Abstract
This work presents an effective approach for manganese-doped Al2O3 ceramic membrane (Mn-doped membrane) fouling control by in-situ confined H2O2 cleaning in wastewater treatment. An Mn-doped membrane with 0.7 atomic percent Mn doping in the membrane layer was used in a membrane bioreactor with the aim to improve the catalytic activity toward oxidation of foulants by H2O2. Backwashing with 1 mM H2O2 solution at a flux of 120 L/m2/h (LMH) for 1 min was determined to be the optimal mode for in-situ H2O2 cleaning, with confined H2O2 decomposition inside the membrane. The Mn-doped membrane with in-situ H2O2 cleaning demonstrated much better fouling mitigation efficiency than a pristine Al2O3 ceramic membrane (pristine membrane). With in-situ H2O2 cleaning, the transmembrane pressure increase (ΔTMP) of the Mn-doped membrane was 22.2 kPa after 24-h filtration, which was 40.5% lower than that of the pristine membrane (37.3 kPa). The enhanced fouling mitigation was attributed to Mn doping, in the Mn-doped membrane layer, that improved the membrane surface properties and confined the catalytic oxidation of foulants by H2O2 inside the membrane. Mn3+/Mn4+ redox couples in the Mn-doped membrane catalyzed H2O2 decomposition continuously to generate reactive oxygen species (ROS) (i.e., HO• and O21), which were likely to be confined in membrane pores and efficiently degraded organic foulants.
1. Introduction
Ceramic membranes have been widely used in the membrane bioreactor (MBR) for wastewater treatment [1]. However, membrane fouling remains a challenge for the wide application of ceramic membrane bioreactors [2]. During membrane filtration, organic foulants in wastewater gradually accumulate on the ceramic membrane surface and block membrane pores, causing severe membrane fouling [3]. To control membrane fouling, membrane cleaning is an essential part of the maintenance of MBR (e.g., backwashing, forward washing and ex-situ cleaning). Fouled membranes are commonly immersed in sodium hypochlorite (NaClO) solutions to remove foulants from membranes in municipal wastewater treatment [4]. However, ex-situ cleaning requires long suspension of MBR, large consumption of chemicals and complex operations. With the aim to reduce the frequency of ex-situ cleaning, in-situ chemical cleaning via backwashing with chemical solutions is employed during filtration [5]. However, traditional in-situ backwashing with chemical agents (e.g., NaClO) poses a high risk of harming the microorganisms in active sludge when the oxidants are backwashed into a mixed liquor [6]. Thus, it is interesting to explore an approach to effectively control membrane fouling but with no harm to microbes and requiring only a short stop time.
On the other hand, membrane surface modification has been studied as a means to mitigate membrane fouling [7,8,9]. With the advantages of strong catalytic activity and a low price, manganese oxides have been considered promising additives for membrane modification [10,11,12]. Byun et al. reported that a ZrO2 ceramic membrane coated with manganese oxide on its surfaces demonstrated a higher anti-fouling tendency during the filtration of real surface water than membranes coated with TiO2 or Fe2O3 [13]. Moreover, manganese oxides have been studied as catalysts and activators for oxidants, such as ozone (O3), peroxymonosulfate (PMS) and hydrogen peroxide (H2O2), in advanced oxidation processes to generate reactive oxygen species (e.g., hydroxyl radical (HO•), superoxide radical (O2•−), and sulfate radical (SO4•−)), which further promote the removal of organic foulants [14,15,16]. Compared with O3 and PMS, H2O2 has a higher water solubility than O3 and constitutes a green and environmentally friendly oxidant because it releases only water as its by-product [17]. Thus, manganese oxide-doped Al2O3 ceramic membrane coupling with in-situ H2O2 cleaning is expected to enhance the removal of organic foulants, providing a novel strategy to control membrane fouling.
Another issue regarding in-situ chemical cleaning is the potential harm from the oxidants to the functional bacteria in activated sludge [18]. In order to avoid the undesirable harm to activated sludge, H2O2 solution could be pumped into the membrane through the inner cavity to control the catalytic oxidation of organic foulants occurring within membrane pores. Confined spaces in membrane pores could achieve catalytic performances that are orders of magnitude faster than those obtained in the bulk phase [19]. We found that the ozone decomposition rate inside the membrane pores was about 428 times faster than that in the bulk phase, which was confirmed as a confinement effect of nano-scale membrane pores [20]. Similarly, it is believed that the confinement effect toward catalytic decomposition of H2O2 within membrane pores could also promote the oxidation of foulants and enhance the cleaning efficiency. Overall, doping manganese oxide in an Al2O3 ceramic membrane active layer is expected to bring about a double-win effect, both enhancing the membrane antifouling performance and promoting fouling mitigation via catalytic oxidation of organic foulants by H2O2 within membrane pores.
In this study, an Al2O3 ceramic membrane doped with Mn2O3 in the membrane active layer (Mn-doped membrane) was applied in MBR for the treatment of real municipal wastewater. The fouling mitigation behavior of the Mn-doped membrane during in-situ H2O2 cleaning confined in membrane pores through the inner cavity was investigated and compared with the conventional Al2O3 ceramic membrane (pristine membrane). Furthermore, we discuss the fouling mitigation mechanisms in terms of surface properties, catalytic activity and the confinement effect of Mn-doped catalytic membranes.
2. Materials and Methods
2.1. Ceramic Membranes and Characterizations
The manganese-doped Al2O3 ceramic membrane (Mn-doped membrane) and Al2O3 ceramic membrane (pristine membrane) were provided by Shenzhen Huayuan Environmental Technology Co., Ltd, Shenzhen, China. Both membranes were composed of a 0.36 mm-thick active layer and 1.1 mm-thick supporting layer. The substrate (supporting layer) of both the Mn-doped membrane and the pristine membrane was made of α-Al2O3 and SiO2. The membrane active layer of the Mn-doped membrane was made of 2% Mn2O3 and 98% α-Al2O3. The pristine membrane was made by the same procedure, without the addition of any manganese oxides.
The surface morphologies and elemental compositions of ceramic membranes were analyzed by a scanning electron microscope combined with an energy dispersive spectrometer (SEM-EDS, SU8010, Hitachi, Tokyo, Japan). The XPS spectra of membrane surfaces were detected by X-ray photoelectron spectroscopy (XPS, PHI 5000 Versa Probe II, ULVAC-PHI, Chigasaki, Japan) using an Al Kα X-ray source.
Catalytic activities of the pristine and Mn-doped membranes toward H2O2 were characterized by the decomposition efficiency of H2O2. To do so, 1 L of 1 mM H2O2 solution was circularly filtrated with ceramic membranes under a membrane flux of 60 LMH. The catalytic stability of membranes was analyzed by cycle tests. For each cycle test, membranes were cleaned by being immersed in 1 L NaClO solution (1000 ppm available chlorine) for 24 h, then immersed in 1 L pure water for another 24 h and finally dried in an oven at 105 °C before the tests. All cycle tests were run in duplicate under a room temperature ranging from 25–28 °C.
