Development of Chitosan/Rice Husk-Based Silica Composite Membranes for Biodiesel Purification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Silica Preparation from Rice Husk
2.3. Preparation of Inorganic–Organic Composite Membrane
2.4. Determination of Porosity and Swelling Degree
2.5. Membrane Pure Water Flux
2.6. Batch Adsorptive Purification
2.7. Biodiesel Filtration
2.8. Determination of Acid Number, Soap Levels, and Free Glycerol Content
2.8.1. Acid Number
2.8.2. Soap Levels
2.8.3. Free Glycerol Content
2.9. Regeneration
3. Results and Discussions
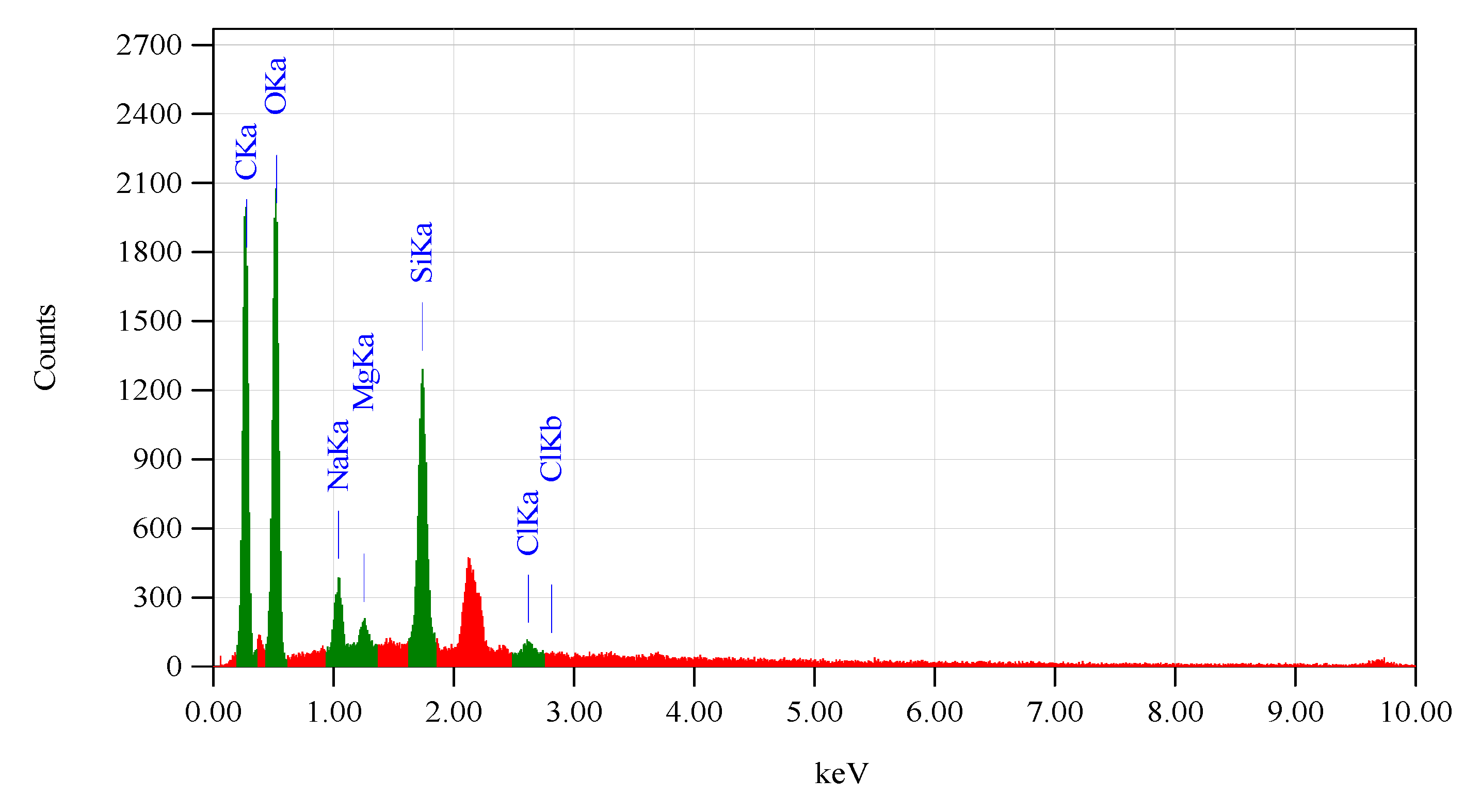
3.1. Isolation of Silica from Rice Husks
3.2. Thickness, Porosity, and Swelling Degree of the Prepared Membrane
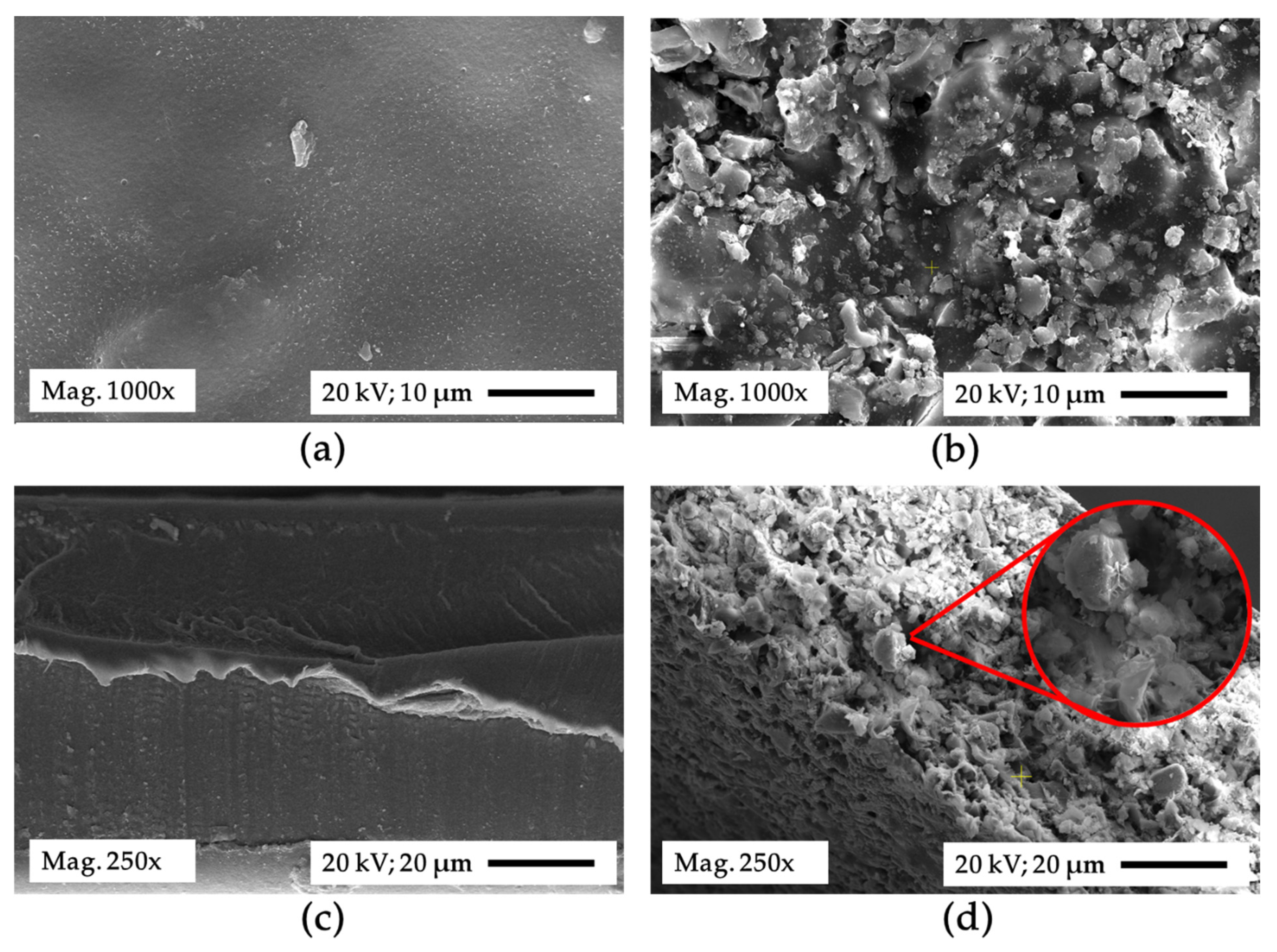
3.3. Membrane Characteristics
3.4. Study of Prepared Membrane on Crude Biodiesel Purification
3.4.1. Effect of Contact Time
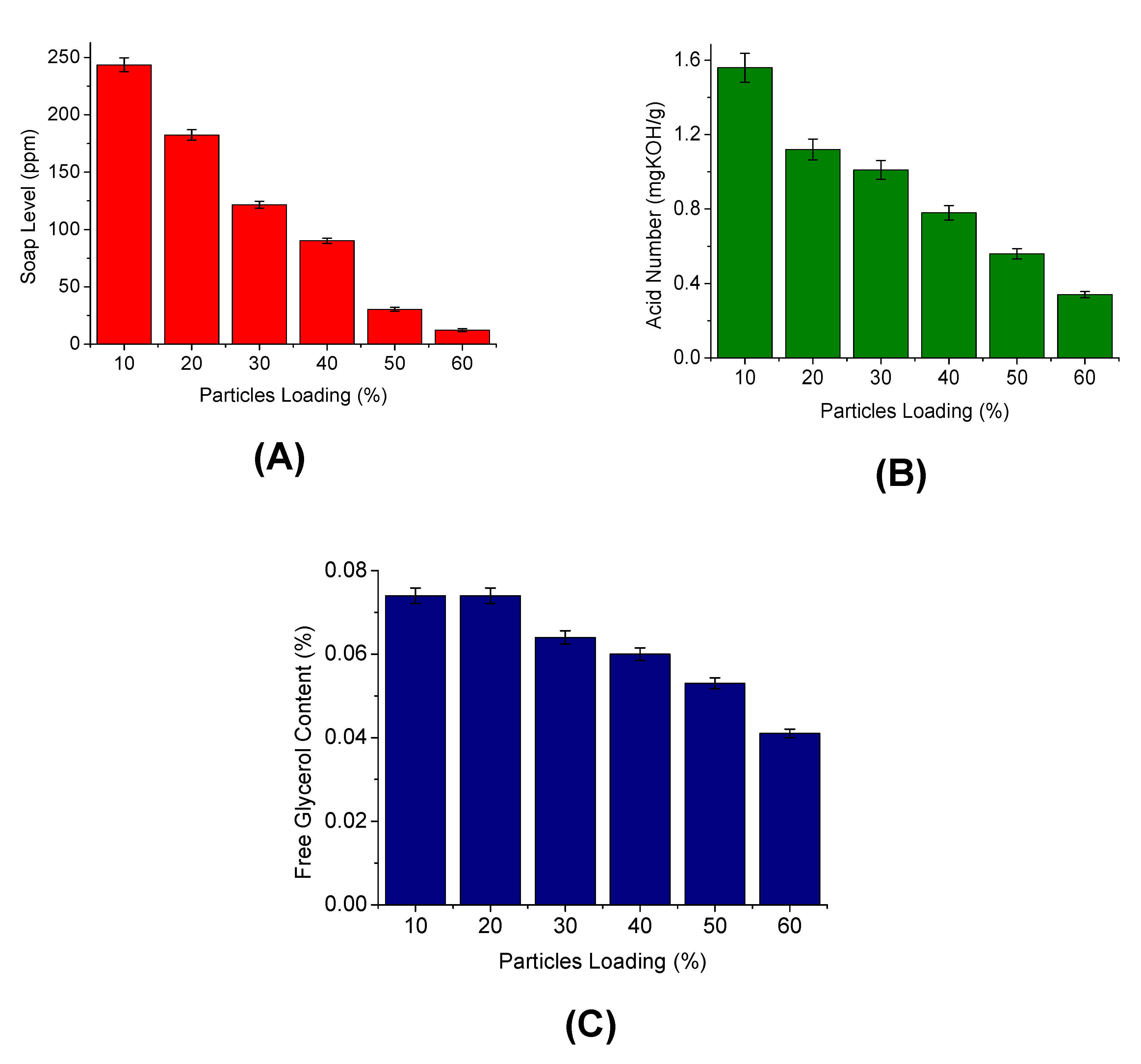
3.4.2. Effect of Silica Particle Loading
3.4.3. Adsorption Isotherm
3.5. Regeneration of the Prepared IOCM
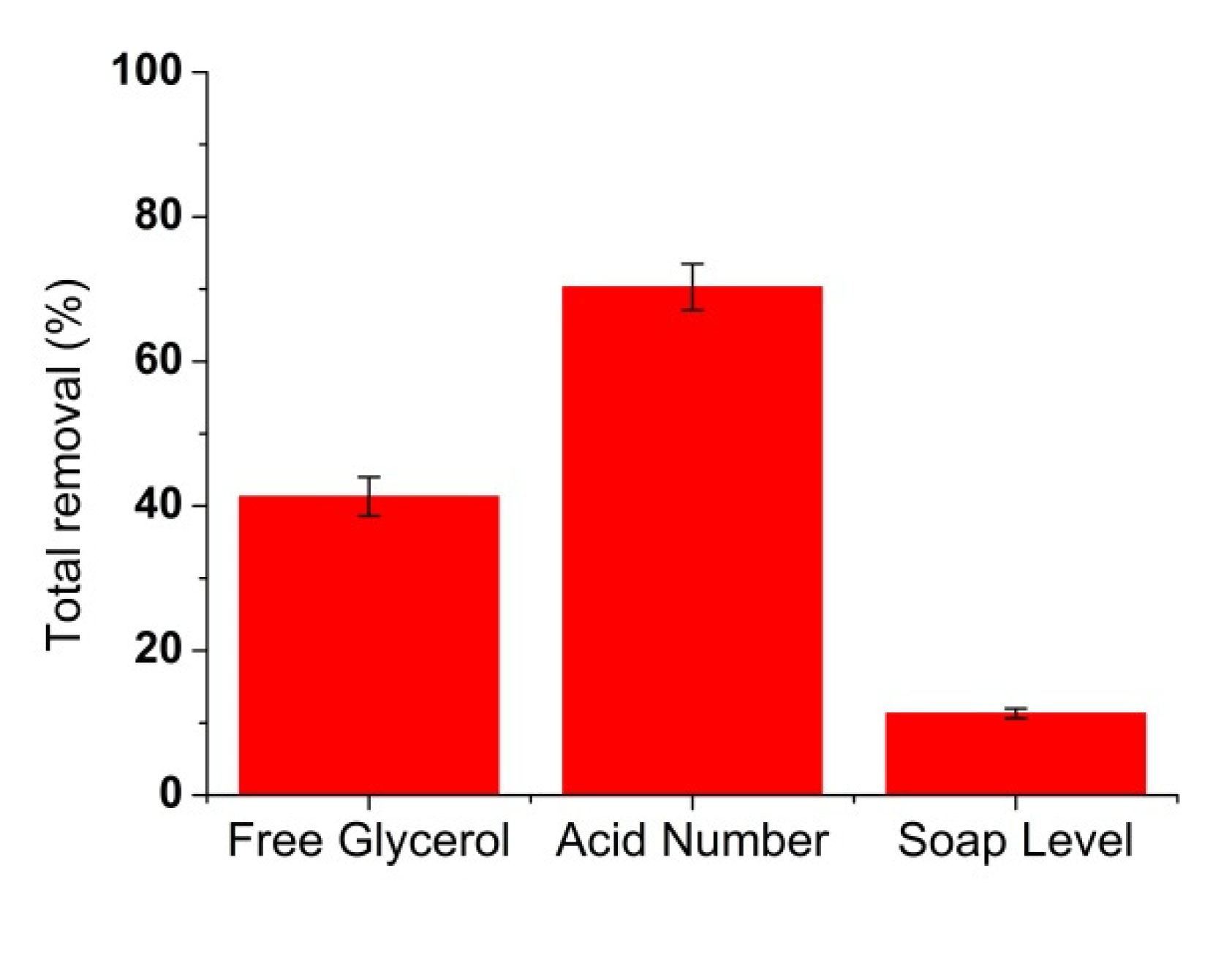
3.6. Biodiesel Filtration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Girish, C.R. A Review of various technologies used for biodiesel production. Int. J. Mech. Prod. Eng. Res. Dev. 2019, 9, 1379–1392. [Google Scholar]
- Ong, H.C.; Milano, J.; Silitonga, A.S.; Hassan, M.H.; Shamsuddin, A.H.; Wang, C.-T.; Mahlia, T.M.I.; Siswantoro, J.; Kusumo, F.; Sutrisno, J. Biodiesel production from Calophyllum inophyllum-Ceiba pentandra oil mixture: Optimization and characterization. J. Clean. Prod. 2019, 219, 183–198. [Google Scholar] [CrossRef]
