Complementary Powerful Techniques for Investigating the Interactions of Proteins with Porous TiO2 and Its Hybrid Materials: A Tutorial Review
Abstract
1. Introduction
2. Materials and Methods
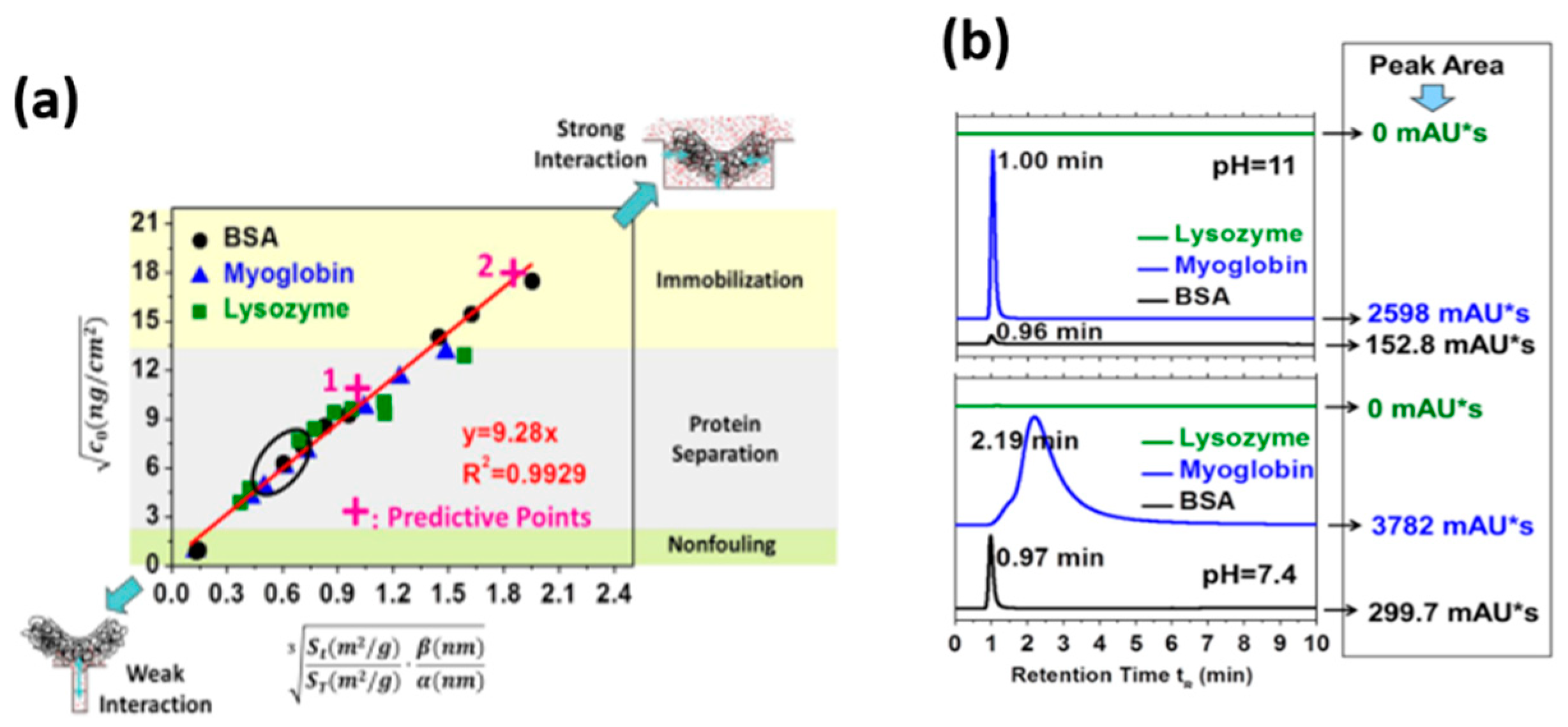
2.1. HPLC-Based Techniques on Biomolecule-TiO2 Interactions
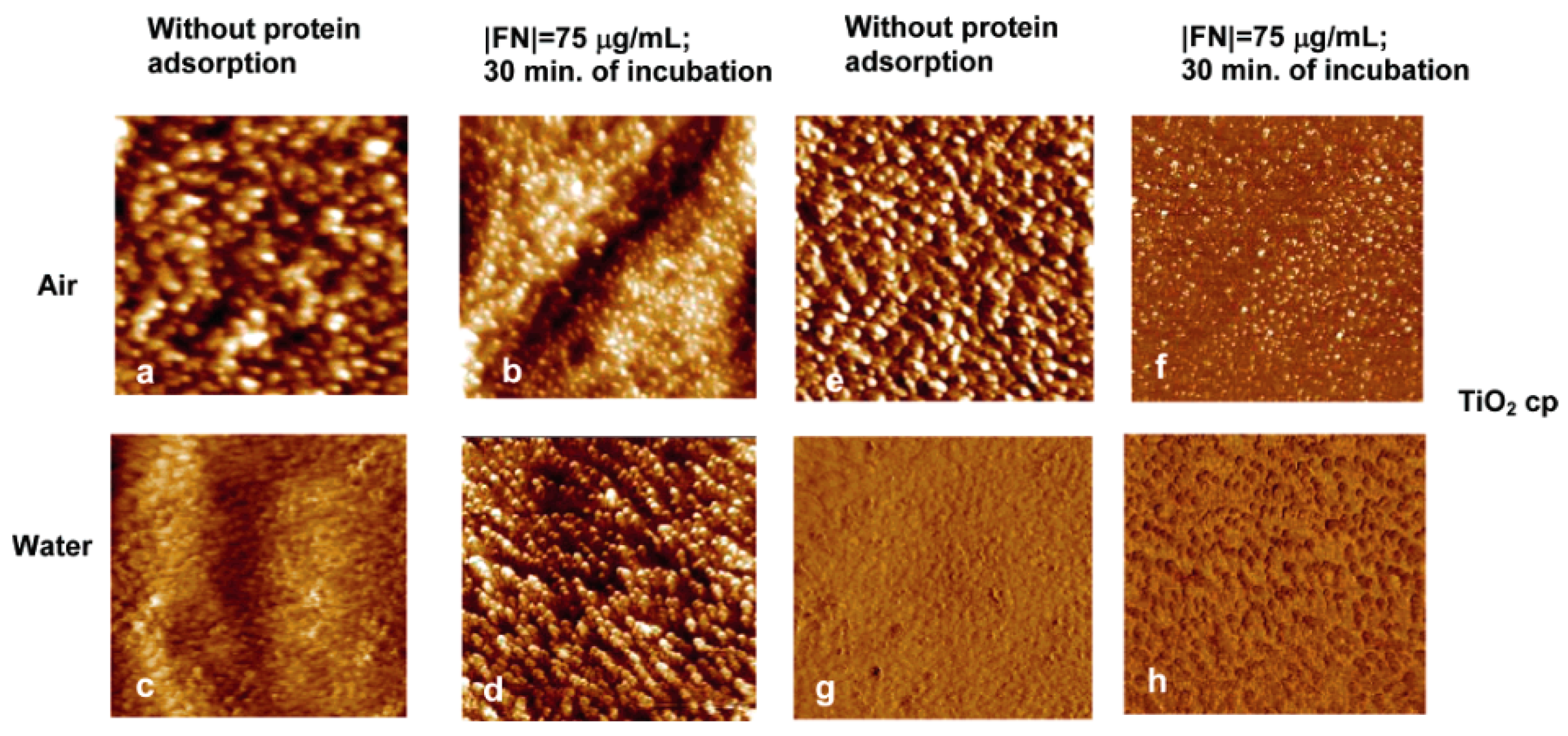
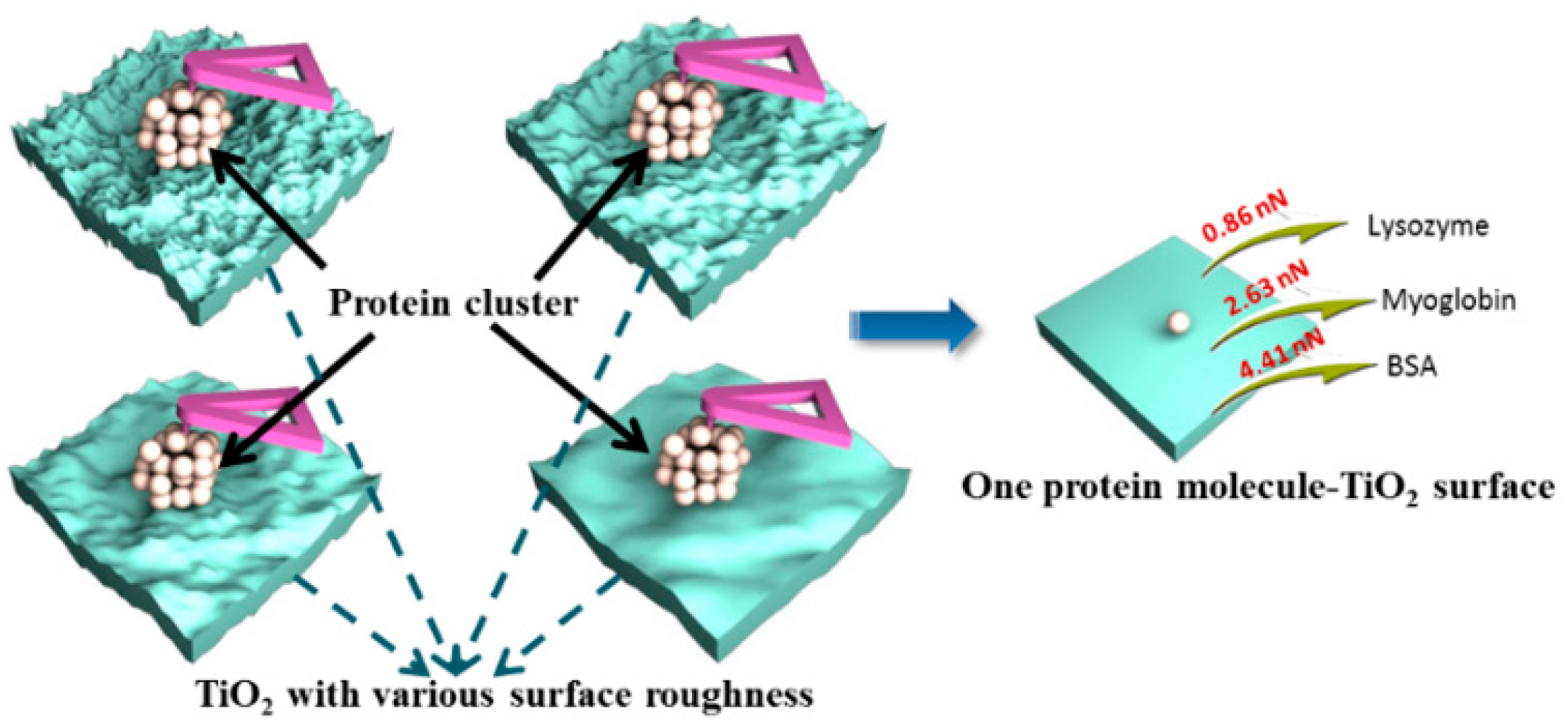
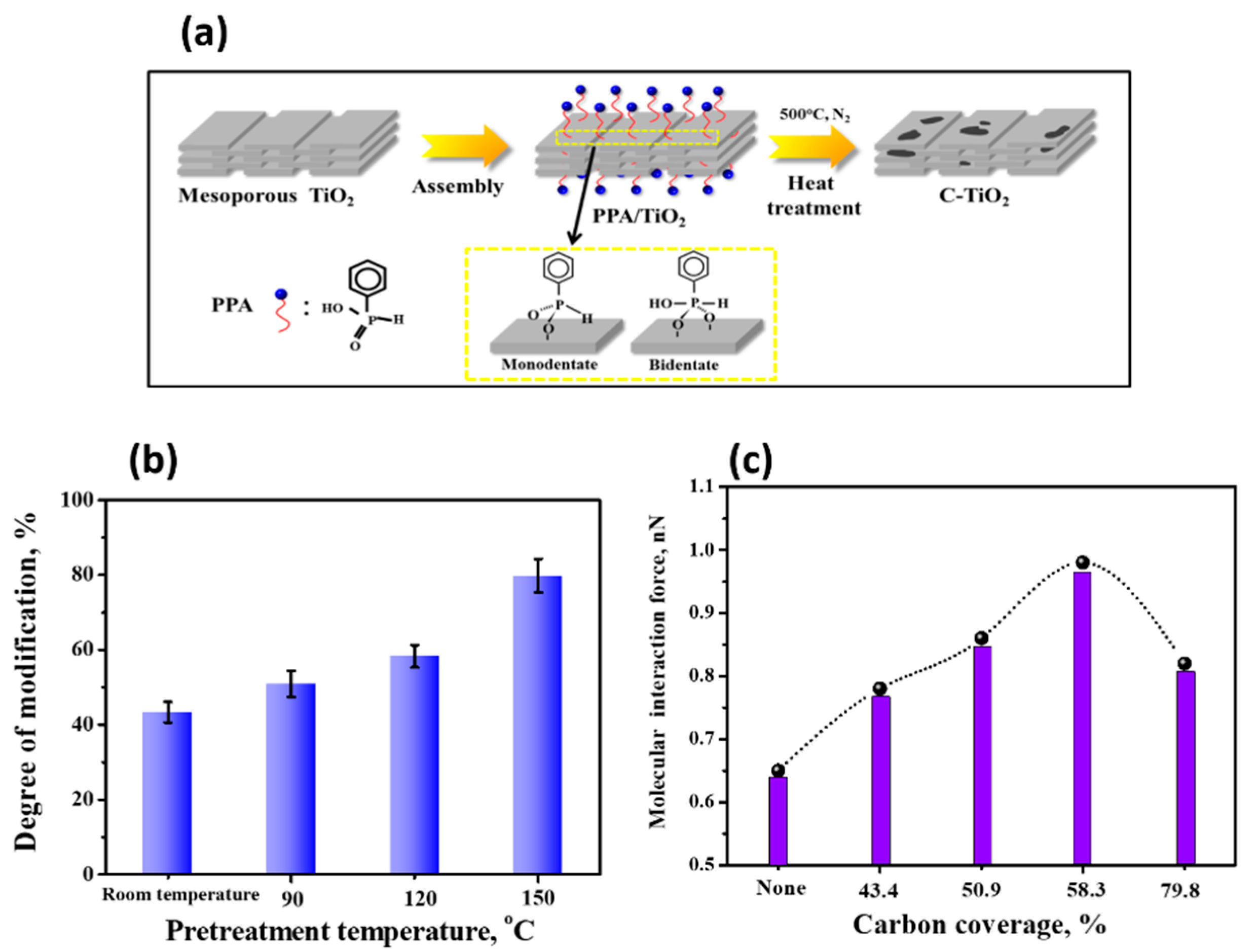
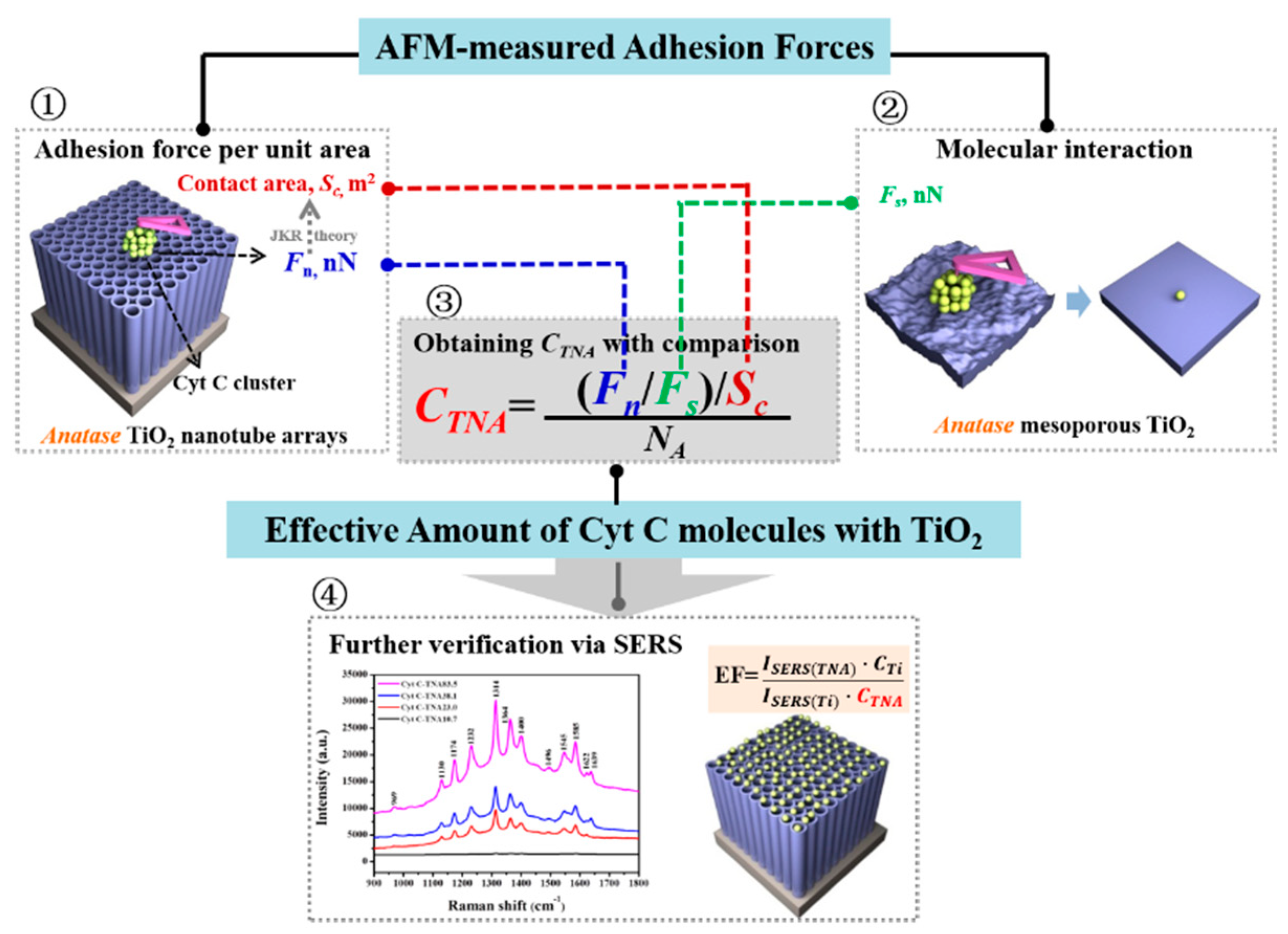
2.2. AFM-Based Techniques on Biomolecule-TiO2 Interactions
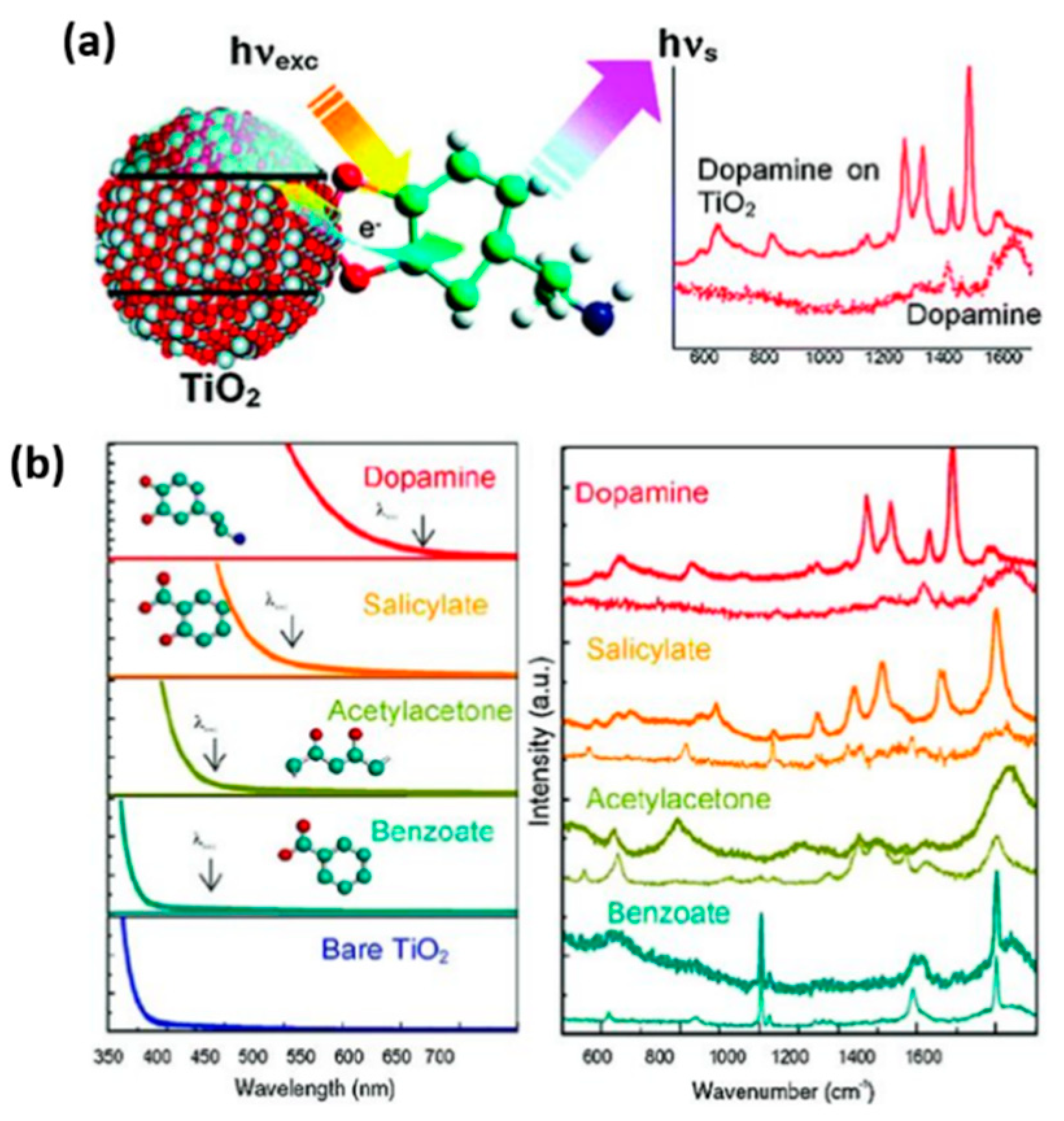
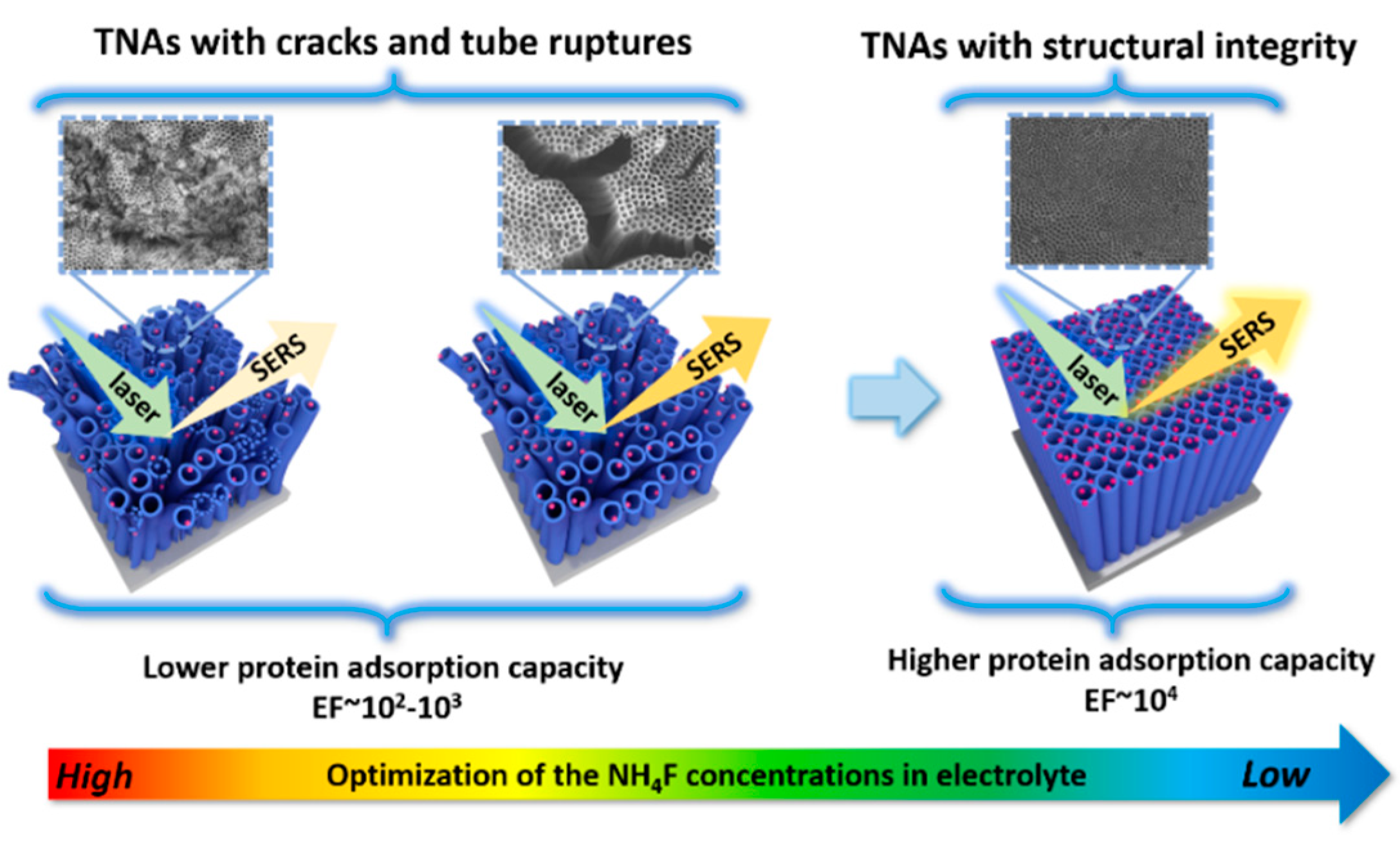
2.3. SERS-Based Techniques on Biomolecule-TiO2 Interactions
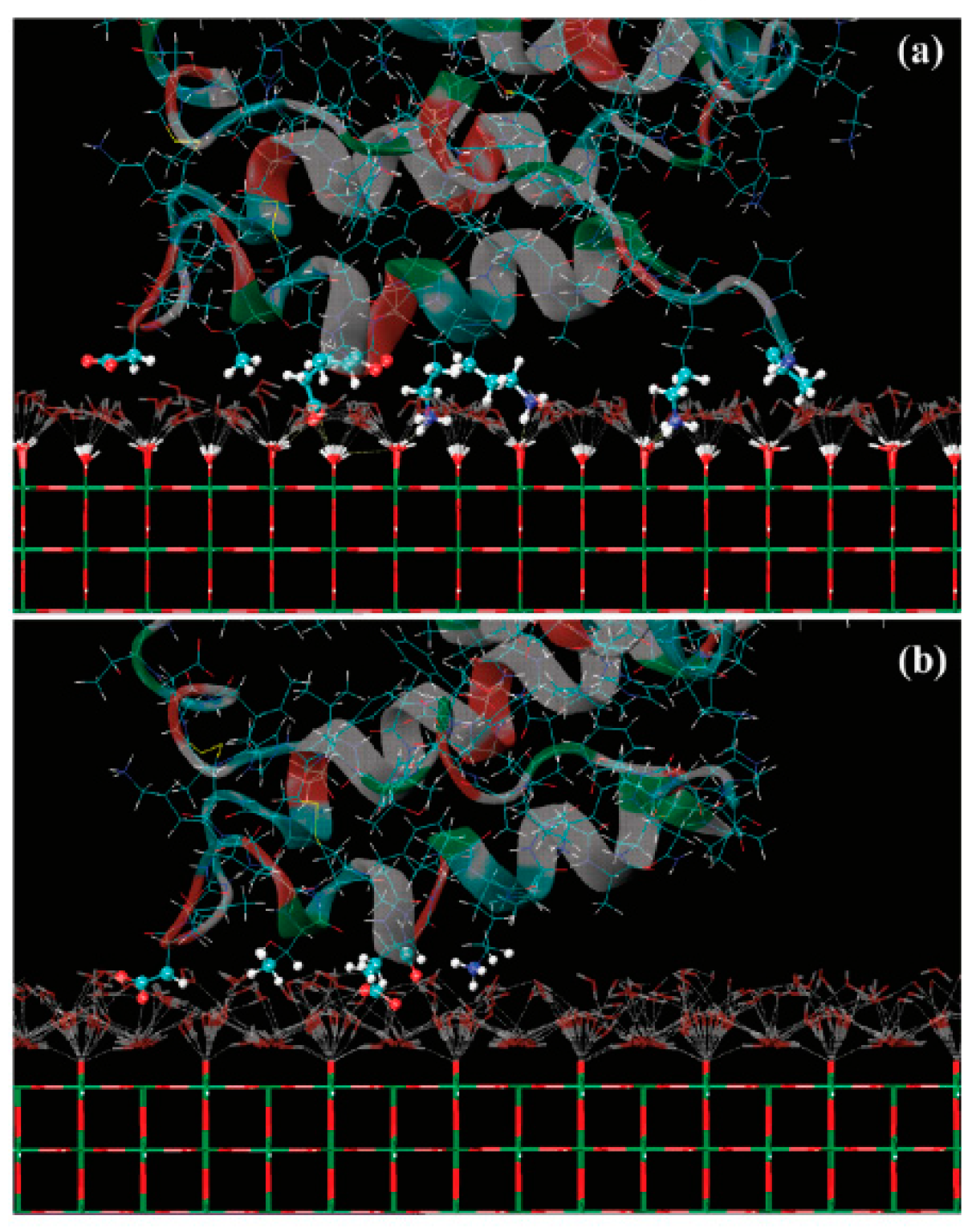
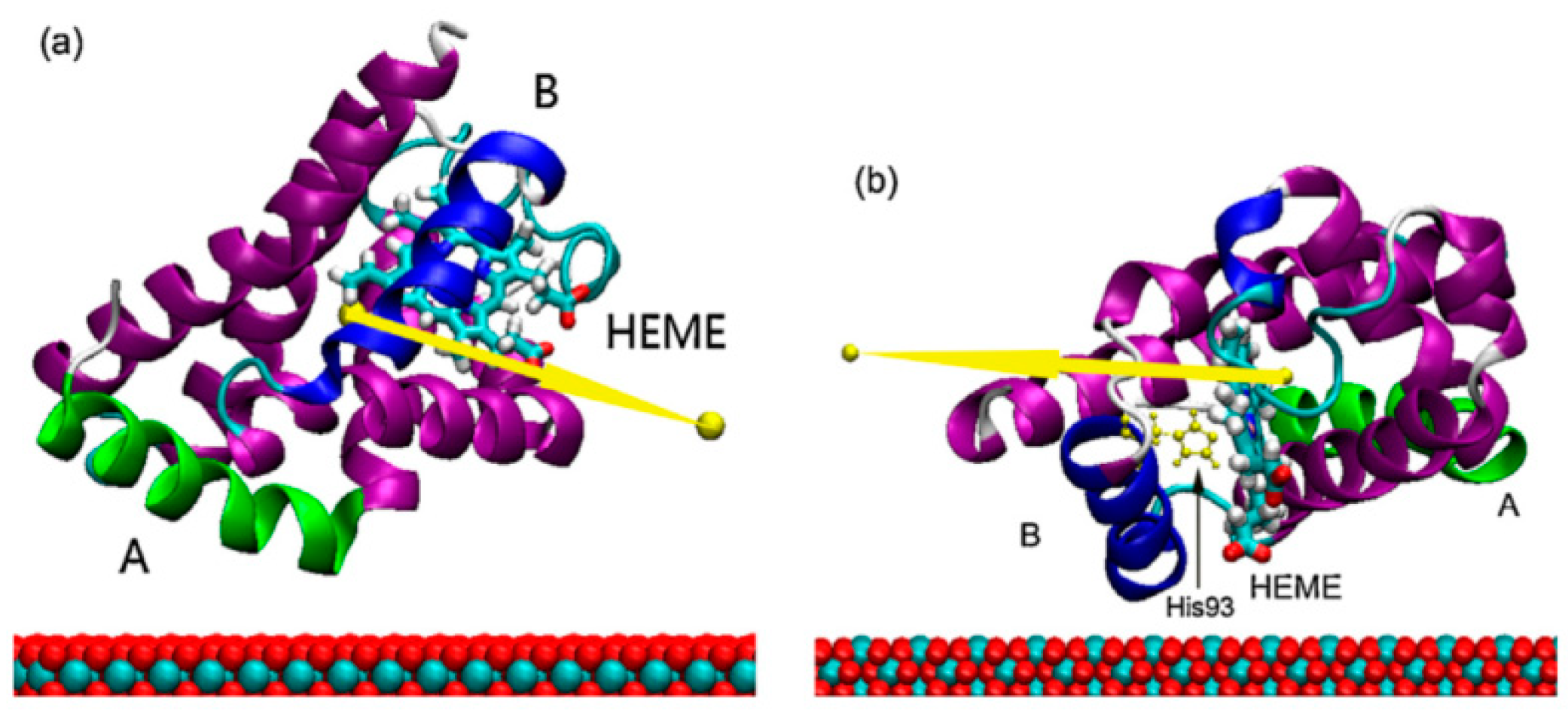
2.4. Molecular Simulations
3. Summary and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Rajh, T.; Dimitrijevic, N.M.; Bissonnette, M.; Koritarov, T.; Konda, V. Titanium dioxide in the service of the biomedical revolution. Chem. Rev. 2014, 114, 10177–10216. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Hartmann, M. Progress in enzyme immobilization in ordered mesoporous materials and related applications. Chem. Soc. Rev. 2013, 42, 3894–3912. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-M.; Ahn, J.-Y.; Jo, M.; Lee, D.-K.; Lis, J.T.; Craighead, H.G.; Kim, S. Selection and elution of aptamers using nanoporous solgel arrays with integrated microheaters. Lab Chip 2009, 9, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Ellingsen, J.E. A study on the mechanism of protein adsorption to TiO2. Biomaterials 1991, 12, 593–596. [Google Scholar] [CrossRef]
- Martin, J.Y.; Schwartz, Z.; Hummert, T.W.; Schraub, D.M.; Simpson, J.; Lankford, J., Jr.; Dean, D.D.; Cochran, D.L.; Boyan, B.D. Effect of titanium surface roughness on proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63). J. Biomed. Mater. Res. 1995, 29, 389–401. [Google Scholar] [CrossRef]
- Limo, M.J.; Sola-Rabada, A.; Boix, E.; Thota, V.; Westcott, Z.C.; Puddu, V.; Perry, C.C. Interactions between Metal Oxides and Biomolecules: From Fundamental Understanding to Applications. Chem. Rev. 2018, 118, 11118–11193. [Google Scholar] [CrossRef]
- Sang, L.-C.; Vinu, A.; Coppens, M.-O. General Description of the Adsorption of Proteins at Their Iso-electric Point in Na-noporous Materials. Langmuir ACS J. Surf. Colloids 2011, 27, 13828–13837. [Google Scholar] [CrossRef]
- Zhuang, W.; Zhang, Y.; Zhu, J.; An, R.; Li, B.; Mu, L.; Ying, H.; Wu, J.; Zhou, J.; Chen, Y.; et al. Influences of geometrical topography and surface chemistry on the stable immobilization of adenosine deaminase on mesoporous TiO2. Chem. Eng. Sci. 2016, 139, 142–151. [Google Scholar] [CrossRef]
- Aramesh, M.; Shimoni, O.; Ostrikov, K.; Prawer, S.; Cervenka, J. Surface charge effects in protein adsorption on nanodiamonds. Nanoscale 2015, 7, 5726–5736. [Google Scholar] [CrossRef] [PubMed]
- Bayne, L.; Ulijn, R.V.; Halling, P.J. Effect of pore size on the performance of immobilised enzymes. Chem. Soc. Rev. 2013, 42, 9000–9010. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Min, Y.; Geng, X. Fast separations of intact proteins by liquid chromatography. J. Sep. Sci. 2012, 35, 3033–3045. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Abel, J.H.; Starger, J.L.; Yi, H. Porosity-Tuned Chitosan–Polyacrylamide Hydrogel Microspheres for Improved Protein Conjugation. Biomacromolecules 2016, 17, 2427–2436. [Google Scholar] [CrossRef]
- Yu, G.; Liu, J.; Zhou, J. Mesoscopic coarse-grained simulations of hydrophobic charge induction chromatography (HCIC) for protein purification. AIChE J. 2015, 61, 2035–2047. [Google Scholar] [CrossRef]
- Alsteens, D.; Gaub, H.E.; Newton, R.; Pfreundschuh, M.; Gerber, C.; Muller, D.J. Atomic force microscopy-based characterization and design of biointerfaces. Nat. Rev. Mater. 2017, 2, 17008. [Google Scholar] [CrossRef]
- Cao, T.; Tang, H.; Liang, X.; Wang, A.; Auner, G.W.; Salley, S.O.; Ng, K.Y.S. Nanoscale investigation on adhesion of E. coli to surface modified silicone using atomic force microscopy. Biotechnol. Bioeng. 2006, 94, 167–176. [Google Scholar] [CrossRef]
