Effect of the Peptization Process and Thermal Treatment on the Sol-Gel Preparation of Mesoporous α-Alumina Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Boehmite Sol
2.2. Characterization
3. Results and Discussion
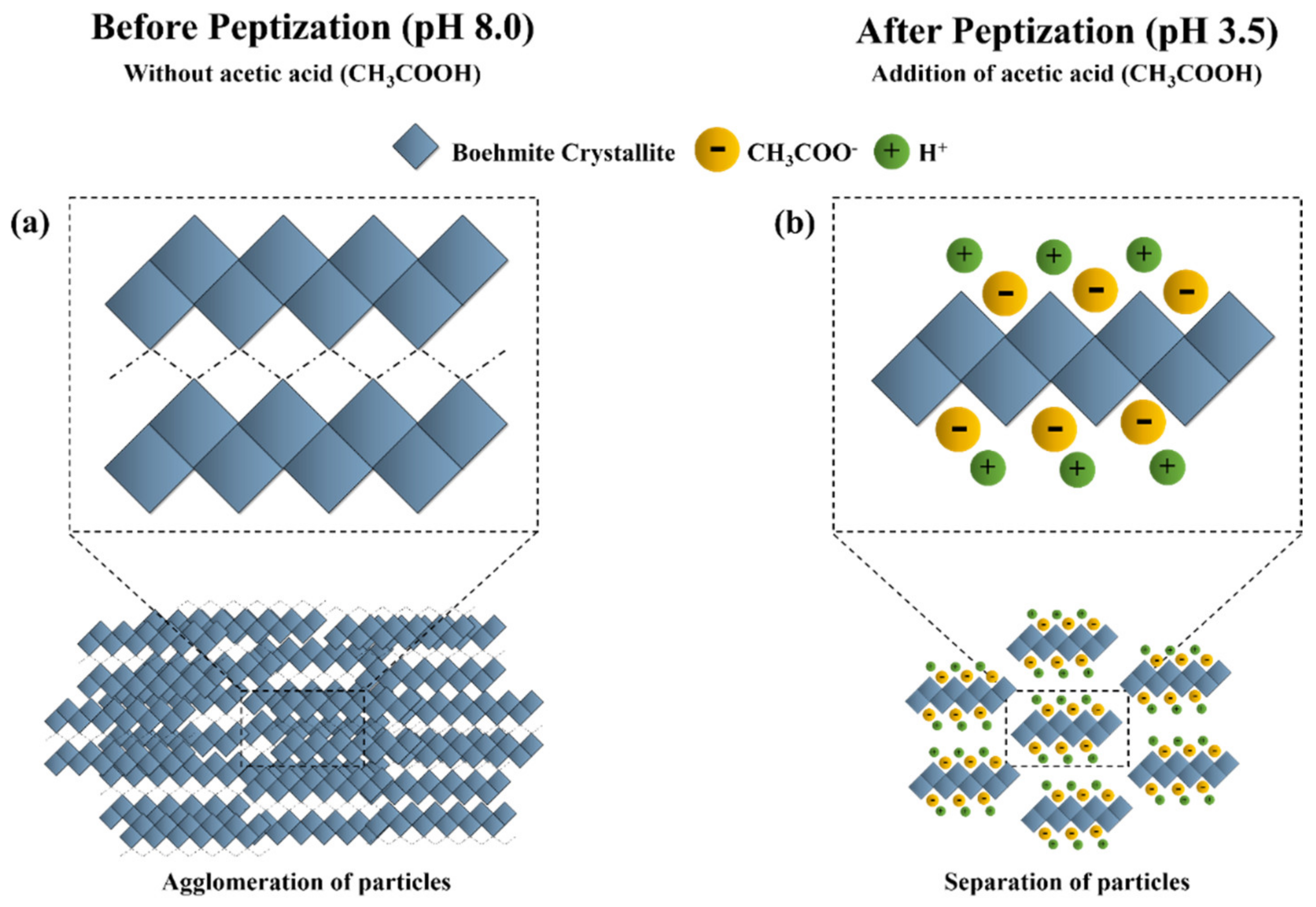
3.1. Effect of Peptization
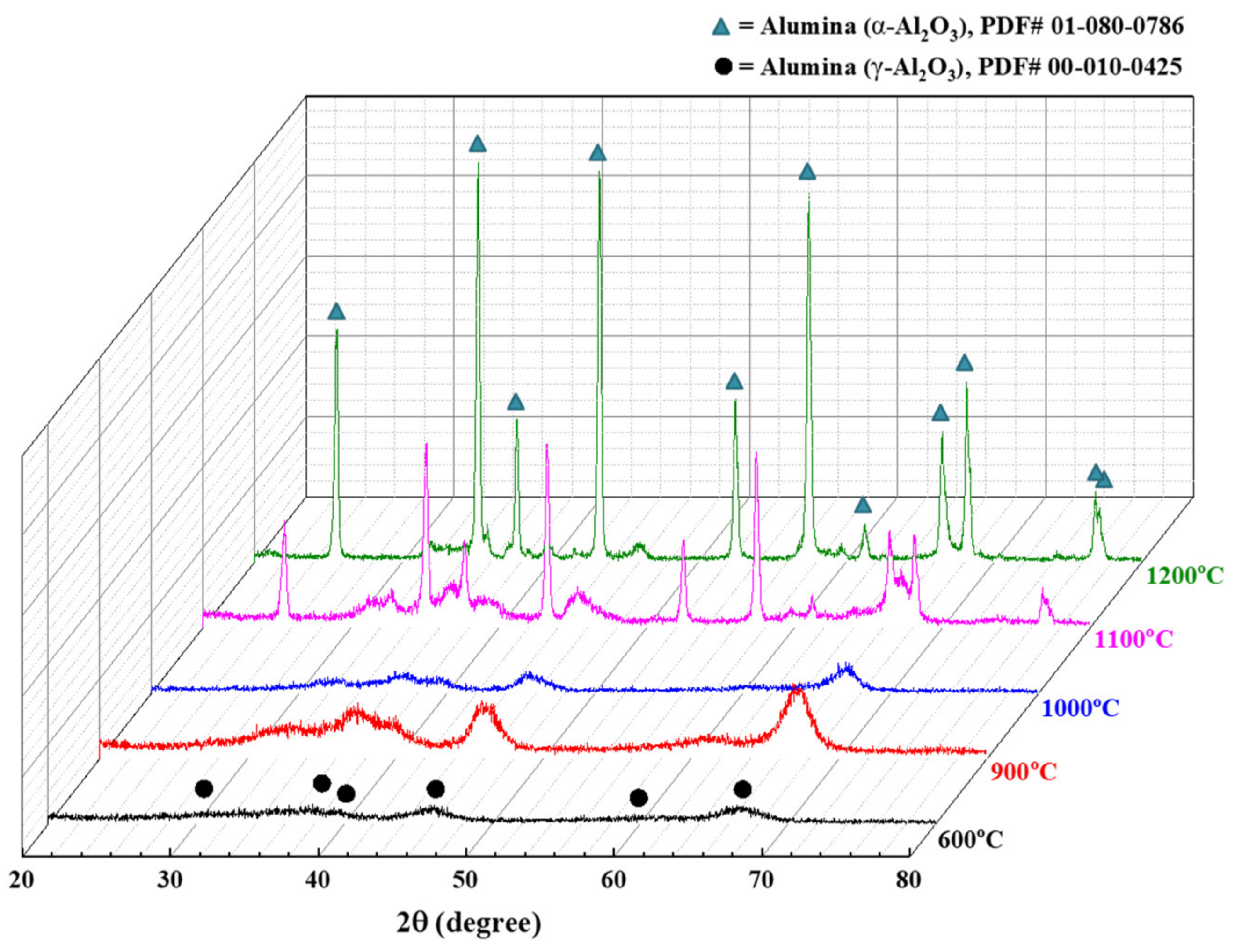
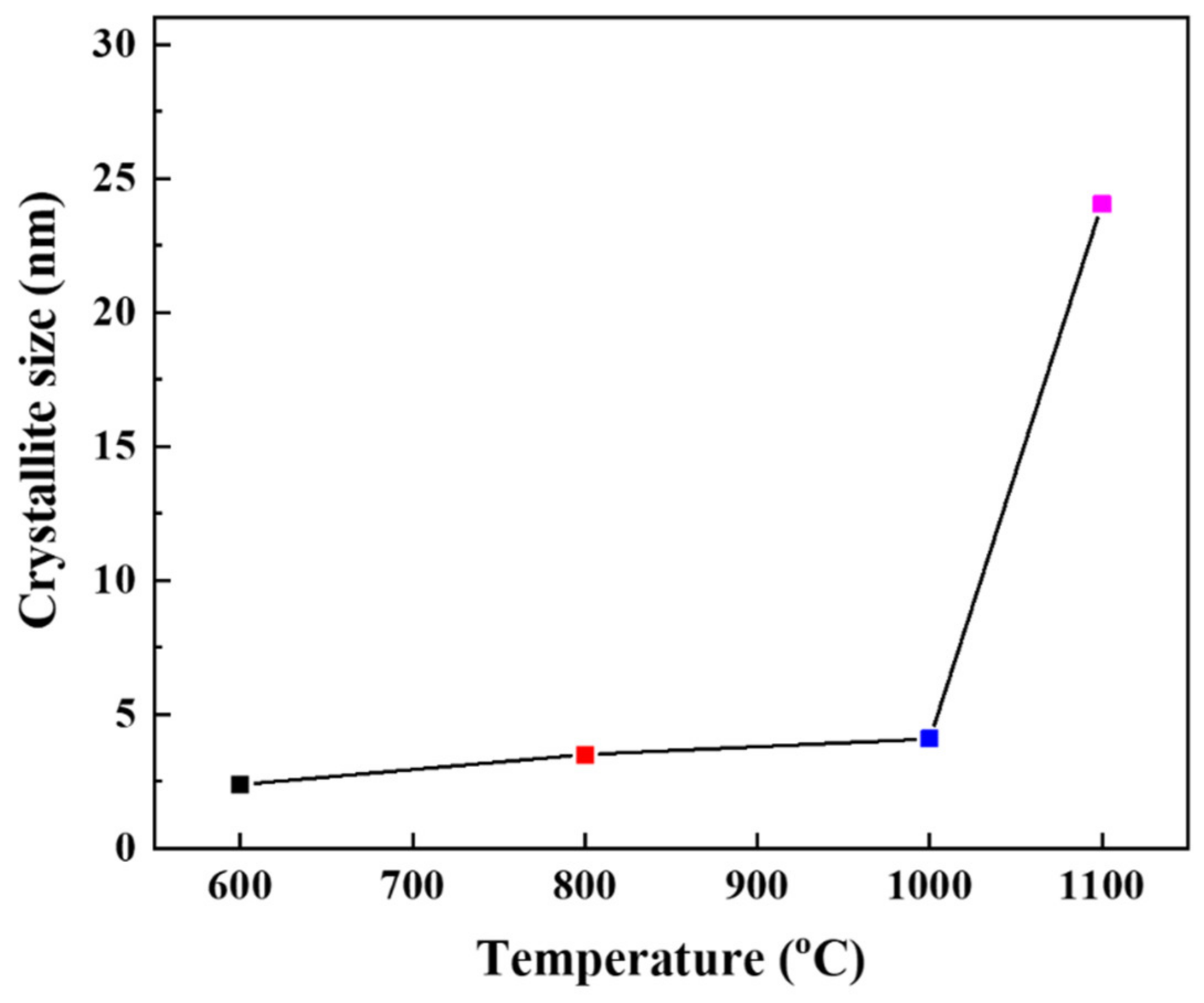
3.2. Effect of Thermal Treatment
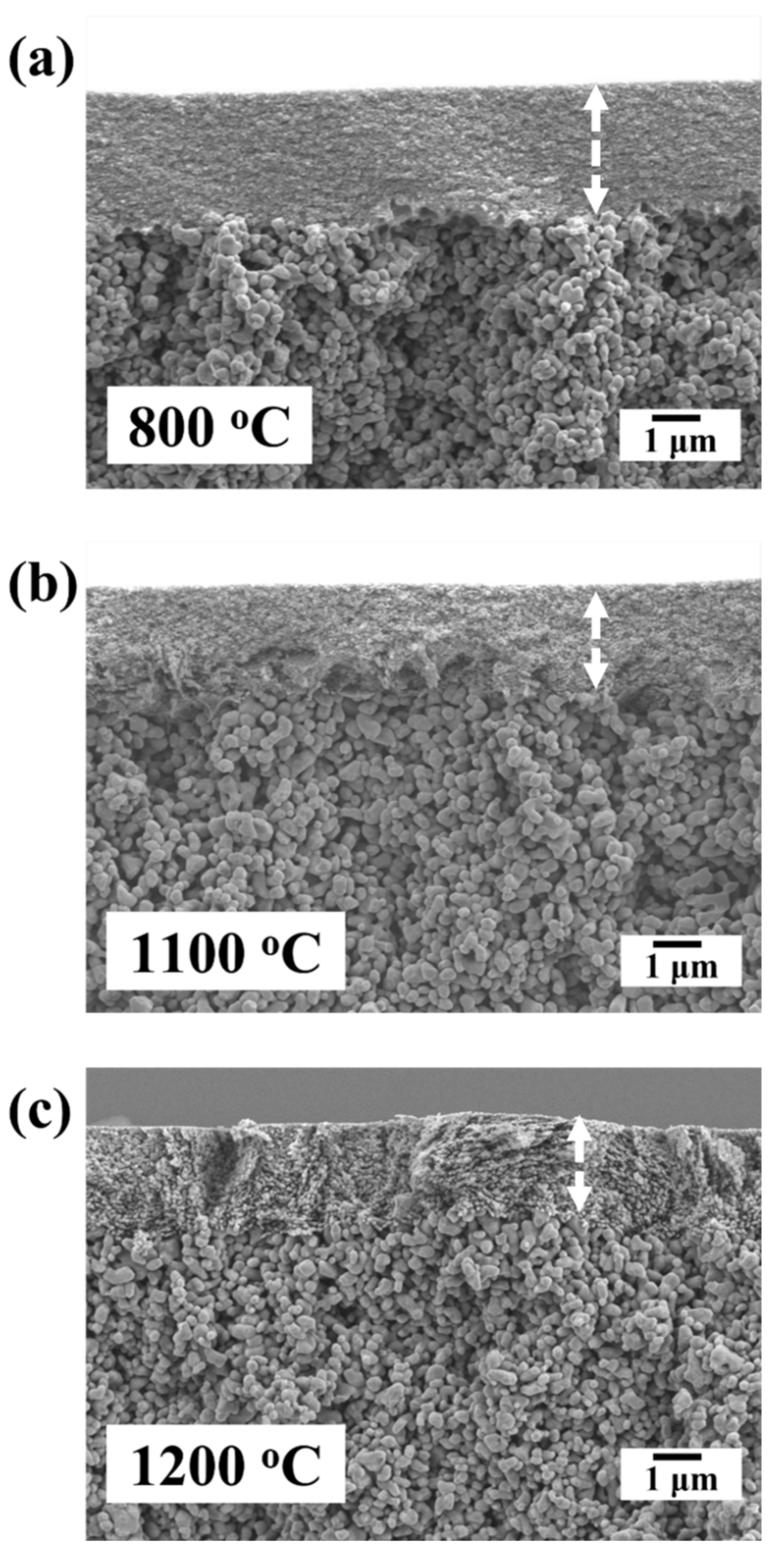
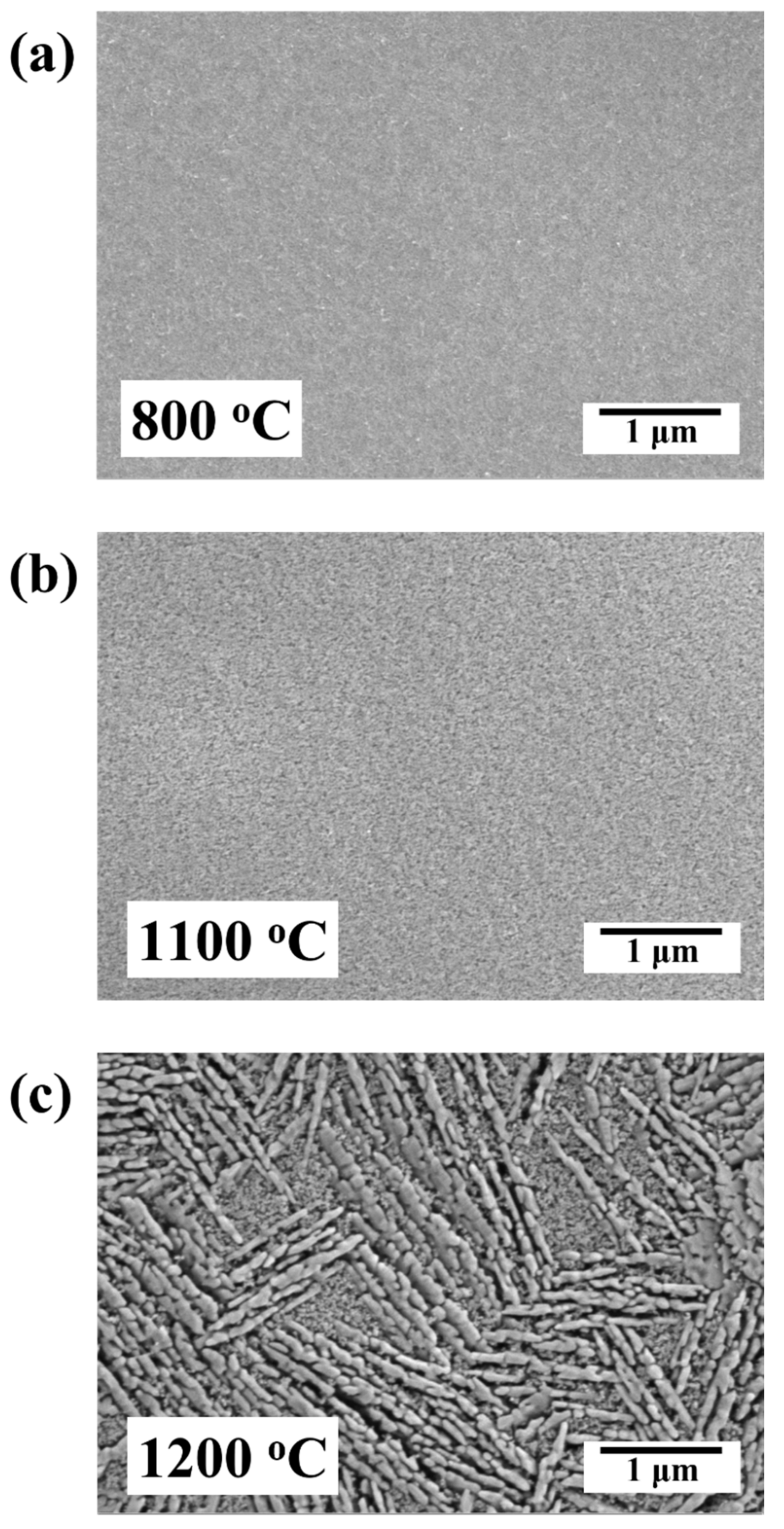
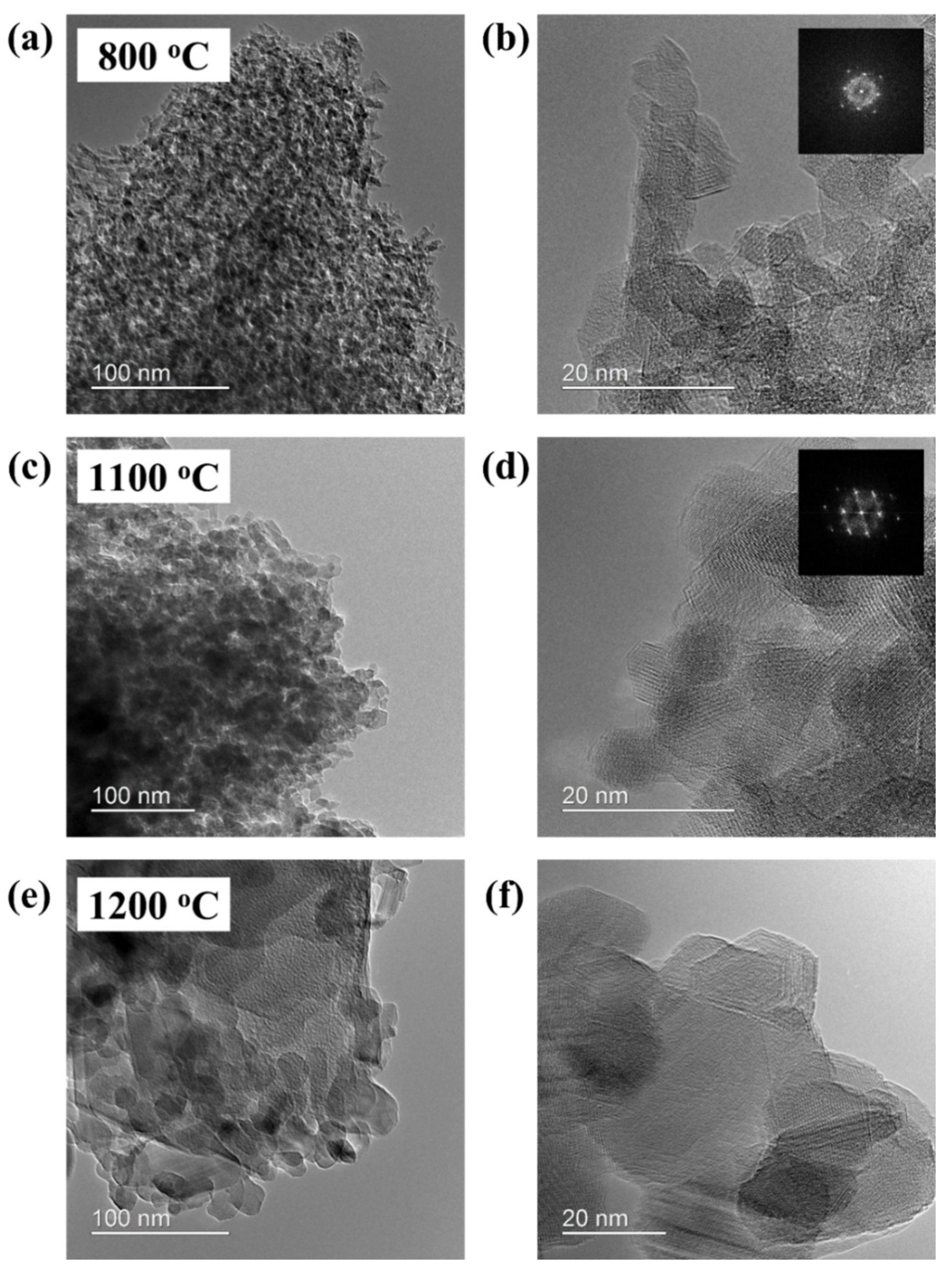
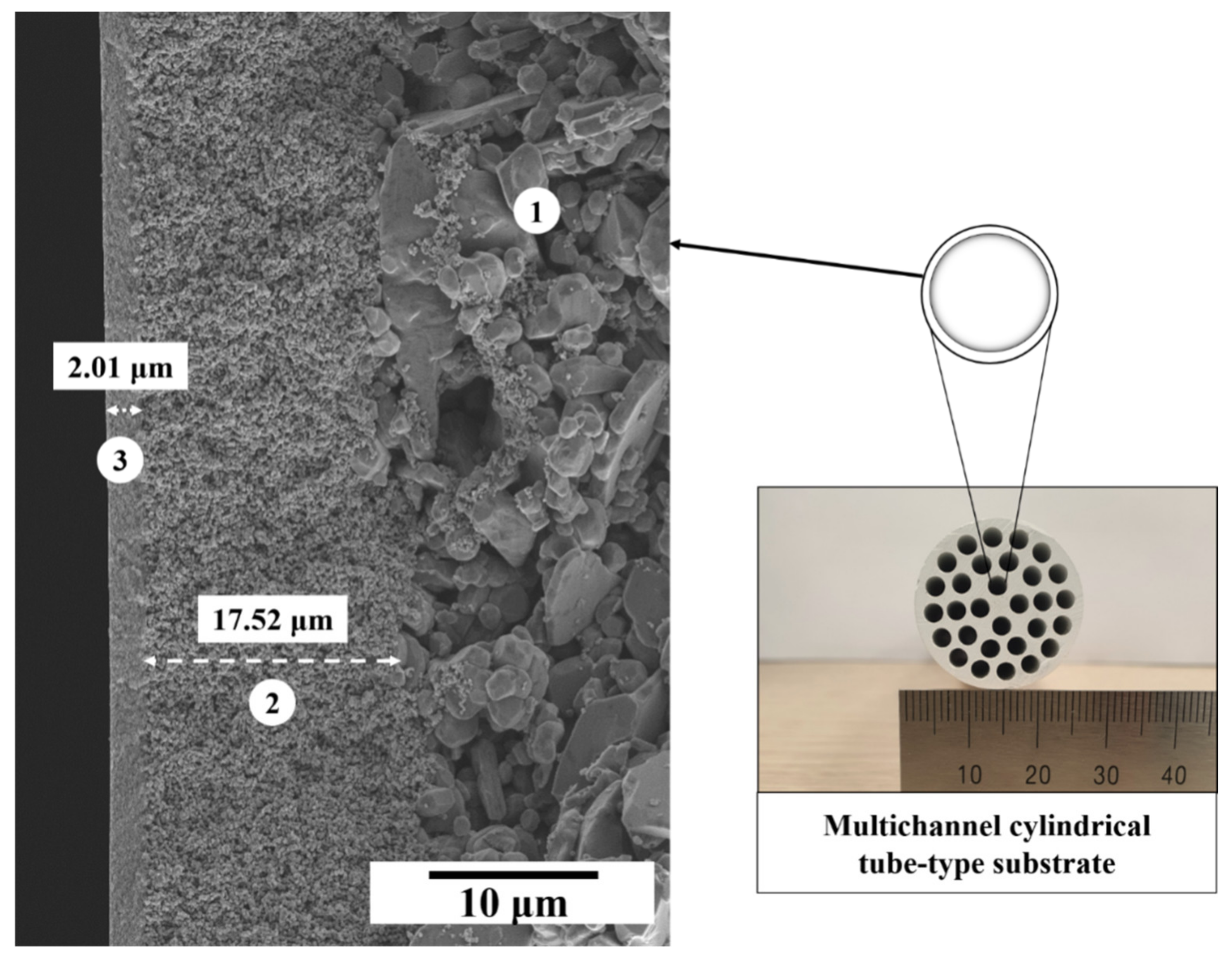
3.3. Microstructural Evolution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van der Bruggen, B.; Vandecasteele, C.; van Gestel, T.; Doyen, W.; Leysen, R. A review of pressure-driven membrane processes in wastewater treatment and drinking water production. Environ. Prog. 2003, 22, 46–56. [Google Scholar] [CrossRef]
- Khemakhem, M.; Khemakhem, S.; Ayedi, S.; Amar, R.B. Study of ceramic ultrafiltration membrane support based on phosphate industry subproduct: Application for the cuttlefish conditioning effluents treatment. Ceram. Int. 2011, 37, 3617–3625. [Google Scholar] [CrossRef]
