Chia Seed Mucilage Edible Films with Origanum vulgare and Satureja montana Essential Oils: Characterization and Antifungal Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
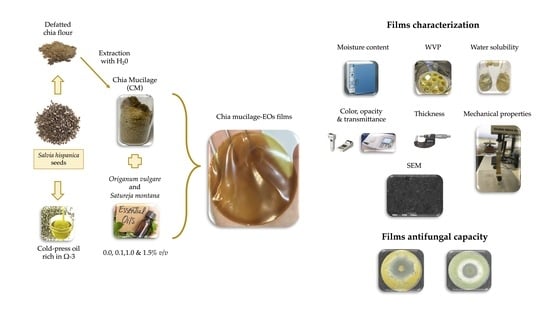
2.2. Defatting of Chia Seeds and Mucilage Extraction
2.3. Production of Chia Mucilage Films Incorporated with Essential Oils
2.4. Characterization of Chia Mucilage EO Films
2.4.1. Moisture Content and Water Solubility (WS)
2.4.2. Water Vapor Permeability (WVP)
2.4.3. Color and Opacity
2.4.4. Light Transmittance
2.4.5. Thickness and Mechanical Properties
2.5. Antifungal Capacity
2.6. Scanning Electron Microscopy (SEM)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Moisture Content, Water Solubility, Thickness and Water Vapor Permeability
3.2. Color and Opacity
3.3. Transmittance
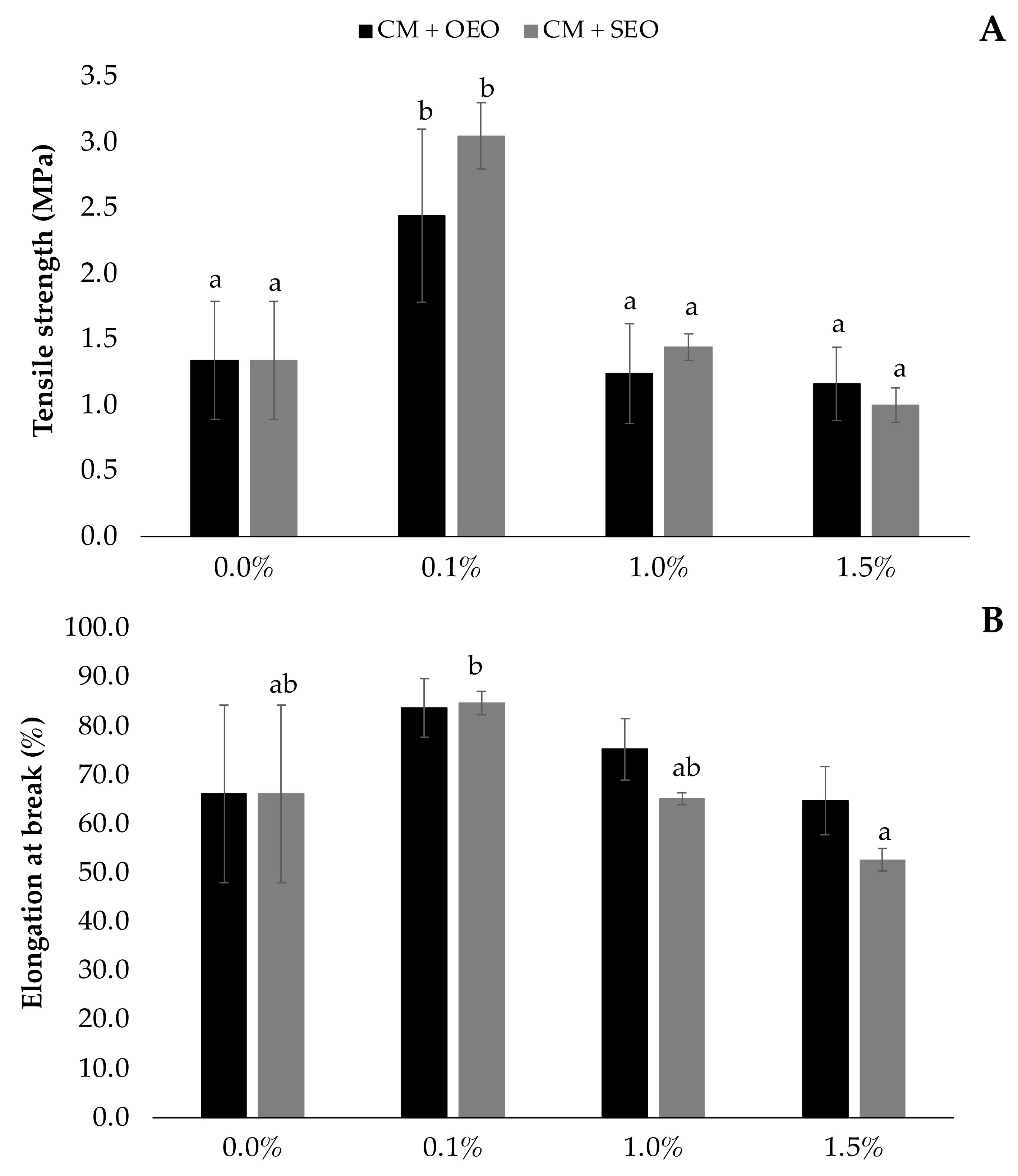
3.4. Mechanical Properties
3.5. Antifungal Capacity
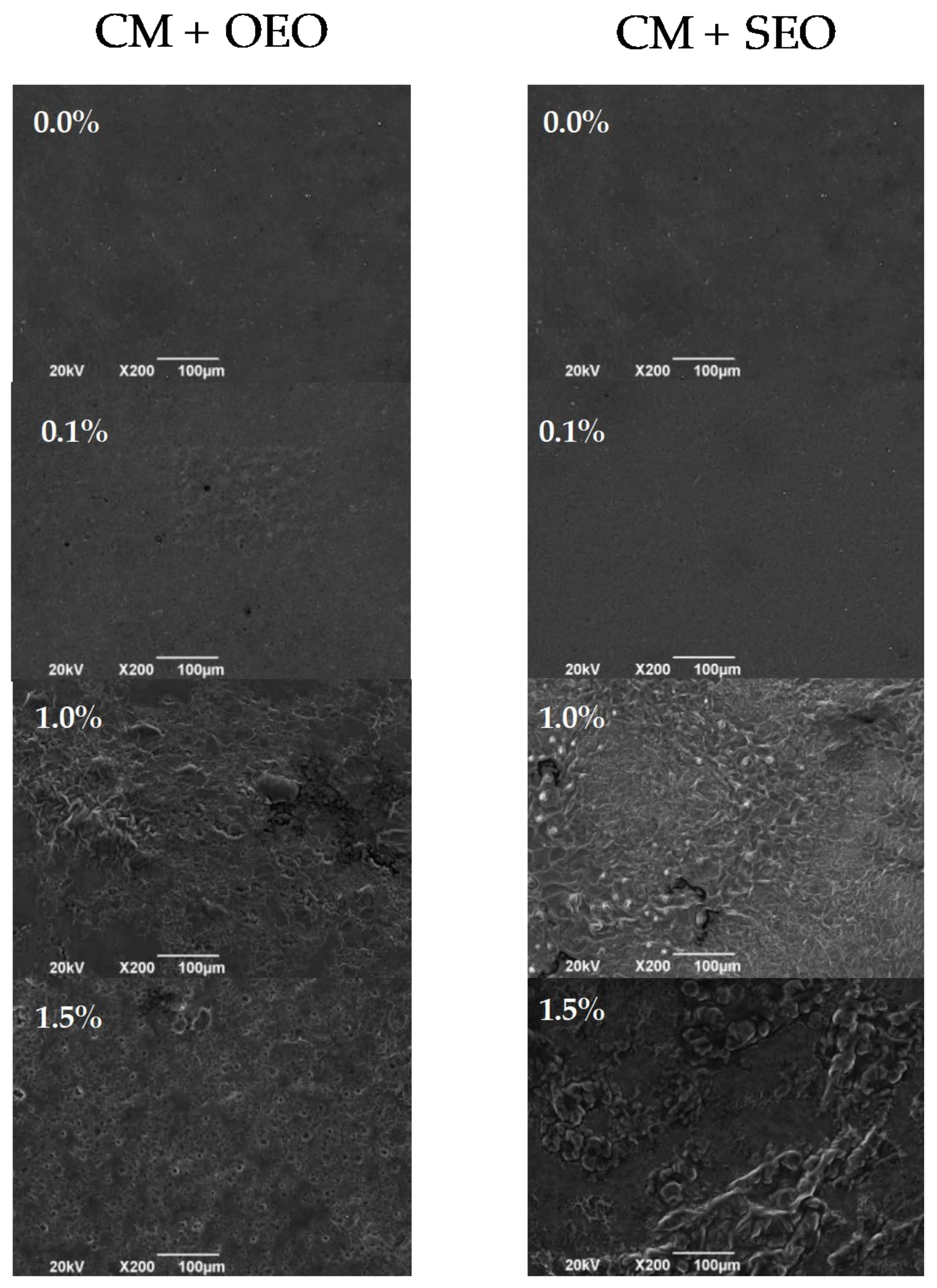
3.6. Scanning Electron Microscopy (SEM)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López De Dicastillo, C.; Rodríguez, F.; Guarda, A.; Galotto, M.J. Antioxidant films based on cross-linked methyl cellulose and native Chilean berry for food packaging applications. Carbohydr. Polym. 2016, 136, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yu, M.; Wang, L. Physical and antimicrobial properties of sodium alginate/carboxymethyl cellulose films incorporated with cinnamon essential oil. Food Packag. Shelf Life 2017, 15, 35–42. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Bio-based composite edible films containing Origanum vulgare L. essential oil. Ind. Crops Prod. 2015, 67, 403–413. [Google Scholar] [CrossRef]
- Urbizo-Reyes, U.; San Martin-gonzález, M.F.; Garcia-Bravo, J.; Liceaga, A.M. Development of chia seed (Salvia hispanica) mucilage films plasticized with polyol mixtures: Mechanical and barrier properties. Int. J. Biol. Macromol. 2020, 163, 854–864. [Google Scholar] [CrossRef] [PubMed]
- Soukoulis, C.; Gaiani, C.; Hoffmann, L. Plant seed mucilage as emerging biopolymer in food industry applications. Curr. Opin. Food Sci. 2018, 22, 28–42. [Google Scholar] [CrossRef]
