Study on Spacing Regulation and Separation Performance of Nanofiltration Membranes of GO
Abstract
:1. Introduction
2. Experimental Section
2.1. Experimental Materials and Reagents
2.2. Modified-BPPO/GO-Membrane Synthesis Method
2.2.1. Preparation of BPPO Membranes
2.2.2. Synthesis of Adipose-Diamine-Modified GO
2.2.3. Preparation of BPPO/AX-GO Composite Membranes
2.3. Characterization of BPPO/AX-GO Composite Membranes
2.4. Membrane-Performance Evaluation
2.4.1. Membrane Flux
2.4.2. Membrane Rejection
3. Results and Discussion
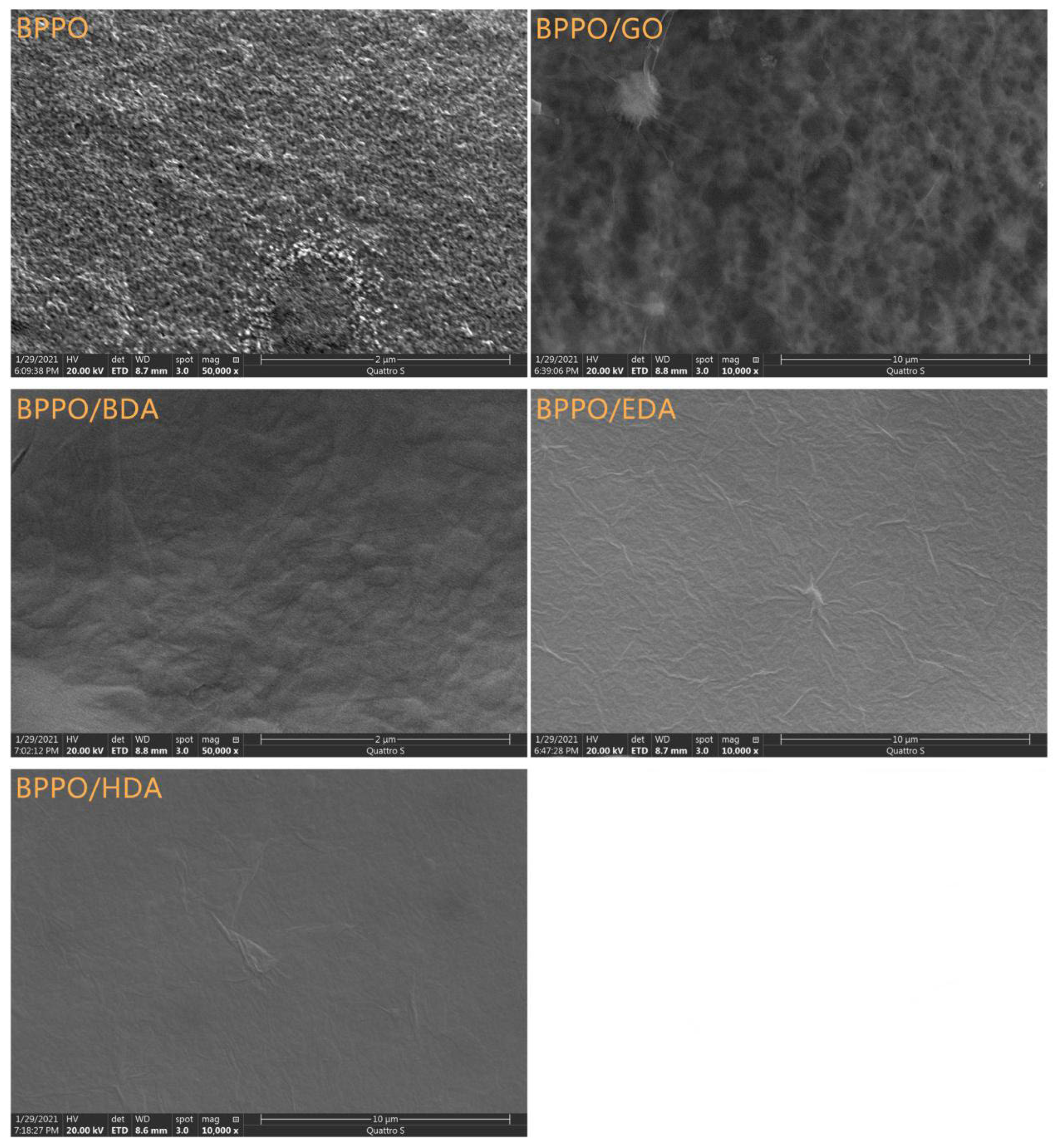
3.1. Microstructure of Composite Membranes
3.1.1. Scanning Electron Microscopy (SEM)
3.1.2. Atomic Force Microscopy (AFM)
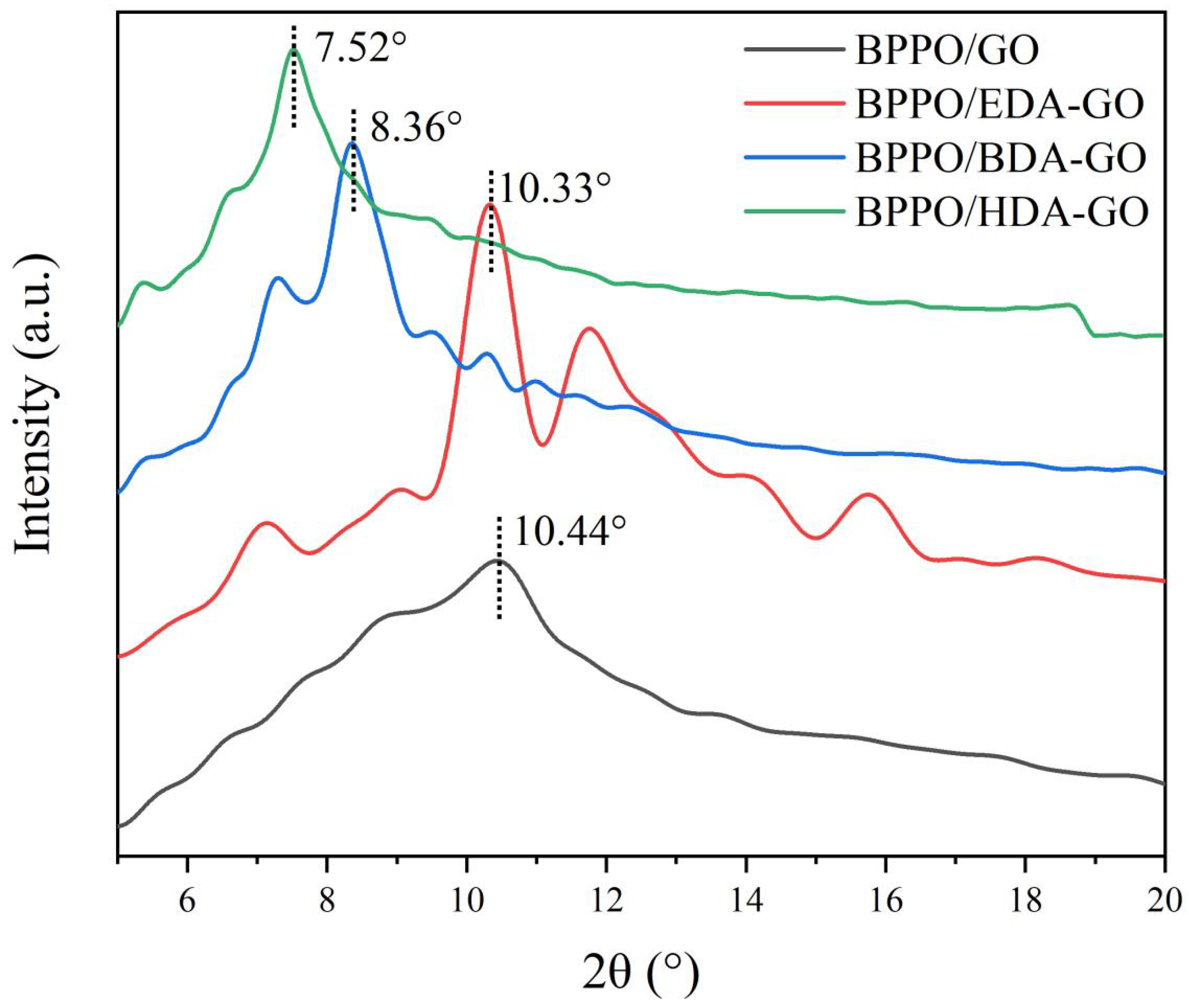
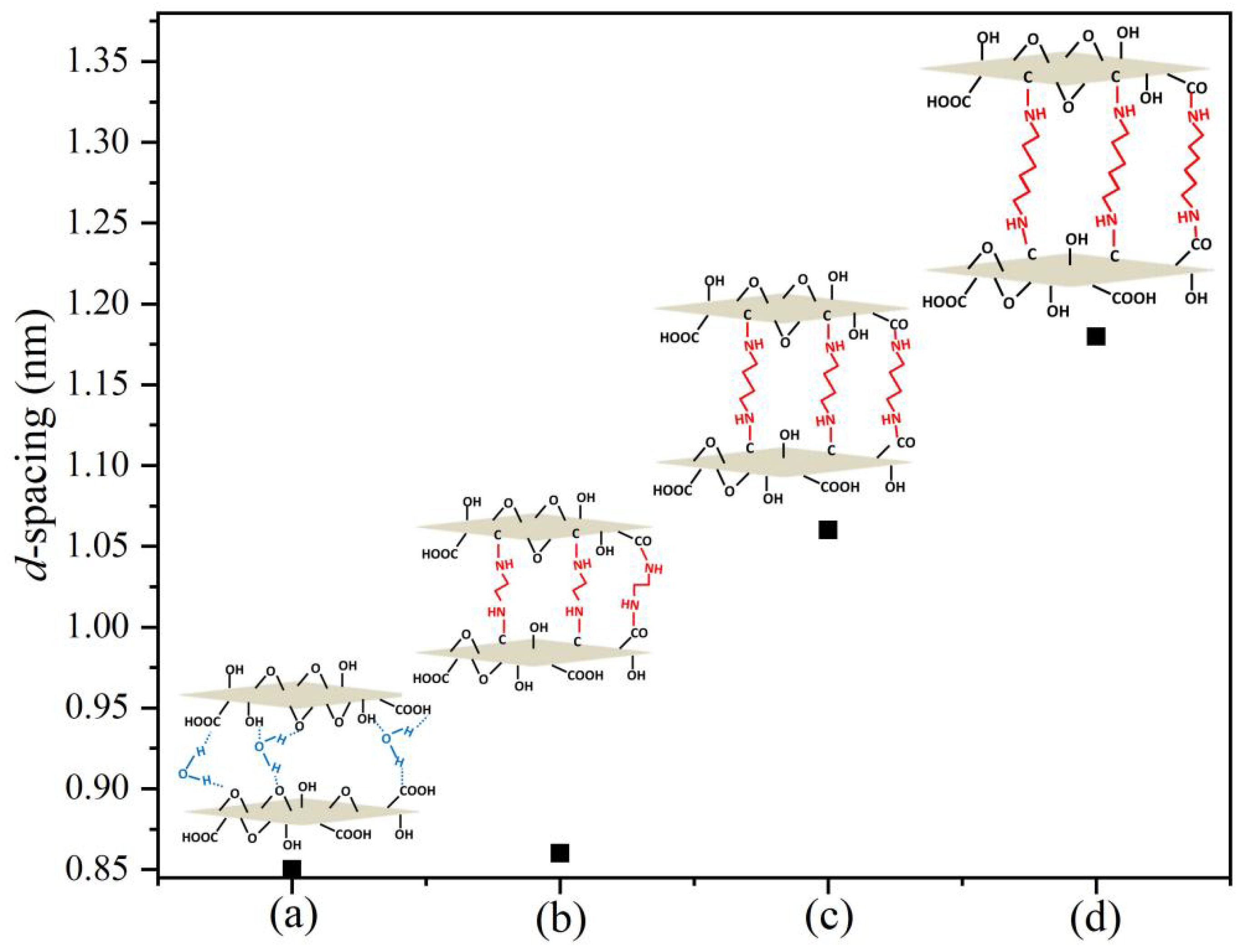
3.2. GO-Nanofiltration-Membrane Spacing
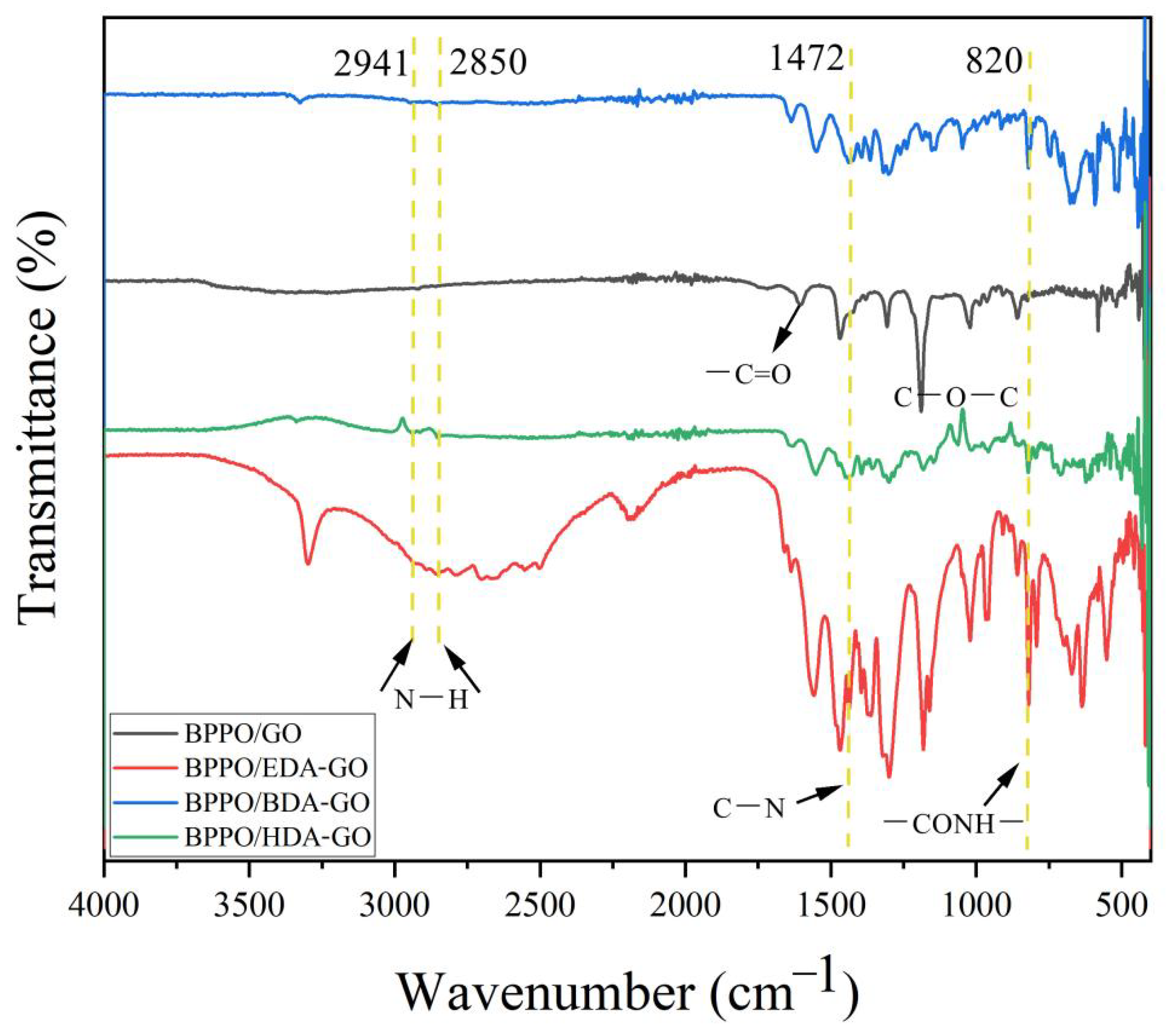
3.3. Fourier-Transform Infrared Characterization
3.4. Membrane Flux and Rejection
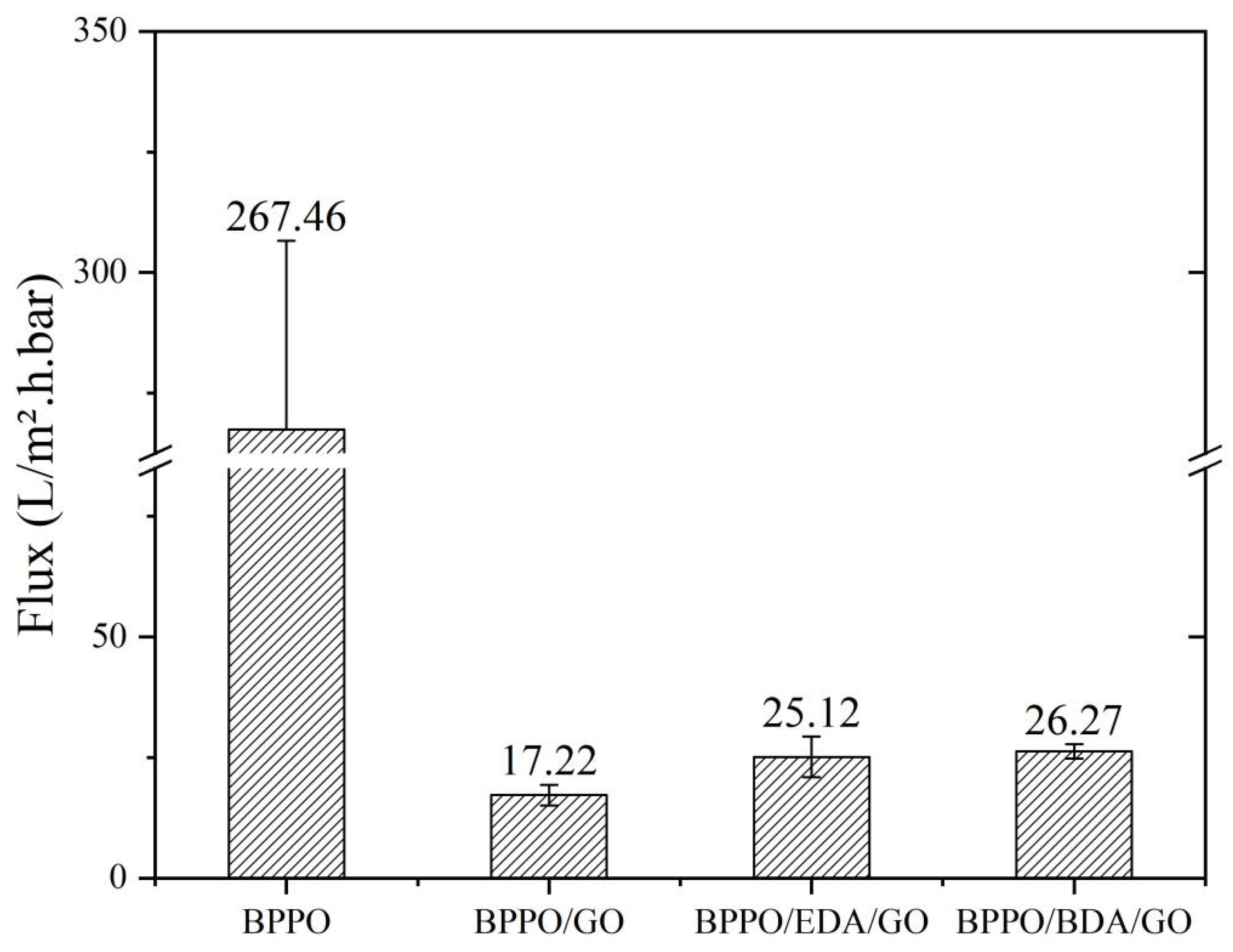
3.4.1. Membrane Flux
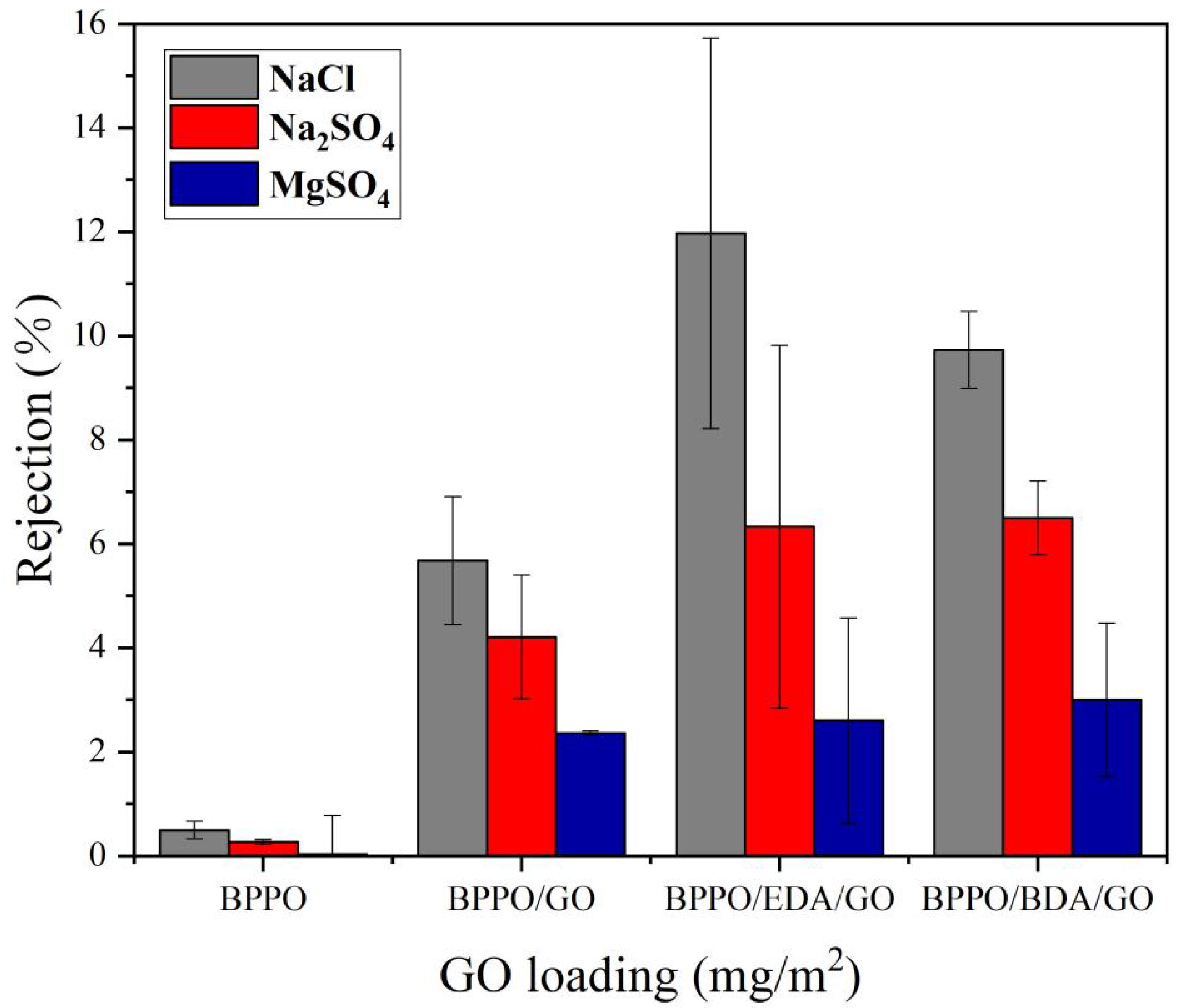
3.4.2. Rejection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geise, G.M.; Park, H.B.; Sagle, A.C.; Freeman, B.D.; McGrath, J.E. Water permeability and water/salt selectivity tradeoff in polymers for desalination. J. Membr. Sci. 2011, 369, 130–138. [Google Scholar] [CrossRef]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Jin, W.; Xu, N. Two-Dimensional-Material Membranes: A New Family of High-Performance Separation Membranes. Angew. Chem. Int. Ed. 2016, 55, 13384–13397. [Google Scholar] [CrossRef]