- Hofs, B.; Ogier, J.; Vries, D.; Beerendonk, E.F.; Cornelissen, E.R. Comparison of ceramic and polymeric membrane permeability and fouling using surface water. Sep. Purif. Technol. 2011, 79, 365–374. [Google Scholar] [CrossRef]
- Facciotti, M.; Boffa, V.; Magnacca, G.; Jørgensen, L.B.; Kristensen, P.K.; Farsi, A.; König, K.; Christensen, M.L.; Yue, Y. Deposition of thin ultrafiltration membranes on commercial SiC microfiltration tubes. Ceram. Int. 2014, 40, 3277–3285. [Google Scholar] [CrossRef]
- Liu, W.; Wang, J. Alpha-Alumina Inorganic Membrane Support and Method of Making the Same. U.S. Patent Application No. 12/001,263, 19 June 2008. [Google Scholar]
- Ebrahimi, M.; Kerker, S.; Schmitz, O.; Schmidt, A.A.; Czermak, P. Evaluation of the fouling potential of ceramic membrane configurations designed for the treatment of oilfield produced water. Sep. Sci. Technol. 2018, 53, 349–363. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, Y.M.; Xu, Z.L.; Cao, Y.; Dong, Z.Q.; Shi, X.L. Preparation, characterization and solvent resistance of γ- Al2O3/α- Al2O3 inorganic hollow fiber nanofiltration membrane. J. Membr. Sci. 2016, 503, 69–80. [Google Scholar] [CrossRef]
- Ma, C.; Chang, Y.; Ye, W.; Duan, L.; Wang, C. Hexagon γ-alumina nanosheets produced with the assistance of supercritical ethanol drying. J. Supercrit. Fluids 2008, 45, 112–120. [Google Scholar] [CrossRef]