The H2O2 concentration was analyzed by the spectrophotometric-iodide method [21]. Free radicals generated in 1 mM H2O2 solution catalyzed by the pristine and Mn-doped membranes were detected by electron paramagnetic resonance (EPR, BRUKER ER070, Karlsruhe, Germany) using 5,5-dimethyl-1-pyrroline N-oxide (DMPO) as the spin trapping agent [22].
2.2. Membrane Bioreactor Filtration Tests
2.2.1. Experimental Setup
As shown in Figure 1, the MBR system consisted of membrane modules, an air aerator and pumps for feed, permeation and cleaning. The membrane tank was 20 cm × 8 cm × 50 cm (L × W × H) and the active volume was 8 L. The surface area of the ceramic membrane was 0.044 m2. The ceramic membrane had two outlets in the diagonal. One was connected with the permeation pump and the other was connected with the chemical feeding pump. The latter outlet was closed during membrane filtration and opened during the chemical cleaning processes. During membrane filtration, an aerator provided air aeration for sufficient mixing of liquor in the membrane tank with an aeration rate of 21 L/h.
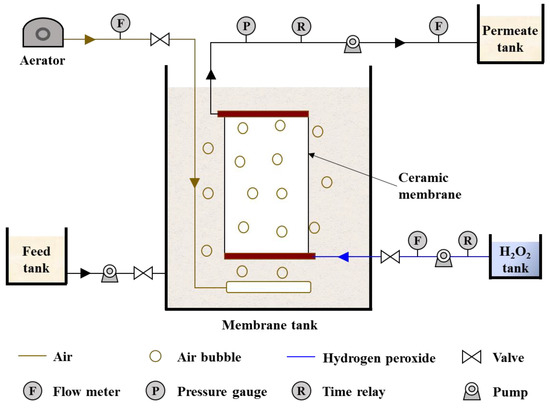
Figure 1.
Schematic diagram of experimental setup.
2.2.2. Optimal In-Situ H2O2 Cleaning Mode Tests
To prevent H2O2 from entering the membrane tank and damaging the activated sludge during in-situ H2O2 cleaning, the optimal dosing mode of H2O2 was explored. The effect of the in-situ H2O2 cleaning mode (i.e., backwash flux and H2O2 dosage) on the concentration of H2O2 in the membrane tank was studied in pure water tests (see experimental setup in Figure 1). The pristine or Mn-doped membranes were vertically fixed within the membrane tank. Prior to membrane filtration, 6 L of pure water (same volume with the fed mixed liquor during membrane fouling test) was fed into the membrane tank. Membrane filtration with pure water was conducted under a flux of 60 LMH for more than 10 min prior to backwashing. After the membrane inner cavity was fully filled with pure water, H2O2 backwash tests were conducted under different modes (i.e., backwashing of H2O2 solution with dosages of 1, 3 or 5 mM at a flux of 120 LMH for 1 min or backwashing of 1 mM H2O2 solution at a flux of 120 or 180 LMH for 1 min). H2O2 solution was backwashed into membranes through the inner cavity by a cleaning pump, while the feed and permeation pumps were closed. At 0, 1, 2, 5, 10, 15, 20 and 30 min of backwashing, water samples were collected from areas near both sides of the ceramic membrane surface using the five-spot sampling method, and the concentration of H2O2 in the water samples was measured. All experiments were conducted in triplicate. In this study, backwashing with 1 mM H2O2 at a flux of 120 LMH for 1 min was determined to be the optimal dosing mode, which resulted in undetected H2O2 in the membrane tank during backwashing.
2.2.3. Fouling Mitigation Tests
The feed of the ceramic membrane reactor was the supernatant of fresh mixed liquor sampled from the aerobic tank in a municipal wastewater treatment plant in Shenzhen, China. The mixed liquid suspended solids (MLSS) of the initial mixed liquor fed into the membrane tank was 6.8 ± 0.1 g/L and the zeta potential was −12.9 ± 1.0 mV. The sludge volume after 30 min sedimentation (SV30) of active sludge was 38.8 ± 1.5%.
Membrane filtration was conducted at a constant flux of 60 LMH with an on/off ratio of 9 min/1 min. The on/off filtration mode was accurately controlled by time relays. A pressure gauge measured and recorded the real-time transmembrane pressure (TMP).
For the membrane fouling mitigation, two kinds of intermittent in-situ cleaning strategies (clean with H2O or 1 mM H2O2) were applied to evaluate the mitigation efficiencies of the pristine membrane and Mn-doped membrane. H2O or 1 mM H2O2 solution was regularly pumped into ceramic membrane pores every six hours under a flux of 120 LMH for 1 min, and then H2O or H2O2 reacted with organic foulants within membrane pores for 10 min after each clean. TMP variations during the membrane filtration were recorded and used as the indicator of membrane fouling. The TMP recovery ratio (R) after each clean was calculated using Equation (1). All filtration experiments ran for 24 h in duplicate under a room temperature ranging from 20–25 °C.
After the filtration experiment, the fouled membranes were rinsed with 100 mL ultrapure water, and then soaked in 500 mL NaClO solution (1000 ppm available chlorine) for 24 h [23,24]. The size-fractionated (≤0.45 μm) cleaning solutions were then analyzed for dissolved organic carbon (DOC). In addition, membrane permeates were collected every six hours during membrane filtration for analysis of water quality (i.e., turbidity, DOC, total nitrogen and total phosphorus). Mixed liquor properties (i.e., MLSS, SV30, zeta potential, specific oxygen uptake rate (SOUR) and DOC) were analyzed after membrane filtration. Detailed analytic methods can be found in our recent papers [23,24].
3. Results and Discussions
3.1. Characterization of Ceramic Membranes
In this study, the Mn element was successfully doped into the membrane layer of the Mn-doped membrane with an atomic percent of 0.7% (Figure 2). The element compositions in supporting layers of the pristine membrane and Mn-doped membrane were almost the same. The average pore sizes of the pristine and Mn-doped membrane active layers were 780 nm (Figure S1a) and 792 nm (Figure S1b), respectively. As shown in Figure 3, the Mn 2p3/2 spectrum showed two distinct peaks at 641.6 eV and 643.2 eV in the Mn-doped membrane layer, which were consistent with the binding energy of Mn(III) and Mn(IV) [25,26,27]. This demonstrated that the doped manganese in the Mn-doped membrane existed as two oxidation states (Mn3+ and Mn4+). The existence of Mn3+/Mn4+ couples would be beneficial for the catalytic activity of Mn-doped membranes [28].
