Supported Liquid Membranes Based on Bifunctional Ionic Liquids for Selective Recovery of Gallium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Synthesis of Bif-ILs
2.2. Preparation of the SLM
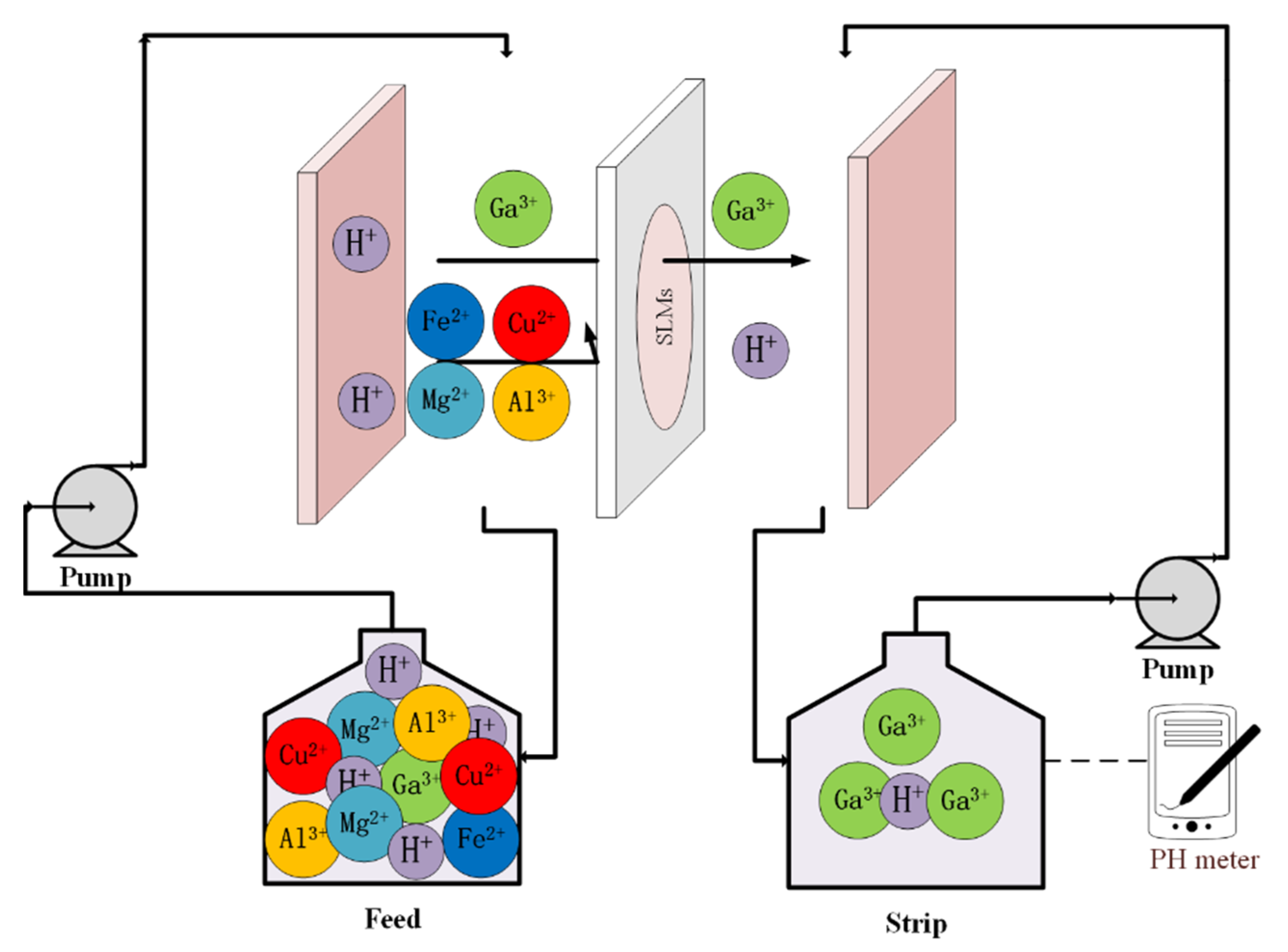
2.3. Solvent Extraction and Supported Liquid Membrane Transport Procedure
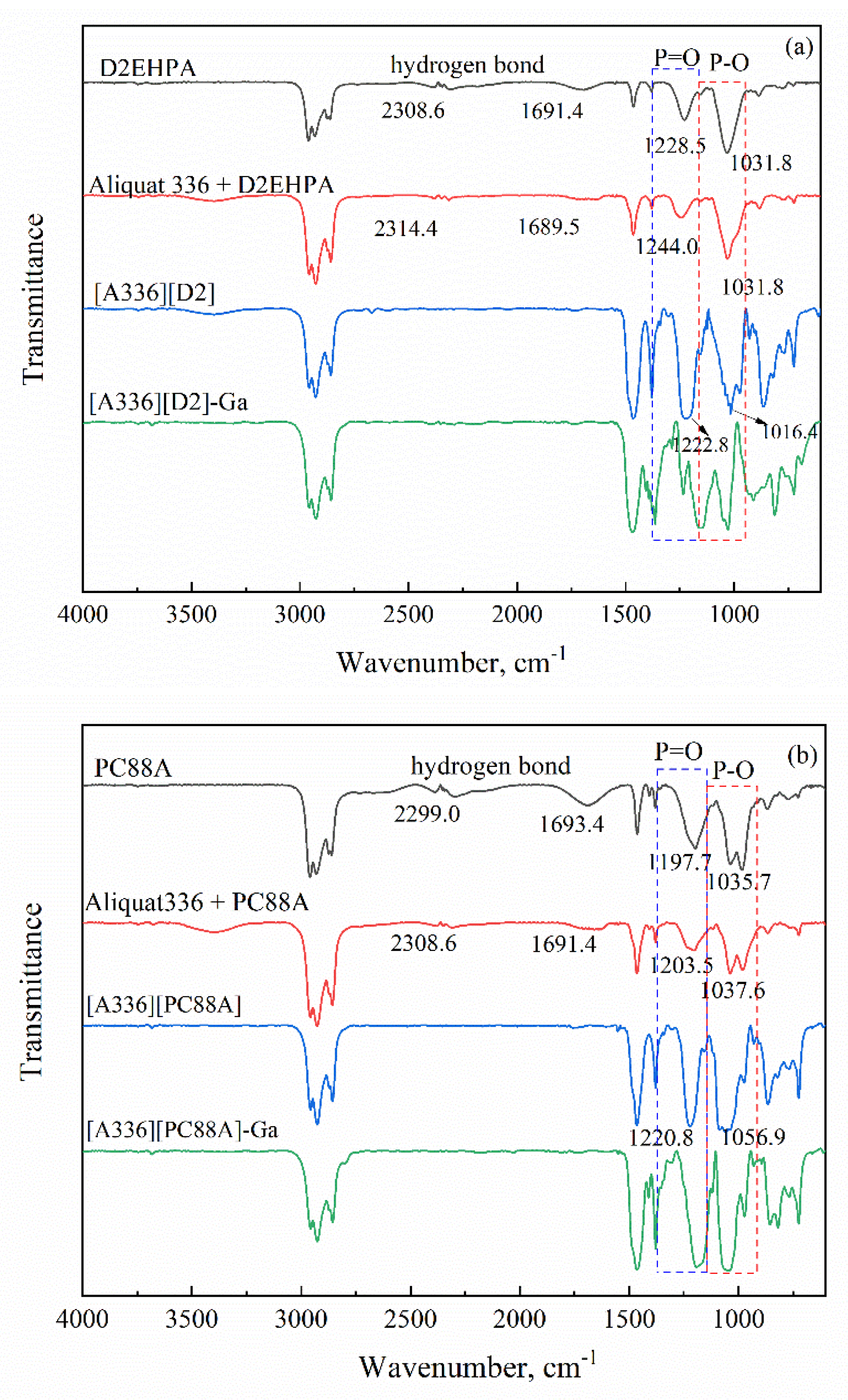
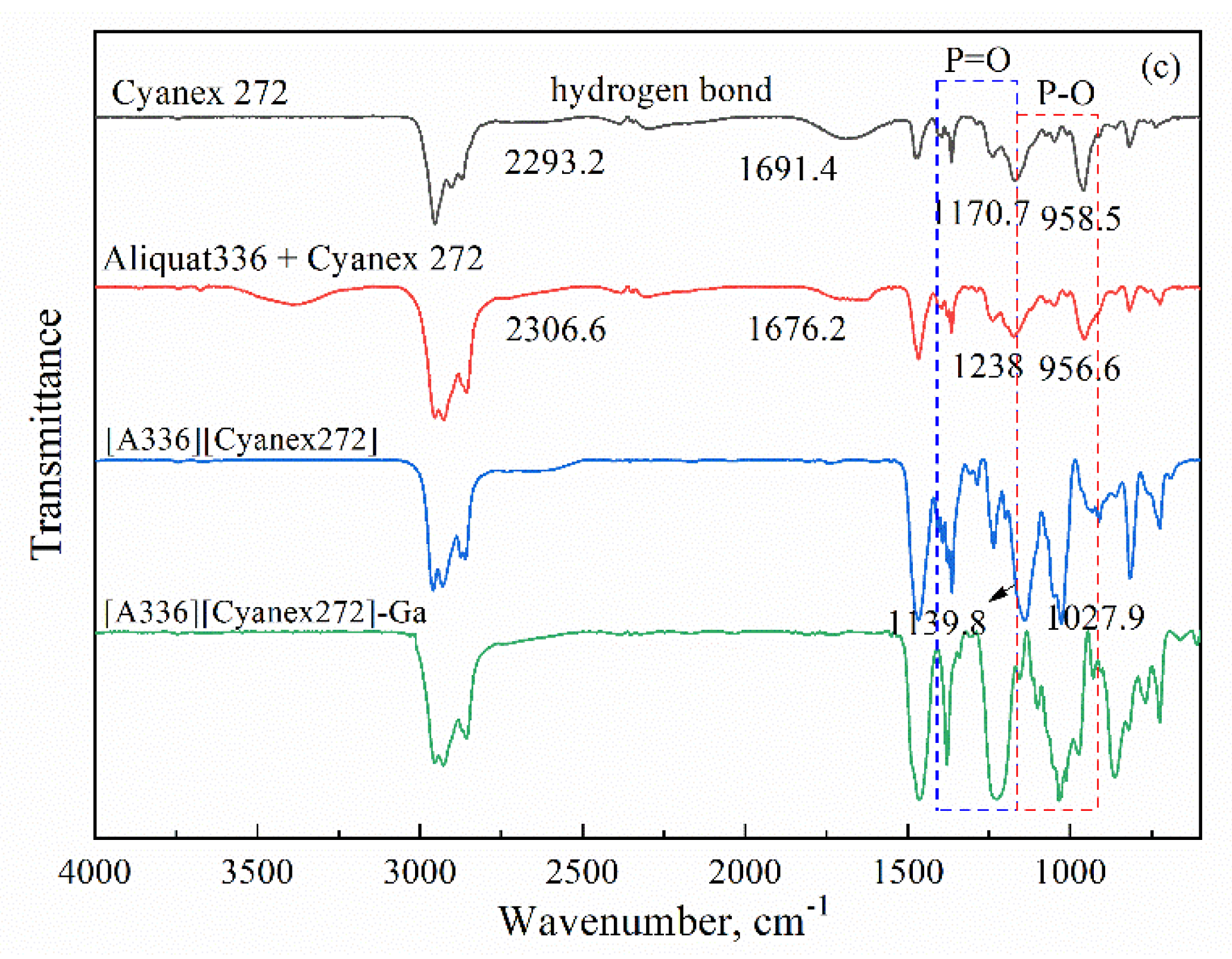
2.4. Characterization and Analysis
3. Results
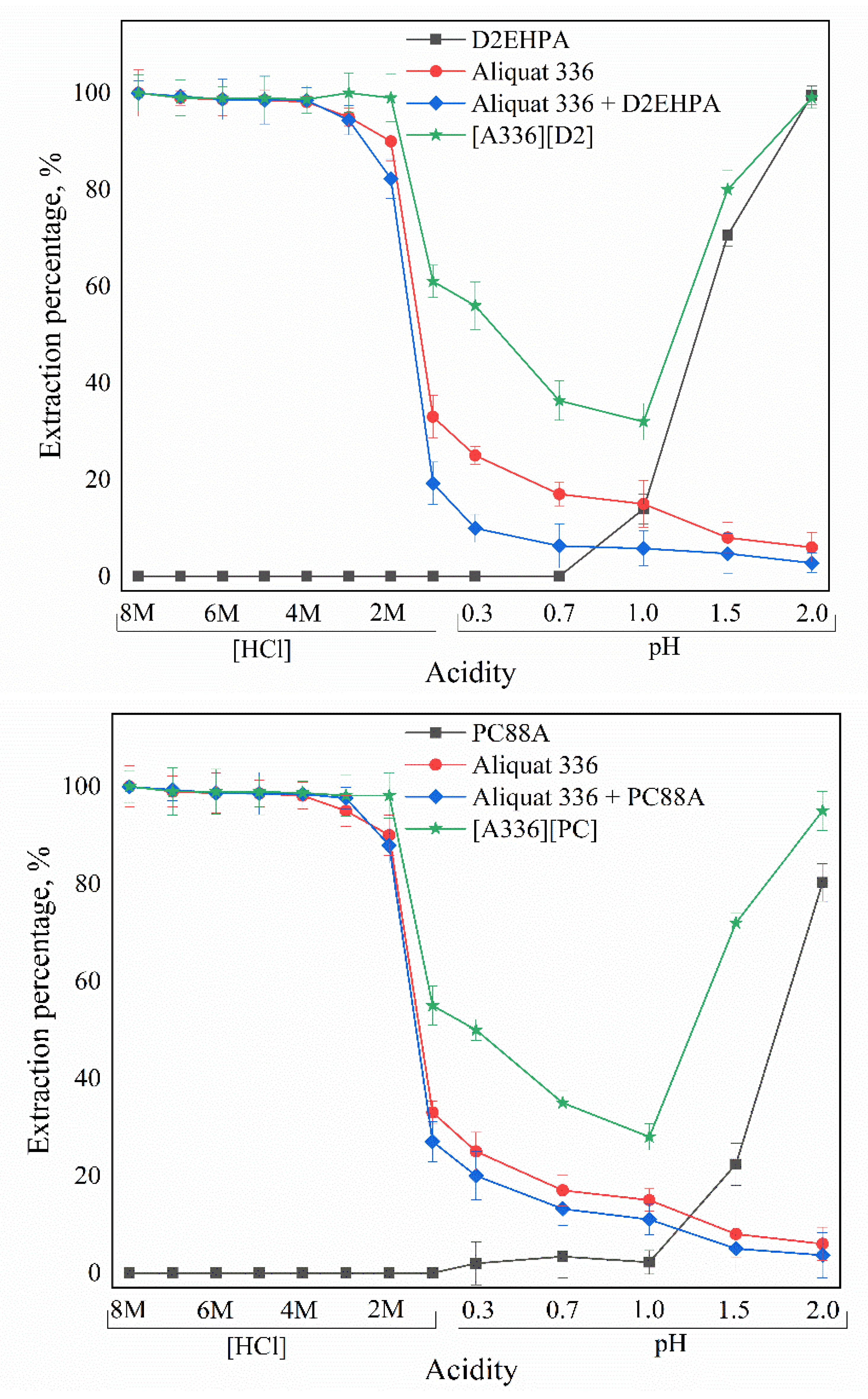
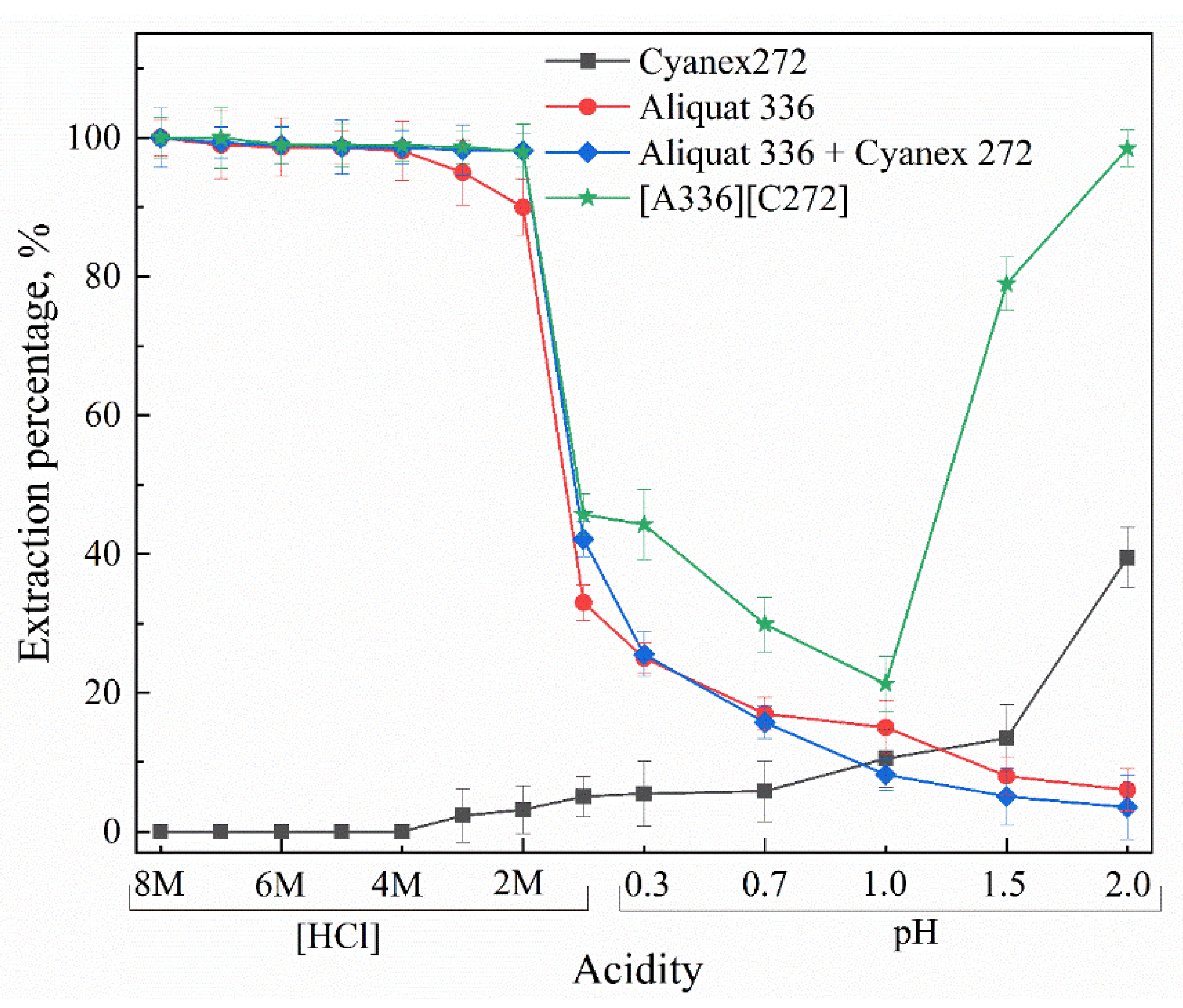
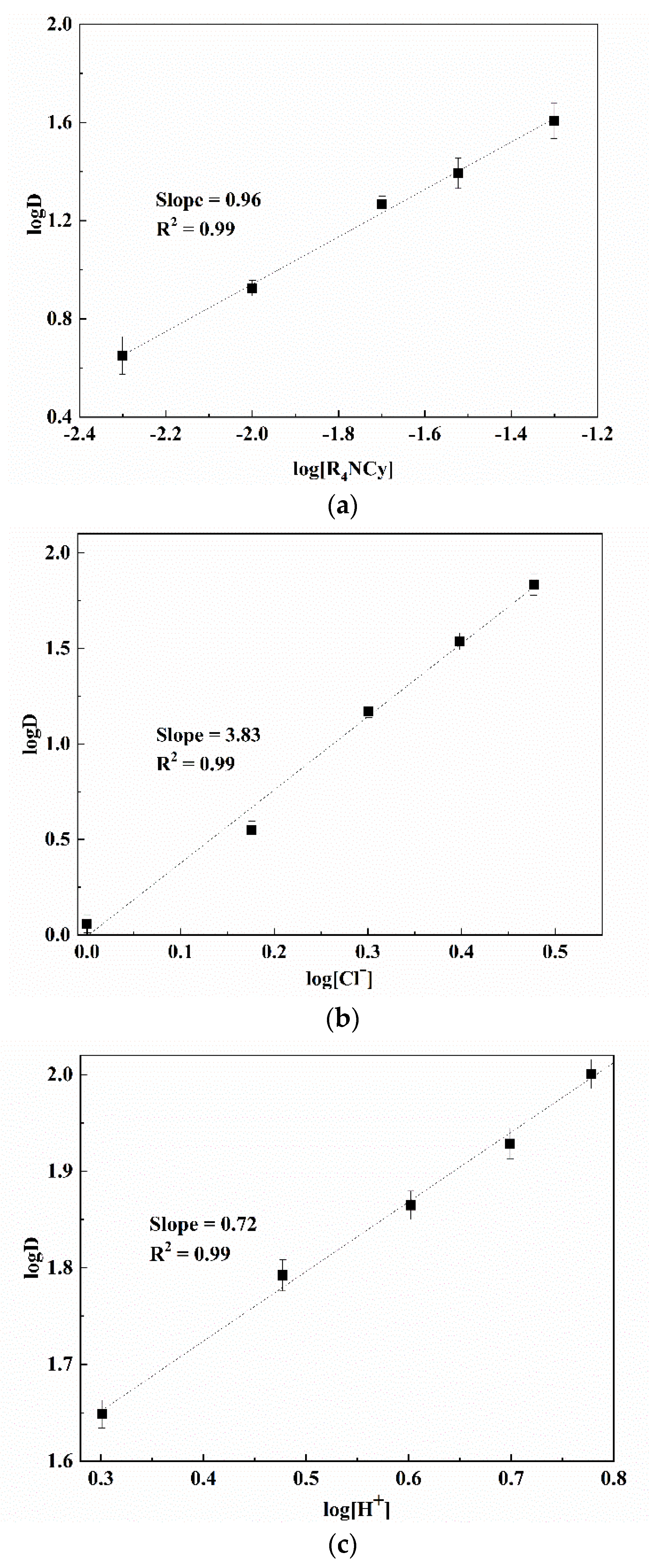
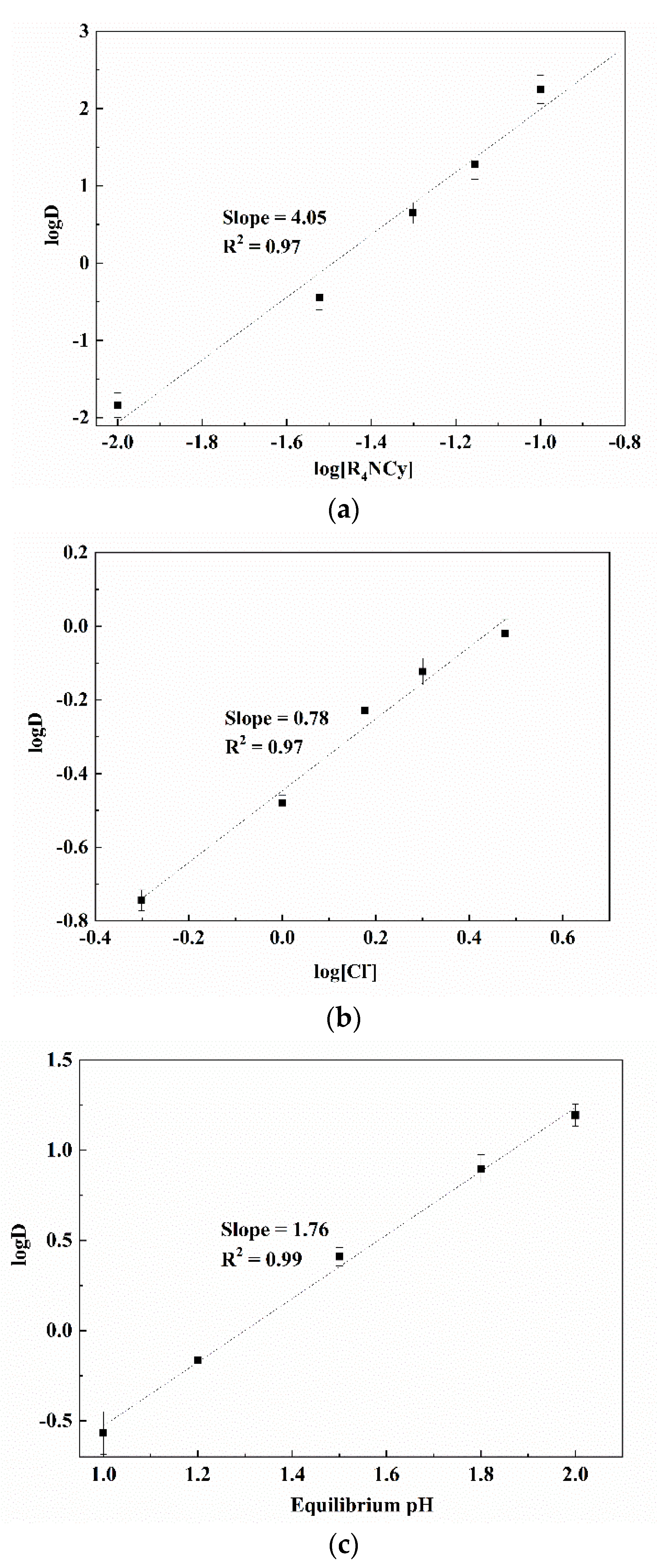
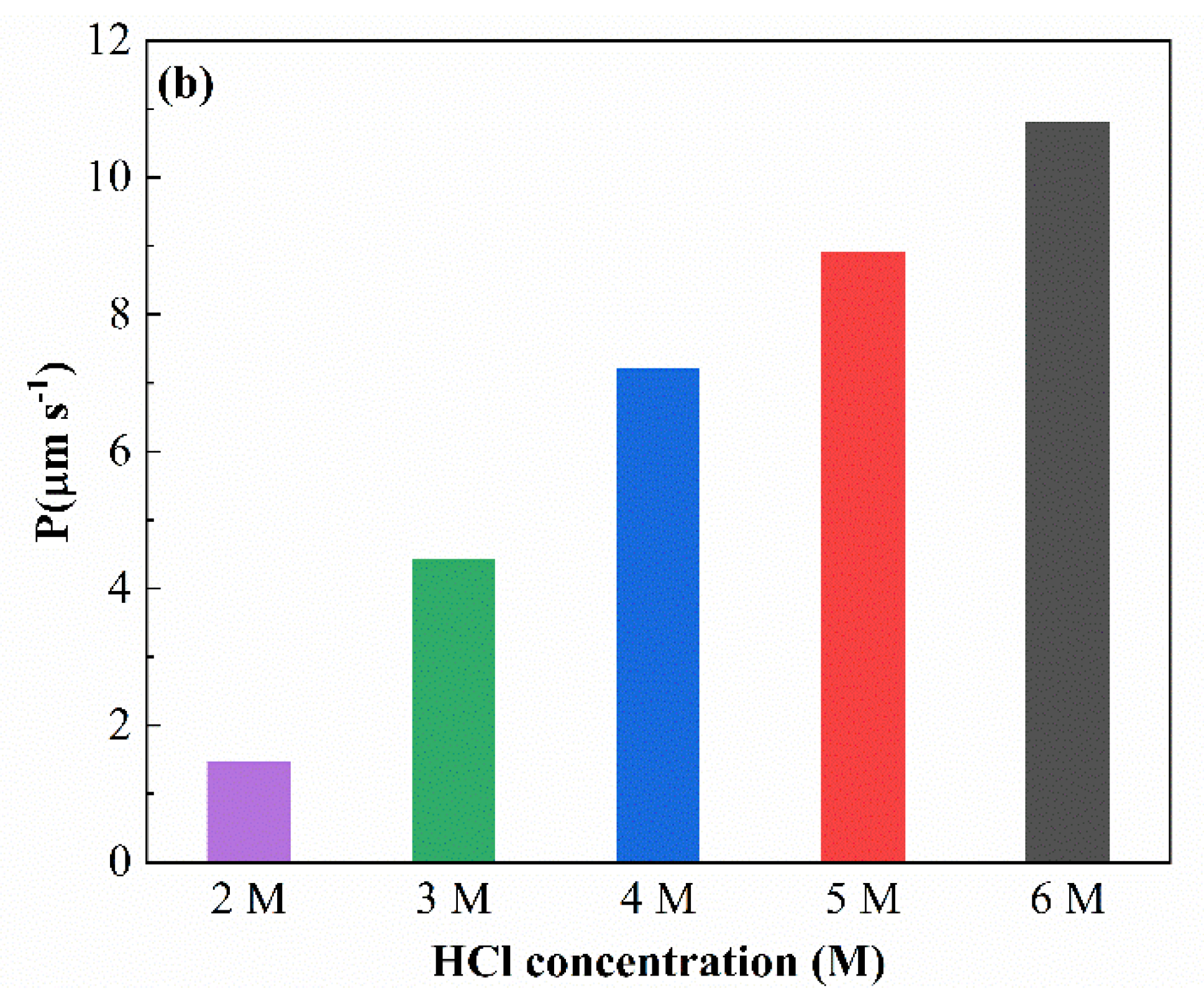
3.1. Parameters and Mechanisms of Ga(III) Extraction Using Bif-ILs
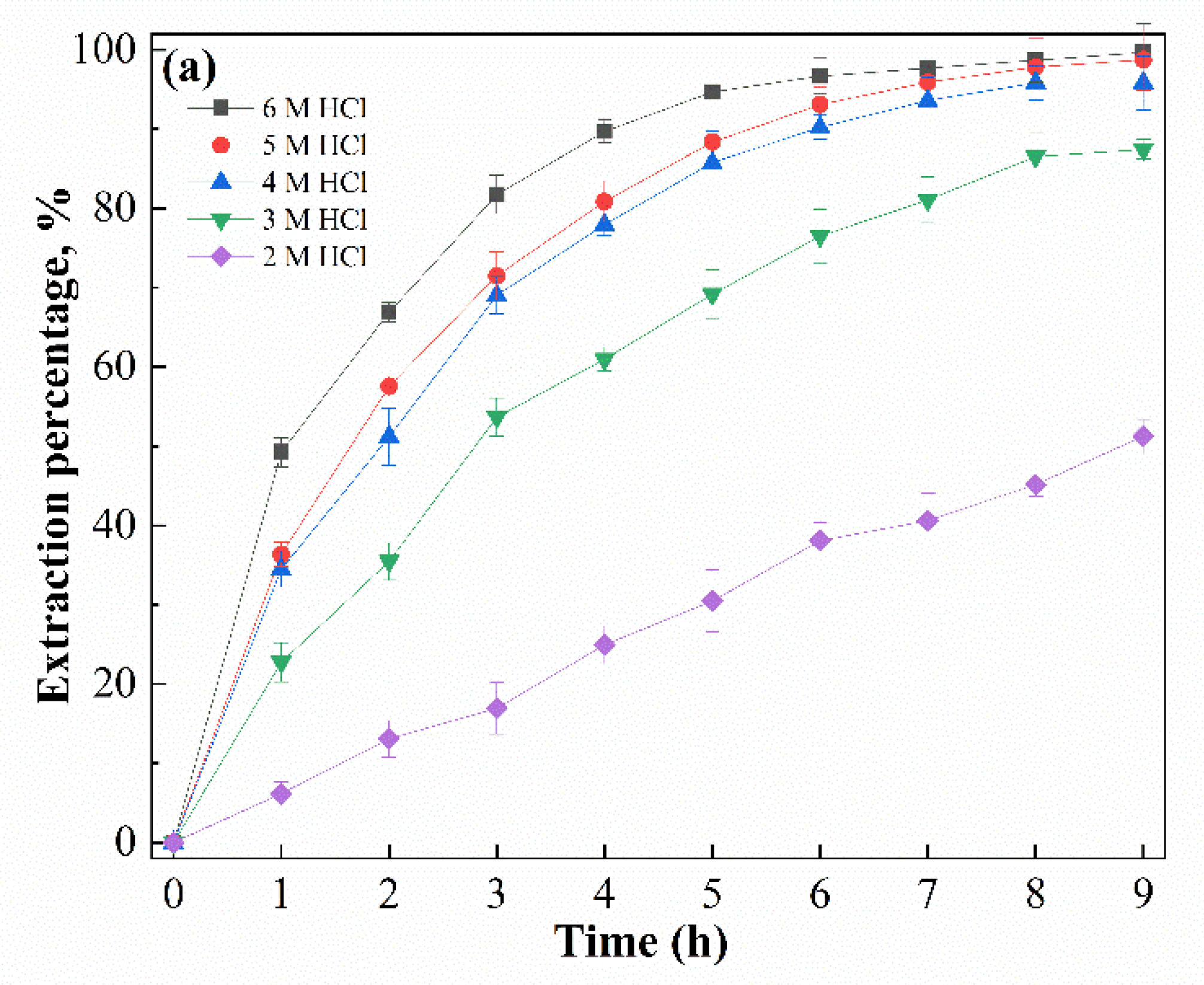
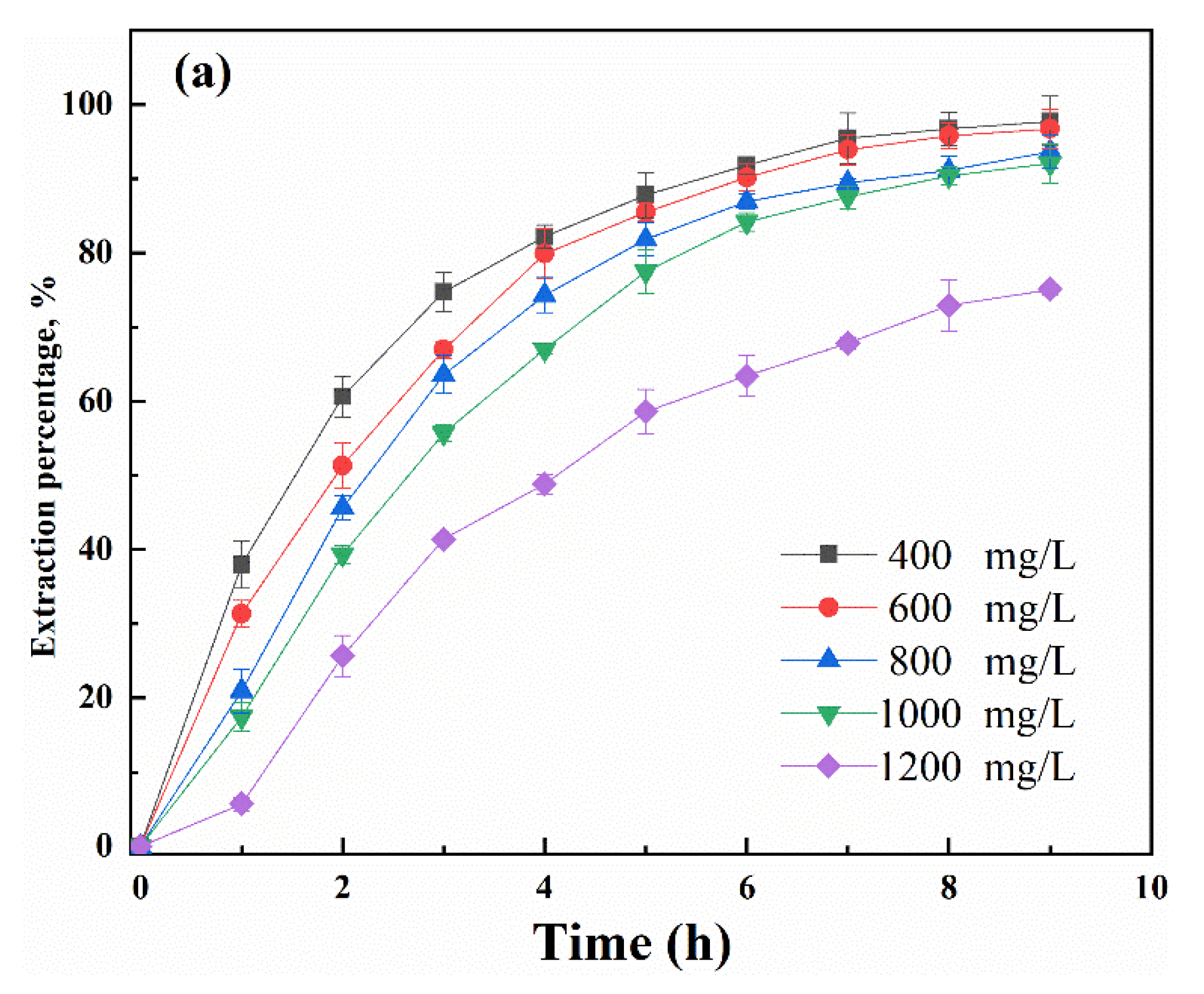
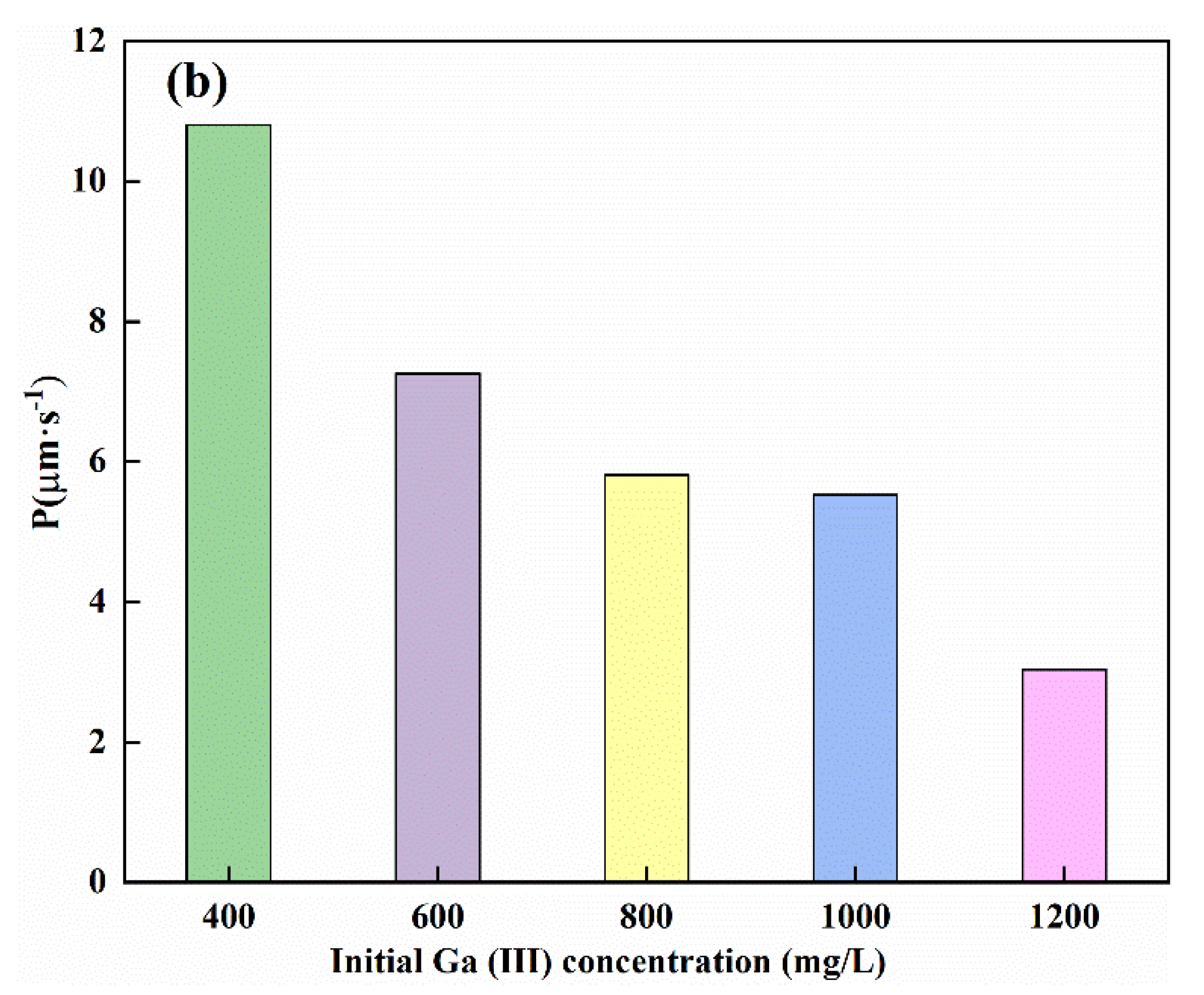
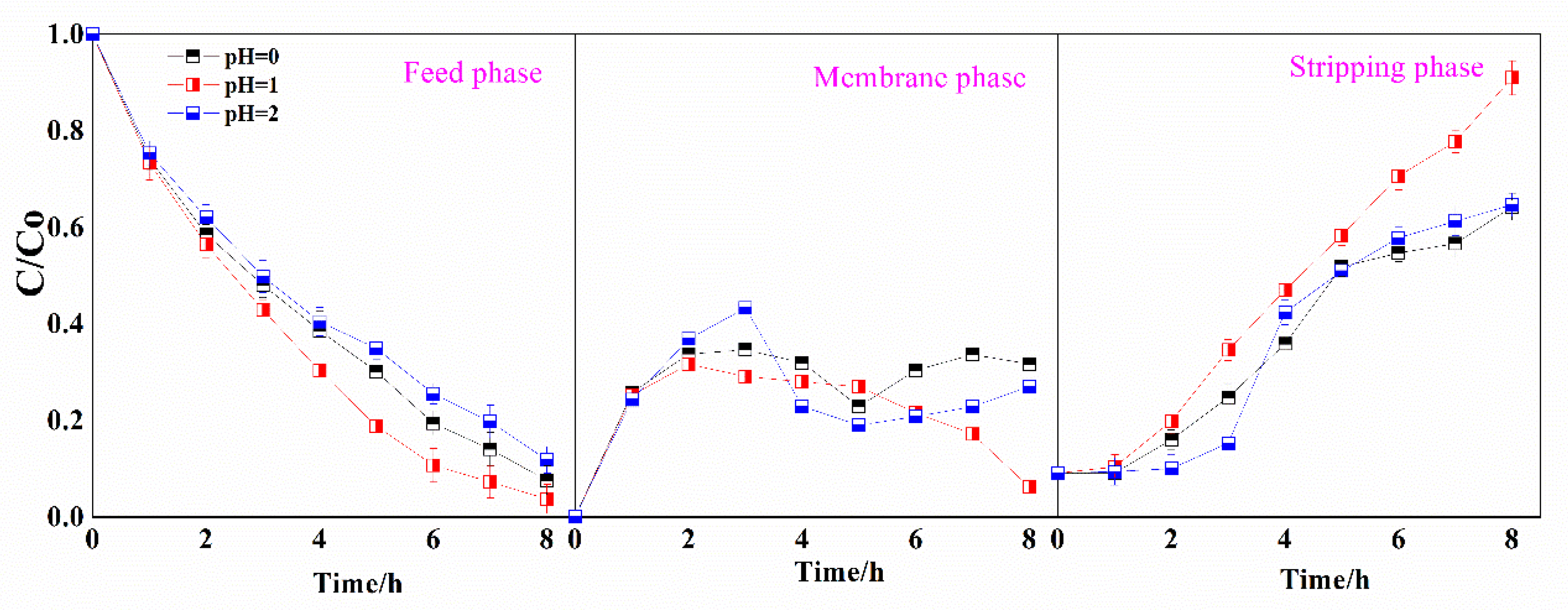
3.2. Mechanistic Investigation of Ga(III) Transport Using Supported Liquid Membrane with Bif-ILs as Carriers
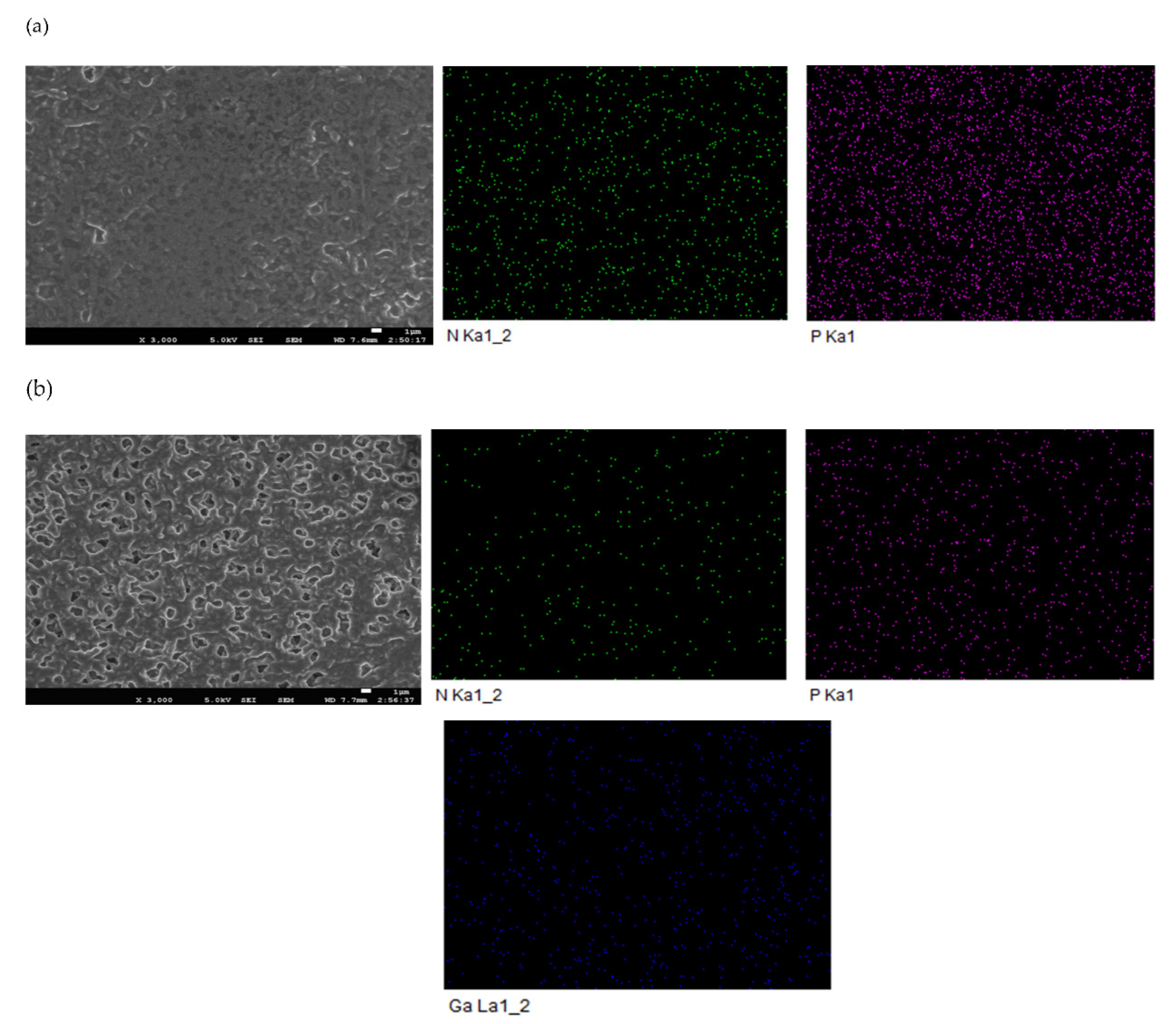
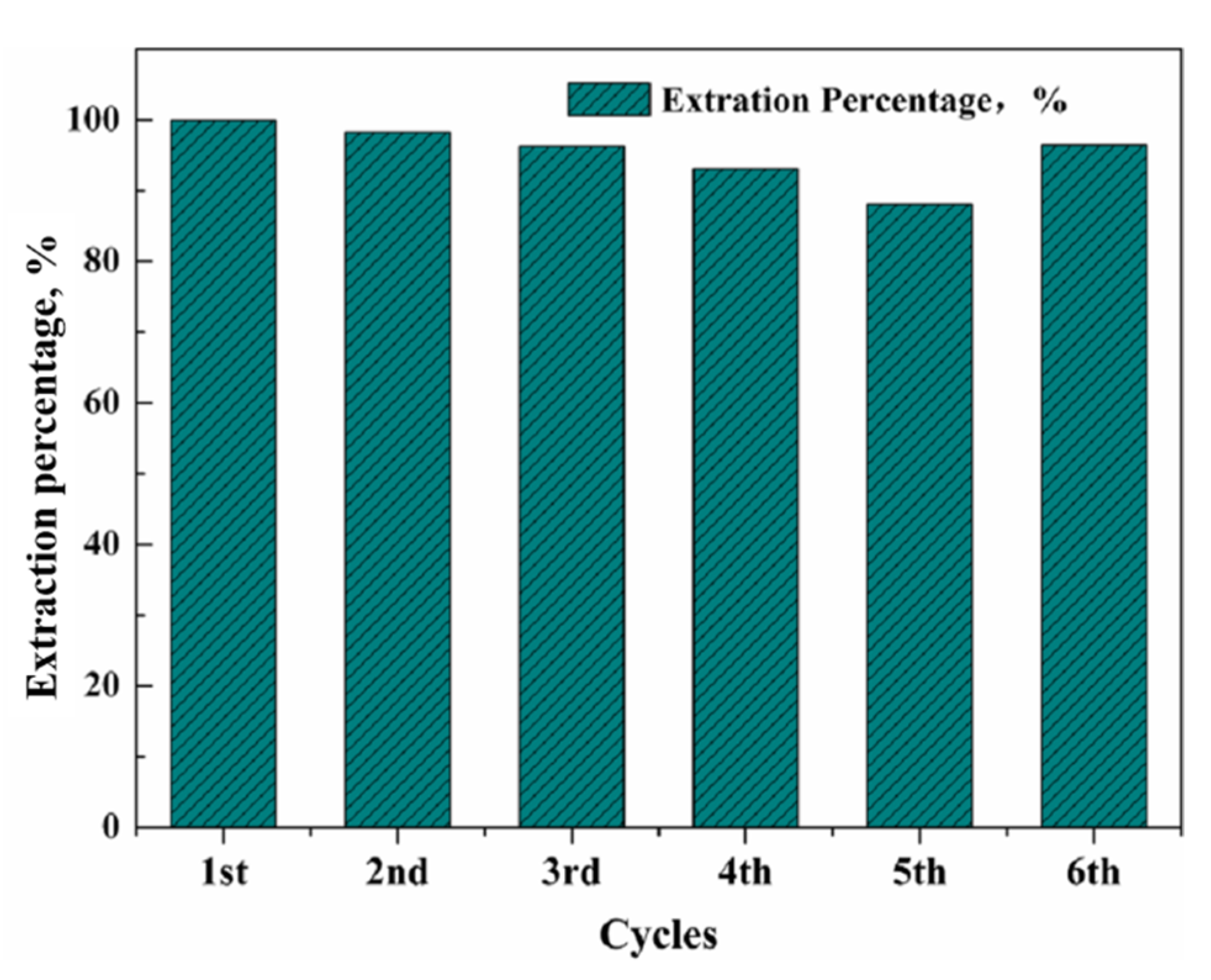
3.3. SLM Stability
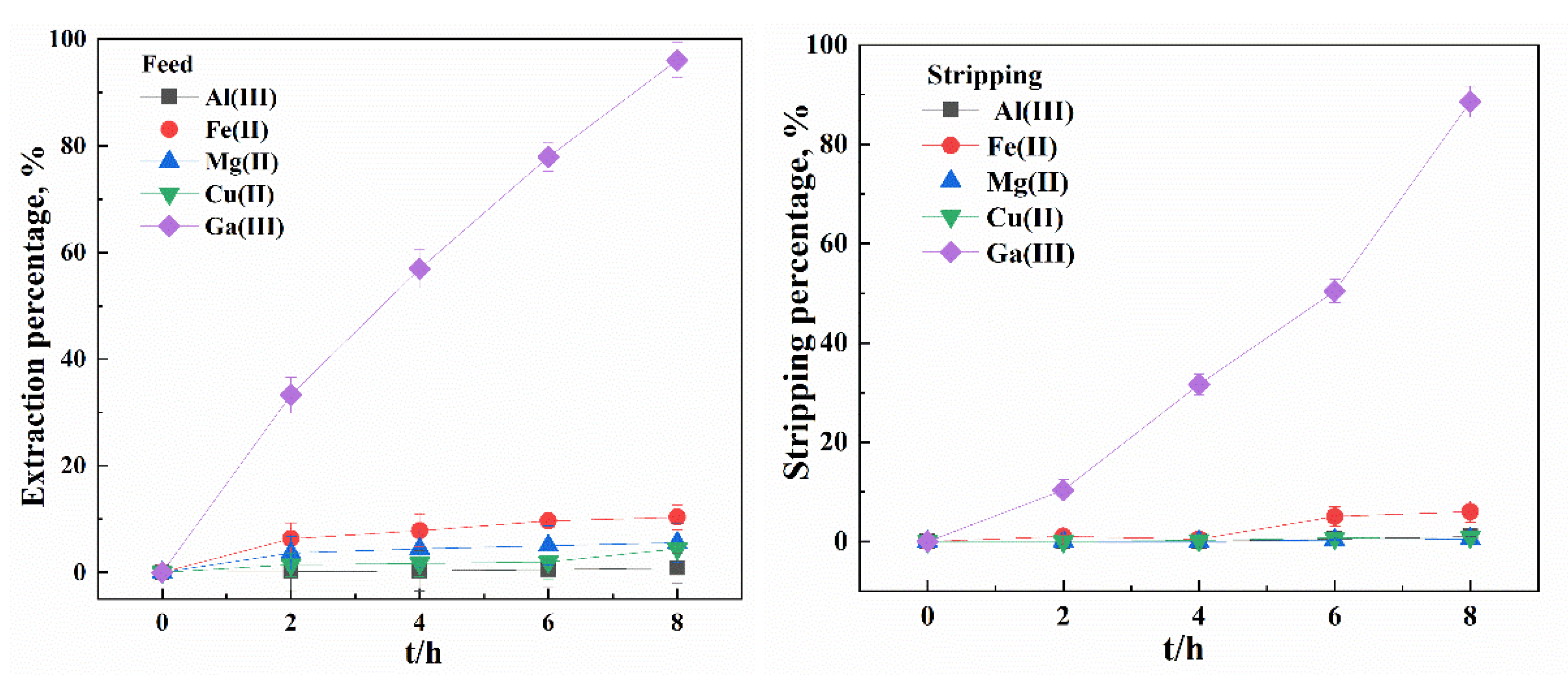
3.4. Selectivity of the Bif-ILs System in Both SX and SLM Processes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, K.; Qiu, L.; Tao, J.; Zhong, X.; Lin, Z.; Wang, R.; Liu, Z. Recovery of gallium from leach solutions of zinc refinery residues by stepwise solvent extraction with N235 and Cyanex 272. Hydrometallurgy 2021, 205, 105722. [Google Scholar] [CrossRef]