- Lin, Y.S.; de Vries, K.J.; Burggraaf, A.J. Thermal stability and its improvement of the alumina membrane top-layers prepared by sol-gel methods. J. Mater. Sci. 1991, 26, 715–720. [Google Scholar] [CrossRef]
- Li, J.G.; Sun, X. Synthesis and sintering behavior of a nanocrystalline α-alumina powder. Acta Mater. 2000, 48, 3103–3112. [Google Scholar] [CrossRef]
- Yuan, Q.; Yin, A.X.; Luo, C.; Sun, L.D.; Zhang, Y.W.; Duan, W.T.; Liu, H.C.; Yan, C.H. Facile synthesis for ordered mesoporous γ-aluminas with high thermal stability. J. Am. Chem. Soc. 2008, 130, 3465–3472. [Google Scholar] [CrossRef]
- Mathieu, Y.; Vidal, L.; Valtchev, V.; Lebeau, B. Preparation of γ- Al2O3 film by high temperature transformation of nanosized γ-AlOOH precursors. New J. Chem. 2009, 33, 2255–2260. [Google Scholar] [CrossRef]
- Lee, H.C.; Kim, H.J.; Rhee, C.H.; Lee, K.H.; Lee, J.S.; Chung, S.H. Synthesis of nanostructured γ-alumina with a cationic surfactant and controlled amounts of water. Microporous Mesoporous Mater. 2005, 79, 61–68. [Google Scholar] [CrossRef]