- Alizadeh Behbahani, B.; Noshad, M.; Jooyandeh, H. Improving oxidative and microbial stability of beef using Shahri Balangu seed mucilage loaded with Cumin essential oil as a bioactive edible coating. Biocatal. Agric. Biotechnol. 2020, 24, 101563. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Mousavi Khaneghah, A. Characterization of novel basil-seed gum active edible films and coatings containing oregano essential oil. Prog. Org. Coat. 2017, 110, 35–41. [Google Scholar] [CrossRef]
- Cuomo, F.; Iacovino, S.; Messia, M.C.; Sacco, P.; Lopez, F. Protective action of lemongrass essential oil on mucilage from chia (Salvia hispanica) seeds. Food Hydrocoll. 2020, 105, 105860. [Google Scholar] [CrossRef]
- Barzegar, H.; Alizadeh Behbahani, B.; Mehrnia, M.A. Quality retention and shelf life extension of fresh beef using Lepidium sativum seed mucilage-based edible coating containing Heracleum lasiopetalum essential oil: An experimental and modeling study. Food Sci. Biotechnol. 2020, 29, 717–728. [Google Scholar] [CrossRef]
- Jouki, M.; Mortazavi, S.A.; Yazdi, F.T.; Koocheki, A. Characterization of antioxidant-antibacterial quince seed mucilage films containing thyme essential oil. Carbohydr. Polym. 2014, 99, 537–546. [Google Scholar] [CrossRef]
- Jouki, M.; Yazdi, F.T.; Mortazavi, S.A.; Koocheki, A. Quince seed mucilage films incorporated with oregano essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Food Hydrocoll. 2014, 36, 9–19. [Google Scholar] [CrossRef]
- Muñoz-Tébar, N.; Molina, A.; Carmona, M.; Berruga, M.I. Use of Chia by-Products Obtained from the Extraction of Seeds Oil for the Development of New Biodegradable Films for the Agri-Food Industry. Foods 2021, 3, 620. [Google Scholar] [CrossRef] [PubMed]
- Valdés, A.; Burgos, N.; Jiménez, A.; Garrigós, M.C. Natural pectin polysaccharides as edible coatings. Coatings 2015, 5, 865–886. [Google Scholar] [CrossRef] [Green Version]