- Seungju, K.; Wang, H.; Lee, Y.M. 2D Nanosheets and Their Composite Membranes for Water, Gas, and Ion Separation. Angew. Chem. 2019, 131, 17674–17689. [Google Scholar]
- Lee, H.K.; Ray, S.S.; Huyen, D.; Kang, W.; Kwon, Y.N. Chemical and surface engineered superhydrophobic patterned membrane with enhanced wetting and fouling resistance for improved membrane distillation performance. J. Membr. Sci. 2021, 629, 119280. [Google Scholar] [CrossRef]
- Yun, C.W.; Yao, M.; Shim, W.G.; Kim, Y.; Tijing, L.D.; Jung, B.; Kim, S.-H.; Shon, H.K. Co-axially electrospun superhydrophobic nanofiber membranes with 3D-hierarchically structured surface for desalination by long-term membrane distillation. J. Membr. Sci. 2021, 623, 119028. [Google Scholar]
- Zhang, J.; Zhou, F.; Li, S.; Wan, Y.; Luo, J. Surface functionalization of nanofiltration membrane by catechol-amine codeposition for enhancing antifouling performance. J. Membr. Sci. 2021, 635, 119451. [Google Scholar] [CrossRef]
- Xu, F.; Dai, L.; Wu, Y.; Xu, Z. Li+/Mg2+ separation by membrane separation: The role of the compensatory effect. J. Membr. Sci. 2021, 636, 119542. [Google Scholar] [CrossRef]