- Raiguel, S.; Dehaen, W.; Binnemans, K. Extraction of gallium from simulated Bayer process liquor by Kelex 100 dissolved in ionic liquids. Dalton Trans. 2020, 49, 3532–3544. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Bruynseels, B.; Zhang, Z.; Binnemans, K. Separation of GaCl3 from AlCl3 by Solid–Liquid Extraction and Stripping Using Anhydrous n-Dodecane and NaCl. Ind. Eng. Chem. Res. 2019, 58, 12459–12464. [Google Scholar] [CrossRef] [PubMed]
- Bossche, A.V.D.; Vereycken, W.; Hoogerstraete, T.V.; Dehaen, W.; Binnemans, K. Recovery of Gallium, Indium, and Arsenic from Semiconductors Using Tribromide Ionic Liquids. ACS Sustain. Chem. Eng. 2019, 7, 14451–14459. [Google Scholar] [CrossRef]
- Syed, S. Recovery of gold from secondary sources—A review. Hydrometallurgy 2012, 115–116, 30–51. [Google Scholar] [CrossRef]
- Roosen, J.; Mullens, S.; Binnemans, K. Chemical immobilization of 8-hydroxyquinoline and 8-hydroxyquinaldine on chitosan-silica adsorbent materials for the selective recovery of gallium from Bayer liquor. Hydrometallurgy 2017, 171, 275–284. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, X.; Lv, T.; Guo, Q.; Wang, L. Comparative study of solvent extraction and supported liquid membrane for the extraction of gallium (III) from chloride solution using organophosphorus acids as extractants. Sep. Sci. Technol. 2020, 55, 3012–3027. [Google Scholar] [CrossRef]
