A Comprehensive Analysis of Inorganic Ions and Their Selective Removal from the Reconstituted Tobacco Extract Using Electrodialysis
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemical and Materials
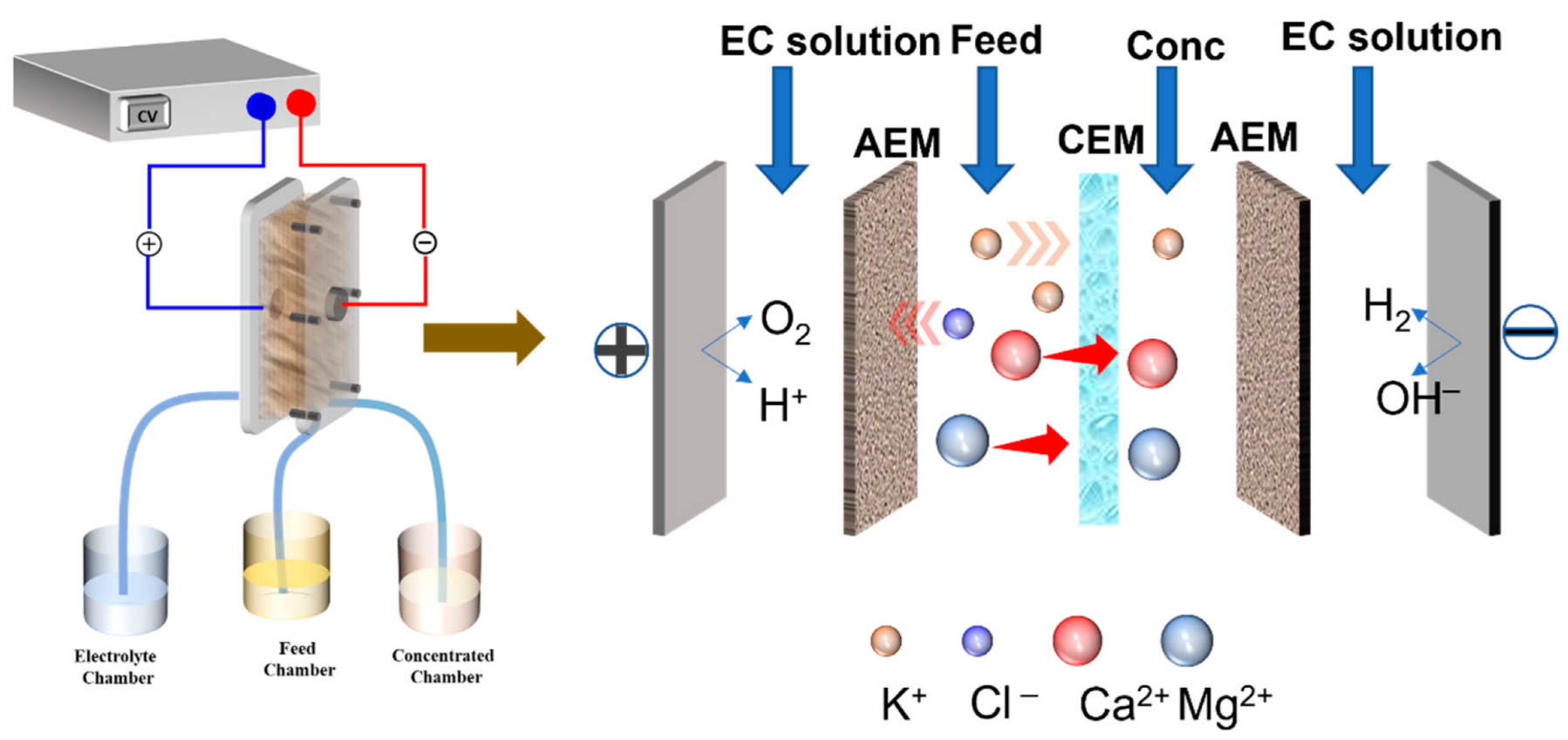
2.2. Electrodialysis Experiment
2.3. Analytical Methods
2.4. Physiochemical Properties of IEMs
3. Results and Discussion
3.1. The Nicotine Content in Different Tobacco Liquids
3.2. Ions Concentration of Tobacco Extract
3.3. ED Analysis at Different pH Values
3.4. Total Sugar/Nicotine and Reducing Sugar/Nicotine at Different pH Values
3.5. The Influence of pH on the Ion’s Removal
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sun, J.-G.; He, J.-W.; Wu, F.-G.; Tu, S.-X.; Yan, T.-J.; Si, H.; Xie, H. Comparative analysis on chemical components and sensory quality of aging flue-cured tobacco from four main tobacco areas of China. Agric. Sci. China 2011, 10, 1222–1231. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Yang, L.; Liu, B.; Lan, M.; Sun, W. Studies on thermal behavior of reconstituted tobacco sheet. Thermochim. Acta 2005, 437, 7–11. [Google Scholar] [CrossRef]
- Potts, R.J.; Bombick, B.R.; Meckley, D.R.; Ayres, P.H.; Pence, D.H. A summary of toxicological and chemical data relevant to the evaluation of cast sheet tobacco. Exp. Toxicol. Pathol. 2010, 62, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, P.; Cong, W. Cation-exchange membrane fouling and cleaning in bipolar membrane electrodialysis of industrial glutamate production wastewater. Sep. Purif. Technol. 2011, 79, 103–113. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, C.; Xu, Y.; Hu, Y. The pyrolysis of cigarette paper under the conditions that simulate cigarette smouldering and puffing. J. Therm. Anal. Calorim. 2011, 104, 1097–1106. [Google Scholar] [CrossRef]
- Seyler, T.H.; Kim, J.G.; Hodgson, J.A.; Cowan, E.A.; Blount, B.C.; Wang, L. Quantitation of Urinary Volatile Nitrosamines from Exposure to Tobacco Smoke. J. Anal. Toxicol. 2013, 37, 195–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narkowicz, S.; Polkowska, Ż.; Kiełbratowska, B.; Namieśnik, J. Environmental Tobacco Smoke: Exposure, Health Effects, and Analysis. Crit. Rev. Environ. Sci. Technol. 2013, 43, 121–161. [Google Scholar] [CrossRef]
- Nowakowski, D.J.; Jones, J. Uncatalysed and potassium-catalysed pyrolysis of the cell-wall constituents of biomass and their model compounds. J. Anal. Appl. Pyrolysis 2008, 83, 12–25. [Google Scholar] [CrossRef]
- Ali, M.; Sreekrishnan, T.R. Aquatic toxicity from pulp and paper mill effluents: A review. Adv. Environ. Res. 2001, 5, 175–196. [Google Scholar] [CrossRef]
- Amuda, O.; Amoo, I.; Ajayi, O. Performance optimization of coagulant/flocculant in the treatment of wastewater from a beverage industry. J. Hazard. Mater. 2006, 129, 69–72. [Google Scholar] [CrossRef]
- Ge, S.; Zhang, Z.; Yan, H.; Irfan, M.; Xu, Y.; Li, W.; Wang, H.; Wang, Y. Electrodialytic Desalination of Tobacco Sheet Extract: Membrane Fouling Mechanism and Mitigation Strategies. Membranes 2020, 10, 245. [Google Scholar] [CrossRef]