- Liao, X.; Wang, Y.; Liao, Y.; You, X.; Yao, L.; Razaqpur, A.G. Effects of different surfactant properties on anti-wetting behaviours of an omniphobic membrane in membrane distillation. J. Membr. Sci. 2021, 634, 119433. [Google Scholar] [CrossRef]
- Tang, Y.; Lin, Y.; Ford, D.M.; Qian, X.; Cervellere, M.R.; Millett, P.C.; Wang, X. A review on models and simulations of membrane formation via phase inversion processes. J. Membr. Sci. 2021, 640, 119810. [Google Scholar] [CrossRef]
- Liu, J.; Guo, H.; Sun, Z.; Li, B. Preparation of photothermal membrane for vacuum membrane distillation with excellent anti-fouling ability through surface spraying. J. Membr. Sci. 2021, 634, 119434. [Google Scholar] [CrossRef]
- Kang, Y.; Xia, Y.; Wang, H.; Zhang, X. 2D Laminar Membranes for Selective Water and Ion Transport. Adv. Funct. Mater. 2019, 29, 1902014. [Google Scholar] [CrossRef]
- Ansari, A.; Galogahi, F.M.; Thiel, D.V.; Helfer, F.; Millar, G.; Soukane, S.; Ghaffour, N. Downstream variations of air-gap membrane distillation and comparative study with direct contact membrane distillation: A modelling approach. Desalination 2022, 526, 115539. [Google Scholar] [CrossRef]
- Davood, G.; Samaneh, B.; Abdolreza, M. Embedded three spinel ferrite nanoparticles in PES-based nano filtration membranes with enhanced separation properties. Main Group Met. Chem. 2021, 45, 1–10. [Google Scholar] [CrossRef]