- Segala, B.N.; Bertuol, D.A.; Tanabe, E.H. Production of polyacrylonitrile nanofibres modified with Cyanex 272 for recovery of gallium from solution. Environ. Technol. 2020, 43, 737–750. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.M.; Ismail, Z.H.; Hamed, M.M. Extraction and separation of Ga(III) from hydrochloric acid solution by Cyanex-921 in sulfonated kerosene. J. Radioanal. Nucl. Chem. Artic. 2018, 317, 969–976. [Google Scholar] [CrossRef]
- Zhao, Z.; Cui, L.; Guo, Y.; Li, H.; Cheng, F. Recovery of gallium from sulfuric acid leach liquor of coal fly ash by stepwise separation using P507 and Cyanex 272. Chem. Eng. J. 2020, 381, 122699. [Google Scholar] [CrossRef]
- Song, S.J.; Le, M.N.; Lee, M.S. Separation of Gallium(III) and Indium(III) by Solvent Extraction with Ionic Liquids from Hydrochloric Acid Solution. Processes 2020, 8, 1347. [Google Scholar] [CrossRef]
- Nayak, S.; Devi, N. Studies on extraction of gallium (III) from chloride solution using Cyphos IL 104 and its removal from photodiodes and red mud. Hydrometallurgy 2017, 171, 191–197. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Lee, M.S. A Review on Separation of Gallium and Indium from Leach Liquors by Solvent Extraction and Ion Exchange. Miner. Process. Extr. Met. Rev. 2018, 40, 278–291. [Google Scholar] [CrossRef]