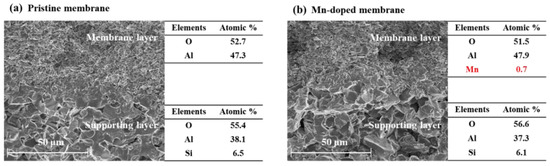
Figure 2.
The cross-section morphologies and element compositions of the pristine membrane and Mn-doped membrane.

Figure 3.
XPS spectra of Mn 2p of the pristine and Mn-doped membranes.
3.2. Catalytic Activity of Ceramic Membranes in H2O2 Decomposition
Figure 4 shows the variation in H2O2 concentration with respect to filtration time with the pristine and Mn-doped membranes in a circular mode. As shown in Figure 4a, less than 1% of H2O2 was decomposed throughout two hours of filtration by the pristine membrane. This indicated that the pristine membrane caused negligible improvement in H2O2 decomposition because of the non-catalytic activity of Al2O3 and SiO2 powders toward H2O2 decomposition (Figure S2a).
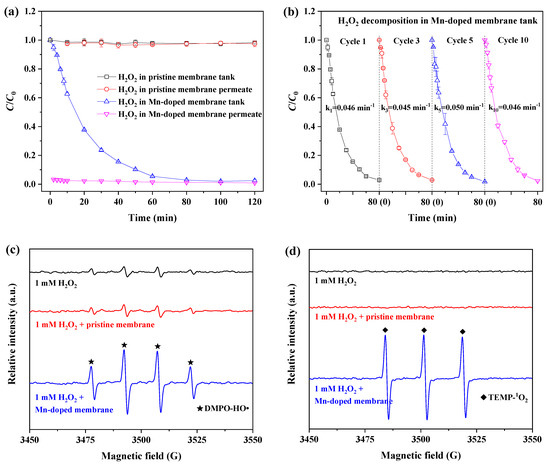
Figure 4.
Catalytic activity of (a) fabricated ceramic membranes in H2O2 decomposition and (b) an Mn-doped membrane cleaned with 1000 ppm NaClO solution (operating conditions: C0 = [H2O2]0 = 1 mM; C is the concentration of H2O2 in the membrane tank or permeation; membrane flux = 60 LMH); EPR spectra of (c) DMPO-HO• and (d) TEMP-1O2 in 1 mM H2O2 solution catalyzed by a pristine or Mn-doped membrane.
In comparison, more than 98% of H2O2 was constantly decomposed in permeation of the Mn-doped membrane, and the concentration of H2O2 was lower than 0.03 mM. The concentration of H2O2 in the tank with the Mn-doped membrane gradually decreased with increasing filtration time, and the decomposition efficiency of H2O2 solution reached 94.6% after one hour of filtration and 97.5% after two hours of filtration. Obviously, the Mn-doped membrane exhibited excellent catalytic activity toward H2O2 decomposition. This was consistent with the finding that Mn2O3 power had very high catalytic activity when it came to H2O2 decomposition (Figure S2b). Moreover, the catalytic activity of the Mn-doped membrane for H2O2 decomposition did not deteriorate after chemical cleaning with NaClO (1000 ppm) (Figure 4b). The decomposition of H2O2 in the tank with the Mn-doped membrane followed first-order kinetics, and the average observed rate constant was 4.6 × 10−2 min−1. There was no significant difference in the observed rate constants for the first, third, fifth and tenth cycle tests. During the chemical cleaning with NaClO (1000 ppm, pH > 9), the concentration of Mn in the NaClO immersion solution was below the detection limit (0.001 mg/L) of flame atomic absorption spectrometry. Mn leaching was not significantly observed during membrane chemical cleaning with NaClO solution at an alkaline pH. These results indicate the high catalytic activity and stability of the Mn-doped membrane when it came to H2O2 decomposition.
It has been reported that ROS are generated from H2O2 decomposition. EPR tests were conducted to confirm the generation of ROS from H2O2 decomposition catalyzed by the pristine and Mn-doped membranes. Four characteristic peaks of DMPO-HO• with an intensity ratio of 1:2:2:1 (Figure 4c) were observed in 1 mM H2O2 solution without catalysis of the pristine or Mn-doped membranes as a result of the self-decomposition of H2O2 at a neutral pH [29,30]. There was no obvious difference in the peak intensities of the DMPO-HO• spectra between the H2O2 solutions with and without the catalysis of the pristine membrane. This result confirmed that the pristine membrane had no catalytic effect on promoting the generation of ROS, consistent with the results of H2O2 decomposition (Figure 4a).
The peak intensities of DMPO-HO• in 1 mM H2O2 solution catalyzed by the Mn-doped membrane were much higher than those in H2O2 solutions with and without catalysis of the pristine membrane (Figure 4c). Moreover, three characteristic peaks of TEMP-1O2 with an intensity ratio of 1:1:1 were significantly observed in a 1 mM H2O2 solution catalyzed by the Mn-doped membrane [31] (Figure 4d), while these were not detectable in 1 mM H2O2 solutions with or without catalysis of the pristine membrane. These results demonstrated the generation and coexistence of HO• and 1O2 in H2O2 solution catalyzed by the Mn-doped membrane [32,33]. This finding directly suggested that Mn doping is a good strategy for enhancing ceramic membrane catalytic activity toward H2O2, thus decomposing H2O2 into more powerful ROS, namely HO• or 1O2 [34].
3.3. Determination of Optimal H2O2 Cleaning Mode for Filtration
As shown in Figure 5, residual H2O2 was detected in the membrane tank when the pristine and Mn-doped membranes were backwashed with H2O2 solution for 1 min under 3 or 5 mM H2O2/120 LMH or 1 mM H2O2/180 LMH. This result indicated that residual H2O2 entered the membrane tank through membrane pores after over-dosage of H2O2, and thus resulted in an increasing H2O2 concentration in the membrane tank. The H2O2 concentration in the membrane tank increased with increasing H2O2 dosage and backwash flux, resulting in an increased risk of harm to activated sludge. Under the same dosing mode of H2O2, H2O2 concentrations in the Mn-doped membrane tank were always much lower than those in the pristine membrane tank. The lower H2O2 concentration in the Mn-doped membrane tank was attributed to the high catalytic activity of the Mn-doped membrane toward H2O2 decomposition (Figure 4). This result demonstrated that Mn doping could reduce the risk of damaging the activated sludge during in-situ H2O2 backwashing by accelerating H2O2 decomposition within the Mn-doped membrane pores.
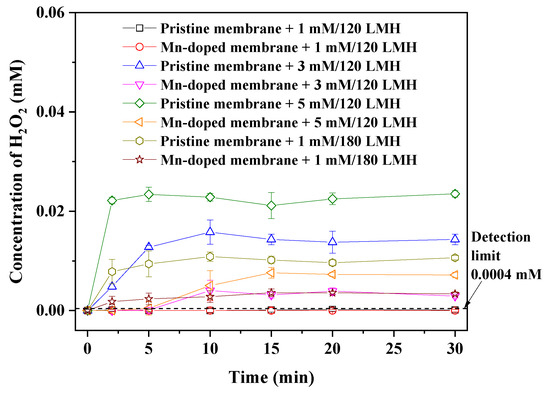
Figure 5.