- Karki, S.; Ingole, P.G. Development of polymer-based new high performance thin-film nanocomposite nanofiltration membranes by vapor phase interfacial polymerization for the removal of heavy metal ions. Chem. Eng. J. 2022, 446, 137303. [Google Scholar] [CrossRef]
- Gohain, M.B.; Pawar, R.R.; Karki, S.; Hazarika, A.; Hazarika, S.; Ingole, P.G. Development of thin film nanocomposite membrane incorporated with mesoporous synthetic hectorite and MSH@UiO-66-NH 2 nanoparticles for efficient targeted feeds separation, and antibacterial performance. J. Membr. Sci. 2020, 609, 118212. [Google Scholar] [CrossRef]
- Heiranian, M.; Farimani, A.B.; Aluru, N.R. Water desalination with a single-layer MoS2 nanopore. Sci. Rep. 2015, 6, 8616. [Google Scholar] [CrossRef]
- Karki, S.; Gohain, M.B.; Yadav, D.; Ingole, P.G. Nanocomposite and bio-nanocomposite polymeric materials/membranes development in energy and medical sector: A review—ScienceDirect. Int. J. Biol. Macromol. 2021, 193, 2121–2139. [Google Scholar] [CrossRef]
- Karki, S.; Ingole, P.G. Graphene-based thin film nanocomposite membranes for separation and purification—ScienceDirect. Compr. Anal. Chem. 2020, 91, 73–97. [Google Scholar]
- Choi, O.; Ingole, P.G.; Park, C.H. Precision-aiming tuning of membranes prepared by NIPS and its performance enhancement. J. Clean. Prod. 2022, 365, 132858. [Google Scholar] [CrossRef]