- Wang, M.S.; Palmer, L.B.; Schwartz, J.D.; Razatos, A. Evaluating Protein Attraction and Adhesion to Biomaterials with the Atomic Force Microscope. Langmuir ACS J. Surf. Colloids 2004, 20, 7753–7759. [Google Scholar] [CrossRef]
- Tsapikouni, T.S.; Missirlis, Y.F. Protein-material interactions: From micro-to-nano scale. Mater. Sci. Eng. B Adv. Funct. Solid State Mater. 2008, 152, 2–7. [Google Scholar] [CrossRef]
- Elter, P.; Lange, R.; Beck, U. Atomic force microscopy studies of the influence of convex and concave nanostructures on the adsorption of fibronectin. Colloids Surf. B Biointerfaces 2012, 89, 139–146. [Google Scholar] [CrossRef]
- Tsapikouni, T.; Missirlis, Y.F. pH and ionic strength effect on single fibrinogen molecule adsorption on mica studied with AFM. Colloids Surf. B Biointerfaces 2007, 57, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Tencer, M.; Charbonneau, R.; Lahoud, N.; Berini, P. AFM study of BSA adlayers on Au stripes. Appl. Surf. Sci. 2007, 253, 9209–9214. [Google Scholar] [CrossRef]
- Dupont-Gillain, C.C.; Fauroux, C.M.J.; Gardner, D.C.J.; Leggett, G.J. Use of AFM to probe the adsorption strength and time-dependent changes of albumin on self-assembled monolayers. J. Biomed. Mater. Res. Part A 2003, 67, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.-C.; Logan, B.E. Interaction Forces Measured Using AFM between Colloids and Surfaces Coated with Both Dextran and Protein. Langmuir ACS J. Surf. Colloids 2006, 22, 4720–4727. [Google Scholar] [CrossRef]
- Valle-Delgado, J.J.; Molina-Bolívar, J.A.; Galisteo-González, F.; Gálvez-Ruiz, M.J.; Feiler, A.; Rutland, M.W. Inter-action Forces between BSA Layers Adsorbed on Silica Surfaces Measured with an Atomic Force Microscope. J. Phys. Chem. B 2004, 108, 5365–5371. [Google Scholar] [CrossRef]
- You, H.X.; Lowe, C.R. AFM studies of protein adsorption. 2. Characterization of immunoglobulin G adsorption by deter-gent washing. J. Colloid Interface Sci. 1996, 182, 586–601. [Google Scholar] [CrossRef]
- Haldavnekar, R.; Venkatakrishnan, K.; Tan, B. Non plasmonic semiconductor quantum SERS probe as a pathway for in vitro cancer detection. Nat. Commun. 2018, 9, 3065. [Google Scholar] [CrossRef]
- Tian, Z.Q. Surface-enhanced Raman spectroscopy: Advancements and applications. J. Raman Spectrosc. 2005, 36, 466–470. [Google Scholar] [CrossRef]
- Palonpon, A.F.; Ando, J.; Yamakoshi, H.; Dodo, K.; Sodeoka, M.; Kawata, S.; Fujita, K. Raman and SERS micros-copy for molecular imaging of live cells. Nat. Protoc. 2013, 8, 677–692. [Google Scholar] [CrossRef]
- Langer, J.; de Aberasturi, J.D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef]
- Yu, Q.; Golden, G. Probing the Protein Orientation on Charged Self-Assembled Monolayers on Gold Nanohole Arrays by SERS. Langmuir ACS J. Surf. Colloids 2007, 23, 8659–8662. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, M.P.; Turek, V.A.; Paget, J.; Kornyshev, A.A.; Edel, J. Self-assembled nanoparticle arrays for multiphase trace analyte detection. Nat. Mater. 2012, 12, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, A.; Gosztola, D.; Schiller, T.; Dimitrijevic, N.M.; Mujica, V.; Martin, D.; Rajh, T. SERS of Semiconducting Nanoparticles (TiO2 Hybrid Composites). J. Am. Chem. Soc. 2009, 131, 6040. [Google Scholar] [CrossRef]
- Tarakeshwar, P.; Finkelstein-Shapiro, D.; Hurst, S.J.; Rajh, T.; Mujica, V. Surface-Enhanced Raman Scattering on Semiconducting Oxide Nanoparticles: Oxide Nature, Size, Solvent, and pH Effects. J. Phys. Chem. C 2011, 115, 8994–9004. [Google Scholar] [CrossRef]
- Schedin, F.; Lidorikis, E.; Lombardo, A.; Kravets, V.G.; Geim, A.K.; Grigorenko, A.N.; Novoselov, K.S.; Ferrari, A.C. Surface-Enhanced Raman Spectroscopy of Graphene. ACS Nano 2010, 4, 5617–5626. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yuan, H.; Fales, A.M.; Tuan, V.-D. pH-sensing nanostar probe using surface-enhanced Raman scattering (SERS): Theoretical and experimental studies. J. Raman Spectrosc. 2013, 44, 980–986. [Google Scholar] [CrossRef]
- Kong, K.V.; Dinish, U.S.; Lau, W.K.O.; Olivo, M. Sensitive SERS-pH sensing in biological media using metal carbonyl functionalized planar substrates. Biosens. Bioelectron. 2014, 54, 135–140. [Google Scholar] [CrossRef]