- Prasad Timilsena, Y.; Adhikari, R.; Kasapis, S.; Adhikari, B. Rheological and microstructural properties of the chia seed polysaccharide. Int. J. Biol. Macromol. 2015, 81, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Dick, M.; Costa, T.M.H.; Gomaa, A.; Subirade, M.; Rios, A.D.O.; Flôres, S.H. Edible film production from chia seed mucilage: Effect of glycerol concentration on its physicochemical and mechanical properties. Carbohydr. Polym. 2015, 130, 198–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caetano, S.; Almeida, N.; Maria, T.; Costa, H.; Brandelli, A.; Rodrigues, E.; Hickmann, S.; Cladera-olivera, F. Characterization of active biodegradable films based on cassava starch and natural compounds. Food Packag. Shelf Life 2018, 16, 138–147. [Google Scholar] [CrossRef]
- Gahruie, H.H.; Ziaee, E.; Eskandari, M.H.; Hosseini, S.M.H. Characterization of basil seed gum-based edible films incorporated with Zataria multiflora essential oil nanoemulsion. Carbohydr. Polym. 2017, 166, 93–103. [Google Scholar] [CrossRef]
- Jouki, M.; Tabatabaei, F.; Ali, S.; Koocheki, A.; Khazaei, N. Effect of quince seed mucilage edible films incorporated with oregano or thyme essential oil on shelf life extension of refrigerated rainbow trout fillets. Int. J. Food Microbiol. 2014, 174, 88–97. [Google Scholar] [CrossRef]
- Sapper, M.; Wilcaso, P.; Santamarina, M.P.; Chiralt, A. Antifungal and functional properties of starch-gellan films containing thyme (Thymus zygis) essential oil. Food Control 2018, 92, 505–515. [Google Scholar] [CrossRef]
- Beikzadeh, S.; Khezerlou, A.; Jafari, S.M.; Pilevar, Z.; Mortazavian, A.M. Seed mucilages as the functional ingredients for biodegradable films and edible coatings in the food industry. Adv. Colloid Interface Sci. 2020, 280, 102164. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Ramos, C.; Serrano, C.; Matos, O.; Neng, N.R.; Nogueira, M.F.; Saraiva, J.A.; Nunes, M.L. Chemical composition and bioactivity of different oregano (Origanum vulgare) extracts and essential oil. J. Sci. Food Agric. 2013, 93, 2707–2714. [Google Scholar] [CrossRef] [PubMed]