- Qin, S.; Liu, D.; Wang, G.; Portehault, D.; Garvey, C.J.; Gogotsi, Y.; Lei, W.; Chen, Y. High and stable ionic conductivity in 2d nanofluidic ion channels between boron nitride layers high and stable ionic conductivity in 2d nanofluidic ion channels between boron nitride layers. J. Am. Chem. Soc. 2019, 139, 6314–6320. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.I.; Ingole, P.G.; Jeon, J.D.; Hong, S.U.; Choi, W.K.; Lee, H.K. Water vapor transport properties of interfacially polymerized thin film nanocomposite membranes modified with graphene oxide and GO-TiO2 nanofillers. Chem. Eng. J. 2019, 373, 1190–1202. [Google Scholar] [CrossRef]
- Yang, J.; Gong, D.; Li, G.; Zeng, G.; Wang, Q.; Zhang, Y.; Liu, G.; Wu, P.; Vovk, E.; Peng, Z.; et al. Self-Assembly of Thiourea-Crosslinked Graphene Oxide Framework Membranes toward Separation of Small Molecules. Adv. Mater. 2018, 30, 1705775. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.J.; Gu, Y.H.; Chen, Y.; Yan, X.; Guo, Y.J.; Lang, W.Z. Ultrahigh permeability of graphene-based membranes by adjusting D-spacing with poly (ethylene imine) for the separation of dye wastewater. Sep. Purif. Technol. 2019, 210, 737–745. [Google Scholar] [CrossRef]