- Das, G.; Mecarini, F.; Gentile, F.; De Angelis, F.; Kumar, H.M.; Candeloro, P.; Liberale, C.; Cuda, G.; Di Fabrizio, E. Nano-patterned SERS substrate: Application for protein analysis vs. temperature. Biosens. Bioelectron. 2009, 24, 1693–1699. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, X.; Ruan, W.; Zhao, B.; Xu, W.; Lombardi, J.R. Adsorption study of 4-MBA on TiO2 nanoparticles by surface-enhanced Raman spectroscopy. J. Raman Spectrosc. 2009, 40, 2004–2008. [Google Scholar] [CrossRef]
- Erol, M.; Du, H.; Sukhishvili, S. Control of Specific Attachment of Proteins by Adsorption of Polymer Layers. Langmuir ACS J. Surf. Colloids 2006, 22, 11329–11336. [Google Scholar] [CrossRef]
- Wang, J.; Anderson, W.; Li, J.; Lin, L.L.; Wang, Y.; Trau, M. A high-resolution study of in situ surface-enhanced Ra-man scattering nanotag behavior in biological systems. J. Colloid Interface Sci. 2019, 537, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Laaksonen, A.; Cao, W.; Ji, X.; Lu, X. AFM Study of pH-Dependent Adhesion of Single Protein to TiO2 Sur-face. Adv. Mater. Interfaces 2019, 6, 1900411. [Google Scholar] [CrossRef]
- Kang, H.; Haynes, C.L. Interactions between Silica-Coated Gold Nanorod Substrates and Hydrophobic Analytes in Colloidal Surface-Enhanced Raman Spectroscopy. J. Phys. Chem. C 2019, 123, 24685–24697. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, S.; Jiang, S. Orientation of Adsorbed Antibodies on Charged Surfaces by Computer Simulation Based on a United-Residue Model. Langmuir ACS J. Surf. Colloids 2003, 19, 3472–3478. [Google Scholar] [CrossRef]
- Xie, Y.; Li, Z.; Zhou, J. Hamiltonian replica exchange simulations of glucose oxidase adsorption on charged surfaces. Phys. Chem. Chem. Phys. 2018, 20, 14587–14596. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.B.; Zhou, J. Understanding the curvature effect of silica nanoparticles on lysozyme adsorption orientation and con-formation: A mesoscopic coarse-grained simulation study. Phys. Chem. Chem. Phys. 2016, 18, 23500–23507. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, F.; Hyltner, E.; Arnebrant, T.; Malmsten, M.; Linse, P. Lysozyme Adsorption to Charged Surfaces. A Monte Carlo Study. J. Phys. Chem. B 2004, 108, 9871–9881. [Google Scholar] [CrossRef]
- Mücksch, C.; Urbassek, H.M. Molecular Dynamics Simulation of Free and Forced BSA Adsorption on a Hydrophobic Graphite Surface. Langmuir ACS J. Surf. Colloids 2011, 27, 12938–12943. [Google Scholar] [CrossRef]
- Ganazzoli, F.; Raffaini, G. Computer simulation of polypeptide adsorption on model biomaterials. Phys. Chem. Chem. Phys. 2005, 7, 3651–3663. [Google Scholar] [CrossRef]
- Thingholm, T.; Jørgensen, T.J.; Jensen, O.N.; Larsen, M.R. Highly selective enrichment of phosphorylated peptides using titanium dioxide. Nat. Protoc. 2006, 1, 1929–1935. [Google Scholar] [CrossRef]
- Dismer, F.; Petzold, M.; Hubbuch, J. Effects of ionic strength and mobile phase pH on the binding orientation of lysozyme on different ion-exchange adsorbents. J. Chromatogr. A 2008, 1194, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.; Manohar, S.; Jagota, A.; Zheng, M. DNA sequence motifs for structure-specific recognition and separation of carbon nanotubes. Nature 2009, 460, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Koga, M.; Kurebayashi, S.; Murai, J.; Saito, H.; Miyazaki, A. Degree of discrepancy between HbA1c and glycemia in variant hemoglobin is smaller when HbA1c is measured by new-type Arkray HPLC compared with old-type HPLC. Clin. Biochem. 2014, 47, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Toumadje, A.; Alcorn, S.W.; Johnson, W.C. Extending CD spectra of proteins to 168 nm improves the analysis for secondary structures. Anal. Biochem. 1992, 200, 321–331. [Google Scholar] [CrossRef]
- Roth, C.M.; Lenhoff, A.M. Electrostatic and vanderwaals contributions to protein adsorption—Computation of equilibrium-constants. Langmuir ACS J. Surf. Colloids 1993, 9, 962–972. [Google Scholar] [CrossRef]