- Ahmed, I.; El-Nadi, Y.; El-Hefny, N. Extraction of gallium(III) from hydrochloric acid by Cyanex 923 and Cyanex 925. Hydrometallurgy 2013, 131–132, 24–28. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Z.; Li, Y.; Wilson, B.; Zeng, L.; Lundström, M. Recovery and separation of gallium(III) and germanium(IV) from zinc refinery residues: Part II: Solvent extraction. Hydrometallurgy 2017, 171, 149–156. [Google Scholar] [CrossRef]
- Huang, C.; Huang, B.; Dong, Y.; Chen, J.; Wang, Y.; Sun, X. Efficient and Sustainable Regeneration of Bifunctional Ionic Liquid for Rare Earth Separation. ACS Sustain. Chem. Eng. 2017, 5, 3471–3477. [Google Scholar] [CrossRef]
- Zeng, Z.; Su, X.; Gao, Y.; Yu, G.; Ni, S.; Su, J.; Sun, X. Separation of lutetium using a novel bifunctional ionic liquid based on phosphonate functionalization. Sep. Purif. Technol. 2021, 264, 118439. [Google Scholar] [CrossRef]
- Azizi, D.; Larachi, F. Behavior of bifunctional phosphonium-based ionic liquids in solvent extraction of rare earth elements-quantum chemical study. J. Mol. Liq. 2018, 263, 96–108. [Google Scholar] [CrossRef]
- Rout, A.; Wellens, S.; Binnemans, K. Separation of rare earths and nickel by solvent extraction with two mutually immiscible ionic liquids. RSC Adv. 2014, 4, 5753–5758. [Google Scholar] [CrossRef] [Green Version]