- Saiful; Ramli, M.; Maulana, I.; Fadli, F.; Yusuf, M. Preparation of mixed matrix polymeric membrane for removing of contaminants in crude biodiesel. Res. J. Chem. Environ. 2018, 22, 15–21. [Google Scholar]
- Silitonga, A.; Mahlia, T.M.I.; Kusumo, F.; Dharma, S.; Sebayang, A.; Sembiring, R.; Shamsuddin, A. Intensification of Reutealis trisperma biodiesel production using infrared radiation: Simulation, optimisation and validation. Renew. Energy 2018, 133, 520–527. [Google Scholar] [CrossRef]
- Silitonga, A.; Masjuki, H.; Ong, H.C.; Sebayang, A.; Dharma, S.; Kusumo, F.; Siswantoro, J.; Milano, J.; Daud, K.; Mahlia, T.M.I.; et al. Evaluation of the engine performance and exhaust emissions of biodiesel-bioethanol-diesel blends using kernel-based extreme learning machine. Energy 2018, 159, 1075–1087. [Google Scholar] [CrossRef]
- Mahlia, T.; Syazmi, Z.; Mofijur, M.; Abas, A.P.; Bilad, M.; Ong, H.C.; Silitonga, A. Patent landscape review on biodiesel production: Technology updates. Renew. Sustain. Energy Rev. 2019, 118, 109526. [Google Scholar] [CrossRef]
- Silitonga, A.; Shamsuddin, A.; Mahlia, T.; Milano, J.; Kusumo, F.; Siswantoro, J.; Dharma, S.; Sebayang, A.; Masjuki, H.; Ong, H.C. Biodiesel synthesis from Ceiba pentandra oil by microwave irradiation-assisted transesterification: ELM modeling and optimization. Renew. Energy 2020, 146, 1278–1291. [Google Scholar] [CrossRef]
- Saiful, S.; Isni, R.N.; Suhud, K. The development of mixed matrix membrane purolite for removing mercury (II) ion in contaminated water. AIP Conf. Proc. 2018, 2049, 020079. [Google Scholar] [CrossRef]
- Saiful, S.; Pratiwi, F.; Maulana, I.; Ramli, M. Mixed Matrix Membrane Adsorbers for Glycerol Removal in Biodiesel. Natural 2012, 12, 8. [Google Scholar]