- Gaisch, H.; Krasna, B.; Schulthess, D. Continuous Method of Denitrating Tobacco Extracts. U.S. Patent 4,622,982, 18 November 1986. [Google Scholar]
- Mattina, C.F. Method of Making Reconstituted Tobacco Having Reduced Nitrates. U.S. Patent No. 3,847,164, 12 November 1974. [Google Scholar]
- Ran, J.; Wu, L.; He, Y.; Yang, Z.; Wang, Y.; Jiang, C.; Ge, L.; Bakangura, E.; Xu, T. Ion exchange membranes: New developments and applications. J. Membr. Sci. 2017, 522, 267–291. [Google Scholar] [CrossRef]
- Strathmann, H. Electrodialysis, a mature technology with a multitude of new applications. Desalination 2010, 264, 268–288. [Google Scholar] [CrossRef]
- Zhao, W.-Y.; Zhou, M.; Yan, B.; Sun, X.; Liu, Y.; Wang, Y.; Xu, T.; Zhang, Y. Waste Conversion and Resource Recovery from Wastewater by Ion Exchange Membranes: State-of-the-Art and Perspective. Ind. Eng. Chem. Res. 2018, 57, 6025–6039. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, M.; Guo, Y.; Mamrol, N.; Yang, X.; Gao, C.; Van der Bruggen, B. Metal-organic framework based membranes for selective separation of target ions. J. Membr. Sci. 2021, 634, 119407. [Google Scholar] [CrossRef]
- Yan, J.; Wang, H.; Fu, R.; Fu, R.; Li, R.; Chen, B.; Jiang, C.; Ge, L.; Liu, Z.; Wang, Y.; et al. Ion exchange membranes for acid recovery: Diffusion Dialysis (DD) or Selective Electrodialysis (SED)? Desalination 2022, 531, 115690. [Google Scholar] [CrossRef]
- Bazinet, L.; DeGrandpré, Y.; Porter, A. Electromigration of tobacco polyphenols. Sep. Purif. Technol. 2005, 41, 101–107. [Google Scholar] [CrossRef]
- Bazinet, L.; DeGrandpré, Y.; Porter, A. Enhanced tobacco polyphenol electromigration and impact on membrane integrity. J. Membr. Sci. 2005, 254, 111–118. [Google Scholar] [CrossRef]
- Zhang, Z.; Ge, S.; Jiang, C.; Zhao, Y.; Wang, Y. Improving the smoking quality of papermaking tobacco sheet extract by using electrodialysis. Membr. Water Treat. 2014, 5, 31–40. [Google Scholar] [CrossRef]
- Ge, S.; Li, W.; Zhang, Z.; Li, C.; Wang, Y. Desalting of tobacco extract using electrodialysis. Membr. Water Treat. 2016, 7, 341–353. [Google Scholar] [CrossRef]
- Li, C.; Ge, S.; Li, W.; Zhang, Z.; She, S.; Huang, L.; Wang, Y. Desalting of papermaking tobacco sheet extract using selective electrodialysis. Membr. Water Treat. 2017, 8, 381–393. [Google Scholar]
- Lindstrand, V.; Sundström, G.; Jönsson, A.-S. Fouling of electrodialysis membranes by organic substances. Desalination 2000, 128, 91–102. [Google Scholar] [CrossRef]
- Lindstrand, V.; Jönsson, A.-S.; Sundström, G. Organic fouling of electrodialysis membranes with and without applied voltage. Desalination 2000, 130, 73–84. [Google Scholar] [CrossRef]