Effect of H2O2 backwash mode on the concentration of H2O2 in the membrane tank.
When the pristine and Mn-doped membranes were backwashed with 1 mM H2O2 at a flux of 120 LMH for 1 min, the concentrations of H2O2 in the pristine and Mn-doped membrane tanks were all below the detection limit (0.0004 mM) of the spectrophotometric-iodide method. Obviously, almost all H2O2 was confined within the membrane under this dosing mode, causing an undetectable change in the H2O2 concentration in the membrane tank (volume of 6 L). Therefore, with the aim to protect activated sludge from being damaged by residual H2O2 in the membrane tank, we conducted backwashing with 1 mM H2O2 at a flux of 120 LMH for 1 min as the optimal dosing mode for in-situ confined H2O2 cleaning.
3.4. Membrane Fouling Mitigation Performance with and without In-Situ H2O2 Cleaning
As seen from Figure 6a, the Mn-doped membrane demonstrated an obviously lower TMP increase rate than the pristine membrane. The TMP of the pristine membrane with H2O cleaning rapidly increased to −42.1 kPa after 24 h of filtration, while the TMP of the Mn-doped membrane with H2O cleaning only increased to −30.4 kPa. After membrane filtration, the total DOC content in the fouling layers of the Mn-doped membrane with H2O cleaning (11.0 mg) was also lower than that of the pristine membrane with H2O cleaning (12.8 mg) (Figure 6c). These results demonstrated the improved antifouling properties of the Mn-doped membrane. With H2O2 cleaning, the TMP of the pristine membrane increased to −40.5 kPa after 24-h filtration, while the TMP of the Mn-doped membrane increased to −25.1 kPa. The total DOC content in the fouling layers decreased from 12.4 mg (the pristine membrane with H2O2 cleaning) to 9.8 mg (the Mn-doped membrane with H2O2 cleaning) (Figure 6c). This suggested that intermittent in-situ H2O2 cleaning efficiently controlled fouling of the Mn-doped membrane but had a slight positive effect on fouling mitigation of the pristine membrane.
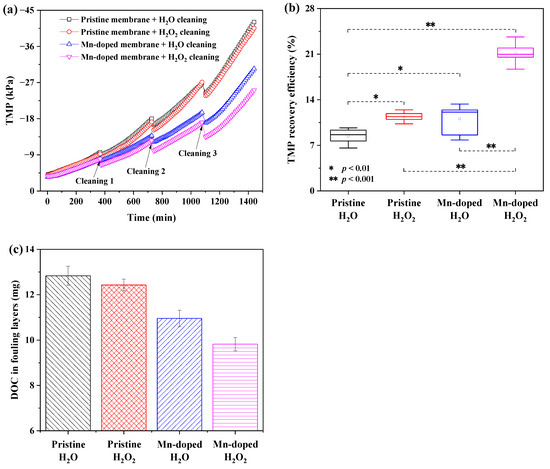
Figure 6.
Membrane fouling mitigation behaviors of different membranes coupling with different in-situ cleaning methods. (a) TMP during filtration and cleaning; (b) TMP recovery efficiencies after cleaning; (c) the total content of DOC in membrane fouling layers after 24 h filtration.
The TMP recovery ratio (R) achieved by each cleaning mode was calculated to investigate the efficiencies of different cleaning strategies. As shown in Figure 6b, the Mn-doped membrane exhibited the highest average TMP recovery efficiency (21.1 ± 1.4%) with H2O2 cleaning, compared to that with H2O cleaning (11.1 ± 2.5%), the pristine membrane with H2O (8.4 ± 1.2%) or H2O2 cleaning (11.4 ± 0.8%). The average TMP recovery efficiencies of membranes with H2O2 cleaning were significantly higher (p < 0.01) than those of membranes with H2O cleaning, indicating that oxidation by H2O2 successfully removed foulants from membranes. Moreover, the Mn-doped membrane with H2O2 cleaning achieved 85.1% higher TMP recovery efficiency than the pristine membrane with H2O2 cleaning. Obviously, Mn doping in the Mn-doped membrane catalyzed the oxidation of organic foulants by H2O2 within the membrane. It is interesting to note that the average TMP recovery efficiency of the Mn-doped membrane with H2O cleaning (11.1 ± 2.5 %) and that of the pristine membrane with H2O2 cleaning (11.4 ± 0.8%) had no significant difference (Figure 6b), but the overall TMP increase and the total DOC content in membrane fouling layers of the former (ΔTMP = 27.7 kPa in 24 h, the total content of DOC = 11.0 mg) were much lower than of the latter (ΔTMP = 37.3 kPa, the total content of DOC = 12.4 mg). This further proved that antifouling properties of membranes were more critical than the cleaning strategy for fouling mitigation when it came to membranes without catalytic activity toward H2O2.
It is worth noting that the H2O2 concentrations in both the pristine membrane and Mn-doped membrane tank were below the detection limit (<0.0004 mM) after in-situ H2O2 cleaning. This result demonstrated that in-situ H2O2 cleaning with the optimal mode of 1 mM H2O2 at a flux of 120 LMH for 1 min caused an undetectable change in the H2O2 concentration in the membrane tank, which was consistent with the results of pure water tests (Figure 5). Moreover, there was no obvious difference of mixed liquor properties (i.e., DOC, MLSS, SV30, zeta potential, SOUR, pH) between four membrane bioreactors tested with H2O or H2O2 cleaning (Table S1). This verified that in-situ H2O2 cleaning with the optimal mode did not affect the mixed liquor properties in the membrane bioreactor. Activated sludge of MBRs was well-protected from being damaged by H2O2. The water quality (i.e., turbidity, DOC, total nitrogen and total phosphorus) of permeation (Figure S3) also showed no significant difference between the pristine membrane and Mn-doped membrane with H2O or H2O2 cleaning.
Overall, coupling the Mn-doped membrane and intermittent in-situ H2O2 cleaning achieved the best fouling mitigation performance with the lowest TMP increase and the highest TMP recovery efficiency, causing no harm to the activated sludge.