- Alves-Silva, J.; Dias, S.M.; Pintado, M.E.; Pérez-álvarez, J.A.; Fernández-López, J.; Viuda-Martos, M. Chemical composition and in vitro antimicrobial, antifungal and antioxidant properties of essential oils obtained from some herbs widely used in Portugal. Food Control 2013, 32, 371–378. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Barros-Velázquez, J. (Ed.) Antimicrobial Food Packaging; Academic Press: Cambridge, MA, USA, 2016; Volume 2001, ISBN 9780128007235. [Google Scholar]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Stević, T.; Berić, T.; Šavikin, K.; Soković, M.; Godevac, D.; Dimkić, I.; Stanković, S. Antifungal activity of selected essential oils against fungi isolated from medicinal plant. Ind. Crops Prod. 2014, 55, 116–122. [Google Scholar] [CrossRef]
- Lukas, B.; Schmiderer, C.; Novak, J. Phytochemistry Essential oil diversity of European Origanum vulgare L. (Lamiaceae). Phytochemistry 2015, 119, 32–40. [Google Scholar] [CrossRef]
- Camiletti, B.X.; Asensio, C.M.; Giménez de la Pecci, M.D.; Lucini, E.I. Natural Control of Corn Postharvest Fungi Aspergillus flavus and Penicillium sp. Using Essential Oils from Plants Grown in Argentina. J. Food Sci. 2014, 79, M2499–M2506. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Grossmann, M.V.E.; Yamashita, F.; Pined, E.A.G. Antimicrobial, mechanical, and barrier properties of cassava starch-chitosan films incorporated with oregano essential oil. J. Agric. Food Chem. 2009, 57, 7499–7504. [Google Scholar] [CrossRef]
- Muñoz-Tebar, N.; González-Navarro, E.J.; López-Díaz, T.M.; Santos, J.; Ortiz de Elguea-Culebras, G.; García-Martínez, M.M.; Molina, A.; Carmona, M.; Berruga, M.I. Biological activity of extracts from aromatic plants as control agents against spoilage molds isolated from sheep cheese. Foods 2021, 7, 1576. [Google Scholar] [CrossRef]
- Muñoz-Tébar, N.; De la Vara, J.A.; Ortiz de Elguea-Culebras, G.; Cano, E.L.; Molina, A.; Carmona, M.; Berruga, M.I. Enrichment of sheep cheese with chia (Salvia hispanica L.) oil as a source of omega-3. LWT 2019, 108, 407–415. [Google Scholar] [CrossRef]
- ASTM E96/E96M-16; Standard Test Methods for Water Vapor Transmission of Materials. ASTM International: West Conshohocken, PA, USA, 2016. Available online: www.astm.org (accessed on 23 November 2021).