- IUPAC. Peptization. In Compendium of Chemical Terminology, 2nd ed.; McNaught, A.D., Wilkinson, A., Eds.; Blackwell Scientific Publications: Oxford, UK, 1997; ISBN 0-9678550-9-8. [Google Scholar] [CrossRef]
- Chen, X.Y.; Lee, S.W. pH-Dependent formation of boehmite (γ-AlOOH) nanorods and nanoflakes. Chem. Phys. Lett. 2007, 438, 279–284. [Google Scholar] [CrossRef]
- Chen, X.Y.; Zhang, Z.J.; Li, X.L.; Lee, S.W. Controlled hydrothermal synthesis of colloidal boehmite (γ-AlOOH) nanorods and nanoflakes and their conversion into γ- Al2O3 nanocrystals. Solid State Commun. 2008, 145, 368–373. [Google Scholar] [CrossRef]
- Thongdee, N.; Watcharamaisakul, S.; Golman, B. Effect of peptizing agent concentration on morphology of unsupported alumina membrane. Key Eng. Mater. 2018, 766, 71–76. [Google Scholar] [CrossRef]
- Raybaud, P.; Digne, M.; Iftimie, R.; Wellens, W.; Euzen, P.; Toulhoat, H. Morphology and surface properties of boehmite (γ-AlOOH): A density functional theory study. J. Catal. 2001, 201, 236–246. [Google Scholar] [CrossRef]
- Lee, B.H.; Lim, H.J.; Lee, I.P.; Ahn, C.Y. Effect of types of peptizing agents used for preparation of alumina sols on the properties of coating films. Korean Chem. Eng. Res. 2016, 54, 767–774. [Google Scholar] [CrossRef][Green Version]