- Liu, T.; Yang, B.; Graham, N.; Yu, W.; Sun, K. Trivalent metal cation cross-linked graphene oxide membranes for NOM removal in water treatment. J. Membr. Sci. 2017, 542, 31–40. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, Y.; Xue, J.; Wang, H. Enhanced antipressure ability through graphene oxide membrane by intercalating g-C3N4 nanosheets for water purification. AlChE J. 2019, 65, e16699. [Google Scholar] [CrossRef]
- Wang, Z.; Tu, Q.; Zheng, S.; Urban, J.J.; Li, S.; Mi, B. Understanding the Aqueous Stability and Filtration Capability of MoS2 Membranes. Nano Lett. 2017, 17, 7289–7298. [Google Scholar] [CrossRef]
- Gao, S.S.; Shang, S.B.; Liu, X.Y.; Li, Z.; Sheng, Y.Q.; Zhang, C.; Yang, C.; Qiu, H.W.; Huo, Y.Y.; Jiang, S.Z. An optical fiber SERS sensor based on GO/AgNPs/rGO sandwich structure hybrid films. RSC Adv. 2016, 6, 81750–81756. [Google Scholar] [CrossRef]
- Wang, F.; Wang, B.; Zhang, X.; Lu, M.; Zhang, Y.; Sun, C.; Peng, W. High Sensitivity Humidity Detection Based on Functional GO/MWCNTs Hybrid Nano-Materials Coated Titled Fiber Bragg Grating. Nanomaterials 2021, 11, 1134. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, V.; Farimani, A.B. Water Desalination with Two-Dimensional Metal-Organic Framework Membranes. Nano Lett. 2019, 19, 8638–8643. [Google Scholar] [CrossRef]
- Ding, L.; Wei, Y.; Wang, Y.; Chen, H.; Caro, J.; Wang, H. A Two-Dimensional Lamellar Membrane: MXene Nanosheet Stacks. Angew. Chem. Int. Ed. 2017, 56, 1825–1829. [Google Scholar] [CrossRef] [PubMed]