- Lee, H.-J.; Hong, M.-K.; Han, S.-D.; Cho, S.-H.; Moon, S.-H. Fouling of an anion exchange membrane in the electrodialysis desalination process in the presence of organic foulants. Desalination 2009, 238, 60–69. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, H.; Zhang, L.; Liu, D.; Ye, X. Extraction of essential oil from discarded tobacco leaves by solvent extraction and steam distillation, and identification of its chemical composition. Ind. Crop. Prod. 2012, 39, 162–169. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, M.; Yang, B.; Jiang, Y.; Rao, G. Identification of polyphenols in tobacco leaf and their antioxidant and antimicrobial activities. Food Chem. 2008, 107, 1399–1406. [Google Scholar] [CrossRef]
- Mikhaylin, S.; Bazinet, L. Fouling on ion-exchange membranes: Classification, characterization and strategies of prevention and control. Adv. Colloid Interface Sci. 2015, 229, 34–56. [Google Scholar] [CrossRef]
- Geise, G.M.; Cassady, H.J.; Paul, D.R.; Logan, B.E.; Hickner, M.A. Specific ion effects on membrane potential and the permselectivity of ion exchange membranes. Phys. Chem. Chem. Phys. 2014, 16, 21673–21681. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Pan, J.; Yang, S.; Zhou, M.; Li, J.; Díaz, A.S.; Van Der Bruggen, B.; Gao, C.; Shen, J. Novel Composite Anion Exchange Membranes Based on Quaternized Polyepichlorohydrin for Electromembrane Application. Ind. Eng. Chem. Res. 2016, 55, 7171–7178. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, Q.; Wang, Y.; Zheng, C.; Wu, L.; Xu, T. In-situ crosslinking of anion exchange membrane bearing unsaturated moieties for electrodialysis. Sep. Purif. Technol. 2015, 156, 226–233. [Google Scholar] [CrossRef]






| Membranes | Thickness (mm) | IECs (mmol g−1) | WU (%) | RM (Ω·cm2) | Transfer Number | Break Stress (MPa) |
|---|---|---|---|---|---|---|
| CJ-MC-2 | 0.200 | 1.50 | 35 | 1.5 | 0.98 | >3.5 |
| CJ-MA-2 | 0.145 | 1.25 | 32 | 1.2 | 0.99 | >3.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, S.; Chen, Q.; Zhang, Z.; She, S.; Xu, B.; Liu, F.; Afsar, N.U. A Comprehensive Analysis of Inorganic Ions and Their Selective Removal from the Reconstituted Tobacco Extract Using Electrodialysis. Membranes 2022, 12, 597. https://doi.org/10.3390/membranes12060597
Ge S, Chen Q, Zhang Z, She S, Xu B, Liu F, Afsar NU. A Comprehensive Analysis of Inorganic Ions and Their Selective Removal from the Reconstituted Tobacco Extract Using Electrodialysis. Membranes. 2022; 12(6):597. https://doi.org/10.3390/membranes12060597
Chicago/Turabian StyleGe, Shaolin, Qian Chen, Zhao Zhang, Shike She, Bingxia Xu, Fei Liu, and Noor Ul Afsar. 2022. "A Comprehensive Analysis of Inorganic Ions and Their Selective Removal from the Reconstituted Tobacco Extract Using Electrodialysis" Membranes 12, no. 6: 597. https://doi.org/10.3390/membranes12060597
APA StyleGe, S., Chen, Q., Zhang, Z., She, S., Xu, B., Liu, F., & Afsar, N. U. (2022). A Comprehensive Analysis of Inorganic Ions and Their Selective Removal from the Reconstituted Tobacco Extract Using Electrodialysis. Membranes, 12(6), 597. https://doi.org/10.3390/membranes12060597