3.5. Fouling Mitigation Mechanisms of Mn-Doped Membrane with In-Situ H2O2 Cleaning
3.5.1. Enhanced Antifouling Properties of Mn-Doped Membrane
All the results indicated that the Mn-doped membrane exhibited a better antifouling performance than the pristine membrane during filtration of real municipal wastewater. This was attributed to the modified surface properties after Mn doping in the membrane layer, including an intensified electric charge and heightened hydrophilicity [35]. In this study, the pristine membrane layer consisted of Al2O3 that was positively charged at pH 7.0, while the Mn-doped membrane layer was doped with Mn2O3 that was negatively charged at the same pH. Mn doping changed the membrane surface charge from positive (pristine membrane) to negative (Mn-doped membrane). Organic foulants, especially biopolymers, were the main contributors to membrane fouling as a result of the high rejection of biopolymers by the pristine and Mn-doped membranes (Figures S4 and S5). Accordingly, the interactions between organic foulants and the ceramic membrane surface changed significantly after Mn doping. The negatively charged surface of the Mn-doped membrane changed the electrostatic attraction force between organic foulants and the pristine membrane to an electrostatic repulsion force [36,37,38]. This inhibited the adsorption and accumulation of organic foulants on the Mn-doped membrane surfaces and further in the pores. Moreover, the pure water contact angle of the Mn-doped membrane (24.1°) was 24.5% lower than that of the pristine membrane (31.9°) (Figure S6). This meant better surface hydrophilicity was achieved by Mn doping. This was in agreement with the analysis of the O 1s spectrum (Figure S7), which showed a greater amount of hydroxyl groups in the Mn-doped membrane. The increase of hydrogen-bond interactions between hydroxyl groups and water made the Mn-doped membrane more hydrophilic [39,40]. Higher hydrophilicity of the Mn-doped membrane weakened the hydrophobic interaction between organic compounds and membrane surface [41,42]. The biopolymer rejection rate by the Mn-doped membrane (49.0%) was lower than that by the pristine membrane (53.9%). Subsequently, fewer organic foulants attached to membrane/pore surfaces (Figure 6c) during membrane filtration, leading to a lower TMP increase rate and mitigating the Mn-doped membrane fouling [13,43]. Usually, hydrophilic membranes require a lower pressure than hydrophobic membranes to obtain the same permeation flux [44,45,46,47]. This is consistent with the fact that the operation TMP of the Mn-doped membrane was lower than that of the pristine membrane at the same flux (Figure S8). Therefore, with a lower operation TMP and slower TMP increase rate, the final TMP of the Mn-doped membrane with H2O cleaning (−30.4 kPa) was 27.8% lower than that of the pristine membrane with H2O cleaning (−42.1 kPa) after 24 h of filtration of real municipal wastewater.
3.5.2. Intensified Catalytic Activity of Mn-Doped Membrane toward H2O2 Decomposition
Coupling the Mn-doped membrane with in-situ H2O2 cleaning exhibited the highest TMP recovery after H2O2 cleaning. This was attributed to the enhanced catalytic activity toward H2O2 decomposition inside the Mn-doped membrane. The existence of Mn3+/Mn4+ couples (i.e., reversible transition between Mn3+ and Mn4+) in the Mn-doped membrane layer (Figure 3) played a crucial role in improving the catalytic activity of the membranes toward H2O2 decomposition and generation of ROS (i.e., HO• and 1O2) [15,28,48]. When H2O2 solution was pumped into the Mn-doped membrane, available Mn3+/Mn4+ couples at the manganese-based catalyst surface effectively catalyzed the decomposition of H2O2 (>98% of 1 mM H2O2 decomposed inside membrane pores (Figure 4a,b)) to continuously produce ROS (i.e., HO• and 1O2 (Figure 4c,d)) (see Equations (3) and (4) in Figure 7) [14,28,33,48]. The oxidizing powers of generated ROS, especially HO•, toward organic matters are much stronger than that of H2O2 [49,50]. The adsorbed organic foulants can be efficiently oxidized by generated ROS into smaller molecules and finally removed from the Mn-doped membrane. Therefore, the total DOC content in the fouling layers of the Mn-doped membrane decreased from 11.0 mg (with H2O cleaning) to 9.8 mg (with H2O2 cleaning) (Figure 6c). However, the pristine membrane consisted of Al2O3 and SiO2 that had no catalytic activity toward H2O2 decomposition (Figure S2a). The decomposition efficiency of H2O2 with the pristine membrane was very low (<1% within two hours) (Figure 4a) and led to a very limited amount of HO• (Figure 4c) mostly from the self-decomposition of 1 mM H2O2 (see Equation (2) in Figure 7). Consequently, the low decomposition rate of H2O2 inside the pristine membrane largely limited the oxidation removal efficiency of organic foulants of the fouled pristine membrane during in-situ H2O2 cleaning with a short stop time (10 min). The total DOC content in the fouling layers of the pristine membrane only decreased from 12.8 mg (with H2O cleaning) to 12.4 mg (with H2O2 cleaning) (Figure 6c). Therefore, the TMP recovery efficiency of the Mn-doped membrane after H2O2 cleaning was significantly higher than that of the pristine membrane.
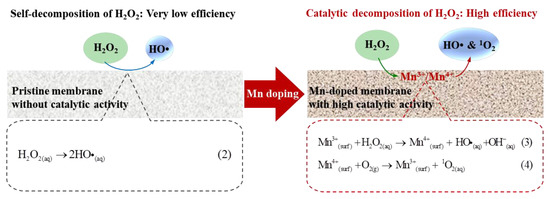
Figure 7.
Schematic diagram of H2O2 decomposition catalyzed by the pristine membrane and Mn-doped membrane.
3.5.3. Confined Catalytic Oxidation Performance within Mn-Doped Membrane Pores
In addition, H2O2 solutions were pumped into the membrane and confined inside the membrane pores almost without detectable residual H2O2 entering the membrane tank. Limited reaction space within the Mn-doped membrane pores further enhanced the oxidation of organic foulants by enriched ROS [51]. It is well-known that the lifetimes of HO• (<1 μs) and 1O2 (~3 μs) in water are extremely short, resulting in elimination before they react with pollutants [52]. In heterogeneous catalytic reaction systems, the concentration of ROS is the highest on the catalyst surface and then rapidly decreases with increasing diffusion length [19]. Assuming the lifetimes (τ) of HO• and 1O2 are 1 μs and 3 μs, respectively, at a neutral pH [53], the diffusion lengths (λL) of HO• and 1O2 in the aqueous phase should be ~96 nm and ~159 nm, respectively, as calculated by (where D is the diffusion coefficient; and ) [54]. Thus, the oxidation efficiency of organic foulants by generated ROS would be significantly improved if the reaction space were restricted far below the diffusion length scale of ROS. This phenomenon was reported in previous studies as a spatial confinement effect [19,20].