- Ang, H.; Hong, L. Polycationic Polymer-Regulated Assembling of 2D MOF Nanosheets for High-Performance Nanofiltration. ACS Appl. Mater. Interfaces 2017, 9, 28079–28088. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kang, Z.; Xu, B.; Fan, L.; Li, G.; Wen, L.; Xin, X.; Xiao, Z.; Pang, J.; Du, X.; et al. Wettability switchable metal-organic framework membranes for pervaporation of water/ethanol mixtures. Inorg. Chem. Commun. 2017, 82, 64–67. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, S.; Guo, Y.; Liu, G.; Jin, W. Separation of mono-/di-valent ions via charged interlayer channels of graphene oxide membranes. J. Membr. Sci. 2021, 645, 120212. [Google Scholar] [CrossRef]
- Shen, J.; Liu, G.; Huang, K.; Chu, Z.; Jin, W.; Xu, N. Subnanometer Two-Dimensional Graphene Oxide Channels for Ultrafast Gas Sieving. ACS Nano 2016, 10, 3398–3409. [Google Scholar] [CrossRef] [PubMed]
- Meng, N.; Zhao, W.; Shamsaei, E.; Wang, G.; Zeng, X.; Lin, X.; Xu, T.; Wang, H.; Zhang, X. A Low-pressure GO nanofiltration membrane crosslinked via Ethylenediamine. J. Membr. Sci. 2017, 548, 363–371. [Google Scholar] [CrossRef]
- Du, H.; Li, J.; Zhang, J.; Su, G.; Li, X.; Zhao, Y. Separation of Hydrogen and Nitrogen Gases with Porous Graphene Membrane. J. Phys. Chem. C 2011, 115, 23261–23266. [Google Scholar] [CrossRef]