- Wi, I.H.; Shin, D.W.; Han, K.S.; Kim, J.H.; Cho, W.S.; Hwang, K.T. Fabrication of boehmite-based UF ceramic membrane. J. Korean Ceram. Soc. 2014, 51, 337–343. [Google Scholar] [CrossRef]
- Schaep, J.; Vandecasteele, C.; Peeters, B.; Luyten, J.; Dotremont, C.; Roels, D. Characteristics and retention properties of a mesoporous γ-Al2O3 membrane for nanofiltration. J. Membr. Sci. 1999, 163, 229–237. [Google Scholar] [CrossRef]
- Kunde, G.B.; Yadav, G.D. Sol–gel synthesis and characterization of defect-free alumina films and its application in the preparation of supported ultrafiltration membranes. J. Sol-Gel Sci. Technol. 2016, 77, 266–277. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, W.; Lin, Y.; Cai, Y.; Qiu, M.; Fan, Y. Preparation of high-flux γ-alumina nanofiltration membranes by using a modified sol-gel method. Microporous Mesoporous Mater. 2015, 214, 195–203. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Wang, S.; Li, Y.; Zhai, Y. Synthesis of γ-alumina via precipitation in ethanol. Mater. Lett. 2008, 62, 3552–3554. [Google Scholar] [CrossRef]
- Riaz, S.; Shamaila, S.; Khan, B.; Naseem, S. Lower temperature formation of alumina thin films through sol-gel route. Surf. Rev. Lett. 2008, 15, 681–688. [Google Scholar] [CrossRef]