- Kusworo, T.D.; Widayat, W.; Utomo, D.P.; Pratama, Y.H.S.; Arianti, R.A.V. Performance evaluation of modified nanohybrid membrane polyethersulfone-nano ZnO (PES-nano ZnO) using three combination effect of PVP, irradiation of ultraviolet and thermal for biodiesel purification. Renew. Energy 2019, 148, 935–945. [Google Scholar] [CrossRef]
- Zhu, T.; Yu, X.; Yi, M.; Wang, Y. Facile covalent crosslinking of zeolitic imidazolate framework/polydimethylsiloxane mixed matrix membrane for enhanced ethanol/water separation performance. ACS Sustain. Chem. Eng. 2020, 8, 12664–12676. [Google Scholar] [CrossRef]
- Govindaraju, R.; Chen, S.-S.; Wang, L.-P.; Chang, H.-M.; Pasawan, M. Significance of Membrane Applications for High-Quality Biodiesel and Byproduct (Glycerol) in Biofuel Industries—Review. Curr. Pollut. Rep. 2021, 7, 128–145. [Google Scholar] [CrossRef]
- Vakili, M.; Deng, S.; Cagnetta, G.; Wang, W.; Meng, P.; Liu, D.; Yu, G. Regeneration of chitosan-based adsorbents used in heavy metal adsorption: A review. Sep. Purif. Technol. 2019, 224, 373–387. [Google Scholar] [CrossRef]
- Saengprachum, N.; Poothongkam., J.; Pengprecha., S. Glycerine Removal in Biodiesel Purification Process by Adsorbent from Rice Husk. Int. J. Eng. Technol. 2017, 2, 4. [Google Scholar]
- Moayedi, H.; Aghel, B.; Abdullahi, M.M.; Nguyen, H.; Rashid, A.S.A. Applications of rice husk ash as green and sustainable biomass. J. Clean. Prod. 2019, 237, 117851. [Google Scholar] [CrossRef]
- Iqhrammullah, M.; Nur, S. Adsorption Behaviour of Hazardous Dye (Methyl Orange) on Cellulose-Acetate Polyurethane Sheets. IOP Conf. Ser. Mater. Sci. Eng. 2020, 845, 012035. [Google Scholar] [CrossRef]