- Dyr, J.E.; Suttnar, J. Separation used for purification of recombinant proteins. J. Chromatogr. B Biomed. Sci. Appl. 1997, 699, 383–401. [Google Scholar] [CrossRef]
- Walkey, C.D.; Chan, W.C.W. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem. Soc. Rev. 2012, 41, 2780–2799. [Google Scholar] [CrossRef]
- Nawrocki, J.; Dunlap, C.; McCormick, A.; Carr, P.J.J. Part I. Chromatography using ultra-stable metal oxide-based stationary phases for HPLC. J. Chromatogr. A 2004, 1028, 1–30. [Google Scholar] [CrossRef]
- Nawrocki, J.; Dunlap, C.; Li, J.; Zhao, J.; McNeff, C.; McCormick, A.; Carr, P.J.J. Part II. Chromatography using ultra-stable metal oxide-based stationary phases for HPLC. J. Chromatogr. A 2004, 1028, 31–62. [Google Scholar] [CrossRef]
- Tsai, P.; Wu, C.-T.; Lee, C.S. Electrokinetic studies of inorganic coated capillaries. J. Chromatogr. B Biomed. Sci. Appl. 1994, 657, 285–290. [Google Scholar] [CrossRef]
- Engholm-Keller, K.; Larsen, M.R. Titanium dioxide as chemo-affinity chromatographic sorbent of biomolecular compounds—Applications in acidic modification-specific proteomics. J. Proteom. 2011, 75, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, M.; Nakamura, H.; Nakajima, T. Titania and zirconia: Possible new ceramic microparticulates for high-performance liquid chromatography. J. Chromatogr. A 1990, 515, 149–158. [Google Scholar] [CrossRef]
- Winkler, J.; Marmé, S. Titania as a sorbent in normal-phase liquid chromatography. J. Chromatogr. A 2000, 888, 51–62. [Google Scholar] [CrossRef]
- Mazanek, M.; Mituloviae, G.; Herzog, F.; Stingl, C.; Hutchins, J.R.A.; Peters, J.-M.; Mechtler, K. Titanium dioxide as a chemo-affinity solid phase in offline phosphopeptide chromatography prior to HPLC-MS/MS analysis. Nat. Protoc. 2006, 2, 1059–1069. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wijeratne, A.B.; Wijesundera, D.N.; Paulose, M.; Ahiabu, I.B.; Chu, W.-K.; Varghese, O.K.; Greis, K.D. Phos-phopeptide Separation Using Radially Aligned Titania Nanotubes on Titanium Wire. ACS Appl. Mater. Interfaces 2015, 7, 11155–11164. [Google Scholar] [CrossRef]
- An, R.; Zhuang, W.; Yang, Z.; Lu, X.; Zhu, J.; Wang, Y.; Dong, Y.; Wu, N. Protein adsorptive behavior on meso-porous titanium dioxide determined by geometrical topography. Chem. Eng. Sci. 2014, 117, 146–155. [Google Scholar] [CrossRef]
- Huangfu, C.; Dong, Y.; Ji, X.; Wu, N.; Lu, X. Mechanistic Study of Protein Adsorption on Mesoporous TiO2 in Aqueous Buffer Solutions. Langmuir ACS J. Surf. Colloids 2019, 35, 11037–11047. [Google Scholar] [CrossRef]
- Kupcik, R.; Macak, J.M.; Rehulkova, H.; Sopha, H.; Fabrik, I.; Anitha, V.C.; Klimentova, J.; Murasova, P.; Bilkova, Z.; Rehulka, P. Amorphous TiO2 Nanotubes as a Platform for Highly Selective Phosphopeptide Enrichment. ACS Omega 2019, 4, 12156–12166. [Google Scholar] [CrossRef]
- Shen, Q.; Cheung, H.-Y. TiO2/SiO2 Core–Shell Composite-Based Sample Preparation Method for Selective Extraction of Phospholipids from Shrimp Waste Followed by Hydrophilic Interaction Chromatography Coupled with Quadrupole Time-of-Flight/Mass Spectrometry Analysis. J. Agric. Food Chem. 2014, 62, 8944–8951. [Google Scholar] [CrossRef]
- Dong, Y.; Laaksonen, A.; Gong, M.; An, R.; Ji, X. Selective Separation of Highly Similar Proteins on Ionic Liquid-Loaded Mesoporous TiO2. Langmuir ACS J. Surf. Colloids 2022, 38, 3202–3211. [Google Scholar] [CrossRef]
- Binnig, G.; Quate, C.F.; Gerber, C.J.P. Atomic force microscope. Phys. Rev. Lett. 1986, 56, 930. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, O.H.; Snel, M.M.; Cambi, A.; Greve, J.; De Grooth, B.G.; Figdor, C.G. Biomolecular Interactions Measured by Atomic Force Microscopy. Biophys. J. 2000, 79, 3267–3281. [Google Scholar] [CrossRef]
- Cacciafesta, P.; Humphris, A.D.L.; Jandt, K.D.; Miles, M.J. Human Plasma Fibrinogen Adsorption on Ultraflat Titanium Oxide Surfaces Studied with Atomic Force Microscopy. Langmuir ACS J. Surf. Colloids 2000, 16, 8167–8175. [Google Scholar] [CrossRef]