- Jiang, D.E.; Cooper, V.R.; Dai, S. Porous Graphene as the Ultimate Membrane for Gas Separation. Nano Lett. 2009, 9, 4019–4024. [Google Scholar] [CrossRef]

| Sample | Mean Roughness/nm | Root Mean Square Roughness/nm |
|---|---|---|
| BPPO | 10.9 | 14.2 |
| BPPO/GO | 19.6 | 24.5 |
| BPPO/BDA-GO | 49.7 | 56.8 |
| BPPO/EDA-GO | 23.9 | 29.6 |
| BPPO/HDA-GO | 15.7 | 22.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, N.; Zhao, P.; Zhou, W.; Yan, J.; Hu, D.; Fang, Y.; Lu, J.; Liu, Q. Study on Spacing Regulation and Separation Performance of Nanofiltration Membranes of GO. Membranes 2022, 12, 803. https://doi.org/10.3390/membranes12080803
Meng N, Zhao P, Zhou W, Yan J, Hu D, Fang Y, Lu J, Liu Q. Study on Spacing Regulation and Separation Performance of Nanofiltration Membranes of GO. Membranes. 2022; 12(8):803. https://doi.org/10.3390/membranes12080803
Chicago/Turabian StyleMeng, Na, Pinping Zhao, Wei Zhou, Jie Yan, Die Hu, Yanqing Fang, Jun Lu, and Qiang Liu. 2022. "Study on Spacing Regulation and Separation Performance of Nanofiltration Membranes of GO" Membranes 12, no. 8: 803. https://doi.org/10.3390/membranes12080803
APA StyleMeng, N., Zhao, P., Zhou, W., Yan, J., Hu, D., Fang, Y., Lu, J., & Liu, Q. (2022). Study on Spacing Regulation and Separation Performance of Nanofiltration Membranes of GO. Membranes, 12(8), 803. https://doi.org/10.3390/membranes12080803