- Iqhrammullah, M.; Mustafa, I. Rahmi the application of chitosan modified polyurethane foam adsorbent. Rasayan J. Chem. 2019, 12, 494–501. [Google Scholar] [CrossRef]
- Iqhrammullah, M.; Saleha, S.; Maulina, F.P.; Idroes, R. Polyurethane film prepared from ball-milled algal polyol particle and activated carbon filler for NH3–N removal. Heliyon 2020, 6, e04590. [Google Scholar] [CrossRef]
- PurnamaWati, F. Preparation of magnetic chitosan using local iron sand for mercury removal. Heliyon 2019, 5, e01731. [Google Scholar] [CrossRef] [Green Version]
- Sargin, I.; Baran, T.; Arslan, G. Environmental remediation by chitosan-carbon nanotube supported palladium nanoparticles: Conversion of toxic nitroarenes into aromatic amines, degradation of dye pollutants and green synthesis of biaryls. Sep. Purif. Technol. 2020, 247, 116987. [Google Scholar] [CrossRef]
- Ghimici, L.; Dinu, I.A. Removal of some commercial pesticides from aqueous dispersions using as flocculant a thymine-containing chitosan derivative. Sep. Purif. Technol. 2018, 209, 698–706. [Google Scholar] [CrossRef]
- Az-Zahra, N.; Rahmi, R.; Lubis, S. Reinforcement of chitosan film using cellulose isolated from grass (imperata cylindrica). J. Phys. Conf. Ser. 2019, 1402, 055039. [Google Scholar] [CrossRef]
- Da Silva, S.R.; De Albuquerque, N.J.A.; De Almeida, R.M.; De Abreu, F.C. Synthesis and Charaterization of Silica-Based Aldehyde Chitosan Hybrid Material for Biodiesel Purification. Materials 2017, 10, 1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Setyawan, N.; Wulanawati, A. Simple extraction of silica nanoparticles from rice husk using technical grade solvent: Effect of volume and concentration. IOP Conf. Ser. Earth Environ. Sci. 2019, 309, 012032. [Google Scholar] [CrossRef]