- Sousa, S.R.; Brás, M.M.; Moradas-Ferreira, P.; Barbosa, M.A. Dynamics of Fibronectin Adsorption on TiO2 Surfaces. Langmuir ACS J. Surf. Colloids 2007, 23, 7046–7054. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Nandgaonkar, A.G.; Wang, Q.; Zhang, J.; Krause, W.E.; Wei, Q.; Lucia, L. Laccase-immobilized bacterial cellulose/TiO2 functionalized composite membranes: Evaluation for photo- and bio-catalytic dye degradation. J. Membr. Sci. 2017, 525, 89–98. [Google Scholar] [CrossRef]
- Scholl, Z.N.; Marszalek, P.E. AFM-Based Single-Molecule Force Spectroscopy of Proteins. In Nanoscale Imaging; Humana Press: New York, NY, USA, 2018; pp. 35–47. [Google Scholar] [CrossRef]
- Petrosyan, R.; Narayan, A.; Woodside, M.T. Single-Molecule Force Spectroscopy of Protein Folding. J. Mol. Biol. 2021, 433, 167207. [Google Scholar] [CrossRef] [PubMed]
- Verdorfer, T.; Bernardi, R.C.; Meinhold, A.; Ott, W.; Luthey-Schulten, Z.; Nash, M.A.; Gaub, H.E. Combining in Vitro and in Silico Single-Molecule Force Spectroscopy to Characterize and Tune Cellulosomal Scaffoldin Mechanics. J. Am. Chem. Soc. 2017, 139, 17841–17852. [Google Scholar] [CrossRef]
- Ma, L.; Cai, Y.; Li, Y.; Jiao, J.; Wu, Z.; O’Shaughnessy, B.; De Camilli, P.; Karatekin, E.; Zhang, Y. Single-molecule force spectroscopy of protein-membrane interactions. eLife 2017, 6, e30493. [Google Scholar] [CrossRef]
- Hoffmann, T.; Dougan, L. Single molecule force spectroscopy using polyproteins. Chem. Soc. Rev. 2012, 41, 4781–4796. [Google Scholar] [CrossRef]
- Huang, W.; Wu, X.; Gao, X.; Yu, Y.; Lei, H.; Zhu, Z.; Shi, Y.; Chen, Y.; Qin, M.; Wang, W.; et al. Maleimide–thiol adducts stabilized through stretching. Nat. Chem. 2019, 11, 310–319. [Google Scholar] [CrossRef]
- Ganbaatar, N.; Imai, K.; Yano, T.-A.; Hara, M. Surface force analysis of glycine adsorption on different crystal surfaces of titanium dioxide (TiO2). Nano Converg. 2017, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Leader, A.; Mandler, D.; Reches, M. The role of hydrophobic, aromatic and electrostatic interactions between amino acid residues and a titanium dioxide surface. Phys. Chem. Chem. Phys. 2018, 20, 29811–29816. [Google Scholar] [CrossRef] [PubMed]
- Vergaro, V.; Carlucci, C.; Cascione, M.; Lorusso, C.; Conciauro, F.; Scremin, B.F.; Congedo, P.M.; Cannazza, G.; Citti, C.; Ciccarella, G.J.N. Nanotechnology, Interaction between human serum albumin and different anatase TiO2 nanoparticles: A nano-bio interface study. Nanomater. Nanotechnol. 2015, 5, 30. [Google Scholar] [CrossRef]
- Dong, Y.; An, R.; Zhao, S.; Cao, W.; Huang, L.; Zhuang, W.; Lu, L.; Lu, X. Molecular Interactions of Protein with TiO2 by the AFM-Measured Adhesion Force. Langmuir ACS J. Surf. Colloids 2017, 33, 11626–11634. [Google Scholar] [CrossRef]
- Dong, Y.; Ji, X.; Laaksonen, A.; Cao, W.; He, H.; Lu, X. Excellent Protein Immobilization and Stability on Heterogeneous C-TiO2 Hybrid Nanostructures: A Single Protein AFM Study. Langmuir ACS J. Surf. Colloids 2020, 36, 9323–9332. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; Silva, F.A.E.; Quental, M.V.; Mondal, D.; Freire, M.G.; Coutinho, J.A.P. Ionic-Liquid-Mediated Extraction and Separation Processes for Bioactive Compounds: Past, Present, and Future Trends. Chem. Rev. 2017, 117, 6984–7052. [Google Scholar] [CrossRef]
- Freire, M.G.; Cláudio, A.F.M.; Araújo, J.M.M.; Coutinho, J.A.P.; Marrucho, I.M.; Lopes, J.N.C.; Rebelo, L.P.N. Aqueous biphasic systems: A boost brought about by using ionic liquids. Chem. Soc. Rev. 2012, 41, 4966–4995. [Google Scholar] [CrossRef]
- Dong, Y.; Laaksonen, A.; Huo, F.; Gao, Q.; Ji, X. Hydrated Ionic Liquids Boost the Trace Detection Capacity of Proteins on TiO2 Support. Langmuir ACS J. Surf. Colloids 2021, 37, 5012–5021. [Google Scholar] [CrossRef]
- Qi, D.; Lu, L.; Wang, L.; Zhang, J. Improved SERS Sensitivity on Plasmon-Free TiO2 Photonic Microarray by Enhancing Light-Matter Coupling. J. Am. Chem. Soc. 2014, 136, 9886–9889. [Google Scholar] [CrossRef] [PubMed]
- Han, X.X.; Ji, W.; Zhao, B.; Ozaki, Y. Semiconductor-enhanced Raman scattering: Active nanomaterials and applications. Nanoscale 2017, 9, 4847–4861. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, I.; Lombardi, J.R. Enhanced Raman Scattering with Dielectrics. Chem. Rev. 2016, 116, 14921–14981. [Google Scholar] [CrossRef] [PubMed]