Organic foulants adsorbed on the membrane/pore surfaces during membrane filtration. When H2O2 was pumped into membrane pores through the inner cavity, ROS (HO• and 1O2) were generated on the Mn-doped membrane/pore surfaces and were restricted within the Mn-doped membrane pores (average pore size of 792 nm (Figure S1b)). The oxidation of organic foulants on the Mn-doped membrane/pore surfaces occurred once ROS were generated. As shown in Figure 8, the confined reaction space within the Mn-doped membrane pores shortened the diffusion lengths of ROS and prevented their elimination, leading to enriched ROS within the membrane pores. Because of the substantially reduced diffusion length and concentrated reactants, ROS reacted with organic foulants within membrane pores with much higher efficiency than they did in the bulk phase [19,55]. In this way, organic foulants were efficiently removed, and membrane fouling was well controlled via in-situ H2O2 cleaning confined in the Mn-doped membrane pores.
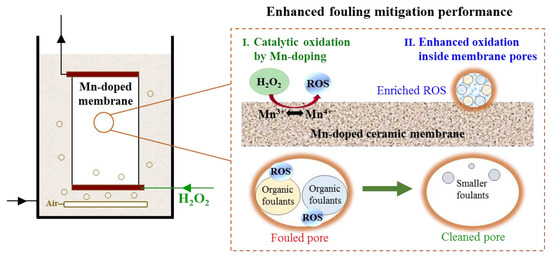
Figure 8.
Schematic diagram of the enhanced catalytic removal of organic foulants within Mn-doped membrane via in-situ H2O2 cleaning.
Overall, coupling the Mn-doped membrane with in-situ H2O2 cleaning achieved the best fouling mitigation performance with the lowest TMP increase, the highest TMP recovery efficiency and the fewest DOC in fouling layers, as a result of improved surface properties and confined catalytic oxidation of organic foulants by H2O2 inside membrane pores.
4. Conclusions
In-situ H2O2 cleaning confined to the inside of the membrane was applied to mitigate membrane fouling in real municipal wastewater treatment. An Mn-doped membrane was used in a membrane bioreactor for wastewater treatment with the aim to improve the membrane catalytic activity toward oxidation of foulants by H2O2. The Mn-doped membrane with in-situ H2O2 cleaning demonstrated a superior fouling mitigation performance to that of a pristine ceramic membrane with no harm to the activated sludge. After 24-h filtration, the ΔTMP of the Mn-doped membrane with H2O2 cleaning was 22.2 kPa, which was 40.5% lower than that of the pristine membrane with H2O2 cleaning (37.3 kPa). The enhanced fouling mitigation was attributed to Mn doping that ameliorated membrane surface properties and catalyzed oxidation of foulants by H2O2 confined in the membrane pores. Mn3+/Mn4+ redox couples in the active layer of the Mn-doped membrane catalyzed H2O2 decomposition continuously to generate ROS (HO• and 1O2), which were confined within the membrane pores and efficiently degraded foulants. Overall, coupling the Mn-doped membrane with confined H2O2 cleaning achieved satisfactory fouling mitigation with a low TMP increase and high TMP recovery efficiency. The findings provide a new strategy for the rational design of antifouling membranes and fouling mitigation processes.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/membranes12010021/s1, Figure S1. The pore size distribution of the (a) pristine and (b) Mn-doped membrane layers; Figure S2. H2O2 decomposition catalyzed by commercial metal oxide particles at a neutral pH. (a) Al2O3 or SiO2; (b) Mn oxides. (Operating conditions: C0 = [H2O2]0 = 1 mM; C is the concentration of H2O2; Mmetal oxides = 1 g/L except MMn oxides = 0.05 g/L; initial pH = 6.8–7.0.); Figure S3. Water quality of the permeation when membranes were in-situ cleaned with H2O or H2O2. (a) Turbidity, (b) DOC, (c) total nitrogen, (d) total phosphorus; Figure S4. MW distribution of DOC in the feed and permeation during membrane filtration at a flux of 60 LMH without backwash. (a) MW distribution of DOC; (b) integral areas of different MW components; (c) rejection rates of different MW components by membranes; Figure S5. MW distribution of DOC in the physically removable fouling layer after 24 h of filtration; Figure S6. Pure water contact angles of the pristine (a) and Mn-doped membranes (b); Figure S7. XPS spectra of O 1s of the pristine and Mn-doped ceramic membranes; Figure S8. Pure water permeabilities of the pristine membrane and Mn-doped membrane; Table S1. Mixed liquor properties after membrane filtration.
Author Contributions
Conceptualization, S.T. and X.Z.; supervision, S.W.H. and X.Z.; experiment operation, data analysis and writing—original draft preparation, S.T.; writing—review and editing, S.T., W.F., S.W.H. and X.Z.; resources and project administration, X.Z.; funding acquisition, X.Z., W.F., S.W.H., J.G., T.S. and T.T. assisted in the operation of membrane bioreactors and the detection of H2O2; L.C. assisted in the detection of free radicals. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Committee of Science and Technology Innovation of Shenzhen (20170935), the China Postdoctoral Science Foundation (2020M680590) and the Committee of Science and Technology Innovation of Shenzhen (JCYJ20180307163205964).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are particularly thankful for the Tsinghua-Berkeley Shenzhen Institute’s support for this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Meng, F.; Zhang, S.; Oh, Y.; Zhou, Z.; Shin, H.S.; Chae, S.R. Fouling in membrane bioreactors: An updated review. Water Res. 2017, 114, 151–180. [Google Scholar] [CrossRef] [PubMed]
- Le-Clech, P.; Chen, V.; Fane, T.A.G. Fouling in membrane bioreactors used in wastewater treatment. J. Membr. Sci. 2006, 284, 17–53. [Google Scholar] [CrossRef]
- Metzger, U.; Le-Clech, P.; Stuetz, R.M.; Frimmel, F.H.; Chen, V. Characterisation of polymeric fouling in membrane bioreactors and the effect of different filtration modes. J. Membr. Sci. 2007, 301, 180–189. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, J.; Tang, C.Y.; Kimura, K.; Wang, Q.; Han, X. Membrane cleaning in membrane bioreactors: A review. J. Membr. Sci. 2014, 468, 276–307. [Google Scholar] [CrossRef]