- Zante, G.; Boltoeva, M.; Masmoudi, A.; Barillon, R.; Trébouet, D. Selective separation of cobalt and nickel using a stable supported ionic liquid membrane. Sep. Purif. Technol. 2020, 252, 117477. [Google Scholar] [CrossRef]
- Lanaridi, O.; Sahoo, A.R.; Limbeck, A.; Naghdi, S.; Eder, D.; Eitenberger, E.; Csendes, Z.; Schnürch, M.; Bica-Schröder, K. Toward the Recovery of Platinum Group Metals from a Spent Automotive Catalyst with Supported Ionic Liquid Phases. ACS Sustain. Chem. Eng. 2021, 9, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Van Roosendael, S.; Regadío, M.; Roosen, J.; Binnemans, K. Selective recovery of indium from iron-rich solutions using an Aliquat 336 iodide supported ionic liquid phase (SILP). Sep. Purif. Technol. 2019, 212, 843–853. [Google Scholar] [CrossRef]
- Alguacil, F.J. Mechanistic investigation of facilitated transport of gold(III) from HCl media using ionic liquid Cyphos IL102 as carrier across a supported liquid membrane. Gold Bull. 2019, 52, 145–151. [Google Scholar] [CrossRef]
- Nosrati, S.; Jayakumar, N.; Hashim, M.; Mukhopadhyay, S. Performance evaluation of vanadium (IV) transport through supported ionic liquid membrane. J. Taiwan Inst. Chem. Eng. 2013, 44, 337–342. [Google Scholar] [CrossRef]