- Peres, E.C.; Slaviero, J.C.; Cunha, A.M.; Hosseini–Bandegharaei, A.; Dotto, G.L. Microwave synthesis of silica nanoparticles and its application for methylene blue adsorption. J. Environ. Chem. Eng. 2018, 6, 649–659. [Google Scholar] [CrossRef]
- Machado, N.B.; Miguez, J.P.; Bolina, I.C.; Salviano, A.B.; Gomes, R.A.; Tavano, O.L.; Luiz, J.H.; Tardioli, P.W.; Cren, C.; Mendes, A.A. Preparation, functionalization and characterization of rice husk silica for lipase immobilization via adsorption. Enzym. Microb. Technol. 2019, 128, 9–21. [Google Scholar] [CrossRef]
- Moeinian, K.; Mehdinia, S.M. Removing Methylene Blue from Aqueous Solutions Using Rice Husk Silica Adsorbent. Pol. J. Environ. Stud. 2019, 28, 2281–2287. [Google Scholar] [CrossRef]
- Lani, N.S.; Ngadi, N.; Inuwa, I.M. New route for the synthesis of silica-supported calcium oxide catalyst in biodiesel production. Renew. Energy 2019, 156, 1266–1277. [Google Scholar] [CrossRef]
- Lani, N.S.; Ngadi, N.; Yahya, N.Y.; Rahman, R.A. Synthesis, characterization and performance of silica impregnated calcium oxide as heterogeneous catalyst in biodiesel production. J. Clean. Prod. 2017, 146, 116–124. [Google Scholar] [CrossRef]
- Li, M.; Xiao, R. Preparation of a dual Pore Structure Activated Carbon from Rice Husk Char as an Adsorbent for CO2 Capture. Fuel Process. Technol. 2018, 186, 35–39. [Google Scholar] [CrossRef]
- Ramli, M.; Saiful, S.; Misriana, R. Free Fatty Acids Purification in Biodiesel with Utilizing Rice Husk Silica. Natural 2013, 13, 3. [Google Scholar]
