
- Pawar, C.M.; Sreenath, S.; Dave, V.; Bavdane, P.P.; Singh, V.; Verma, V.; Nagarale, R.K. Chemically stable and high acid recovery anion exchange membrane. Polymer 2022, 251, 124915. [Google Scholar] [CrossRef]
- Deng, T.; Zeng, X.J.; Zhang, C.Y.; Wang, Y.X.; Zhang, W. Constructing proton selective pathways using MOFs to enhance acid recovery efficiency of anion exchange membranes. Chem. Eng. J. 2022, 445, 136752. [Google Scholar] [CrossRef]
- Zhang, P.P.; Wu, Y.Y.; Liu, W.Y.; Cui, P.; Huang, Q.; Ran, J. Construction of two-dimensional anion exchange membranes to boost acid recovery performances. J. Membr. Sci. 2021, 618, 118692. [Google Scholar] [CrossRef]
- Chen, C.D.; Xie, H.X.; Jiang, Y.Y.; Chen, Y.W.; Liang, Y.R.; Ruzetuoheti, G.; Liao, S.J.; Li, X.H.; Wei, B.W. Influence of hydrophobic components tuning of poly (aryl ether sulfone)s ionomers based anion exchange membranes on diffusion dialysis for acid recovery. J. Membr. Sci. 2021, 636, 119562. [Google Scholar] [CrossRef]
- Sharma, J.; Misra, S.K.; Kulshrestha, V. Internally cross-linked poly(2,6-dimethyl-1,4-phenylene ether) based anion exchange membrane for recovery of different acids by diffusion dialysis. Chem. Eng. J. 2021, 414, 128776. [Google Scholar] [CrossRef]
- Khan, M.I.; Shanableh, A.; Khraisheh, M.; AlMomani, F. Synthesis of Porous BPPO-Based Anion Exchange Membranes for Acid Recovery via Diffusion Dialysis. Membranes 2022, 12, 95. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.G.; Wu, B.; Zhu, Y.; Irfan, M.; Afsar, N.U.; Ge, L.; Xu, T.W. Self-organized nanostructured anion exchange membranes for acid recovery. Chem. Eng. J. 2020, 382, 1228382. [Google Scholar] [CrossRef]


| Ligands | U-C(50) | U-C(60) | U-C(70) | U-C(80) |
|---|---|---|---|---|
| 2-NH2-BDC | 0.322 g | 0.322 g | 0.322 g | 0.322 g |
| H4BTEC | 0.091 g | 0.072 g | 0.054 g | 0.036 g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Afsar, N.U.; Chen, X.; Wu, Y.; Chen, Y.; Shao, F.; Song, J.; Yao, S.; Xia, R.; Qian, J.; et al. Negatively Charged MOF-Based Composite Anion Exchange Membrane with High Cation Selectivity and Permeability. Membranes 2022, 12, 601. https://doi.org/10.3390/membranes12060601
Li X, Afsar NU, Chen X, Wu Y, Chen Y, Shao F, Song J, Yao S, Xia R, Qian J, et al. Negatively Charged MOF-Based Composite Anion Exchange Membrane with High Cation Selectivity and Permeability. Membranes. 2022; 12(6):601. https://doi.org/10.3390/membranes12060601
Chicago/Turabian StyleLi, Xiaohuan, Noor Ul Afsar, Xiaopeng Chen, Yifeng Wu, Yu Chen, Feng Shao, Jiaxian Song, Shuai Yao, Ru Xia, Jiasheng Qian, and et al. 2022. "Negatively Charged MOF-Based Composite Anion Exchange Membrane with High Cation Selectivity and Permeability" Membranes 12, no. 6: 601. https://doi.org/10.3390/membranes12060601
APA StyleLi, X., Afsar, N. U., Chen, X., Wu, Y., Chen, Y., Shao, F., Song, J., Yao, S., Xia, R., Qian, J., Wu, B., & Miao, J. (2022). Negatively Charged MOF-Based Composite Anion Exchange Membrane with High Cation Selectivity and Permeability. Membranes, 12(6), 601. https://doi.org/10.3390/membranes12060601