- Zante, G.; Boltoeva, M.; Masmoudi, A.; Barillon, R.; Trébouet, D. Lithium extraction from complex aqueous solutions using supported ionic liquid membranes. J. Membr. Sci. 2019, 580, 62–76. [Google Scholar] [CrossRef]
- Fortuny, A.; Coll, M.T.; Sastre, A.M. Use of methyltrioctyl/decylammonium bis 2,4,4-(trimethylpentyl)phosphinate ionic liquid (ALiCY IL) on the boron extraction in chloride media. Sep. Purif. Technol. 2012, 97, 137–141. [Google Scholar] [CrossRef]
- Tran, T.T.; Azra, N.; Iqbal, M.; Lee, M.S. Synthesis of succinimide based ionic liquids and comparison of extraction behavior of Co(II) and Ni(II) with bi-functional ionic liquids synthesized by Aliquat336 and organophosphorus acids. Sep. Purif. Technol. 2020, 238, 116496. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Meng, S.; Li, D. The extraction of rare earths using mixtures of acidic phosphorus-based reagents or their thio-analogues. J. Chem. Technol. Biotechnol. 2006, 81, 761–766. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, Y.; Guo, W.; Liu, D.; Ding, Y. Enhanced homogeneous liquid–liquid extraction for the selective recovery of sc(iii) by novel ucst-type ionic liquids. ACS Sustain. Chem. Eng. 2021, 9, 9932–9940. [Google Scholar] [CrossRef]