- Ha, J.H.; Abbas Bukhari, S.Z.; Lee, J.; Song, I.H. The membrane properties of alumina-coated alumina support layers and alumina-coated diatomite–kaolin composite support layers. Adv. Appl. Ceram. 2018, 117, 1–8. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, W.; Page, K.L.; Pearce, C.I.; Bowden, M.E.; Graham, T.R.; Shen, Z.; Li, P.; Wang, Z.; Kerisit, S.; et al. Size and Morphology Controlled Synthesis of Boehmite Nanoplates and Crystal Growth Mechanisms. Cryst. Growth Des. 2018, 18, 3596–3606. [Google Scholar] [CrossRef]
- He, Z.; Ng, T.C.A.; Lyu, Z.; Gu, Q.; Zhang, L.; Ng, H.Y.; Wang, J. Alumina double-layered ultrafiltration membranes with enhanced water flux. Colloids Surf. A Physicochem. Eng. Asp. 2020, 587, 124324. [Google Scholar] [CrossRef]
- Langford, J.I.; Wilson, A.J.C. Seherrer after Sixty Years: A Survey and Some New Results in the Determination of Crystallite Size. J. Appl. Crystallogr. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Gommes, C.J. Ostwald ripening of confined nanoparticles: Chemomechanical coupling in nanopores. Nanoscale 2019, 11, 7386–7393. [Google Scholar] [CrossRef]
- Qiu, M.; Feng, J.; Fan, Y.; Xu, N. Pore evolution model of ceramic membrane during constrained sintering. J. Mater. Sci. 2009, 44, 689–699. [Google Scholar] [CrossRef]