- Iqhrammullah, M.; Marlina, M.; Khalil, H.P.S.A.; Kurniawan, K.H.; Suyanto, H.; Hedwig, R.; Karnadi, I.; Olaiya, N.G.; Abdullah, C.K.; Abdulmadjid, S.N. Characterization and Performance Evaluation of Cellulose Acetate–Polyurethane Film for Lead II Ion Removal. Polymers 2020, 12, 1317. [Google Scholar] [CrossRef] [PubMed]
- Iqhrammullah, M.; Suyanto, H.; Pardede, M.; Karnadi, I.; Kurniawan, K.H.; Chiari, W.; Abdulmadjid, S.N. Cellulose acetate-polyurethane film adsorbent with analyte enrichment for in-situ detection and analysis of aqueous Pb using Laser-Induced Breakdown Spectroscopy (LIBS). Environ. Nanotechnol. Monit. Manag. 2021, 16, 100516. [Google Scholar] [CrossRef]
- Terzioğlu, P.; Yücel, S.; Rabagah, T.M.; Özçimen, D. Characterization of Wheat Hull and Wheat Hull Ash as a Potential Source of SiO2. BioResources 2013, 8, 4406–4420. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.K.; Jain, R.; Varshney, S. Removal of Reactofix golden yellow 3 RFN from aqueous solution using wheat husk—An agricultural waste. J. Hazard. Mater. 2007, 142, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Habaki, H.; Hayashi, T.; Sinthupinyo, P.; Egashira, R. Purification of glycerol from transesterification using activated carbon prepared from Jatropha Shell for biodiesel production. J. Environ. Chem. Eng. 2019, 7, 103303. [Google Scholar] [CrossRef]
- Jr, N.S.; Frantz, T.S.; Lütke, S.F.; Arabidian, V.C.; Jr, T.R.S.C.; Pinto, L.A.A. Treatment of industrial glycerol from biodiesel production by adsorption operation: Kinetics and thermodynamics analyses. Chem. Eng. Commun. 2018, 206, 1388–1398. [Google Scholar] [CrossRef]