- Nguyen, T.T.N.; Nguyen, V.N.H.; Liu, Y.; Lee, M.S. Analysis of the interaction in the mixture of organophosphorus acids and Aliquat 336 through the measurement of dielectric constant and viscosity. J. Mol. Liq. 2020, 315, 113738. [Google Scholar] [CrossRef]
- Jean, E.; Villemin, D.; Hlaibi, M.; Lebrun, L. Heavy metal ions extraction using new supported liquid membranes containing ionic liquid as carrier. Sep. Purif. Technol. 2018, 201, 1–9. [Google Scholar] [CrossRef]
- Shahrezaei, F.; Shamsipur, M.; Gholivand, M.B.; Zohrabi, P.; Babajani, N.; Abri, A.; Zonouz, A.M.; Shekaari, H. A highly selective green supported liquid membrane by using a hydrophobic deep eutectic solvent for carrier-less transport of silver ions. Anal. Methods 2020, 12, 4682–4690. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.A.; Hashim, M.A.; Nabi, F. Ionic liquids in supported liquid membrane technology. Chem. Eng. J. 2011, 171, 242–254. [Google Scholar] [CrossRef]
- Zante, G.; Boltoeva, M.; Masmoudi, A.; Barillon, R.; Trébouet, D. Supported ionic liquid and polymer inclusion membranes for metal separation. Sep. Purif. Rev. 2021, 51, 100–116. [Google Scholar] [CrossRef]
| Chemicals (Abbreviations) | Structures |
|---|---|
| Cyanex 272 | 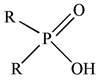 |
| PC88A | 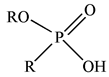 |
| D2EHPA | 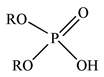 |
| Aliquat 336 | 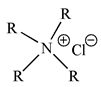 |