- Yue, X.; Yoong, K.K.K.; How, Y.N. Membrane fouling mitigation by NaClO-assisted backwash in anaerobic ceramic membrane bioreactors for the treatment of domestic wastewater. Bioresour. Technol. 2018, 268, 622–632. [Google Scholar] [CrossRef]
- Sun, M.; Hou, B.; Wang, S.; Zhao, Q.; Zhang, H. Effects of a NaClO shock on MBR performance under continuous operating conditions. Environ. Sci. Water Res. Technol. 2021, 7, 396–404. [Google Scholar] [CrossRef]
- Lee, J.; Ha, J.-H.; Song, I.-H.; Park, J.-W. Effect of SiO2 coating on alumina microfiltration membranes on flux performance in membrane fouling process. J. Ceram. Soc. Jpn. 2019, 127, 35–43. [Google Scholar] [CrossRef]
- Wang, S.; Tian, J.; Wang, Q.; Xiao, F.; Gao, S.; Shi, W.; Cui, F. Development of CuO coated ceramic hollow fiber membrane for peroxymonosulfate activation: A highly efficient singlet oxygen-dominated oxidation process for bisphenol a degradation. Appl. Catal. B Environ. 2019, 256, 117783. [Google Scholar] [CrossRef]
- Zhu, Y.; Quan, X.; Chen, F.; Fan, X.; Feng, Y. CeO2-TiO2 coated ceramic membrane with catalytic ozonation capability for treatment of tetracycline in drinking water. Sci. Adv. Mater. 2012, 4, 1191–1199. [Google Scholar] [CrossRef]
- Corneal, L.M.; Baumann, M.J.; Masten, S.J.; Davies, S.H.R.; Tarabara, V.V.; Byun, S. Mn oxide coated catalytic membranes for hybrid ozonation-membrane filtration: Membrane microstructural characterization. J. Membr. Sci. 2011, 369, 182–187. [Google Scholar] [CrossRef]
- Corneal, L.M.; Masten, S.J.; Davies, S.H.R.; Tarabara, V.V.; Byun, S.; Baumann, M.J. AFM, SEM and EDS characterization of manganese oxide coated ceramic water filtration membranes. J. Membr. Sci. 2010, 360, 292–302. [Google Scholar] [CrossRef]
- Davies, S.H.; Baumann, M.J.; Byun, S.; Corneal, L.M.; Tarabara, V.V.; Masten, S.J. Fabrication of catalytic ceramic membranes for water filtration. Water Sci. Technol. Water Supply 2010, 10, 81–86. [Google Scholar] [CrossRef]
- Byun, S.; Davies, S.H.; Alpatova, A.L.; Corneal, L.M.; Baumann, M.J.; Tarabara, V.V.; Masten, S.J. Mn oxide coated catalytic membranes for a hybrid ozonation-membrane filtration: Comparison of Ti, Fe and Mn oxide coated membranes for water quality. Water Res. 2011, 45, 163–170. [Google Scholar] [CrossRef]
- Han, Y.F.; Chen, F.; Zhong, Z.; Ramesh, K.; Chen, L.; Jian, D.; Ling, W.W. Complete oxidation of low concentration ethanol in aqueous solution with H2O2 on nanosized Mn3O4/SBA-15 catalyst. Chem. Eng. J. 2007, 134, 276–281. [Google Scholar] [CrossRef]
- Hasan, M.A.; Zaki, M.I.; Pasupulety, L.; Kumari, K. Promotion of the hydrogen peroxide decomposition activity of manganese oxide catalysts. Appl. Catal. A Gen. 1999, 181, 171–179. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, H. Mn-based catalysts for sulfate radical-based advanced oxidation processes: A review. Environ. Int. 2019, 133, 105141. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, Z.; Zhang, X. Coupling in-situ ozonation with ferric chloride addition for ceramic ultrafiltration membrane fouling mitigation in wastewater treatment: Quantitative fouling analysis. J. Membr. Sci. 2018, 555, 307–317. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, M.; Hedtke, T.; Deshmukh, A.; Kim, J.H. Mechanism of heterogeneous Fenton reaction kinetics enhancement under nanoscale spatial confinement. Environ. Sci. Technol. 2020, 54, 10868–10875. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, X. Characteristics of ozone decomposition inside ceramic membrane pores as nano-reactors. Water Sci. Technol. 2014, 3, 421–428. [Google Scholar] [CrossRef]
- Wang, A.; Qu, J.; Ru, J.; Liu, H.; Ge, J. Mineralization of an azo dye Acid Red 14 by electro-Fenton’s reagent using an activated carbon fiber cathode. Dye. Pigment 2005, 65, 227–233. [Google Scholar] [CrossRef]
- Li, L.; Abe, Y.; Kanagawa, K.; Usui, N.; Imai, K.; Mashino, T.; Mochizuki, M.; Miyata, N. Distinguishing the 5,5-dimethyl-1-pyrroline N-oxide (DMPO)-OH radical quenching effect from the hydroxyl radical scavenging effect in the ESR spin-trapping method. Anal. Chim. Acta 2004, 512, 121–124. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, Z.; Liu, J.; Zhang, X. Double-win effects of in-situ ozonation on improved filterability of mixed liquor and ceramic UF membrane fouling mitigation in wastewater treatment. J. Membr. Sci. 2017, 533, 112–120. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, Z.; Zhang, X. New insight into the effect of mixed liquor properties changed by pre-ozonation on ceramic UF membrane fouling in wastewater treatment. Chem. Eng. J. 2016, 314, 670–680. [Google Scholar] [CrossRef]
- Kwon, B.; Park, J.H.; Jang, S.C.; Oh, S.G. Synthesis of alumina-grafted manganese oxide particles using surfactants through coprecipitation method and their thermal properties. Bull. Korean Chem. Soc. 2013, 34, 3559–3564. [Google Scholar] [CrossRef][Green Version]
- Mitran, G.; Chen, S.; Seo, D.K. Role of oxygen vacancies and Mn4+/Mn3+ ratio in oxidation and dry reforming over cobalt-manganese spinel oxides. Mol. Catal. 2019, 483, 110704. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Banerjee, D. Interpretation of XPS Mn(2p) spectra of Mn oxyhydroxides and constraints on the mechanism of MnO2 precipitation. Am. Mineral. 1998, 83, 305–315. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, Y.; Song, C.; Ma, Z.; Gao, Y. A novel redox-precipitation method for the preparation of α-MnO2 with a high surface Mn4+ concentration and its activity toward complete catalytic oxidation of o-xylene. Catal. Today 2013, 201, 32–39. [Google Scholar] [CrossRef]
- Nie, Y.; Zhang, L.; Li, Y.Y.; Hu, C. Enhanced Fenton-like degradation of refractory organic compounds by surface complex formation of LaFeO3 and H2O2. J. Hazard. Mater. 2015, 294, 195–200. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, L.; Sun, L.; Zhu, S.; Chen, W. Efficient removal of dyes using heterogeneous Fenton catalysts based on activated carbon fibers with enhanced activity. Chem. Eng. Sci. 2013, 101, 424–431. [Google Scholar] [CrossRef]