- Iqhrammullah, M.; Hedwig, R.; Karnadi, I.; Kurniawan, K.H.; Olaiya, N.G.; Haafiz, M.K.M.; Khalil, H.P.S.A.; Abdulmadjid, S.N. Filler-Modified Castor Oil-Based Polyurethane Foam for the Removal of Aqueous Heavy Metals Detected Using Laser-Induced Breakdown Spectroscopy (LIBS) Technique. Polymers 2020, 12, 903. [Google Scholar] [CrossRef] [Green Version]
- Safitri, E.; Humaira, H.; Murniana, M.; Nazaruddin, N.; Iqhrammullah, M.; Sani, N.M.; Esmaeili, C.; Susilawati, S.; Mahathir, M.; Nazaruddin, S.L. Optical pH Sensor Based on Immobilization Anthocyanin from Dioscorea alata L. onto Polyelectrolyte Complex Pectin–Chitosan Membrane for a Determination Method of Salivary pH. Polymers 2021, 13, 1276. [Google Scholar] [CrossRef]
- Rahmi; Iqhrammullah, M.; Audina, U.; Husin, H.; Fathana, H. Adsorptive removal of Cd (II) using oil palm empty fruit bunch-based charcoal/chitosan-EDTA film composite. Sustain. Chem. Pharm. 2021, 21, 100449. [Google Scholar] [CrossRef]
- Kanehashi, S.; Chen, G.Q.; Danaci, D.; Webley, P.A.; Kentish, S.E. Can the addition of carbon nanoparticles to a polyimide membrane reduce plasticization? Sep. Purif. Technol. 2017, 183, 333–340. [Google Scholar] [CrossRef]
- Li, W.; Chuah, C.Y.; Nie, L.; Bae, T.-H. Enhanced CO2/CH4 selectivity and mechanical strength of mixed-matrix membrane incorporated with NiDOBDC/GO composite. J. Ind. Eng. Chem. 2019, 74, 118–125. [Google Scholar] [CrossRef]
- Ebisike, K.; Okoronkwo, A.E.; Alaneme, K.K. Synthesis and characterization of Chitosan–silica hybrid aerogel using sol-gel method. J. King Saud Univ.-Sci. 2018, 32, 550–554. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Saiful; Rahmi; Marlina. Minireview: Membrane forward osmosis as alternative method in water treatment. AIP Conf. Proc. 2021, 2360, 050033. [Google Scholar] [CrossRef]