- Cong, S.; Liu, X.; Jiang, Y.; Zhang, W.; Zhao, Z. Surface Enhanced Raman Scattering Revealed by Interfacial Charge-Transfer Transitions. Innovation 2020, 1, 100051. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Babur, E.; Ozdemir, M.; Gieseking, R.L.; Dede, Y.; Tamer, U.; Schatz, G.C.; Facchetti, A.; Usta, H.; Demirel, G. Nanostructured organic semiconductor films for molecular detection with surface-enhanced Raman spectroscopy. Nat. Mater. 2017, 16, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Shoute, L.C.T.; Loppnow, G.R. Excited-state dynamics of alizarin-sensitized TiO2 nanoparticles from resonance Raman spectroscopy. J. Chem. Phys. 2002, 117, 842–850. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, X.; Ruan, W.; Zhao, B.; Xu, W.; Lombardi, J.R. Observation of Enhanced Raman Scattering for Molecules Adsorbed on TiO2 Nanoparticles: Charge-Transfer Contribution. J. Phys. Chem. C 2008, 112, 20095–20098. [Google Scholar] [CrossRef]
- Yang, L.; Gong, M.; Jiang, X.; Yin, D.; Qin, X.; Zhao, B.; Ruan, W. Investigation on SERS of different phase structure TiO2 nanoparticles. J. Raman Spectrosc. 2015, 46, 287–292. [Google Scholar] [CrossRef]
- Yang, L.; Qin, X.; Jiang, X.; Gong, M.; Yin, D.; Zhang, Y.; Zhao, B. SERS investigation of ciprofloxacin drug molecules on TiO2 nanoparticles. Phys. Chem. Chem. Phys. 2015, 17, 17809–17815. [Google Scholar] [CrossRef]
- Chen, L.; Cai, L.; Ruan, W.; Zhao, B. Surface-Enhanced Raman Spectroscopy: Protein Application. Encycl. Anal. Chem. 2018, 1–25. [Google Scholar] [CrossRef]
- Li, X.; Chen, G.; Yang, L.; Jin, Z.; Liu, J. Multifunctional Au-Coated TiO2 Nanotube Arrays as Recyclable SERS Substrates for Multifold Organic Pollutants Detection. Adv. Funct. Mater. 2010, 20, 2815–2824. [Google Scholar] [CrossRef]
- Ling, Y.; Zhuo, Y.; Huang, L.; Mao, D. Using Ag-embedded TiO2 nanotubes array as recyclable SERS substrate. Appl. Surf. Sci. 2016, 388, 169–173. [Google Scholar] [CrossRef]
- Roguska, A.; Kudelski, A.; Pisarek, M.; Lewandowska, M.; Dolata, M.; Janik-Czachor, M. Raman investigations of TiO2 nanotube substrates covered with thin Ag or Cu deposits. J. Raman Spectrosc. 2009, 40, 1652–1656. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, J.; Lai, W.; Yang, X.; Meng, J.; Su, L.; Gu, C.; Jiang, T.; Pun, E.Y.B.; Shao, L.; et al. Irreversible accumulated SERS behavior of the molecule-linked silver and silver-doped titanium dioxide hybrid system. Nat. Commun. 2020, 11, 1785. [Google Scholar] [CrossRef] [PubMed]
- Han, X.X.; Koehler, C.; Kozuch, J.; Kuhlmann, U.; Paasche, L.; Sivanesan, A.; Weidinger, I.M.; Hildebrandt, P. Potential-Dependent Surface-Enhanced Resonance Raman Spectroscopy at Nanostructured TiO2: A Case Study on Cytochrome b(5). Small 2013, 9, 4175–4181. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, I. Enhancing Raman Scattering without Plasmons: Unprecedented Sensitivity Achieved by TiO2 Shell-Based Resonators. J. Am. Chem. Soc. 2013, 135, 5541–5544. [Google Scholar] [CrossRef]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 Nanotubes: Synthesis and Applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Mazare, A.; Schmuki, P. One-Dimensional Titanium Dioxide Nanomaterials: Nanotubes. Chem. Rev. 2014, 114, 9385–9454. [Google Scholar] [CrossRef] [PubMed]
- Öner, I.H.; Querebillo, C.J.; David, C.; Gernert, U.; Walter, C.; Driess, M.; Leimkühler, S.; Ly, K.H.; Weidinger, I.M.; Oener, H.I. High Electromagnetic Field Enhancement of TiO2 Nanotube Electrodes. Angew. Chem. Int. Ed. 2018, 57, 7225–7229. [Google Scholar] [CrossRef]
- Liu, L.; Pan, F.; Liu, C.; Huang, L.; Li, W.; Lu, X. TiO2 Nanofoam–Nanotube Array for Surface-Enhanced Raman Scattering. ACS Appl. Nano Mater. 2018, 1, 6563–6566. [Google Scholar] [CrossRef]
- Dong, Y.; Ji, X.; Laaksonen, A.; Cao, W.; An, R.; Lu, L.; Lu, X. Determination of the small amount of proteins inter-acting with TiO2 nanotubes by AFM-measurement. Biomaterials 2019, 192, 368–376. [Google Scholar] [CrossRef]
- Shaik, U.P.; Hamad, S.; Mohiddon, A.; Soma, V.R.; Krishna, M.G. Morphologically manipulated Ag/ZnO nanostructures as surface enhanced Raman scattering probes for explosives detection. J. Appl. Phys. 2016, 119, 093103. [Google Scholar] [CrossRef]