- Cha, M.; Boo, C.; Song, I.H.; Park, C. Investigating the potential of ammonium retention by graphene oxide ceramic nanofiltration membranes for the treatment of semiconductor wastewater. Chemosphere 2022, 286, 131745. [Google Scholar] [CrossRef]
- Shang, R.; Goulas, A.; Tang, C.Y.; Serra, X.d.; Rietveld, L.C.; Heijman, S.G.J. Atmospheric pressure atomic layer deposition for tight ceramic nanofiltration membranes: Synthesis and application in water purification. J. Membr. Sci. 2017, 528, 163–170. [Google Scholar] [CrossRef]
- Ren, J.; Li, Z.; Wong, F.S. A new method for the prediction of pore size distribution and MWCO of ultrafiltration membranes. J. Membr. Sci. 2006, 279, 558–569. [Google Scholar] [CrossRef]
- Wang, L.; Song, L. Flux decline in crossflow microfiltration and ultrafiltration: Experimental verification of fouling dynamics. J. Membr. Sci. 1999, 160, 41–50. [Google Scholar] [CrossRef]


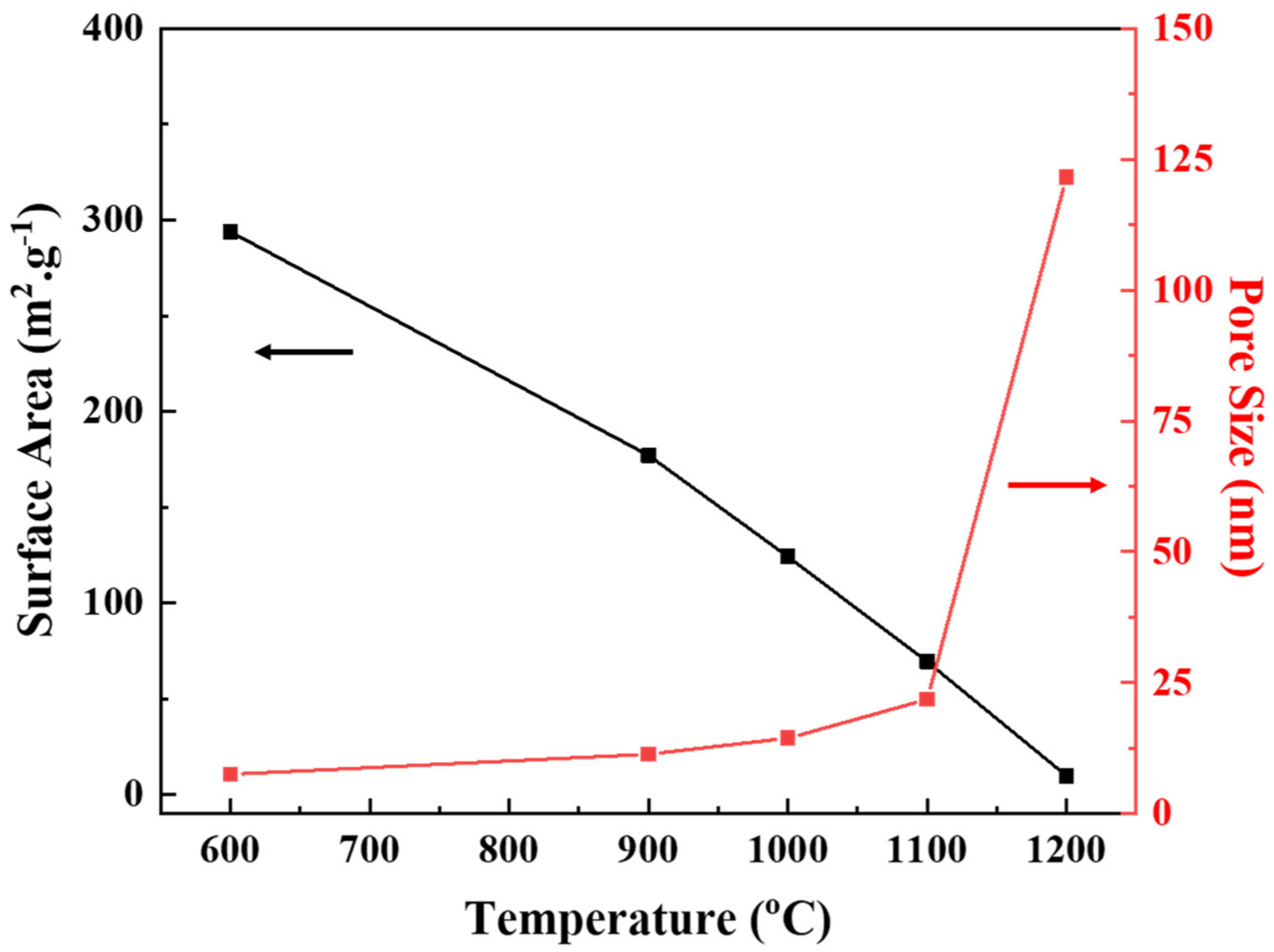

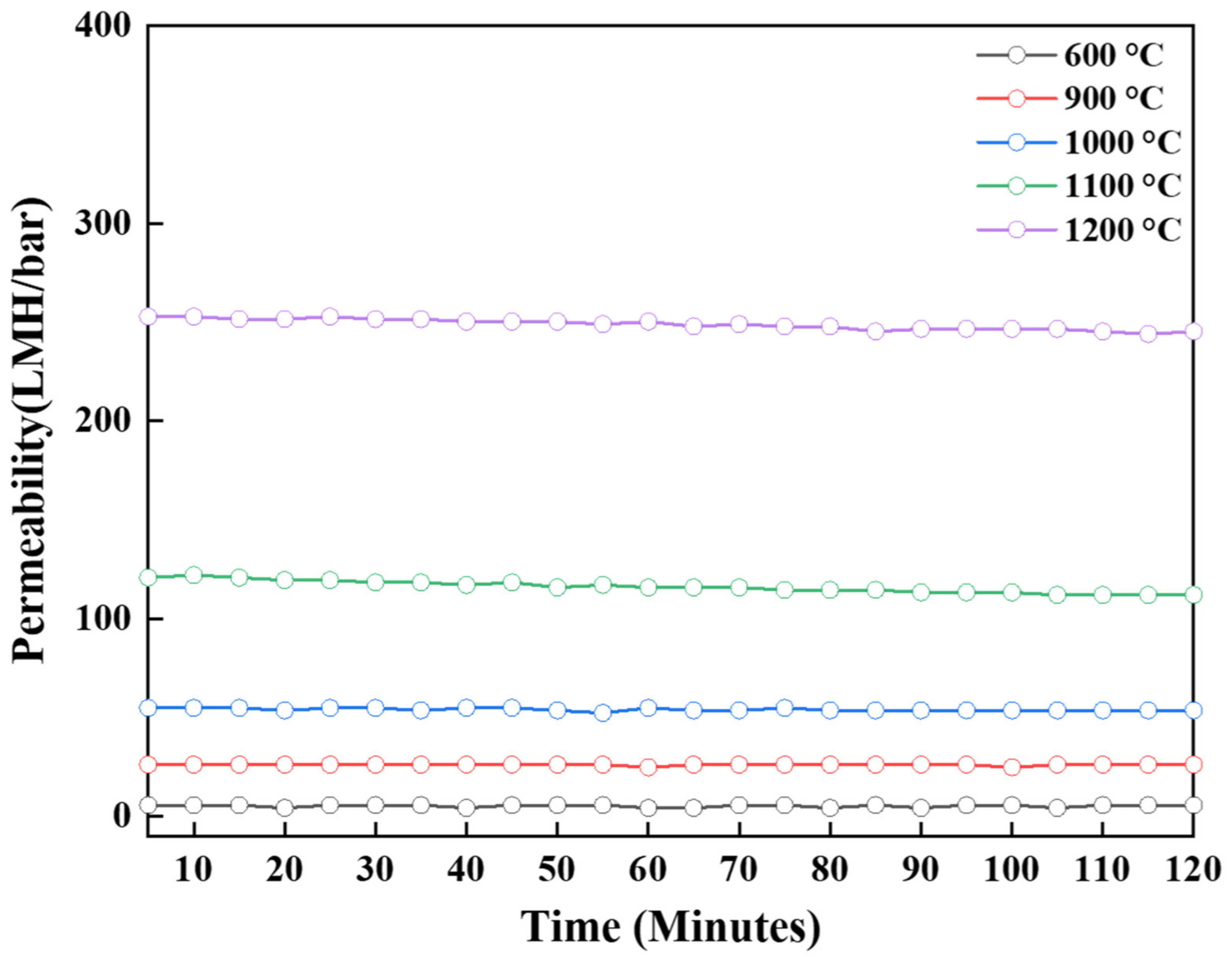
| Temperature (°C) | Phase | Specific Surface Area (m2/g) | Pore Size (nm) | Permeability (LMH/bar) |
|---|---|---|---|---|
| 600 | γ | 293.84 | 7.53 | 5.65 |