- Rahmi, R.; Lubis, S.; Az-Zahra, N.; Puspita, K.; Iqhrammullah, M. Synergetic photocatalytic and adsorptive removals of metanil yellow using TiO2/grass-derived cellulose/chitosan (TiO2/GC/CH) film composite. Int. J. Eng. 2021, 34, 1827–1836. [Google Scholar] [CrossRef]






| Isotherm Model | Equation | Parameters | |
|---|---|---|---|
| Langmuir | 0.996 | ||
| 1.057 | |||
| 7.749 | |||
| 0.005 | |||
| Freundlich | 0.997 | ||
| 0.853 | |||
| 1.054 | |||
| 0.045 | |||
| BET | 0.995 | ||
| 1.113 | |||
| 2.288 | |||
| 0.035 | |||
| 2.186 | |||
| Sips | 0.999 | ||
| 1.77 × 10−8 | |||
| 0.037 | |||
| 1.00048 | |||
| 0.871 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saiful; Riana, U.; Ramli, M.; Iqrammullah, M.; Raharjo, Y.; Wibisono, Y. Development of Chitosan/Rice Husk-Based Silica Composite Membranes for Biodiesel Purification. Membranes 2022, 12, 435. https://doi.org/10.3390/membranes12040435
Saiful, Riana U, Ramli M, Iqrammullah M, Raharjo Y, Wibisono Y. Development of Chitosan/Rice Husk-Based Silica Composite Membranes for Biodiesel Purification. Membranes. 2022; 12(4):435. https://doi.org/10.3390/membranes12040435
Chicago/Turabian StyleSaiful, Ulfa Riana, Muliadi Ramli, Muhammad Iqrammullah, Yanuardi Raharjo, and Yusuf Wibisono. 2022. "Development of Chitosan/Rice Husk-Based Silica Composite Membranes for Biodiesel Purification" Membranes 12, no. 4: 435. https://doi.org/10.3390/membranes12040435
APA StyleSaiful, Riana, U., Ramli, M., Iqrammullah, M., Raharjo, Y., & Wibisono, Y. (2022). Development of Chitosan/Rice Husk-Based Silica Composite Membranes for Biodiesel Purification. Membranes, 12(4), 435. https://doi.org/10.3390/membranes12040435