- Qian, L.H.; Yan, X.Q.; Fujita, T.; Inoue, A.; Chen, M. Surface enhanced Raman scattering of nanoporous gold: Smaller pore sizes stronger enhancements. Appl. Phys. Lett. 2007, 90, 153120. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, N.; Ji, X.; Laaksonen, A.; Lu, X.; Zhang, S. Excellent Trace Detection of Proteins on TiO2 Nanotube Substrates through Novel Topography Optimization. J. Phys. Chem. C 2020, 124, 27790–27800. [Google Scholar] [CrossRef]
- Ding, Q.; Wang, J.; Chen, X.; Liu, H.; Li, Q.; Wang, Y.; Yang, S. Quantitative and Sensitive SERS Platform with Analyte En-richment and Filtration Function. Nano Lett. 2020, 20, 7304–7312. [Google Scholar] [CrossRef] [PubMed]
- Agafilushkina, S.N.; Zukovskaja, O.; Dyakov, S.A.; Weber, K.; Sivakov, V.; Popp, J.; Cialla-May, D.; Osminkina, L.A. Ra-man Signal Enhancement Tunable by Gold-Covered Porous Silicon Films with Different Morphology. Sensors 2020, 20, 5634. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yi, R.; Zhai, X.; Bian, R.; Gao, Y.; Cai, D.; Liu, J.; Huang, X.; Lu, G.; Li, H.; et al. A flexible SERS-active film for studying the effect of non-metallic nanostructures on Raman enhancement. Nanoscale 2018, 10, 16895–16901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tian, Q.; Chen, Z.; Zhao, C.; Chai, H.; Wu, Q.; Li, W.; Chen, X.; Deng, Y.; Song, Y. Arrayed nanopore silver thin films for surface-enhanced Raman scattering. RSC Adv. 2020, 10, 23908–23915. [Google Scholar] [CrossRef]
- Hurst, S.J.; Fry, H.C.; Gosztola, D.J.; Rajh, T. Utilizing Chemical Raman Enhancement: A Route for Metal Oxide Sup-port-Based Biodetection. J. Phys. Chem. C 2011, 115, 620–630. [Google Scholar] [CrossRef]
- Jiang, X.; Xu, L.; Ji, W.; Wang, W.; Du, J.; Yang, L.; Song, W.; Han, X.; Zhao, B. One plus one greater than Two: Ultrasensitive Surface-Enhanced Raman scattering by TiO2/ZnO heterojunctions based on Electron-Hole separation. Appl. Surf. Sci. 2022, 584, 152609. [Google Scholar] [CrossRef]
- Das, S.; Saxena, K.; Goswami, L.P.; Gayathri, J.; Mehta, D.S. Mesoporous Ag–TiO2 based nanocage like structure as sensitive and recyclable low-cost SERS substrate for biosensing applications. Opt. Mater. 2022, 125, 111994. [Google Scholar] [CrossRef]
- Xu, W.; Mao, N.; Zhang, J. Graphene: A Platform for Surface-Enhanced Raman Spectroscopy. Small 2013, 9, 1206–1224. [Google Scholar] [CrossRef]
- Nordness, O.; Brennecke, J.F. Ion Dissociation in Ionic Liquids and Ionic Liquid Solutions. Chem. Rev. 2020, 120, 12873–12902. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Laaksonen, A.; Liu, C.; Lu, X.; Ji, X. The peculiar effect of water on ionic liquids and deep eutectic solvents. Chem. Soc. Rev. 2018, 47, 8685–8720. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Liu, J.; Zhou, J. Molecular Simulations of Cytochrome c Adsorption on a Bare Gold Surface: Insights for the Hindrance of Electron Transfer. J. Phys. Chem. C 2015, 119, 20773–20781. [Google Scholar] [CrossRef]
- Quan, X.; Liu, J.; Zhou, J. Multiscale modeling and simulations of protein adsorption: Progresses and perspectives. Curr. Opin. Colloid Interface Sci. 2018, 41, 74–85. [Google Scholar] [CrossRef]
- Guo, C.; Wu, C.; Chen, M.; Zheng, T.; Chen, N.; Cummings, P.T. Molecular modeling of fibronectin adsorption on topographically nanostructured rutile (110) surfaces. Appl. Surf. Sci. 2016, 384, 36–44. [Google Scholar] [CrossRef]
- Kang, Y.; Li, X.; Tu, Y.; Wang, Q.; Ågren, H. On the Mechanism of Protein Adsorption onto Hydroxylated and Non-hydroxylated TiO2 Surfaces. J. Phys. Chem. C 2010, 114, 14496–14502. [Google Scholar] [CrossRef]
- Zheng, T.; Zhang, Y.; Wu, C.; Zhou, L.; Cummings, P.T. Molecular investigations of tripeptide adsorption onto TiO2 surfaces: Synergetic effects of surface nanostructure, hydroxylation and bioactive ions. Appl. Surf. Sci. 2020, 512, 145713. [Google Scholar] [CrossRef]