| 900 | γ + δ | 177.01 | 11.35 | 26.12 |
| 1000 | γ + θ | 124.17 | 14.43 | 53.55 |
| 1100 | α | 69.35 | 21.8 | 111.94 |
| 1200 | α | 9.66 | 121.68 | 245.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naseer, D.; Ha, J.-H.; Lee, J.; Park, C.; Song, I.-H. Effect of the Peptization Process and Thermal Treatment on the Sol-Gel Preparation of Mesoporous α-Alumina Membranes. Membranes 2022, 12, 313. https://doi.org/10.3390/membranes12030313
Naseer D, Ha J-H, Lee J, Park C, Song I-H. Effect of the Peptization Process and Thermal Treatment on the Sol-Gel Preparation of Mesoporous α-Alumina Membranes. Membranes. 2022; 12(3):313. https://doi.org/10.3390/membranes12030313
Chicago/Turabian StyleNaseer, Danyal, Jang-Hoon Ha, Jongman Lee, Chanhyuk Park, and In-Hyuck Song. 2022. "Effect of the Peptization Process and Thermal Treatment on the Sol-Gel Preparation of Mesoporous α-Alumina Membranes" Membranes 12, no. 3: 313. https://doi.org/10.3390/membranes12030313
APA StyleNaseer, D., Ha, J.-H., Lee, J., Park, C., & Song, I.-H. (2022). Effect of the Peptization Process and Thermal Treatment on the Sol-Gel Preparation of Mesoporous α-Alumina Membranes. Membranes, 12(3), 313. https://doi.org/10.3390/membranes12030313