- Costa, M.J.; Cerqueira, M.A.; Ruiz, H.A.; Fougnies, C.; Richel, A.; Vicente, A.A.; Teixeira, J.A.; Aguedo, M. Use of wheat bran arabinoxylans in chitosan-based films: Effect on physicochemical properties. Ind. Crops Prod. 2015, 66, 3050311. [Google Scholar] [CrossRef] [Green Version]
- Casariego, A.; Souza, B.W.S.; Cerqueira, M.A.; Teixeira, J.A.; Cruz, L.; Díaz, R.; Vicente, A.A. Chitosan/clay films’ properties as affected by biopolymer and clay micro/nanoparticles’ concentrations. Food Hydrocoll. 2009, 23, 1895–1902. [Google Scholar] [CrossRef] [Green Version]
- Ortiz de Elguea-Culebras, G.; Bourbon, A.I.; Costa, M.J.; Muñoz-Tebar, N.; Carmona, M.; Molina, A.; Sánchez-Vioque, R.; Berruga, M.I.; Vicente, A.A. Optimization of a chitosan solution as potential carrier for the incorporation of Santolina chamaecyparissus L. solid by-product in an edible vegetal coating on ‘Manchego’ cheese. Food Hydrocoll. 2019, 89, 272–282. [Google Scholar] [CrossRef] [Green Version]
- ASTM D882-10; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2010. Available online: www.astm.org (accessed on 2 November 2020).
- Hasheminya, S.M.; Mokarram, R.R.; Ghanbarzadeh, B.; Hamishekar, H.; Kafil, H.S.; Dehghannya, J. Development and characterization of biocomposite films made from kefiran, carboxymethyl cellulose and Satureja Khuzestanica essential oil. Food Chem. 2019, 289, 443–452. [Google Scholar] [CrossRef]
- Go, E.J.; Song, K.B. Effect of java citronella essential oil addition on the physicochemical properties of Gelidium corneum-chitosan composite films. Food Sci. Biotechnol. 2020, 29, 909–915. [Google Scholar] [CrossRef]
- Hosseini, M.H.; Razavi, S.H.; Mousavi, M.A. Antimicrobial, physical and mechanical properties of chitosan-based films incorporated with thyme, clove and cinnamon essential oils. J. Food Process. Preserv. 2009, 33, 727–743. [Google Scholar] [CrossRef]
- Aguirre, A.; Borneo, R.; Leon, A.E. Antimicrobial, mechanical and barrier properties of triticale protein films incorporated with oregano essential oil. Food Biosci. 2013, 1, 2–9. [Google Scholar] [CrossRef]
- Nisar, T.; Wang, Z.C.; Yang, X.; Tian, Y.; Iqbal, M.; Guo, Y. Characterization of citrus pectin films integrated with clove bud essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Int. J. Biol. Macromol. 2018, 106, 670–680. [Google Scholar] [CrossRef]
- Shojaee-Aliabadi, S.; Hosseini, H.; Mohammadifar, M.A.; Mohammadi, A.; Ghasemlou, M.; Ojagh, S.M.; Hosseini, S.M.; Khaksar, R. Characterization of antioxidant-antimicrobial κ-carrageenan films containing Satureja hortensis essential oil. Int. J. Biol. Macromol. 2013, 52, 116–124. [Google Scholar] [CrossRef]