- Wu, C.; Chen, M.; Xing, C. Molecular Understanding of Conformational Dynamics of a Fibronectin Module on Rutile (110) Surface. Langmuir ACS J. Surf. Colloids 2010, 26, 15972–15981. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Skelton, A.A.; Chen, M.; Vlček, L.; Cummings, P.T. Modeling the Interaction between Integrin-Binding Pep-tide (RGD) and Rutile Surface: The Effect of Cation Mediation on Asp Adsorption. Langmuir ACS J. Surf. Colloids 2012, 28, 2799–2811. [Google Scholar] [CrossRef]
- Yang, C.; Peng, C.; Zhao, D.; Liao, C.; Zhou, J.; Lu, X. Molecular simulations of myoglobin adsorbed on rutile (1 1 0) and (0 0 1) surfaces. Fluid Phase Equilibr. 2014, 362, 349–354. [Google Scholar] [CrossRef]
- Wu, X.; Hao, P.; He, F.; Yao, Z.; Zhang, X. Molecular dynamics simulations of BSA absorptions on pure and for-mate-contaminated rutile (110) surface. Appl. Surf. Sci. 2020, 533, 147574. [Google Scholar] [CrossRef]
- Wu, X.; Wang, C.; Hao, P.; He, F.; Yao, Z.; Zhang, X. Adsorption properties of albumin and fibrinogen on hydrophilic/hydrophobic TiO2 surfaces: A molecular dynamics study. Colloids Surf. B Biointerfaces 2021, 207, 111994. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.L.; Sheng, S.X.; Wang, R.M.; Sun, M.T. Tip-Enhanced Raman Spectroscopy. Anal. Chem. 2016, 88, 9328–9346. [Google Scholar] [CrossRef] [PubMed]
- Verma, P. Tip-Enhanced Raman Spectroscopy: Technique and Recent Advances. Chem. Rev. 2017, 117, 6447–6466. [Google Scholar] [CrossRef]
- Kushiro, K.; Lee, C.H.; Takai, M. Simultaneous characterization of protein-material and cell-protein interactions using dynam-ic QCM-D analysis on SAM surfaces. Biomater. Sci. 2016, 6, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.W.; Sut, T.N.; Jeon, W.-Y.; Yoon, B.K.; Jackman, J.A. On/off switching of lipid bicelle adsorption on titanium oxide controlled by sub-monolayer molecular surface functionalization. Appl. Mater. Today 2022, 27, 101444. [Google Scholar] [CrossRef]
- Fabre, H.; Mercier, D.; Galtayries, A.; Portet, D.; Delorme, N.; Bardeau, J.-F. Impact of hydrophilic and hydrophobic function-alization of flat TiO2/Ti surfaces on proteins adsorption. Appl. Surf. Sci. 2018, 432, 15–21. [Google Scholar] [CrossRef]
- Chen, X.; Ferrigno, R.; Yang, A.J.; Whitesides, G.M. Redox Properties of Cytochrome c Adsorbed on Self-Assembled Monolayers: A Probe for Protein Conformation and Orientation. Langmuir ACS J. Surf. Colloids 2002, 18, 7009–7015. [Google Scholar] [CrossRef]
- Tollin, G.; Salamon, Z.; Hruby, V.J. Techniques: Plasmon-waveguide resonance (PWR) spectroscopy as a tool to study lig-and-GPCR interactions. Trends Pharmacol. Sci. 2003, 24, 655–659. [Google Scholar] [CrossRef]
- Kovacs, N.; Patko, D.; Orgovan, N.; Kurunczi, S.; Ramsden, J.J.; Vonderviszt, F.; Horvath, R. Optical Anisotropy of Flagellin Layers: In Situ and Label-Free Measurement of Adsorbed Protein Orientation Using OWLS. Anal. Chem. 2013, 85, 5382–5389. [Google Scholar] [CrossRef]
- Patko, D.; Cottier, K.; Hamori, A.; Horvath, R. Single beam grating coupled interferometry: High resolution miniaturized la-bel-free sensor for plate based parallel screening. Opt. Express 2012, 20, 23162–23173. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Lin, W.; Laaksonen, A.; Ji, X. Complementary Powerful Techniques for Investigating the Interactions of Proteins with Porous TiO2 and Its Hybrid Materials: A Tutorial Review. Membranes 2022, 12, 415. https://doi.org/10.3390/membranes12040415
Dong Y, Lin W, Laaksonen A, Ji X. Complementary Powerful Techniques for Investigating the Interactions of Proteins with Porous TiO2 and Its Hybrid Materials: A Tutorial Review. Membranes. 2022; 12(4):415. https://doi.org/10.3390/membranes12040415
Chicago/Turabian StyleDong, Yihui, Weifeng Lin, Aatto Laaksonen, and Xiaoyan Ji. 2022. "Complementary Powerful Techniques for Investigating the Interactions of Proteins with Porous TiO2 and Its Hybrid Materials: A Tutorial Review" Membranes 12, no. 4: 415. https://doi.org/10.3390/membranes12040415
APA StyleDong, Y., Lin, W., Laaksonen, A., & Ji, X. (2022). Complementary Powerful Techniques for Investigating the Interactions of Proteins with Porous TiO2 and Its Hybrid Materials: A Tutorial Review. Membranes, 12(4), 415. https://doi.org/10.3390/membranes12040415