- Wang, Z.; Deb, A.; Srivastava, V.; Iftekhar, S.; Sillanp, M. Investigation of textural properties and photocatalytic activity of PbO/TiO2 and Sb2O3/TiO2 towards the photocatalytic degradation Benzophenone-3 UV filter. Sep. Purif. Technol. 2019, 228, 115763. [Google Scholar] [CrossRef]
- Debnath, B.; Roy, A.S.; Kapri, S.; Bhattacharyya, S. Efficient dye degradation catalyzed by manganese oxide nanoparticles and the role of cation valence. ChemistrySelect 2016, 1, 4265–4273. [Google Scholar] [CrossRef]
- Zhang, L.; Nie, Y.; Hu, C.; Hu, X. Decolorization of methylene blue in layered manganese oxide suspension with H2O2. J. Hazard. Mater. 2011, 190, 780–785. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Sun, B.; He, M.; Chi, H.; Ma, J. Highly efficient simultaneous catalytic degradation and defluorination of perfluorooctanoic acid by the H2O2-carbon/MnO2 system generating O2 and OH synchronously. Appl. Catal. B Environ. 2020, 277, 119219. [Google Scholar] [CrossRef]
- Xing, W.; Zhong, Z.; Jing, W.; Fan, Y. Controlling of membrane fouling based on membrane interface interactions. CIESC J. 2013, 64, 173–181. [Google Scholar]
- Breite, D.; Went, M.; Prager, A.; Schulze, A. The critical zeta potential of polymer membranes: How electrolytes impact membrane fouling. Rsc Adv. 2016, 6, 180–189. [Google Scholar] [CrossRef]
- Breite, D.; Went, M.; Thomas, I.; Prager, A.; Schulze, A. Particle adsorption on a polyether sulfone membrane: How electrostatic interactions dominate membrane fouling. RSC Adv. 2016, 6, 65383–65391. [Google Scholar] [CrossRef]
- Moritz, T.; Benfer, S.; Arki, P.; Tomandl, G. Influence of the surface charge on the permeate flux in the dead-end filtration with ceramic membranes. Sep. Purif. Technol. 2001, 25, 501–508. [Google Scholar] [CrossRef]
- Jun, D.U.; Qi, W.U.; Zhong, S.; Xin, G.U.; Liu, J.; Guo, H.; Zhang, W.; Peng, H.; Zou, J. Effect of hydroxyl groups on hydrophilic and photocatalytic activities of rare earth doped titanium dioxide thin films. J. Rare Earth. 2015, 33, 148–153. [Google Scholar]
- Li, N.; Zhang, J.; Tian, Y.; Zhang, J.; Zhan, W.; Zhao, J.; Ding, Y.; Zuo, W. Hydrophilic modification of polyvinylidene fluoride membranes by ZnO atomic layer deposition using nitrogen dioxide/diethylzinc functionalization. J. Membr. Sci. 2016, 514, 241–249. [Google Scholar] [CrossRef]
- Chen, X.; Luo, J.; Qi, B.; Cao, W.; Wan, Y. NOM fouling behavior during ultrafiltration: Effect of membrane hydrophilicity. J. Water Process Eng. 2015, 7, 1–10. [Google Scholar] [CrossRef]
- Wei, D.; Tao, Y.; Zhang, Z.; Liu, L.; Zhang, X. Effect of in-situ ozonation on ceramic UF membrane fouling mitigation in algal-rich water treatment. J. Membr. Sci. 2016, 498, 116–124. [Google Scholar] [CrossRef]
- Seidel, A.; Elimelech, M. Coupling between chemical and physical interactions in natural organic matter (NOM) fouling of nanofiltration membranes: Implications for fouling control. J. Membr. Sci. 2002, 203, 245–255. [Google Scholar] [CrossRef]
- Gu, Q.; Ng, T.C.A.; Zain, I.; Liu, X.; Zhang, L.; Zhang, Z.; Lyu, Z.; He, Z.; Ng, H.Y.; Wang, J. Chemical-grafting of graphene oxide quantum dots (GOQDs) onto ceramic microfiltration membranes for enhanced water permeability and anti-organic fouling potential. Appl. Surf. Sci. 2020, 502, 128–144. [Google Scholar] [CrossRef]
- Khan, M.I.; Mondal, A.N.; Tong, B.; Jiang, C.; Emmanuel, K.; Yang, Z.; Wu, L.; Xu, T. Development of BPPO-based anion exchange membranes for electrodialysis desalination applications. Desalination 2016, 391, 61–68. [Google Scholar] [CrossRef]
- Khan, M.I.; Zheng, C.; Mondal, A.N.; Hossain, M.M.; Wu, B.; Emmanuel, K.; Wu, L.; Xu, T. Preparation of anion exchange membranes from BPPO and dimethylethanolamine for electrodialysis. Desalination 2017, 402, 10–18. [Google Scholar] [CrossRef]
- Li, D.; Wu, J.; Yang, S.; Zhang, W.; Niu, X.; Chen, Y.H.; Ran, F. Hydrophilicity and anti-fouling performance of polyethersulfone membrane modified by grafting block glycosyl copolymers via surface initiated electrochemically mediated atom transfer radical polymerization. New J. Chem. 2018, 42, 2692–2701. [Google Scholar] [CrossRef]
- Lee, Y.N.; Lago, R.M.; Fierro, J.L.G.; González, J. Hydrogen peroxide decomposition over Ln1-xAxMnO3 (Ln=La or Nd and A=K or Sr) perovskites. Appl. Catal. A Gen. 2001, 215, 245–256. [Google Scholar] [CrossRef]
- He, X.; Li, B.; Wang, P.; Ma, J. Novel H2O2-MnO2 system for efficient physico-chemical cleaning of fouled ultrafiltration membranes by simultaneous generation of reactive free radicals and oxygen. Water Res. 2019, 167, 115111. [Google Scholar] [CrossRef]
- Nagy, I.Z.; Floyd, R.A. Hydroxyl free radical reactions with amino acids and proteins studied by electron spin resonance spectroscopy and spin-trapping. Biochim. Biophys. Acta. 1984, 790, 238–250. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, G.; Liu, H.; Qu, J. Confining free radicals in close vicinity to contaminants enables ultrafast Fenton-like processes in the interspacing of MoS2 membranes. Angew. Chem. Int. Edit. 2019, 58, 8134–8138. [Google Scholar] [CrossRef]
- Pryor, W.A. Oxy-radicals and related species: Their formation, lifetimes, and reactions. Annu. Rev. Physiol. 1986, 48, 657–667. [Google Scholar] [CrossRef]
- Müller, P.; Ahmad, M. Light-activated cryptochrome reacts with molecular oxygen to form a flavin–superoxide radical pair consistent with magnetoreception. J. Biol. Chem. 2011, 286, 21033–21040. [Google Scholar] [CrossRef]
- Bielski, B.H.J.; Cabelli, D.E.; Arudi, R.L. Reactivity of HO2/O2− radicals in aqueous solution. J. Phys. Chem. Ref. Data 1985, 14, 1041–1100. [Google Scholar] [CrossRef]
- Sui, M.; She, L.; Sheng, L.; Wei, J.; Zhang, L.; Huang, S. Ordered mesoporous manganese oxide as catalyst for hydrogen peroxide oxidation of norfloxacin in water. Chin. J. Catal. 2013, 34, 536–541. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).