| BIf-IL Aliquat336–Cyanex 272 (R4NCy) | 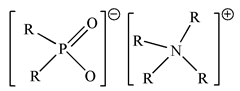 |
| BIf-IL Aliquat336–PC88A (R4NPC) | 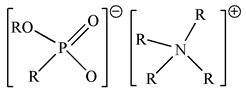 |
| BIf-IL Aliquat336–D2EHPA (R4ND2) | 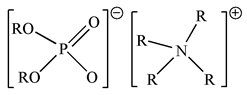 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Ye, Y.; Tan, Y.; Zhu, K.; Liu, X.; Tian, H.; Guo, Q.; Wang, L.; Zhao, S.; Liu, Y. Supported Liquid Membranes Based on Bifunctional Ionic Liquids for Selective Recovery of Gallium. Membranes 2022, 12, 376. https://doi.org/10.3390/membranes12040376
Zhou H, Ye Y, Tan Y, Zhu K, Liu X, Tian H, Guo Q, Wang L, Zhao S, Liu Y. Supported Liquid Membranes Based on Bifunctional Ionic Liquids for Selective Recovery of Gallium. Membranes. 2022; 12(4):376. https://doi.org/10.3390/membranes12040376
Chicago/Turabian StyleZhou, Haitao, Yuxi Ye, Yuefei Tan, Kailun Zhu, Xinmin Liu, Hongjing Tian, Qingjie Guo, Lingyun Wang, Shuju Zhao, and Yang Liu. 2022. "Supported Liquid Membranes Based on Bifunctional Ionic Liquids for Selective Recovery of Gallium" Membranes 12, no. 4: 376. https://doi.org/10.3390/membranes12040376
APA StyleZhou, H., Ye, Y., Tan, Y., Zhu, K., Liu, X., Tian, H., Guo, Q., Wang, L., Zhao, S., & Liu, Y. (2022). Supported Liquid Membranes Based on Bifunctional Ionic Liquids for Selective Recovery of Gallium. Membranes, 12(4), 376. https://doi.org/10.3390/membranes12040376