- González Sandoval, D.; Luna Sosa, B.; Martínez-Ávila, G.C.G.; Rodríguez Fuentes, H.; Avendaño Abarca, V.H.; Rojas, R. Formulation and characterization of edible films based on organic mucilage from Mexican Opuntia ficus-indica. Coatings 2019, 9, 506. [Google Scholar] [CrossRef] [Green Version]
- Ares, G.; Deliza, R. Studying the influence of package shape and colour on consumer expectations of milk desserts using word association and conjoint analysis. Food Qual. Prefer. 2010, 21, 930–937. [Google Scholar] [CrossRef]
- Ahmad, M.; Benjakul, S.; Prodpran, T.; Agustini, T.W. Physico-mechanical and antimicrobial properties of gelatin film from the skin of unicorn leatherjacket incorporated with essential oils. Food Hydrocoll. 2012, 28, 189–199. [Google Scholar] [CrossRef]
- Noshirvani, N.; Ghanbarzadeh, B.; Gardrat, C.; Rezaei, M.R.; Hashemi, M.; Le Coz, C.; Coma, V. Cinnamon and ginger essential oils to improve antifungal, physical and mechanical properties of chitosan-carboxymethyl cellulose films. Food Hydrocoll. 2017, 70, 36–45. [Google Scholar] [CrossRef]
- Martucci, J.F.; Gende, L.B.; Neira, L.M.; Ruseckaite, R.A. Oregano and lavender essential oils as antioxidant and antimicrobial additives of biogenic gelatin films. Ind. Crops Prod. 2015, 71, 205–213. [Google Scholar] [CrossRef]
- Pagno, C.H.; Klug, T.V.; Costa, T.M.H.; De Oliveira Rios, A.; Flôres, S.H. Physical and antimicrobial properties of quinoa flour-based films incorporated with essential oil. J. Appl. Polym. Sci. 2016, 133, 1–9. [Google Scholar] [CrossRef]
- Nordin, N.; Othman, S.H.; Rashid, S.A.; Basha, R.K. Effects of glycerol and thymol on physical, mechanical, and thermal properties of corn starch films. Food Hydrocoll. 2020, 106, 105884. [Google Scholar] [CrossRef]
- Ocak, B. Properties and characterization of thyme essential oil incorporated collagen hydrolysate films extracted from hide fleshing wastes for active packaging. Environ. Sci. Pollut. Res. 2020, 27, 29019–29030. [Google Scholar] [CrossRef]
- Atarés, L.; Bonilla, J.; Chiralt, A. Characterization of sodium caseinate-based edible films incorporated with cinnamon or ginger essential oils. J. Food Eng. 2010, 100, 678–687. [Google Scholar] [CrossRef]
- Souza, A.C.; Goto, G.E.O.; Mainardi, J.A.; Coelho, A.C.V.; Tadini, C.C. Cassava starch composite films incorporated with cinnamon essential oil: Antimicrobial activity, microstructure, mechanical and barrier properties. LWT Food Sci. Technol. 2013, 54, 346–352. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Mousavi Khaneghah, A.; Ghaderi Ghahfarrokhi, M.; Eş, I. Basil-seed gum containing Origanum vulgare subsp. viride essential oil as edible coating for fresh cut apricots. Postharvest Biol. Technol. 2017, 125, 26–34. [Google Scholar] [CrossRef]
- Stark, J.; Tan, H.S. Natamycin. In Food Preservatives; Russell, N.J., Gould, G.W., Eds.; Springer: Boston, MA, USA, 2003; pp. 179–195. [Google Scholar]

| Parameter | Concentration (%) | Films | |
|---|---|---|---|
| CM + OEO | CM + SEO | ||
| Moisture (%) | 0.0 1 | 22.44 ± 1.19 a | 22.44 ± 1.19 a |
| 0.1 | 24.80 ± 0.95 ab | 25.73 ± 0.52 ab | |
| 1.0 | 25.31 ± 0.21 ab | 27.60 ± 1.72 bc | |
| 1.5 | 27.69 ± 2.05 b | 30.83 ± 2.76 c | |
| Water solubility (%) | 0.0 1 | 64.45 ± 3.90 a | 64.45 ± 3.90 a |
| 0.1 | 64.98 ± 0.57 a | 65.39 ± 1.00 a | |
| 1.0 | 67.53 ± 0.30 a | 68.64 ± 0.21 a | |
| 1.5 | 75.11 ± 0.66 b | 75.60 ± 0.30 b | |
| Thickness (mm) | 0.0 1 | 0.123 ± 0.010 b | 0.123 ± 0.010 b |
| 0.1 | 0.075 ± 0.007 a | 0.082 ± 0.001 a | |
| 1.0 | 0.085 ± 0.001 a | 0.090 ± 0.004 a | |
| 1.5 | 0.092 ± 0.002 a | 0.096 ± 0.004 a | |
| WVP 2 × 10−10 (g/s m Pa) | 0.0 1 | 0.58 ± 0.03 b | 0.58 ± 0.03 b |
| 0.1 | 0.14 ± 0.03 a | 0.14 ± 0.02 a | |
| 1.0 | 0.16 ± 0.03 a | 0.18 ± 0.03 a | |
| 1.5 | 0.17 ± 0.03 a | 0.20 ± 0.01 a | |
| Parameter | Concentration (%) | Films | |
|---|---|---|---|
| CM + OEO | CM + SEO | ||
| L* | 0.01 | 56.42 ± 4.15 | 56.42 ± 4.15 |
| 0.1 | 53.03 ± 0.96 | 51.27 ± 1.90 | |
| 1.0 | 50.64 ± 2.33 | 53.27 ± 1.28 | |
| 1.5 | 50.18 ± 1.45 | 50.32 ± 1.57 | |
| a* | 0.0 1 | 5.45 ± 0.32 a | 5.45 ± 0.32 a |
| 0.1 | 6.35 ± 0.70 ab | 6.93 ± 1.04 b | |
| 1.0 | 7.90 ± 1.63 bc | 6.55 ± 0.54 b | |
| 1.5 | 8.66 ± 0.74 c | 8.74 ± 0.79 c | |
| b* | 0.0 1 | 37.39 ± 4.16 | 37.39 ± 4.16 |
| 0.1 | 37.98 ± 0.41 | 38.13 ± 0.34 | |
| 1.0 | 36.57 ± 1.58 | 36.38 ± 1.17 | |
| 1.5 | 36.31 ± 1.44 | 36.83 ± 0.61 | |
| ∆E | 0.0 1 | 54.40 ± 5.71 | 54.40 ± 5.71 |
| 0.1 | 57.45 ± 0.92 A | 64.33 ± 1.55 B | |
| 1.0 | 58.68 ± 2.37 A | 65.14 ± 0.84 B | |
| 1.5 | 59.01 ± 1.06 A | 63.00 ± 1.50 B | |
| Opacity (%) | 0.0 1 | 26.87 ± 0.75 a | 26.87 ± 0.75 a |
| 0.1 | 28.80 ± 0.11 ab | 28.09 ± 0.50 ab | |
| 1.0 | 29.73 ± 3.18 ab | 29.60 ± 0.75 ab | |
| 1.5 | 31.48 ± 0.32 b | 30.86 ± 2.41 b | |
| Mold | Parameter | Concentration (%) | Films | |
|---|---|---|---|---|
| CM + OEO | CM + SEO | |||
| Aspergillus flavus CECT 2687 | Halo (mm) | 0.0 1 | nd 2 | nd 2 |
| 0.1 | nd 2 | nd 2 | ||
| 1 | 31.35 ± 2.83 a | 28.94 ± 1.19 a | ||
| 1.5 | 47.90 ± 4.49 b | 41.62 ± 1.70 b | ||
| % inhibition | 0.0 1 | nd 2 | nd 2 | |
| 0.1 | nd 2 | nd 2 | ||
| 1 | 34.83 ± 3.15 a | 32.15 ± 1.32 a | ||
| 1.5 | 53.22 ± 4.99 b | 46.25 ± 1.89 b | ||
| Aspergillus puulauensis 1AO5 | Halo (mm) | 0.0 1 | nd 2 | nd 2 |
| 0.1 | nd 2 | nd 2 | ||
| 1 | 51.56 ± 0.95 aA | 43.13 ± 1.41 aA | ||
| 1.5 | 64.86 ± 0.06 bA | 69.89 ± 0.82 bB | ||
| % inhibition | 0.0 1 | nd 2 | nd 2 | |
| 0.1 | nd 2 | nd 2 | ||
| 1 | 57.29 ± 1.06 aB | 47.92 ± 1.57 aA | ||
| 1.5 | 72.07 ± 0.06 bA | 77.66 ± 0.91 bB | ||
| Penicillium commune 301 | Halo (mm) | 0.01 | nd 2 | nd 2 |
| 0.1 | nd 2 | nd 2 | ||
| 1 | 39.28 ± 0.26 aB | 34.47 ± 0.23 aA | ||
| 1.5 | 62.16 ± 2.34 b | 54.56 ± 5.20 b | ||
| % inhibition | 0.0 1 | nd 2 | nd 2 | |
| 0.1 | nd 2 | nd 2 | ||
| 1 | 43.65 ± 0.29 aB | 38.30 ± 0.25 aA | ||
| 1.5 | 69.06 ± 2.60 b | 60.63 ± 5.78 b | ||
| Penicillium crustosum 1AO1 | Halo (mm) | 0.0 1 | nd 2 | nd 2 |
| 0.1 | nd 2 | nd 2 | ||
| 1 | 28.95 ± 0.41 a | 26.44 ± 1.72 | ||
| 1.5 | 39.45 ± 2.40 b | 38.01 ± 3.91 | ||
| % inhibition | 0.0 1 | nd 2 | nd 2 | |
| 0.1 | nd 2 | nd 2 | ||
| 1 | 32.16 ± 0.46 a | 29.38 ± 1.91 | ||
| 1.5 | 43.84 ± 2.67 b | 42.23 ± 4.34 | ||
| Penicillium verrucosum CECT 2906 | Halo (mm) | 0.0 1 | nd 2 | nd 2 |
| 0.1 | nd 2 | nd 2 | ||
| 1 | 43.19 ± 4.08 | 33.53 ± 3.97 a | ||
| 1.5 | 56.43 ± 6.74 | 50.38 ± 1.99 b | ||
| % inhibition | 0.0 1 | nd 2 | nd 2 | |
| 0.1 | nd 2 | nd 2 | ||
| 1 | 47.99 ± 4.53 | 37.25 ± 4.41 a | ||
| 1.5 | 62.70 ± 7.49 | 55.98 ± 2.21 b | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Tébar, N.; Carmona, M.; Ortiz de Elguea-Culebras, G.; Molina, A.; Berruga, M.I. Chia Seed Mucilage Edible Films with Origanum vulgare and Satureja montana Essential Oils: Characterization and Antifungal Properties. Membranes 2022, 12, 213. https://doi.org/10.3390/membranes12020213
Muñoz-Tébar N, Carmona M, Ortiz de Elguea-Culebras G, Molina A, Berruga MI. Chia Seed Mucilage Edible Films with Origanum vulgare and Satureja montana Essential Oils: Characterization and Antifungal Properties. Membranes. 2022; 12(2):213. https://doi.org/10.3390/membranes12020213
Chicago/Turabian StyleMuñoz-Tébar, Nuria, Manuel Carmona, Gonzalo Ortiz de Elguea-Culebras, Ana Molina, and María Isabel Berruga. 2022. "Chia Seed Mucilage Edible Films with Origanum vulgare and Satureja montana Essential Oils: Characterization and Antifungal Properties" Membranes 12, no. 2: 213. https://doi.org/10.3390/membranes12020213
APA StyleMuñoz-Tébar, N., Carmona, M., Ortiz de Elguea-Culebras, G., Molina, A., & Berruga, M. I. (2022). Chia Seed Mucilage Edible Films with Origanum vulgare and Satureja montana Essential Oils: Characterization and Antifungal Properties. Membranes, 12(2), 213. https://doi.org/10.3390/membranes12020213